Key Points
Question
Is a family history of atrial fibrillation associated with a higher incidence of atrial fibrillation or worse outcomes?
Findings
In a nationwide population-based study of more than 23 million people in Taiwan, individuals who had first-degree relatives with atrial fibrillation had a mildly increased risk of new atrial fibrillation. Once atrial fibrillation had been diagnosed, family history was not associated with more major adverse cardiovascular events.
Meaning
Family history may be helpful in the diagnosis but not the management of atrial fibrillation.
Abstract
Importance
The heritability of atrial fibrillation (AF), the contribution of genetic and environmental factors, and the association of a family history of AF with prognosis are unclear.
Objectives
To measure genetic and environmental factors in the familial aggregation of AF and to estimate the association of a family history of AF with major adverse cardiovascular events (MACE).
Design, Setting, and Participants
In this Taiwanese nationwide population-based study among more than 23 million people, a custom data set was obtained using the data of all patients having a diagnosis of AF recorded between January 1996 and December 2013 in the Taiwan National Health Insurance Research Database. The study population comprised all 23 422 955 individuals registered with the database in 2013, of whom 177 770 had a diagnosis of AF and were included in the heritability estimation. From the latter, a subgroup of patients having newly diagnosed AF with a first-degree relative affected by AF between 2000 and 2010 were selected and matched 1:4 to controls without a family history for estimating MACE-free survival. The dates of analysis were January 2010 to December 2013.
Main Outcomes and Measures
The prevalence and relative risk of AF in relatives of patients with AF, as well as the relative contributions of heritability and shared and nonshared environmental factors to AF susceptibility. Also measured was MACE-free survival after AF was diagnosed.
Results
In total, 1510 patients (204 [13.5%] female; mean [SD] age, 57.9 [9.2] years) had newly diagnosed AF with a first-degree relative affected by AF. Individuals with a first-degree relative affected by AF had a relative risk of 1.92 (95% CI, 1.84-1.99) for AF. The accountability for the phenotypic variance of AF was 19.9% for genetic factors (heritability), 3.5% for shared environmental factors, and 76.6% for nonshared environmental factors. After matching for age, sex, hypertension, type 2 diabetes, previous stroke, and anticoagulation, incident AF patients with vs without an affected first-degree relative had similar MACE-free survival.
Conclusions and Relevance
Genetic and environmental factors were associated with AF, with nonshared environmental factors accounting for three-fourths of the phenotypic variance in Taiwan. Patients having AF with a first-degree relative affected by AF did not have more MACE. Therefore, family history may not be particularly informative in the diagnosis or management of AF.
This nationwide population-based study measures genetic and environmental factors in the familial aggregation of atrial fibrillation and estimates the association of a family history of atrial fibrillation with major adverse cardiovascular events.
Introduction
Atrial fibrillation (AF) is the most common clinically significant dysrhythmia, which can result in thromboembolic events, heart failure, and even death. The pathophysiological mechanisms responsible for AF are multifactorial. Investigators have reported that genetic variants underlie specific types of AF. Genome-wide association studies also support a role of genetic factors in the pathogenesis of AF. However, the exact roles of candidate genes associated with these polymorphisms in the development of AF are controversial. The apparent gap in the observed and estimated genetic contributions may be due to unidentified risk alleles or, more plausibly, the overestimation of heritability.
The findings of several studies have suggested that AF aggregates within a family. For example, in a subgroup of 2243 offspring in the Framingham Heart Study, a parental history of AF was shown to be associated with an odds ratio of 1.85 for AF events in the offspring. In addition, an Icelandic study reported a similar relative risk (RR) of 1.77 for AF in first-degree relatives of patients. A Danish study compared the risk of AF between monozygotic and dizygotic twins and estimated a heritability of 62%. These studies support a varying degree of the familial aggregation of AF, which may be attributable to genetic or shared environmental factors.
If a family history of AF is associated with the incidence of the disease, it is plausible to hypothesize that there may be an association between such a family history and prognosis. A recent analysis of the incidence in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) revealed that, once AF developed, similar risks of thromboembolism were associated with a family history of AF compared with no family history. However, other complications of AF were not evaluated.
Some important questions remain with regard to the familial risk of AF. First, the distribution of the phenotypic variance in AF is unclear. The weight of the genetic component on the occurrence of AF has never been measured for the whole population, to our knowledge. Second, to date, the risk of major adverse cardiovascular events (MACE) has not been estimated between patients with vs without a family history of AF in a matched manner. Therefore, we conducted this study to (1) estimate the RR of AF in individuals with affected relatives of specific kinship; (2) describe and measure the relative contributions of genetic, shared, and nonshared environmental factors to susceptibility to AF; and (3) compare MACE-free survival of patients having AF with vs without a family history of AF in essentially the entire population of Taiwan in 2013.
Methods
Study Population
This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital, Taipei, Taiwan. Patient informed consent was waived because all research data in this study were anonymous. The National Health Insurance covered more than 99.5% of all Taiwanese residents in 2013. We obtained a custom data set containing the data of all patients having a diagnosis of AF recorded between January 1996 and December 2013 in the Taiwan National Health Insurance Research Database (NHIRD). The dates of analysis were January 2010 to December 2013.
The methods of genealogy reconstruction using recorded family relationships in the NHIRD have been described previously. Briefly, by analyzing the birth certificate and the dependent, insurance, and employment status, we were able to establish family relationships (parents, offspring, full siblings, twins, and spouses). A family was defined as a cluster of individuals who were related to each other by blood or by at least one common blood relative. Among 29 505 197 beneficiaries included in the registry, 7 856 663 were registered by themselves without any relationships to other people over a span of 19 years. The rest of the beneficiaries were clustered into 4 042 209 families, with a mean family size of 5.4 persons.
To estimate the association of first-degree relatives affected by AF and MACE, we matched incident AF patients with a family history to those incident AF patients without a family history. All newly diagnosed patients having AF with an affected first-degree relative between 2000 and 2010 were selected. A 1:4 matched cohort of AF cases without affected first-degree relatives was constructed according to calendar year of initial diagnosis, CHA2DS2-VASc parameters (congestive heart failure, hypertension, age, type 2 diabetes, stroke, transient ischemic attack, vascular disease, and sex), and medication use within the first year after the diagnosis of AF and used as a control to compare their MACE-free survival.
Case Definition of AF
Ascertainment of AF was based on an International Classification of Diseases, Ninth Revision (ICD-9) code of 427.31. To improve the diagnostic accuracy, we defined patients as having AF only when they had code 427.31 as their discharge diagnosis or when they had more than 2 outpatient visits with the same code. The same registry and definition of AF have been applied in our group’s previous studies.
Definition of MACE
MACE were defined as an admission with a primary diagnosis of stroke (ICD-9 codes 430-437), myocardial infarction (ICD-9 code 410), or coronary angioplasty (ICD-9 procedure code 360). Similar codes and definitions have been applied and validated in other investigations using data from the NHIRD. Because the cause of death was not available, we did not include cardiovascular mortality as one of our MACE definitions.
Covariates
We considered age, sex, occupation, income level, urbanization of residence, and family size to be potential confounders or to potentially affect familial associations. Therefore, they were included as covariates.
Statistical Analysis
The prevalence of AF was calculated for the general population and for individuals with an affected first-degree relative as of July 1, 2013. We calculated the RR of AF as the adjusted prevalence ratio between first-degree relatives of an individual with AF and the general population. The RRs estimated in this study were essentially relative recurrence risks according to the original definition by Risch, which is the prevalence ratio between individuals with a specific type of affected relative and the general population.
The RRs and tetrachoric correlations were calculated for individuals with an affected first-degree relative of any kinship and for individual first-degree relatives (parents, offspring, full siblings, and twins) and spouses. Because the kinship and sex of the affected relative may have also affected the familial risk, models were fitted separately according to kinship and relatives. Twins were calculated separately from the sibling analysis, and the RRs were estimated for the number of affected first-degree kinships (father, mother, son, daughter, brother, and sister). In this model, we compared the risk of AF in individuals having 1 or 2 affected first-degree relatives with the risk in the general population.
Heritability was defined as the proportion of the phenotypic variance attributable to genetic factors. Familial transmission was defined as a measure of the combined contribution of genetic and environmental factors to susceptibility to AF. The standard ACE model (additive genetic, common environmental factors shared by family members, and nonshared environmental factors) was applied and is described in the eAppendix in the Supplement. Spouses were used as controls to estimate the contribution of shared environmental factors to the phenotypic variance, assuming that they shared the family environment with no close genetic relationship. It should be noted that spouses affected only shared adult environment but not early life factors. The liability of AF for the affected individuals was greater than a critical threshold (threshold liability model), the value of which could be determined from the prevalence of AF among the affected and general populations.
An adjusted MACE-free survival analysis was carried out using a multivariable Cox proportional hazards regression model to determine the association of affected first-degree relatives with long-term MACE among patients having AF in the cohort of incident AF patients and their matched controls. Follow-up started from the date of initial diagnosis until the occurrence of MACE or deregistration at the end of 2013. All-cause mortality was considered a competing risk to estimate subdistribution hazard in a secondary analysis. All analyses were performed using statistical software (SAS, version 9.3; SAS Institute Inc). Two-sided P = .05 was considered statistically significant.
Results
Prevalence of AF Among Patients With Affected First-Degree Family Members vs the General Population
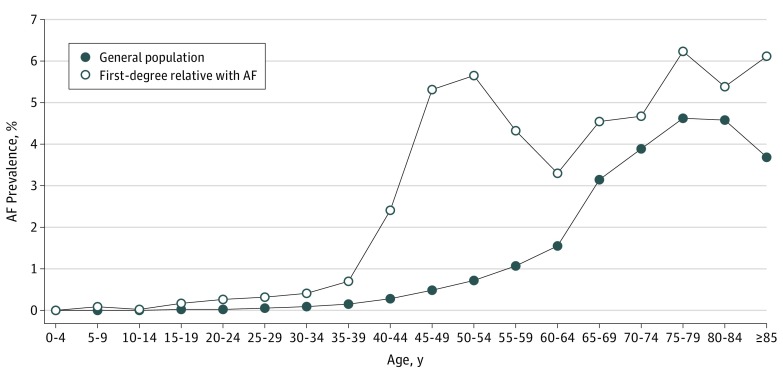
The study population comprised all 23 422 955 individuals registered with the database in 2013, of whom 177 770 had a diagnosis of AF and were included in the heritability estimation, giving a crude prevalence of 0.8%. There were slightly more men (98 874 [0.9%]) than women (78 896 [0.7%]), with a ratio of men to women of 1.15:1. In the general population, 300 239 individuals (1.3%) had at least one first-degree relative with AF (Table 1), including 279 756 with affected parents, 10 973 with affected offspring, 11 253 with affected siblings, and 44 with affected twins (some had more than one relative affected). The age-specific prevalence of AF was higher among those with affected first-degree relatives with AF than among the general population. Both groups had a peak of AF prevalence at an age older than 70 years, while patients with a family history had another earlier, separate peak at age 40 to 50 years (Figure).
Table 1. Baseline Characteristics of Individuals Having Affected First-Degree Relatives With AF and in the General Population.
| Variable | First-Degree Relative With AF | General Population |
|---|---|---|
| No. | 300 239 | 23 422 955 |
| Male, No. (%) | 176 151 (58.7) | 11 519 413 (49.2) |
| Age, mean (SD), y | 43.7 (14.9) | 39.0 (20.9) |
| AF, No. (%) | 3509 (1.2) | 177 770 (0.8) |
Abbreviation: AF, atrial fibrillation.
Figure. Age-Specific Prevalence of AF in Individuals Having Affected First-Degree Relatives With AF and in the General Population.
AF indicates atrial fibrillation.
RRs for AF in Patients With Affected First-Degree Relatives
Compared with the general population, individuals with one type of a first-degree relative affected by AF had an RR of 1.92 (95% CI, 1.84-1.99) for AF, and those with 2 or more types had an RR of 3.63 (95% CI, 3.06-4.32). The sex of the affected relative did not seem to affect the RR for AF. For example, the RRs for patients with an affected male vs female relative were 2.20 (95% CI, 2.09-2.30) vs 1.79 (95% CI, 1.71-1.88). The RRs were 2.25 (95% CI, 1.88-2.68) for those with an affected sibling, 1.81 (95% CI, 1.74-1.89) for those with an affected parent, and 2.39 (95% CI, 2.26-2.52) for those with an affected offspring (Table 2). A family history of AF was associated with a higher RR for AF in the younger patients than in the older patients. The RRs for AF were 3.20 (95% CI, 2.75-3.71) for those younger than 40 years, 2.10 (95% CI, 1.98-2.20) for those aged 40 to 59 years, and 1.81 (95% CI, 1.73-1.90) for those 60 years or older.
Table 2. Relative Risk of and Tetrachoric Correlation for AF in First-Degree Relatives.
| Sex of Affected Relative | Sex of Individual | No. of Cases | Prevalence, %a | Relative Risk (95% CI)b | Tetrachoric Correlation (95% CI)b |
|---|---|---|---|---|---|
| Any Relative | |||||
| Male | Male | 1248 | 1.2 | 2.20 (2.06-2.35) | 0.10 (0.09-0.10) |
| Female | 701 | 0.9 | 2.01 (1.87-2.16) | 0.11 (0.10-0.11) | |
| All | 1949 | 1.1 | 2.20 (2.09-2.31) | 0.10 (0.10-0.11) | |
| Female | Male | 1352 | 1.7 | 1.79 (1.70-1.88) | 0.18 (0.18-0.19) |
| Female | 335 | 0.7 | 1.59 (1.40-1.81) | 0.11 (0.10-0.11) | |
| All | 1687 | 1.4 | 1.79 (1.71-1.88) | 0.17 (0.16-0.17) | |
| All | Male | 2493 | 1.4 | 1.93 (1.85-2.01) | 0.11 (0.11-0.12) |
| Female | 1016 | 0.8 | 1.84 (1.73-1.96) | 0.08 (0.07-0.08) | |
| All | 3509 | 1.2 | 1.96 (1.88-2.04) | 0.10 (0.10-0.11) | |
| Parent | |||||
| Male | Male | 784 | 0.8 | 2.03 (1.89-2.17) | 0.30 (0.29-0.31) |
| Female | 139 | 0.2 | 1.43 (1.21-1.69) | 0.22 (0.20-0.23) | |
| All | 923 | 0.6 | 1.98 (1.86-2.11) | 0.29 (0.28-0.29) | |
| Female | Male | 1264 | 1.7 | 1.78 (1.69-1.88) | 0.36 (0.35-0.37) |
| Female | 224 | 0.5 | 1.46 (1.28-1.66) | 0.31 (0.30-0.33) | |
| All | 1488 | 1.3 | 1.77 (1.69-1.86) | 0.36 (0.35-0.36) | |
| All | Male | 1973 | 1.2 | 1.83 (1.76-1.91) | 0.32 (0.32-0.33) |
| Female | 349 | 0.3 | 1.42 (1.28-1.58) | 0.26 (0.25-0.27) | |
| All | 2322 | 0.8 | 1.81 (1.74-1.89) | 0.32 (0.31-0.32) | |
| Offspring | |||||
| Male | Male | 362 | 12.2 | 2.72 (2.47-2.99) | 0.33 (0.32-0.34) |
| Female | 535 | 9.8 | 2.28 (2.11-2.47) | 0.36 (0.35-0.37) | |
| All | 897 | 10.7 | 2.50 (2.35-2.65) | 0.34 (0.33-0.35) | |
| Female | Male | 65 | 6.5 | 1.84 (1.47-2.32) | 0.20 (0.18-0.23) |
| Female | 105 | 6.6 | 1.92 (1.60-2.30) | 0.29 (0.27-0.31) | |
| All | 170 | 6.6 | 1.93 (1.67-2.23) | 0.25 (0.23-0.26) | |
| All | Male | 426 | 10.8 | 2.53 (2.32-2.77) | 0.31 (0.30-0.31) |
| Female | 639 | 9.1 | 2.22 (2.06-2.38) | 0.35 (0.34-0.35) | |
| All | 1065 | 9.7 | 2.39 (2.26-2.52) | 0.32 (0.32-0.33) | |
| Full Sibling | |||||
| Male | Male | 121 | 2.6 | 2.45 (1.97-3.04) | 0.36 (0.34-0.38) |
| Female | 31 | 0.9 | 1.68 (1.19-2.38) | 0.32 (0.29-0.35) | |
| All | 152 | 1.8 | 2.27 (1.88-2.74) | 0.35 (0.34-0.37) | |
| Female | Male | 26 | 1.6 | 1.91 (1.31-2.79) | 0.33 (0.30-0.36) |
| Female | 9 | 0.7 | 3.14 (1.37-7.19) | 0.25 (0.25-0.06) | |
| All | 35 | 1.2 | 2.13 (1.49-3.03) | 0.31 (0.29-0.34) | |
| All | Male | 147 | 2.3 | 2.34 (1.93-2.83) | 0.36 (0.34-0.37) |
| Female | 40 | 0.8 | 1.88 (1.36-2.61) | 0.31 (0.29-0.34) | |
| All | 187 | 1.7 | 2.25 (1.88-2.68) | 0.35 (0.33-0.36) | |
| Twin | |||||
| Male | Male | 4 | 16.7 | 17.37 (3.9-77.40) | 0.71 (0.63-0.80) |
| Female | 0 | 0 | NA | NA | |
| All | 4 | 13.3 | 14.78 (3.38-64.71) | 0.70 (0.62-0.79) | |
| Female | Male | 0 | NA | NA | NA |
| Female | 0 | NA | NA | NA | |
| All | 0 | NA | NA | NA | |
| All | Male | 4 | 14.3 | 14.14 (3.36-59.51) | 0.70 (0.61-0.78) |
| Female | 0 | NA | NA | NA | |
| All | 4 | 9.1 | 12.15 (2.94-50.21) | 0.65 (0.57-0.73) | |
| Spouse | |||||
| Female | Male | 1311 | 5.4 | 1.18 (1.12-1.24) | 0.25 (0.24-0.25) |
| Male | Female | 1819 | 2.8 | 1.14 (1.09-1.19) | 0.23 (0.22-0.23) |
| All | All | 3130 | 3.5 | 1.15 (1.10-1.20) | 0.22 (0.21-0.22) |
Abbreviations: AF, atrial fibrillation; NA, not applicable (because of no AF cases with affected twin).
Prevalence was equal to recurrence risk.
Adjusted for age, sex, occupation, income level, urbanization of residence, and family size.
Age at Onset of AF
The age distribution of patients having AF with vs without a family history of AF is shown in eFigure 1 in the Supplement. In general, AF rarely occurred before age 25 years, and most of the patients had an age at onset of 50 years or older. Patients with a family history of AF tended to develop AF earlier (median age at onset, 59 years) than those without a family history (median age at onset, 69 years) (P < .001).
Familial Resemblance and the Heritability of AF
Using the threshold liability model, we estimated that the accountability for the phenotypic variance of AF was 19.9% for genetic factors (heritability), 3.5% for shared environmental factors, and 76.6% for nonshared environmental factors. In other words, familial transmission of AF was 23.4%. Given the previously estimated parameters, the probability for sporadic AF was 82.8% (eFigure 2 in the Supplement).
MACE-Free Survival in Patients Having AF With a Family History of AF
We selected a subgroup of 1510 patients (204 [13.5%] female; mean [SD] age, 57.9 [9.2] years) having newly diagnosed AF with a first-degree relative affected by AF between 2000 and 2010. For estimating MACE-free survival, a 1:4 matched cohort of AF cases without affected first-degree relatives was constructed according to CHA2DS2-VASc parameters and medication use (Table 3). The mean follow-up times were 5.49 vs 5.56 years in those patients with vs without affected first-degree relatives. The RRs for MACE among the 2 groups were then compared in patients with vs without first-degree affected relatives. The RRs in patients who had a first-degree relative with AF were 0.79 (95% CI, 0.61-1.03) for ischemic stroke, 0.58 (95% CI, 0.30-1.14) for acute myocardial infarction, 0.73 (95% CI, 0.49-1.08) for coronary revascularization, and 0.86 (95% CI, 0.76-1.00) for composite MACE (Table 4). eFigure 3 in the Supplement shows that newly diagnosed patients having AF with vs without an affected first-degree relative had similar MACE-free survival. After considering all-cause mortality as a competing risk, the hazard ratio for MACE was 0.91 (95% CI, 0.78-1.06).
Table 3. Characteristics of Patients Having AF With vs Without First-Degree Relatives With AF.
| Variable | Before Matching | After Matching | ||||
|---|---|---|---|---|---|---|
| With First-Degree Relative With AF | Without First-Degree Relative With AF | P Value | With First-Degree Relative With AF | Without First-Degree Relative With AF | P Value | |
| Patients with AF, No. | 1576 | 242 491 | NA | 1510 | 6040 | NA |
| CHA2DS2-VASc parameters within the first year after the diagnosis of AF, No. (%) | ||||||
| Age at onset of AF, y | ||||||
| ≥75 | 46 (2.9) | 101 942 (42.0) | <.001 | 45 (3.0) | 180 (3.0) | >.99 |
| 65-74 | 236 (15.0) | 68 662 (28.3) | 219 (14.5) | 876 (14.5) | ||
| <65 | 1294 (82.1) | 71 887 (29.7) | 1246 (82.5) | 4984 (82.5) | ||
| Female | 215 (13.6) | 109 048 (45.0) | <.001 | 204 (13.5) | 816 (13.5) | >.99 |
| Heart failure | 131 (8.3) | 51 547 (21.3) | <.001 | 117 (7.8) | 468 (7.8) | >.99 |
| Hypertension | 544 (34.5) | 143 388 (59.1) | <.001 | 511 (33.8) | 2044 (33.8) | >.99 |
| Stroke, TIA, or thromboembolism | 79 (5.0) | 32 670 (13.5) | <.001 | 64 (4.2) | 256 (4.2) | >.99 |
| Peripheral vascular disease | 24 (1.5) | 8240 (3.4) | <.001 | 12 (0.8) | 48 (0.8) | >.99 |
| Diabetes | 284 (18.0) | 66 351 (27.4) | <.001 | 258 (17.1) | 1032 (17.1) | >.99 |
| Medication use within the first year after the diagnosis of AF, No. (%) | ||||||
| Warfarin sodium | 44 (2.8) | 7404 (3.1) | .58 | 30 (2.0) | 120 (2.0) | >.99 |
| Dabigatran etexilate | 2 (0.1) | 180 (0.1) | .45 | 1 (0.1) | 4 (0.1) | >.99 |
| Aspirin | 140 (8.9) | 20 505 (8.5) | .54 | 118 (7.8) | 472 (7.8) | >.99 |
| Clopidogrel bisulfate | 23 (1.5) | 5225 (2.2) | .06 | 12 (0.8) | 48 (0.8) | >.99 |
| ACEI or ARB | 62 (3.9) | 16 148 (6.7) | <.001 | 42 (2.8) | 168 (2.8) | >.99 |
| β-Blocker | 113 (7.2) | 17 257 (7.1) | .93 | 90 (6.0) | 360 (6.0) | >.99 |
| Statin | 26 (1.7) | 4579 (1.9) | .49 | 12 (0.8) | 48 (0.8) | >.99 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; CHA2DS2-VASc, congestive heart failure, hypertension, age, diabetes, stroke, transient ischemic attack, vascular disease, and sex; NA, not applicable; TIA, transient ischemic attack.
Table 4. Relative Risk of Adverse Cardiovascular Events in Incident AF Patients With vs Without First-Degree Relatives With AF.
| Variable | With First-Degree Relative With AF (n = 1510) |
Without First-Degree Relative With AF (n = 6040) |
Relative Risk (95% CI) | ||
|---|---|---|---|---|---|
| No. | Prevalence, % | No. | Prevalence, % | ||
| Ischemic stroke | 66 | 4.37 | 319 | 5.28 | 0.79 (0.61-1.03) |
| Acute myocardial infarction | 10 | 0.66 | 66 | 1.09 | 0.58 (0.30-1.14) |
| Coronary revascularization | 30 | 1.99 | 158 | 2.62 | 0.73 (0.49-1.08) |
| Admission for heart failure | 82 | 5.43 | 294 | 4.87 | 1.09 (0.85-1.39) |
| Cardiogenic shock | 2 | 0.13 | 6 | 0.10 | 1.26 (0.25-6.22) |
| Malignant dysrhythmia | 8 | 0.53 | 39 | 0.65 | 0.79 (0.37-1.69) |
| Composite MACE | 198 | 13.11 | 882 | 14.60 | 0.86 (0.74-1.00) |
Abbreviations: AF, atrial fibrillation; MACE, major adverse cardiovascular events.
Discussion
In this study, we investigated the familial aggregation of AF in a population of more than 23 million people. The analysis yielded 4 main findings. First, patients with an affected first-degree relative were 1.92 times more likely to have AF than the general population. Second, genetic and shared environmental factors both had a minor role in the pathogenesis of AF, although nonshared environmental factors accounted for two-thirds of the phenotypic variance for susceptibility to AF. Third, age at onset of AF was 10 years younger in patients with a family history of AF than in those without a family history. Fourth and most important, among patients with AF, a family history of AF was not associated with an increased risk of MACE after matching for CHA2DS2-VASc parameters.
The moderate familial aggregation of AF found in this study is consistent with previous family studies. However, the estimated familial risk of AF was dependent on the age of the studied population and the type of AF being studied. For example, previous family studies have reported higher RRs in younger patients with lone AF. The RRs reported in this study were calculated from the whole population and therefore included all age groups and all types of AF. We found that a family history of AF was associated with approximately twice the risk of AF than in the general population and demonstrated that having 2 or more affected first-degree relatives was associated with a higher RR of 3.63. The magnitude of the RR was associated with the genetic distance of the affected kinship, with twins having the highest RR of 12.15. These findings support the existence of a familial aggregation of AF, although the magnitude of the risk was lower than previously reported. In addition, we found that AF in patients with a family history of AF tended to have an age at onset 10 years earlier than those without a family history. Therefore, a family history was associated with an increased overall risk of AF and an earlier onset of disease. It should be noted that the familial risk is higher in individuals who are younger, manifest closer relatedness, or have more affected relatives. Taken together, these findings suggest that AF is a multifactorial or complex disorder. Although there was no significantly different family risk between the sexes, a slightly stronger male transmission also implied that AF was a complex trait (the Carter effect).
An increased risk of AF in patients with an affected family member suggests that AF is a heritable condition; however, both genetic and shared environmental factors contributed to the familial risk of AF. Assuming that spouses share the familial environment but not genetics with other family members, they can be used to estimate the relative contribution of shared environmental factors to susceptibility to AF. We found that only approximately 20% of the phenotypic variance was due to genetic factors and that shared environmental factors only contributed marginally, whereas nonshared environmental factors accounted for more than two-thirds of the phenotypic variance. Compared with a 44% genetic contribution of the phenotypic variance in systemic lupus erythematosus, the decreased heritability of AF would be considered a minor component. Given that multiple comorbidities of AF, such as hypertension, diabetes, hyperthyroidism, and chronic kidney disease, have substantial heritability, the genetic contribution of AF per se may be lower than the estimated 20% after subtracting the association of concomitant diseases and structural changes. However, we were not able to test this hypothesis due to a lack of complete clinical data for the entire population, and further studies are needed to clarify this issue.
Numerous environmental risk factors for AF have been reported, including diet, alcohol or coffee consumption, air pollution, endurance sports, and smoking. Most important, some comorbidities (eg, hypertension, heart failure, valvular diseases, obesity, sleep apnea, hyperthyroidism, chronic kidney disease, and chronic obstructive pulmonary disease) should also be considered environmental factors. The interaction between these environmental factors is complex and beyond the scope of the present study.
Many clinical characteristics have been proposed to predict the prognosis of AF, including heart failure events, diabetes, smoking, and lung conditions. To our knowledge, the present investigation is the first large study to examine whether a family history of AF is associated with stroke or coronary events. The results showed that patients having AF with an affected first-degree relative with AF had a risk of MACE similar to that of patients without an affected first-degree relative with AF. This finding suggests that a family history of AF may be irrelevant in choosing the management strategies for AF in terms of stroke prevention.
The validity of this study is strengthened by the use of the NHIRD, which contains information on the entire population of Taiwan. In addition, the definitions of AF and MACE used in this study have been validated.
Limitations
Our study has some limitations. First, lone AF was not analyzed separately because it is difficult to identify lone AF cases using administrative data, although it is expected to have a higher heritability than common AF. Nevertheless, few patients have lone AF, and our primary objective was to measure the extent to which genetic factors have a role in common AF. The association of catheter ablation with AF was not available in the data set. Second, heritability was estimated using a threshold liability model, which assumed that cases of AF resulting from an underlying liability were normally distributed in the population. However, validation of this model has been verified in studies on schizophrenia, bipolar disorder, and major depression using Danish and Swedish population databases. In addition, there was no formal validation of AF diagnosis or familial relationship in the registry, although identical methods have been applied in multiple studies. Third, our nationwide genealogy reconstruction did not cover the entire population. Some individuals, especially mobile populations (eg, foreign workers or Taiwanese nationals living abroad), tend not to have recorded family relationships in the NHIRD. However, we believe that this limitation did not adversely affect our results because the procedure of reporting familial relationships is not related to AF or a family history of AF. Fourth, using spouses as a reference may have resulted in an underestimation of heritability because assortative mating was not considered. Fifth, our estimations were based on the population in Taiwan, and these findings may not be applicable to other populations. Therefore, additional studies in other populations are required to clarify the generalizability of our observations. Sixth, several residual risk factors for MACE other than CHA2DS2-VASc parameters and medication use, such as renal function, were not considered in the Cox proportional hazards regression model. In addition, we did not have complete original claims data for individuals without AF; therefore, we were not able to include factors like comorbidity and service use in the model. Seventh, because a lack of differentiation exists between various forms of AF (secondary vs primary, rheumatic vs nonrheumatic, and paroxysmal vs persistent) and given the observation that AF occurred much earlier in patients having first-degree relatives with AF, it is premature to conclude that genetics does not have a pivotal role in the pathophysiology of AF.
Conclusions
In this nationwide population-based study, AF was found to aggregate in families. Despite this observation, nonshared environmental factors seemed to have a stronger contribution to the phenotypic variance of AF than genetic factors. Age at onset of AF in patients having a first-degree relative with AF was much earlier than in those not having a first-degree relative with AF. However, patients having AF with vs without a first-degree relative affected by AF had a similar risk of stroke and coronary events. These findings provide valuable information for future studies on genetic and familial risks of AF, and they suggest that a family history of AF may not be particularly useful in the management of patients with AF.
eAppendix. Supplemental Appendix
eFigure 1. The Distribution of Diagnosis Age of AF in Those With Family History of AF and in Those Without
eFigure 2. The Distribution of Phenotypic Variance of AF Estimated by the Threshold Liability Model
eFigure 3. MACE-Free Survival of Newly Diagnosed AF Patients With or Without FDR Affected by AF
References
- 1.Passman R, Bernstein RA. New appraisal of atrial fibrillation burden and stroke prevention. Stroke. 2016;47(2):570-576. [DOI] [PubMed] [Google Scholar]
- 2.Kolek MJ, Muehlschlegel JD, Bush WS, et al. . Genetic and clinical risk prediction model for postoperative atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8(1):25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreasen L, Nielsen JB, Olesen MS. Genetic aspects of lone atrial fibrillation: what do we know? Curr Pharm Des. 2015;21(5):667-678. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg MA, Kaplan RC, Siscovick DS, et al. . Genetic variants related to height and risk of atrial fibrillation: the Cardiovascular Health Study. Am J Epidemiol. 2014;180(2):215-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnani JW, Rienstra M, Lin H, et al. . Atrial fibrillation: current knowledge and future directions in epidemiology and genomics. Circulation. 2011;124(18):1982-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aleong RG, MacRae CA, Ellinor PT. Heritability and genetics of atrial fibrillation. Curr Cardiovasc Risk Rep. 2007;1(5):414-419. doi: 10.1007/s12170-007-0068-7 [DOI] [Google Scholar]
- 7.Oyen N, Ranthe MF, Carstensen L, et al. . Familial aggregation of lone atrial fibrillation in young persons. J Am Coll Cardiol. 2012;60(10):917-921. [DOI] [PubMed] [Google Scholar]
- 8.Zöller B, Ohlsson H, Sundquist J, Sundquist K. High familial risk of atrial fibrillation/atrial flutter in multiplex families: a nationwide family study in Sweden. J Am Heart Assoc. 2012;2(1):e003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gundlund A, Christiansen MN, Hansen ML, et al. . Familial clustering and subsequent incidence of atrial fibrillation among first-degree relatives in Denmark. Europace. 2016;18(5):658-664. [DOI] [PubMed] [Google Scholar]
- 10.Fox CS, Parise H, D’Agostino RB Sr, et al. . Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291(23):2851-2855. [DOI] [PubMed] [Google Scholar]
- 11.Arnar DO, Thorvaldsson S, Manolio TA, et al. . Familial aggregation of atrial fibrillation in Iceland. Eur Heart J. 2006;27(6):708-712. [DOI] [PubMed] [Google Scholar]
- 12.Christophersen IE, Ravn LS, Budtz-Joergensen E, et al. . Familial aggregation of atrial fibrillation: a study in Danish twins. Circ Arrhythm Electrophysiol. 2009;2(4):378-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gundlund A, Fosbøl EL, Kim S, et al. ; ORBIT-AF Investigators . Family history of atrial fibrillation is associated with earlier-onset and more symptomatic atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J. 2016;175:28-35. [DOI] [PubMed] [Google Scholar]
- 14.Kuo CF, Grainge MJ, Valdes AM, et al. . Familial aggregation of systemic lupus erythematosus and coaggregation of autoimmune diseases in affected families. JAMA Intern Med. 2015;175(9):1518-1526. [DOI] [PubMed] [Google Scholar]
- 15.Kuo CF, Grainge MJ, See LC, et al. . Familial aggregation of gout and relative genetic and environmental contributions: a nationwide population study in Taiwan. Ann Rheum Dis. 2015;74(2):369-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao TF, Liu CJ, Wang KL, et al. . Using the CHA2DS2-VASc score for refining stroke risk stratification in “low-risk” Asian patients with atrial fibrillation. J Am Coll Cardiol. 2014;64(16):1658-1665. [DOI] [PubMed] [Google Scholar]
- 17.Chang SH, Wu LS, Chiou MJ, et al. . Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. 2014;13(1):123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan YH, Kuo CT, Yeh YH, et al. . Thromboembolic, bleeding, and mortality risks of rivaroxaban and dabigatran in Asians with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2016;68(13):1389-1401. [DOI] [PubMed] [Google Scholar]
- 19.Chan YH, Yeh YH, See LC, et al. . Acute kidney injury in Asians with atrial fibrillation treated with dabigatran or warfarin. J Am Coll Cardiol. 2016;68(21):2272-2283. [DOI] [PubMed] [Google Scholar]
- 20.Lai CH, Lai WW, Chiou MJ, et al. . Outcomes of percutaneous coronary intervention in patients with rheumatoid arthritis and systemic lupus erythematosus: an 11-year nationwide cohort study. Ann Rheum Dis. 2016;75(7):1350-1356. [DOI] [PubMed] [Google Scholar]
- 21.Risch N. Linkage strategies for genetically complex traits, I: multilocus models. Am J Hum Genet. 1990;46(2):222-228. [PMC free article] [PubMed] [Google Scholar]
- 22.Reich T, James JW, Morris CA. The use of multiple thresholds in determining the mode of transmission of semi-continuous traits. Ann Hum Genet. 1972;36(2):163-184. [DOI] [PubMed] [Google Scholar]
- 23.Rice TK. Familial resemblance and heritability. Adv Genet. 2008;60:35-49. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.So Y, Lin G, Johnston G Using the PHREG procedure to analyze competing-risks data. https://support.sas.com/rnd/app/stat/papers/2014/competingrisk2014.pdf. Accessed May 2017.
- 26.Lubitz SA, Yin X, Fontes JD, et al. . Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010;304(20):2263-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265(5181):2037-2048. [DOI] [PubMed] [Google Scholar]
- 28.Kantarci OH, Barcellos LF, Atkinson EJ, et al. . Men transmit MS more often to their children vs women: the Carter effect. Neurology. 2006;67(2):305-310. [DOI] [PubMed] [Google Scholar]
- 29.Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol. 1994;74(3):236-241. [DOI] [PubMed] [Google Scholar]
- 30.Shih CJ, Ou SM, Chao PW, et al. . Risks of death and stroke in patients undergoing hemodialysis with new-onset atrial fibrillation: a competing-risk analysis of a nationwide cohort. Circulation. 2016;133(3):265-272. [DOI] [PubMed] [Google Scholar]
- 31.Zöller B, Ohlsson H, Sundquist J, Sundquist K. Family history as a risk factor for recurrent hospitalization for lone atrial fibrillation: a nationwide family study in Sweden. BMC Cardiovasc Disord. 2012;12:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wray NR, Gottesman II. Using summary data from the Danish national registers to estimate heritabilities for schizophrenia, bipolar disorder, and major depressive disorder. Front Genet. 2012;3:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chao TF, Liu CJ, Liao JN, et al. . The use of oral anticoagulants for stroke prevention in atrial fibrillation patients with history of intra-cranial hemorrhage. Circulation. 2016;133(16):1540-1547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplemental Appendix
eFigure 1. The Distribution of Diagnosis Age of AF in Those With Family History of AF and in Those Without
eFigure 2. The Distribution of Phenotypic Variance of AF Estimated by the Threshold Liability Model
eFigure 3. MACE-Free Survival of Newly Diagnosed AF Patients With or Without FDR Affected by AF