This birth cohort study examined trends in dementia incidence and concomitant trends in cardiovascular comorbidities among individuals aged 70 years or older who were enrolled in the Einstein Aging Study between 1993 and 2015.
Key Points
Question
Has the incidence of all-cause dementia decreased in successive birth cohorts in a representative sample of community-residing adults in Bronx County, New York?
Findings
Using a birth cohort analysis to disentangle age from cohort effects, this study found that dementia incidence rates decreased significantly among individuals born after 1929. Adjustment for age, sex, educational level, and prevalence of cardiovascular comorbidities did not explain the observed trend.
Meaning
Additional studies are needed to determine whether decreasing dementia incidence will offset the demographic shift to older ages.
Abstract
Importance
Trends in dementia incidence rates have important implications for planning and prevention. To better understand incidence trends over time requires separation of age and cohort effects, and few prior studies have used this approach.
Objectives
To examine trends in dementia incidence and concomitant trends in cardiovascular comorbidities among individuals aged 70 years or older who were enrolled in the Einstein Aging Study between 1993 and 2015.
Design, Setting, and Participants
In this birth cohort analysis of all-cause dementia incidence in persons enrolled in the Einstein Aging Study from October 20, 1993, through November 17, 2015, a systematically recruited, population-based sample of 1348 participants from Bronx County, New York, who were 70 years or older without dementia at enrollment and at least one annual follow-up was studied. Poisson regression was used to model dementia incidence as a function of age, sex, educational level, race, and birth cohort, with profile likelihood used to identify the timing of significant increases or decreases in incidence.
Exposures
Birth year and age.
Main Outcomes and Measures
Incident dementia defined by consensus case conference based on annual, standardized neuropsychological and neurologic examination findings, using criteria from the DSM-IV.
Results
Among 1348 individuals (mean [SD] baseline age, 78.5 [5.4] years; 830 [61.6%] female; 915 [67.9%] non-Hispanic white), 150 incident dementia cases developed during 5932 person-years (mean [SD] follow-up, 4.4 [3.4] years). Dementia incidence decreased in successive birth cohorts. Incidence per 100 person-years was 5.09 in birth cohorts before 1920, 3.11 in the 1920 through 1924 birth cohorts, 1.73 in the 1925 through 1929 birth cohorts, and 0.23 in cohorts born after 1929. Change point analyses identified a significant decrease in dementia incidence among those born after July 1929 (95% CI, June 1929 to January 1930). The relative rate for birth cohorts before July 1929 vs after was 0.13 (95% CI, 0.04-0.41). Prevalence of stroke and myocardial infarction decreased across successive birth cohorts, whereas diabetes prevalence increased. Adjustment for these cardiovascular comorbidities did not explain the decreased dementia incidence rates for more recent birth cohorts.
Conclusions and Relevance
Analyses confirm decreasing dementia incidence in this population-based sample. Whether decreasing incidence will contribute to reduced burden of dementia given the aging of the population is not known.
Introduction
The world population older than 80 years is expected to increase more than 3-fold by 2050. As the population ages, dementia and Alzheimer disease are consuming an increasing share of resources. Worldwide dementia prevalence is more than 47 million, and 7 million new cases develop each year. Dementia prevalence has been projected to double every 20 years and to reach 115 million by the year 2050. Prevalence of Alzheimer disease, the most common cause of dementia, is expected to reach 106 million by 2050.
The projected increase in dementia prevalence is driven by shifting demographics toward an older population. However, evidence suggests that dementia prevalence may have decreased in recent decades. Decreasing incidence rates may explain the observed decreases in prevalence and would suggest a decrease in the projected burden of disease in the coming decades. Earlier studies have found no significant changes in dementia incidence through the 1980s. Studies of more recent periods suggest that dementia incidence may be decreasing in the United States and Western Europe, although the opposite trend has been reported for Wales. Confirming that rates of dementia onset are decreasing has important implications for health care planning and resource allocation. Furthermore, a strong body of evidence supports the role of cardiovascular disease in cognitive decline and dementia, and estimates indicate that more than half of all cases of Alzheimer disease are attributable to modifiable cardiovascular risk factors. Establishing whether decreasing incidence is attributable to decreasing rates of cardiovascular disease would underscore the need for continuing prevention efforts that target vascular risk factors to reduce the burden of dementia.
Since 1993, the Einstein Aging Study (EAS) has systematically recruited and followed up noninstitutionalized individuals 70 years and older from Bronx County, New York. Incident dementia has consistently been ascertained using standard diagnostic criteria. Confirming temporal trends in incidence requires separation of age effects from cohort effects. Our goals were to determine whether there is evidence of a decrease in dementia incidence across sequential birth cohorts in the EAS population and to examine whether trends in the prevalence of cardiovascular disease explained the observed dementia trends. We thus conducted a cohort analysis by year of birth for the EAS cohort. This approach allowed for more precise separation of age and cohort effects than would analyses that compare cohorts recruited decades apart.
Methods
Study Population
Between 1993 and 2004, Health Care Financing Administration/Centers for Medicaid & Medicare Services (HCFA/CMS) rosters of Medicare-eligible persons 70 years or older were used by the EAS to develop sampling frames of community-residing individuals in Bronx County. Since 2004, New York City Board of Elections registered voter lists for Bronx County have been used because of changes in HCFA/CMS policies. Individuals were mailed introductory letters, which were followed by a brief telephone screening interview. Final screening and enrollment were completed at the EAS clinic. The overlap of the HCFA/CMS and voter lists was estimated to be 90%. The demographic characteristics of participants enrolled using either list were similar. Methods for recruitment, telephone screening procedures, and consenting protocols have not changed since 1993, and retention rates and duration of follow-up have also remained similar. Written informed consent was obtained using protocols approved by the Institutional Review Board of the Albert Einstein College of Medicine. Data included a unique study identification number for each person.
Eligibility criteria were age 70 years or older, fluent in English, and no dementia at study entry. Comparison with US Census data indicates that the cohort is representative of the Bronx County community with respect to sex and educational level. Among 2133 EAS participants enrolled since 1993, this analysis excluded 382 who died and 403 who were unavailable for follow-up before their first annual visit, leaving an analysis sample of 1348 EAS participants enrolled from October 20, 1993, through November 17, 2015, who had at least one annual follow-up. Those in the analysis sample and those excluded were similar at baseline with regard to mean age (78.5 vs 78.9 years; t test, P = .97), sex (female, 61.6% vs 62.3%; χ2 test, P = .74), and global cognition based on the Blessed Information Memory Concentration (BIMC) test (2.42 vs 3.12; t test, P = .81).
Follow-up Assessments
Annual assessments included a clinical neurologic examination, comprehensive neuropsychological assessments, medical history, blood pressure, anthropometrics, and psychosocial assessments. Prevalence of myocardial infarction, stroke, and diabetes was ascertained by self-report of physician diagnosis. Global cognitive performance was assessed using the BIMC test. Depression was assessed using the Geriatric Depression Scale.
Neurologic Examination and Dementia Diagnosis
The standardized neurologic examination was adapted from the Unified Parkinson’s Disease Rating Scale. The neurologist assigned a Hachinski Ischemic Score and a Clinical Dementia Rating and provided a clinical impression of the presence or absence of dementia.
Dementia diagnosis was based on standardized clinical criteria from the DSM-IV and required impairment in memory plus at least one additional cognitive domain, accompanied by evidence of functional decline. Diagnoses were assigned at consensus case conferences, which included comprehensive review of cognitive test results, relevant neurologic signs and symptoms, and functional status. Memory impairment was defined as scores of 1.5 SDs or more below the age-adjusted mean for the Logical Memory test or a score of 24 or less on the Free and Cued Selective Reminding Test–Free Recall. Functional impairment was determined using the Lawton Brody Scale, clinical evaluation, and informant questionnaires. To ensure that diagnostic criteria were uniform over time, all individuals evaluated before the release of DSM-IV in 1994 were retrospectively reconferenced according to DSM-IV criteria.
A subset of individuals who participate in the EAS receive autopsies, providing an important quality control for diagnostic accuracy. A clinical diagnosis of dementia in the cohort has a positive predictive value of 96% for significant pathologic findings.
Statistical Analysis
After exploratory analyses of cohort demographic characteristics and crude dementia incidence rates as a function of age and date of birth, we fit locally weighted scatterplot smoothing (LOESS) functions using generalized additive models to graphically identify patterns in the age-specific and birth cohort–specific dementia incidence rates. Poisson regression was used to model the incidence as a function of age, sex, race, educational level, and birth cohort, with change points used to identify the timing of noticeable increases or decreases in incidence. Profile likelihood was used to estimate the change points, with a change point allowed to possibly fall any time between 1915 and 1935. The possibility of a second change point was considered by fitting a model with 2 change points and using likelihood ratio tests to select the best model. For the comparison of a model with 2 change points versus 1 change point, we used a P value of .025 as the threshold for selecting the larger model. We also considered a model with a log-linear trend in incidence as a function of birth year. Additional models included history of myocardial infarction, stroke, and diabetes to ascertain whether trends in prevalence of these conditions might account for changes in dementia incidence. All analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc).
Results
Among the 1348 individuals in the analysis cohort (mean [SD] baseline age, 78.5 [5.4] years; 830 [61.6%] female; 915 [67.9%] non-Hispanic white), there were 150 incident cases of dementia during 5932 person-years of follow-up (Table 1). The most recent birth cohort included a higher proportion of African American and Hispanic individuals, reflecting changes in the surrounding community. Mean years of education was slightly higher in the more recent birth cohorts. Baseline global cognitive status (BIMC) reflects that individuals were free of dementia at baseline, with slightly better baseline global performance among individuals born in more recent years, consistent with their younger age and higher educational level.
Table 1. Descriptive Characteristics by Birth Cohorta.
| Characteristic | Birth Cohort | ||||
|---|---|---|---|---|---|
| Before 1920 (n = 369) |
1920-1924 (n = 285) |
1925-1929 (n = 344) |
After 1929 (n = 350) |
Total (N = 1348) |
|
| Age, y | 83.7 (4.5) | 80.2 (3.4) | 75.8 (4.0) | 74.4 (3.0) | 78.5 (5.4) |
| Follow-up, y | 3.9 (2.9) | 4.9 (3.5) | 5.2 (4.0) | 3.8 (3.1) | 4.4 (3.4) |
| Female, No. (%) | 225 (61.0) | 173 (60.7) | 206 (59.9) | 226 (64.6) | 830 (61.6) |
| Race, No. (%) | |||||
| Non-Hispanic white | 292 (79.1) | 200 (70.2) | 226 (65.7) | 197 (56.3) | 915 (67.9) |
| Non-Hispanic black | 66 (17.9) | 81 (28.4) | 94 (27.3) | 119 (34.0) | 360 (26.7) |
| Hispanic | 5 (1.4) | 3 (1.1) | 20 (5.8) | 23 (6.6) | 51 (3.8) |
| Educational level, y | 12.7 (3.6) | 13.3 (3.6) | 13.9 (3.4) | 14.6 (3.3) | 13.6 (3.5) |
| GDS scoreb | 2.8 (2.3) | 2.5 (2.5) | 2.4 (2.4) | 1.9 (2.1) | 2.4 (2.3) |
| BIMC test scorec | 2.8 (2.4) | 2.6 (2.4) | 2.3 (2.3) | 1.9 (2.0) | 2.4 (2.3) |
Abbreviations: BIMC, Blessed Information Memory Concentration; GDS, Geriatric Depression Scale.
Data are presented as mean (SD) unless otherwise indicated.
Scores range from 0 to 15, with higher scores reflecting depression.
Scores range from 0 to 32, with higher scores reflecting worse cognition.
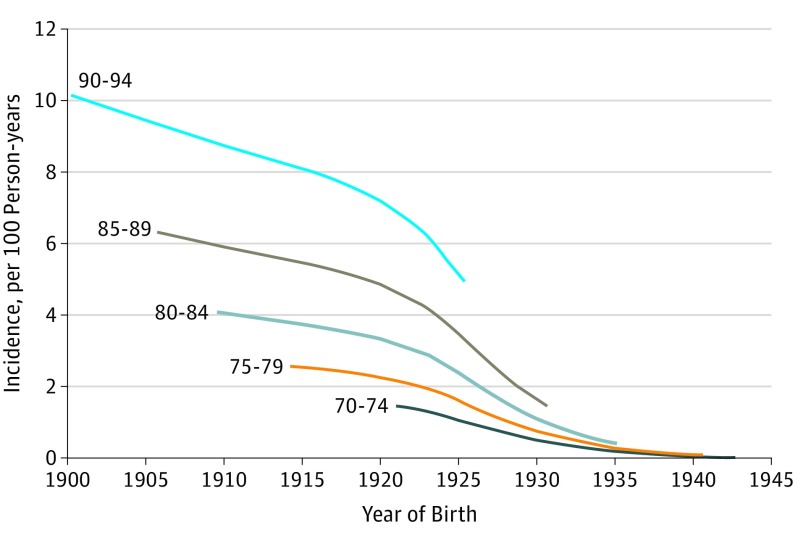
Table 2 gives the crude dementia incidence rates as a function of age and dates of birth. Overall, the expected trend for increasing dementia incidence with increasing age was evident. Within each age range, dementia incidence rates were lower across sequential birth cohorts, particularly after 1929. Figure 1 displays LOESS plots of age-specific dementia incidence. The plot demonstrates the expected association of increasing incidence with increasing age. Figure 1 also shows a consistent pattern within each age group of decreasing incidence in sequential birth years, with an accelerated decrease in incidence rates apparent for those born in the middle to late 1920s.
Table 2. Crude Dementia Incidence by Age at Diagnosis and Year of Birth.
| Variable | Year of Birth | ||||
|---|---|---|---|---|---|
| Before 1920 | 1920-1924 | 1925-1929 | After 1929 | Total | |
| Patients ≤74 y of age | |||||
| No. with dementia | ND | 0 | 4 | 0 | 4 |
| No. of cases | ND | 7.6 | 343.3 | 427.0 | 777.9 |
| No. of person-years | ND | 0 | 1.21 | 0 | 0.51 |
| Patients 75-79 y of age | |||||
| No. of dementia cases | 0 | 10 | 8 | 2 | 20 |
| No. of person-years | 114.0 | 294.9 | 648.5 | 663.5 | 1721.0 |
| Rate per 100 person-years | 0 | 3.39 | 1.23 | 0.30 | 1.16 |
| Patients 80-84 y of age | |||||
| No. of dementia cases | 12 | 18 | 13 | 1 | 44 |
| No. of person-years | 512.9 | 541.0 | 598.8 | 231.6 | 1885.3 |
| Rate per 100 person-years | 2.34 | 3.33 | 2.17 | 0.43 | 2.33 |
| Patients 85-89 y of age | |||||
| No. of dementia cases | 37 | 11 | 6 | 0 | 54 |
| No. of person-years | 553.4 | 443.0 | 199.3 | 1.5 | 1197.1 |
| Rate per 100 person-years | 6.69 | 2.48 | 3.01 | 0 | 4.51 |
| Patients 90-94 y of age | |||||
| No. of dementia cases | 21 | 4 | 0 | ND | 25 |
| No. of person-years | 210.9 | 86.7 | 0.9 | ND | 308.5 |
| Rate per 100 person-years | 9.96 | 4.14 | 0 | ND | 8.10 |
| Patients ≥95 y of age | |||||
| No. of dementia cases | 3 | ND | ND | ND | 3 |
| No. of person-years | 42.0 | ND | ND | ND | 42.0 |
| Rate per 100 person-years | 7.14 | ND | ND | ND | 7.14 |
| Total | |||||
| No. of dementia cases | 73 | 43 | 31 | 3 | 150 |
| No. of person-years | 1434.3 | 1383.2 | 1790.9 | 1323.6 | 5931.9 |
| Rate per 100 person-years | 5.09 | 3.11 | 1.73 | 0.23 | 2.53 |
Abbreviation: ND, no data (birth cohorts for which there were no observations in the specified age group).
Figure 1. Crude Dementia Incidence in the Einstein Aging Study Cohort as a Function of Date of Birth and Age.
Data were smoothed using locally weighted scatterplot smoothing (LOESS) methods.
To further explore this trend, we fit Poisson regression models with change points to identify birth years when there was a significant change in incidence rates. Models adjusted for age, sex, race, and educational level indicated a significant change point for individuals born after July 1929 (95% CI, June 1929 to January 1930). Model fit was not improved by addition of a second change point (likelihood ratio for the model with 1 change point was −647.671 and for 2 change points was −645.601; likelihood ratio test with 2 degrees of freedom, P = .13). Furthermore, a model for a simpler, log-linear trend in incidence by birth cohort did not fit the data as well as the change point model with the same number of parameters, as indicated by a smaller log-likelihood value. To illustrate this trend, we used the Poisson model to estimate dementia incidence for a white woman aged 80 years born before or after the change point at July 1929. Before July 1929, dementia incidence was 2.26 per 100 person-years (95% CI, 1.67-3.07) for an 80-year-old white woman. For a white woman aged 80 years born after July 1929, the incidence rate was 0.29 per 100 person-years (95% CI, 0.09-0.92). The relative rate for a white woman aged 80 years born before vs after July 1929 was 0.13 (95% CI, 0.04-0.41), reflecting a more than 85% lower incidence in the later birth cohorts. Because individuals older than 85 years are not represented in the later birth cohorts, we performed a sensitivity analysis restricted to follow-up before the age of 85 years. Results were unchanged from those in the overall cohort.
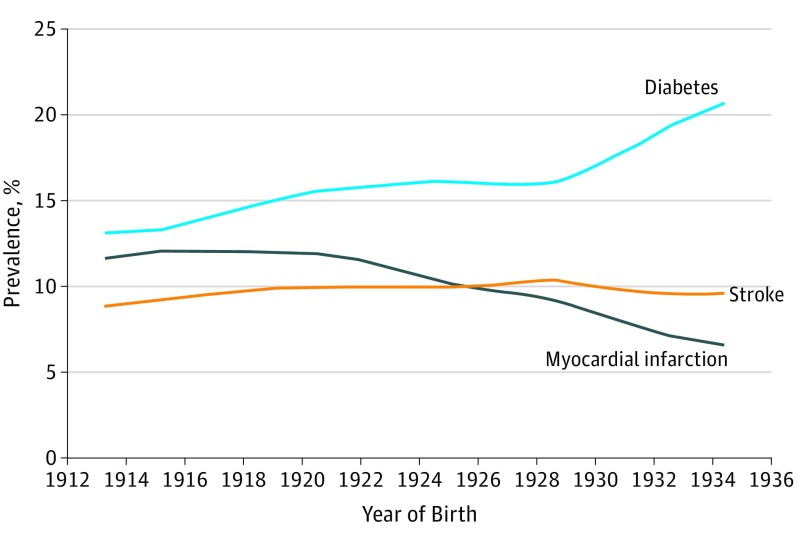
To explore whether decreasing rates of cardiovascular disease and cardiovascular risk factors may have affected the dementia incidence rates, we explored age-specific trends for stroke, myocardial infarction, and diabetes by year (Figure 2). The observed trends in the EAS cohort are consistent with national data from the United States showing reductions in cardiovascular disease rates across the past decades. The age-specific prevalence of myocardial infarction and stroke has decreased in our cohort across sequential birth cohorts. In contrast to the favorable myocardial infarction and stroke trends, we observed a notable increase in diabetes prevalence in later birth cohorts that is also consistent with national trends. However, when the Poisson models of dementia incidence by birth year were further adjusted for prevalence of myocardial infarction, stroke, or diabetes, the results remained unchanged and a significant decrease in dementia incidence among individuals born after mid-1929 remained.
Figure 2. Prevalence of Myocardial Infarction, Stroke, and Diabetes at the Age of 80 Years by Year of Birth, Einstein Aging Study Cohort.
Locally weighted scatterplot smoothing (LOESS) curves of prevalence as a function of date of birth are shown.
Discussion
This cohort analysis of age-specific dementia incidence rates by birth year demonstrated decreased incidence of dementia in more recent birth cohorts among older individuals in Bronx County, New York. The decrease appears to have occurred for individuals born after mid-1929. The observed relative rate of incidence for those born after vs before July 1929 was 0.13 per 100 person-years. These results are consistent with previous studies that have suggested decreased dementia incidence in more recent periods and no significant trends before the 1990s.
Our analytic approach differs from prior studies in that we examined incidence according to year of birth, whereas most prior studies have compared dementia rates in cohorts in a specified age bracket enrolled during different periods (eg, decades). Our birth cohort approach based on individual years of birth allows for a more precise separation of age and cohort effects, which might be difficult to disentangle when the age bands or enrollment periods studied are wide. Despite this difference, the EAS results are very consistent with earlier work, suggesting that any decrease in dementia incidence has been during more recent decades.
The Rotterdam Study reported a 25% decrease in all-cause dementia rates between 1990 and 2000. The timing of this decrease coincides with our observed reduction in age-specific incidence among individuals born after 1929 because the earlier Rotterdam cohort was born between 1900 and 1930, whereas the later cohort was born between 1910 and 1940. Although the reduction in incidence did not reach statistical significance in the Rotterdam Study, the suggestion of a true decrease in dementia rates is strengthened by their observation of less cerebral small vessel disease and larger total brain volumes among individuals enrolled in the more recent period. The EAS was unable to confirm whether cohort differences in brain pathologic findings may underlie the observed decrease in dementia incidence.
More recent data from the Framingham cohort demonstrate a 20% decrease in dementia incidence rates since the late 1970s among those older than 60 years. Similarly, Rocca et al reported a small but statistically significant downward trend of 3% per year in all-cause and Alzheimer disease dementia among individuals aged 70 to 94 years during 1985 to 1994. A similar decrease in Alzheimer disease incidence was observed among individuals 65 years and older in the Chicago Health and Aging Project cohort between 1997 and 2008, although the trend did not reach statistical significance.
Earlier studies have found no decrease in dementia incidence rates for cohorts followed up before 1990. This finding is consistent with our results indicating no decrease in dementia incidence rates among individuals aged 70 to 84 years who were born from 1910 to 1930. Data from Rochester, Minnesota, indicate random fluctuations in dementia incidence before the mid-1980s, with no evidence of a decrease, and suggest an increase in rates between 1965 and 1974. There was no evidence of a birth cohort effect on dementia incidence for individuals born between 1885 and 1930, although birth cohorts after 1930 were not examined. Consistent with our findings, a birth cohort analysis of 2 cohorts in western Pennsylvania demonstrated decreasing rates of cognitive decline with increasing age for individuals born between 1932 and 1943 vs earlier birth cohorts.
Prior studies have varied with regard to the method for ascertaining dementia incidence. The application of consistent diagnostic procedures over time is critical to the interpretation of temporal trends. A strength of our study is that dementia diagnoses were based on information from standardized study assessments and diagnostic criteria applied to all participants across the study period. Similarly, the Framingham, Rotterdam, and Chicago study results are based on clinical evaluations and diagnostic criteria applied uniformly over time. In contrast, the studies from the Rochester cohort relied on diagnostic codes from administrative databases. Despite these important differences, there appears to be consistent evidence across diverse cohorts that dementia incidence may be decreasing.
Decreasing incidence rates are consistent with previous reports of decreased dementia prevalence during the past 10 to 20 years in the United States and Western Europe. Decreased prevalence may result from decreasing rates of disease onset, increasing rates of cure or remission, or decreasing rates of survival after dementia diagnosis. Given the current lack of treatments to reverse Alzheimer disease or dementia and data suggesting that survival among patients with dementia has increased, it is likely that the favorable prevalence trends are attributable to decreased risk of dementia onset. Consistent with this hypothesis, a recent analysis of administrative data from Canada found decreasing dementia incidence concurrent with increasing prevalence between 2005 and 2013.
Some researchers have suggested that trends toward increasing level of education may underlie decreases in dementia incidence and prevalence. Although there was a shift toward higher education in our cohort, this trend did not explain our findings. Similarly, adjustment for education did not attenuate the cohort effects for decreased cognitive decline with age observed by Dodge et al. Improved nutrition over successive birth cohorts might also have affected dementia risk. We were not able to assess this possibility because the EAS has not routinely ascertained dietary data.
Vascular risk factors increase the risk of developing dementia, and the incidence of stroke has decreased and the control of several cardiovascular risk factors has improved in the United States during the past several decades. Although the decreasing dementia incidence that we observed may be partially related to decreases in the prevalence of cardiovascular comorbidities, these factors did not explain the observed results. Failure to detect an effect of these trends may have occurred because our analysis was based on self-reported medical history and may not reflect the overall burden of vascular disease in the population.
We observed an adverse trend for the prevalence of diabetes that may portend increasing dementia incidence in the coming decades. The increasing prevalence of diabetes among EAS participants in later birth cohorts may reflect better treatment and survival among individuals with diabetes born in more recent decades. The trend is consistent with data on the worldwide increase in diabetes prevalence and given evidence that links diabetes with dementia risk. This increase in diabetes prevalence may adversely affect dementia rates in the future.
We conducted a sensitivity analysis to determine whether our observed change point in dementia incidence was explained by the demographic shift in the racial/ethnic composition of the EAS cohort. In an analysis restricted to only white individuals, results were similar to those observed in the total sample, suggesting that shifting demographic characteristics did not explain our results.
Strengths and Limitations
A strength of our study is that the EAS is a community-based sample that is representative of the surrounding population. Community-based samples provide critical information regarding secular trends that cannot be assessed in referral samples of individuals with cognitive complaints. A limitation of our study is the small number of dementia cases for later birth years. Alzheimer disease is the leading cause of dementia in the elderly population, accounting for 60% to 80% of dementia cases. Thus, although a decrease in all-cause dementia is likely to reflect changes in Alzheimer disease rates, we could not address this issue because sample size constraints precluded our ability to analyze dementia subtypes. Nevertheless, the observed decrease in incidence after mid-1929 was robust in analyses that adjusted for differences in demographic characteristics and prevalence of cardiovascular comorbidity.
Conclusions
Future analyses in larger cohorts are required to confirm whether dementia incidence is decreasing. If the trend is confirmed, it remains to be seen whether decreasing dementia incidence will offset the ongoing demographic shift to older ages given that dementia incidence increases exponentially after 60 years of age. In addition, despite advances in prevention and control of cardiovascular disease in recent decades, the future influence of the current diabetes epidemic on dementia incidence remains unknown. The observed trends highlight the need for continued efforts toward prevention and control of diabetes and vascular risk.
References
- 1.United Nations World population prospects: the 2010. revision. https://esa.un.org/unpd/wpp/Publications/Files/WPP2017_KeyFindings.pdf. Updated 2016. Accessed June 1, 2017.
- 2.World Health Organization; Alzheimer’s Disease International . Dementia: A Public Health Priority. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 3.Langa KM. Is the risk of Alzheimer’s disease and dementia declining? Alzheimers Res Ther. 2015;7(1):34. doi: 10.1186/s13195-015-0118-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kokmen E, Chandra V, Schoenberg BS. Trends in incidence of dementing illness in Rochester, Minnesota, in three quinquennial periods, 1960-1974. Neurology. 1988;38(6):975-980. [DOI] [PubMed] [Google Scholar]
- 5.Kokmen E, Beard CM, O’Brien PC, Offord KP, Kurland LT. Is the incidence of dementing illness changing? a 25-year time trend study in Rochester, Minnesota (1960-1984). Neurology. 1993;43(10):1887-1892. [DOI] [PubMed] [Google Scholar]
- 6.Rorsman B, Hagnell O, Lanke J. Prevalence and incidence of senile and multi-infarct dementia in the Lundby study: a comparison between the time periods 1947-1957 and 1957-1972. Neuropsychobiology. 1986;15(3-4):122-129. [DOI] [PubMed] [Google Scholar]
- 7.Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7(1):80-93. doi: 10.1016/j.jalz.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523-532. doi: 10.1056/NEJMoa1504327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. Is dementia incidence declining? trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78(19):1456-1463. doi: 10.1212/WNL.0b013e3182553be6 [DOI] [PubMed] [Google Scholar]
- 10.Abdulrahman GO., Jnr Alzheimer’s disease: current trends in Wales. Oman Med J. 2014;29(4):280-284. doi: 10.5001/omj.2014.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu C, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol. 2015;12(5):267-277. doi: 10.1038/nrcardio.2014.223 [DOI] [PubMed] [Google Scholar]
- 12.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788-794. doi: 10.1016/S1474-4422(14)70136-X [DOI] [PubMed] [Google Scholar]
- 13.Katz MJ, Lipton RB, Hall CB, et al. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26(4):335-343. doi: 10.1097/WAD.0b013e31823dbcfc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Census Bureau American FactFinder. http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml?_ts=498596416732. Accessed January 1, 2011.
- 15.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797-811. [DOI] [PubMed] [Google Scholar]
- 16.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS); recent evidence and development of a shorter version In: Brink TL, ed. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: Haworth Press; 1986:165-173. [Google Scholar]
- 17.Fahn S, Elton R. Unified Parkinson’s Disease Rating Scale In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson’s Disease. Vol 2 Florham Park, NJ: Macmillan Healthcare Information; 1987:153-163. [Google Scholar]
- 18.Hachinski VC, Lassen NA, Marshall J. Multi-infarct dementia: a cause of mental deterioration in the elderly. Lancet . 1974;2(7874):207-210. [DOI] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Associaiton Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.Wechsler D. Memory Scale - Revised. New York, NY: Psychological Corporation; 1987. [Google Scholar]
- 22.Buschke H. Cued recall in amnesia. J Clin Neuropsychol. 1984;6(4):433-440. [DOI] [PubMed] [Google Scholar]
- 23.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186. [PubMed] [Google Scholar]
- 24.Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83(403):596-610. [Google Scholar]
- 25.Hastie TJ, Tibshirani RJ. Generalized Additive Models. London, England: Chapman & Hall/CRC; 1990. [Google Scholar]
- 26.Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Stat Med. 2000;19(11-12):1555-1566. doi: 10.1002/(SICI)1097-0258(20000615/30)19:11/123.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- 27.Koton S, Schneider AL, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312(3):259-268. doi: 10.1001/jama.2014.7692 [DOI] [PubMed] [Google Scholar]
- 28.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25-e146. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Yan F, Li G, et al. Is the dementia rate increasing in Beijing? prevalence and incidence of dementia 10 years later in an urban elderly population. Acta Psychiatr Scand. 2007;115(1):73-79. [DOI] [PubMed] [Google Scholar]
- 30.Hebert LE, Bienias JL, Aggarwal NT, et al. Change in risk of Alzheimer disease over time. Neurology. 2010;75(9):786-791. doi: 10.1212/WNL.0b013e3181f0754f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dodge HH, Zhu J, Lee CW, Chang CC, Ganguli M. Cohort effects in age-associated cognitive trajectories. J Gerontol A Biol Sci Med Sci. 2014;69(6):687-694. doi: 10.1093/gerona/glt181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee Y. The recent decline in prevalence of dementia in developed countries: implications for prevention in the Republic of Korea. J Korean Med Sci. 2014;29(7):913-918. doi: 10.3346/jkms.2014.29.7.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu YT, Fratiglioni L, Matthews FE, et al. Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol. 2016;15(1):116-124. doi: 10.1016/S1474-4422(15)00092-7 [DOI] [PubMed] [Google Scholar]
- 34.Daviglus ML, Bell CC, Berrettini W, et al. National Institutes of Health State-of-the-Science Conference statement: preventing Alzheimer disease and cognitive decline. Ann Intern Med. 2010;153(3):176-181. doi: 10.7326/0003-4819-153-3-201008030-00260 [DOI] [PubMed] [Google Scholar]
- 35.Qiu C, von Strauss E, Bäckman L, Winblad B, Fratiglioni L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology. 2013;80(20):1888-1894. doi: 10.1212/WNL.0b013e318292a2f9 [DOI] [PubMed] [Google Scholar]
- 36.Kosteniuk JG, Morgan DG, O’Connell ME, et al. Simultaneous temporal trends in dementia incidence and prevalence, 2005-2013: a population-based retrospective cohort study in Saskatchewan, Canada. Int Psychogeriatr. 2016;28(10):1643-1658. doi: 10.1017/S1041610216000818 [DOI] [PubMed] [Google Scholar]
- 37.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387(10027):1513-1530. doi: 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737-1746. [DOI] [PubMed] [Google Scholar]