Key Points
Question
What is the effectiveness and safety study of standard-dose nonvitamin K antagonist oral anticoagulants (NOACs) and warfarin among patients with atrial fibrillation with 1 stroke risk factor?
Findings
In this nationwide observational cohort study, no significant differences of the NOACs compared with warfarin across strata were evident for the principal effectiveness end point of ischemic stroke/systemic embolism. The end point of “any bleeding” was significantly lower for patients treated with apixaban and dabigatran and not significantly different for patients treated with rivaroxaban vs warfarin; however, there was possible residual confounding across these comparisons that was unmasked with falsification outcomes.
Meaning
Our data show no significant differences of the NOACs compared with treatment with warfarin for ischemic stroke/systemic embolism, but for “any bleeding,” this was lower for treatment with apixaban and dabigatran.
Abstract
Importance
The randomized clinical trials comparing nonvitamin K antagonist oral anticoagulants (NOACs) vs warfarin largely focused on recruiting high-risk patients with atrial fibrillation with more than 2 stroke risk factors, with only the trials testing dabigatran or apixaban including few patients with 1 stroke risk factor. Despite this, regulatory approvals of all NOACs have been based on stroke prevention for patients with atrial fibrillation with 1 or more stroke risk factors.
Objective
To compare the effectiveness and safety study of standard-dose NOACs (dabigatran at 150 mg twice daily, rivaroxaban at 20 mg once daily, and apixaban at 5 mg twice daily) and warfarin in patients with atrial fibrillation with 1 low-risk, nonsex-related stroke risk factor.
Design, Setting, and Participants
This nationwide observational cohort study used data from Danish registries to determine the inverse probability of treatment-weighted comparative effectiveness and safety of standard-dose NOACs (dabigatran at 150 mg twice daily, rivaroxaban at 20 mg once daily, and apixaban at 5 mg twice daily) compared with treatment with warfarin among 14 020 patients with atrial fibrillation with 1 low-risk, nonsex- related stroke risk factor.
Main Outcomes and Measures
Rates of ischemic stroke/systemic embolism, death, and bleeding.
Results
Of 14 020 participants, 5151 (36.7%) were women, and the median age for participants was 66.5 years. For the principal effectiveness end point of ischemic stroke/systemic embolism, no significant differences of the NOACs compared with treatment with warfarin across strata were evident. For the end point of “any bleeding,” this was significantly lower for treatment with apixaban (hazard ratio [HR], 0.35; 95% CI, 0.17-0.72) and dabigatran (HR, 0.48; 95% CI, 0.30-0.77) compared with warfarin in the main analysis, and was not significantly different for treatment with rivaroxaban vs warfarin (HR, 0.84; 95% CI, 0.49-1.44). There was broad consistency across most subgroups in the sensitivity analyses and whether 1- or 2.5-year follow-up periods were analyzed. However, falsification end points generally did not falsify, indicating the possible presence of residual confounding across these comparisons, presumably related to selective prescribing and unobserved covariates.
Conclusions and Relevance
In this Danish cohort study of patients with atrial fibrillation and a single stroke risk factor, there was no difference between NOACs compared with treatment with warfarin in terms of the risk of having an ischemic stroke/systemic embolism. For “any bleeding,” this was lower for treatment with apixaban and dabigatran compared with warfarin. These data do not allow for a definitive statement of the comparative effectiveness or safety of NOACs because of the possible residual confounding that was unmasked with falsification outcomes.
This observational cohort study examines the differences between treating patients with atrial fibrillation with 1 stroke risk factor with nonvitamin K antagonist oral anticoagulants vs warfarin.
Introduction
In the respective randomized clinical trials of nonvitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in atrial fibrillation (AF), the focus was on recruiting patients with AF with 2 or more stroke risk factors, and only the trials testing treatment with dabigatran or apixaban included a minority of patients with 1 stroke risk factor. To our knowledge, specific randomized clinical trials including patients with AF with only 1 stroke risk factor have not been performed, and there is a low likelihood for such trials to be conducted. The objective of this study was to investigate the comparative effectiveness and safety of standard-dose NOACs (dabigatran at 150 mg twice daily, rivaroxaban at 20 mg once daily, and apixaban at 5 mg twice daily) compared with treatment with warfarin among such patients in the Danish registries.
Methods
We conducted an observational cohort study of Danish citizens initiating OAC treatment for stroke prevention in AF with 1 stroke risk factor. To align with previous randomized clinical trials, we focused on standard-dose NOAC agents only and used Danish nationwide databases (eMethods in the Supplement).
The study was based on new users of OACs with no history of treatment for other indications than AF. For this analysis, we only recruited patients with 1 nonsex-related stroke risk factor that was assigned 1 point in the CHA2DS2-VASc score (ie, congestive heart failure or left ventricular disease, hypertension, 65 years ≤ age <75 years, and diabetes or vascular diseases such as myocardial infarction, aortic plaque or peripheral aortic disease), thus excluding patients with prior strokes, systemic embolisms/transient ischemic attacks, or those who were younger than 75 years.
Nonvitamin K antagonist oral anticoagulants were restricted to standard doses: apixaban at 5 mg twice daily, dabigatran at 150 mg twice daily, and rivaroxaban at 20 mg once daily. To establish an OAC-naive cohort, we excluded patients previously treated with OAC-inclusive doses approved for other indications within 1 year. Eventually, we excluded patients with prior diagnoses of valvular AF or a venous thromboembolism. Also, patients with contraindications for standard NOAC dose regimens because of renal impairment were excluded. This population formed the main cohort for the analyses. Sensitivity analyses were reported on the subgroup with prior diagnoses of AF. To increase homogeneity across patient groups, we also performed a sensitivity analysis in which patients with prior heart failure, chronic pulmonary disease, or cancer were excluded.
End Points and Variable Definitions
Clinical end points were extracted from hospital discharge codes with follow-up until April 30, 2016 (eTable 1 in the Supplement). Primary and secondary discharge codes from nonemergency wards were extracted. We only analyzed end points obtained as discharge codes from hospitalizations and did not include codes from ambulatory visits.
Efficiency was investigated by the occurrence of ischemic stroke or systemic embolism. All-cause death was included as a lone end point. The safety of the treatments was investigated by the following bleeding events: intracranial, gastrointestinal, traumatic intracranial, and clinically relevant nonmajor bleeding reported in total as “any bleeding” and more specifically for intracranial (including traumatic) and gastrointestinal bleeding (see eTable 1 in the Supplement for International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] discharge codes).
A patient’s baseline comorbidities and comedications when treatment was initiated were ascertained (Table 1) (eTable 1 in the Supplement). The overall risk of bleeding was assessed by the hypertension, abnormal renal and liver function, stroke, bleeding, labile international normalized ratio, elderly, drugs or alcohol (HAS-BLED) score (eTable 2 in the Supplement). The Danish registries are well-validated with a sufficiently high positive predictive value (>80%) of included comorbidities and outcomes.
Table 1. Participant Characteristics at Treatment Initiation According to Treatment.
| Characteristic | No. (%) | ||||
|---|---|---|---|---|---|
| Apixaban | Dabigatran | Rivaroxaban | Warfarin | All | |
| No. of patients | 1470 | 3272 | 1604 | 7674 | 14 020 |
| Women | 589 (40.1) | 1160 (35.5) | 609 (38.0) | 2793 (36.4) | 5151 (36.7) |
| Age, median (IQR) | 67.4 (62.5-70.9) | 66.2 (61.3-69.8) | 67.2 (62.4-70.7) | 66.2 (60.5-70.4) | 66.5 (61.1-70.4) |
| Heart failure or LVD | 31 (2.1) | 90 (2.8) | 17 (1.1) | 232 (3.0) | 370 (2.6) |
| Hypertension | 411 (28.0) | 1134 (34.7) | 471 (29.4) | 2430 (31.7) | 4446 (31.7) |
| 65≤ age <75 y | 963 (65.5) | 1884 (57.6) | 1037 (64.7) | 4435 (57.8) | 8319 (59.3) |
| Diabetes | 44 (3.0) | 96 (2.9) | 41 (2.6) | 271 (3.5) | 452 (3.2) |
| Vascular disease | 21 (1.4) | 68 (2.1) | 38 (2.4) | 306 (4.0) | 433 (3.1) |
| Prior AF diagnosis | 1042 (70.9) | 2300 (70.3) | 1037 (64.4) | 4114 (53.6) | 8489 (60.5) |
| Cancer | 206 (14.0) | 345 (10.5) | 217 (13.5) | 1079 (14.1) | 1847 (13.2) |
| HAS-BLED score, mean (SD)a | 1.5 (0.6) | 1.5 (0.6) | 1.5 (0.6) | 1.5 (0.7) | 1.5 (0.7) |
| Hepatic dysfunction | <0.3 (<5) | <0.1 (<5) | 0.3 (5) | 0.4 (29) | 0.3 (39) |
| Alcohol | 38 (2.6) | 83 (2.5) | 55 (3.4) | 264 (3.4) | 440 (3.1) |
| CPD | 128 (8.7) | 237 (7.2) | 115 (7.2) | 665 (8.7) | 1145 (8.2) |
| Previous bleeding | 128 (8.6) | 224 (6.8) | 128 (8.0) | 521 (6.8) | 999 (7.1) |
| Aspirin | 342 (23.3) | 960 (29.3) | 437 (27.2) | 2316 (30.2) | 4055 (28.9) |
| Ticagralor | <0.3 (<5) | 0.4 (12) | <0.2 (<5) | 0.4 (31) | 0.3 (49) |
| Clopidogrel | 28 (1.9) | 55 (1.7) | 34 (2.1) | 181 (2.4) | 298 (2.1) |
| β-blockers | 914 (62.2) | 2175 (66.5) | 931 (58.0) | 4408 (57.4) | 8428 (60.1) |
| NSAIDs | 339 (23.1) | 806 (24.6) | 386 (24.1) | 2053 (26.8) | 3584 (25.6) |
| Statins | 359 (24.4) | 817 (25.0) | 387 (24.1) | 2053 (26.8) | 3616 (25.8) |
| ACE/ARB inhibiters | 396 (26.8) | 1000 (30.6) | 430 (26.8) | 2118 (26.8) | 3944 (28.1) |
| Loop diuretics | 97 (6.6) | 200 (6.1) | 94 (5.9) | 680 (8.9) | 1071 (7.6) |
| Concomitant cardiovascular drugs, No.b | |||||
| None | 641 (43.6) | 1288 (39.4) | 627 (39.1) | 3019 (39.3) | 5575 (39.8) |
| 1 | 341 (23.2) | 727 (22.2) | 394 (24.6) | 1713 (22.3) | 3175 (22.6) |
| 2 | 259 (17.6) | 602 (18.4) | 298 (18.6) | 1403 (18.3) | 2562 (18.3) |
| 3 | 127 (8.6) | 369 (11.3) | 177 (11.0) | 901 (11.7) | 1574 (11.2) |
| ≥4 | 102 (6.9) | 286 (8.7) | 108 (6.7) | 638 (8.3) | 1134 (8.1) |
Abbreviation: ACE/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; AF, atrial fibrillation; CPD, chronic pulmonary disease; HAS-BLED, hypertension, abnormal renal and liver function, stroke, bleeding, labile international normalized ratio, elderly, drugs or alcohol; IQR, interquartile range; NSAIDs, non-steroidal anti-inflammatory drugs; LVD, left ventricle disease.
HAS-BLED: score ranging from 0 to 9, which reflects the risk of bleeding in patients with atrial fibrillation undergoing anticoagulant therapy (eTable 2 in the Supplement).
Cardiovascular drugs covers all Anatomical Therapeutic Chemical codes in groups B and C.
Statistical Analysis
A time to event analysis was used to compare the risk of an end point between treatment groups, measuring risk time from the patient receiving their initial prescription and until the relevant event, emigration, death, or end of follow-up (whichever came first). An intent-to-treat approach was applied for the main analyses. This was supplemented by a continuous treatment analysis by censoring the follow-up if the patient was prescribed another treatment than what was initiated.
Crude incidence rates were calculated as the number of events divided by the person-time. Cox regressions with a robust variance estimator were used to compare event rates between the treatment groups, with warfarin as the primary reference. To address confounding by indication of treatment, an inverse probability of treatment weighted (IPTW) analysis was applied. We used weights that enabled estimates representing mean population treatment effects. The underlying propensity models included the following treatment predictors: age (continuous); binary indicators for sex, prior bleeding, vascular disease, hypertension, diabetes, renal disease, chronic pulmonary disease, heart failure, cancer, and a recent prescription for aspirin, β-blockers, nonsteroidal anti-inflammatory drugs, statins, or loop diuretics; and the HAS-BLED score.
Balances between treatment populations were evaluated by the standardized differences of all baseline covariates, using a threshold of 0.1 to indicate an imbalance.
To evaluate the potential for residual confounding, falsification analysis was performed by applying the propensity–weighted cohort in the analyses on (falsification) end points that a priori should be expected not to be associated with the effects of treatment. For this study, we considered pneumonia, hip fractures, cancer, and urinary tract infections.
We repeated the analyses on the subgroup with a hospital discharge diagnosis for AF either before or within 30 days of first receiving a prescription. In addition, as there may be confounding because of differences in the health status of patients, we also reported results of analyses in which patients with high-mortality conditions (heart failure, cancer, or chronic pulmonary disorder) at the time treatment was initiated were excluded. Eventually, the results of the IPTW analysis were compared with a trimmed analysis that removed 5% of the extreme weights as well as with an ordinary crude and adjusted analysis (data not shown).
The analyses on the entire population were supplemented by stratified analyses on the populations with 1 of the frequent risk factors who were older than 65 and patients with hypertension; additionally, the results of the analyses were reported for men and women separately. Stata/MP, version 14 (StataCorp) and R version 3.1.1 (The R Foundation) were used for the statistical analysis. A 2-tailed P value of less than .05 was considered statistically significant.
Ethical Considerations
No ethical approval is required for anonymous register studies in Denmark. The study was approved by the Danish Data Protection Agency (J. No. File No. 2012-41-0633).
Results
We identified 240 360 unique OAC users in the inclusion period, 59% of whom (n = 142 307) either prevalently used vitamin K antagonists or had an indication for treatment because of other reasons than nonvalvular AF. Of the remaining OAC users (n = 98 053), we excluded 22% (n = 21 456) because of treatment with reduced-dose NOACs or because of the presence of prior discharge codes for renal impairment. Thus, the study population covers 76 597 new users of OAC treatment for AF, with either standard-dose NOACs or warfarin. Of these, 82% (n = 62 577) were excluded because they had either 0 or more than 1 nonsex-related stroke risk factor or because their risk factors had scored a 2-point CHA2DS2-VASc score.
The final study population (n = 14 020) was distributed according to treatment type: warfarin (n = 7674 [54.7%]), dabigatran (n = 3272 [23.3%]), rivaroxaban( n = 1604 [11.4%]), and apixaban (n = 1470 [10.5%]) (eFigure 1 in the Supplement). The mean (SD) follow-up was 2.6 (1.6) years, with the shortest follow-up for the group treated with apixaban (mean [SD] follow-up, 1.1 [0.7] years) reflecting its later market introduction.
Baseline information about the initial study population before weighing is shown in Table 1. The 5151 women (36.7%) included in the study population were separated into treatment groups: 1160 (35.5%) were treated with dabigatran, 589 (40.1%) were treated with apixaban, 2793 (36.4%) were treated with warfarin, and 609 (38.0%) were treated with rivaroxaban. All patients had 1 nonsex-related and 1 point-valued stroke risk factor according to the CHA2DS2-VASc score. The dominant factor for inclusion was being 65 to 74 years (n = 8319, 59.3%). Patients initiated with dabigatran or warfarin were slightly younger; 1884 patients (57.6%) were treated with dabigatran and 4435 (57.8%) were older than 65 years compared with 963 patients (65.5%) treated with apixaban and 1037 patients (64.7%) treated with rivaroxaban who were older than 65 years. Hypertension, which affected 4446 participants (31.7%), was lowest among the 411 patients (28.0%) initiated with apixaban and 471 patients (29.4%) treated with rivaroxaban and highest among the 1134 patients (37.4%) using dabigatran. The remaining 1255 patients (8.9%) were included because they received a diagnosis of vascular diseases (eg, myocardial infarction, peripheral artery disease, or aortic plaques), diabetes, or heart failure. Diabetes was the primary reason among the NOACs, whereas they were distributed more evenly among patients treated with warfarin. Notably, 8750 patients (62.4%) had a prescription history with either 0 or only 1 drug within the Anatomical Therapeutic Chemical chapters B or C, which cover multiple cardiovascular indications. Apixaban had the highest proportion (n = 982, 66.8%) and warfarin (n = 4732, 61.6%) and dabigatran (n = 2015, 61.6%) had a lower proportion. Approximately 1134 patients (8%) were intensively treated, with 4 or more different drugs prescribed, ranging from 108 patients (6.7%) in the group treated with rivaroxaban to 286 patients (8.7%) in the group treated with dabigatran.
After weighing the study populations using the IPTW method, all baseline differences were less than 0.08 standardized differences at maximum (eFigure 2 in the Supplement). An inspection of individual propensity score distributions showed a sufficient overlap between treatment populations to obtain a valid comparison (eFigures 3-5 in the Supplement).
Effectiveness: Stroke and Systemic Embolism
Event count and crude or weighted rates for the different NOACs and warfarin are shown in Table 2, for 1 and 2.5 years follow-up. Falsification analysis, described below, found that these end points did not falsify, indicating residual confounding. Survival analyses are first described below.
Table 2. Number of Events and Crude and Weighted Event Rates According to Treatmenta.
| Apixaban | Dabigatran | Rivaroxaban | Warfarin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Rate | Events | Rate | Events | Rate | Events | Rate | |||||
| Crude | Weighted | Crude | Weighted | Crude | Weighted | Crude | Weighted | |||||
| 1-y Follow-up | ||||||||||||
| Ischemic stroke/SE | 10 | 0.68 | 0.83 | 22 | 0.70 | 0.65 | 14 | 1.09 | 1.20 | 57 | 0.79 | 0.81 |
| All-cause death | 19 | 1.62 | 1.52 | 51 | 1.62 | 1.84 | 26 | 2.01 | 1.67 | 249 | 3.45 | 3.11 |
| Any bleeding | 8 | 0.68 | 0.57 | 23 | 0.74 | 0.73 | 17 | 1.33 | 1.38 | 109 | 1.52 | 1.53 |
| Intracranial/gastrointestinal bleeding | <5 | 0.09 | 0.06 | 6 | 0.19 | 0.16 | <5 | 0.23 | 0.29 | 39 | 0.54 | 0.54 |
| 2.5-y Follow-up | ||||||||||||
| Ischemic stroke/SE | 14 | 0.85 | 0.85 | 41 | 0.61 | 0.58 | 16 | 0.75 | 0.81 | 109 | 0.70 | 0.69 |
| All-cause death | 22 | 1.32 | 1.21 | 91 | 1.33 | 1.48 | 49 | 2.28 | 2.10 | 428 | 2.71 | 2.47 |
| Any bleeding | 11 | 0.67 | 0.56 | 44 | 0.65 | 0.65 | 22 | 1.04 | 1.09 | 202 | 1.30 | 1.31 |
| Intracranial/gastrointestinal bleeding | <5 | 0.12 | 0.12 | 17 | 0.25 | 0.21 | 6 | 0.28 | 0.30 | 72 | 0.46 | 0.45 |
Abbreviations: IPTW, inverse probability of treatment weighed; SE, systemic embolism.
Crude rates are events divided by person time per 100 years; weighted rates are based on IPTW population and express population mean treatment rates per 100 years. Main analysis.
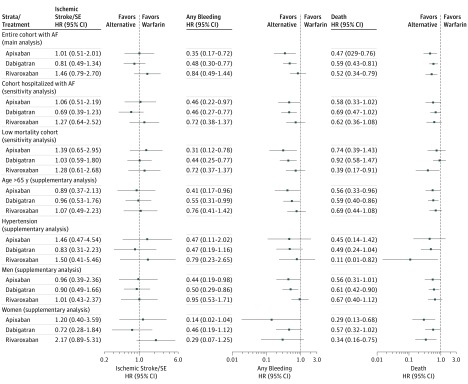
For the principal effectiveness end point of ischemic stroke/systemic embolism, 103 events (80.5% as primary diagnoses) were observed within the first year, representing a rate between 0.65 and 1.20 per 100 person-years among the IPTW population. The estimated effect sizes in terms of hazard ratios (HR) of the NOACs ranged between 0.81 (95% CI, 0.49-1.34) and 1.46 (0.79-2.70), with all treatment groups displaying statistically nonsignificant differences compared with warfarin (Figure 1). The primary effectiveness outcome was almost exclusively composed of ischemic stroke, and only 3 events were systemic embolism (data not shown).
Figure 1. Propensity Weighted Cox Hazard Ratios for 1-Year Follow-Up for Nonvitamin K Antagonist Anticoagulants Compared With Warfarian for Effectiveness, Safety, and Death End Points.
The corresponding results for a follow-up of 2.5 years are provided in eFigure 8. AF indicates atrial fibrillation; SE, systemic embolism.
In sensitivity analyses restricted to patients with a history of AF discharge codes or to the cohort with a low mortality for the composite end point of ischemic stroke/systemic embolism at 1 year, the findings from the main analysis were generally in agreement, and no statistically significant differences between NOAC agents compared with warfarin were observed. The number of events and the event rates for the sensitivity analyses on the configuration of the study cohorts are provided in Table 3.
Table 3. Number of Events and Crude and Weighted Event Rates at 1-Year Follow-up According to Treatment for Main End Points and Selected Study Cohortsa.
| End Point/Cohort | Apixaban | Dabigatran | Rivaroxaban | Warfarin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Rate | Events | Rate | Events | Rate | Events | Rate | |||||
| Crude | Weighted | Crude | Weighted | Crude | Weighted | Crude | Weighted | |||||
| Ischemic Stroke/SE | ||||||||||||
| Main analysis cohort | 10 | 0.86 | 0.83 | 22 | 0.70 | 0.65 | 14 | 1.09 | 1.20 | 57 | 0.79 | 0.81 |
| Cohort hospitalized with AF | 9 | 1.01 | 1.07 | 17 | 0.69 | 0.66 | 11 | 1.25 | 1.29 | 41 | 0.94 | 0.96 |
| Low mortality cohort | 9 | 1.00 | 1.00 | 19 | 0.75 | 0.79 | 10 | 0.97 | 0.92 | 38 | 0.68 | 0.70 |
| All-Cause Death | ||||||||||||
| Main analysis cohort | 19 | 1.62 | 1.52 | 51 | 1.62 | 1.84 | 26 | 2.01 | 1.67 | 249 | 3.45 | 3.11 |
| Cohort hospitalized with AF | 14 | 1.56 | 1.40 | 36 | 1.46 | 1.60 | 15 | 1.69 | 1.50 | 107 | 2.44 | 2.33 |
| Low mortality cohort | 11 | 1.21 | 0.96 | 26 | 1.02 | 1.14 | 6 | 0.58 | 0.50 | 69 | 1.24 | 1.24 |
| Any Bleeding | ||||||||||||
| Main analysis cohort | 8 | 0.68 | 0.57 | 23 | 0.74 | 0.73 | 17 | 1.33 | 1.38 | 109 | 1.52 | 1.53 |
| Cohort hospitalized with AF | 8 | 0.90 | 0.79 | 18 | 0.74 | 0.72 | 12 | 1.36 | 1.24 | 69 | 1.58 | 1.59 |
| Low mortality cohort | 5 | 0.55 | 0.44 | 15 | 0.59 | 0.58 | 11 | 1.06 | 1.02 | 70 | 1.26 | 1.32 |
Abbreviations: AF, atrial fibrillation; IPTW, inverse probability of treatment weighed; SE, systemic embolism.
Crude rates are events divided by person time per 100 years; weighted rates are based on IPTW population and express population mean treatment rates per 100 years.
All-Cause Mortality
There was an apparent significant lower risk of all-cause mortality, death, across all treatment groups compared with warfarin, although effect sizes shifted toward unity and became nonsignificant when restricted to patients who were hospitalized with AF (Figure 1).
When restricting the study population to patients without conditions associated with increased mortality (ie, excluding patients with heart failure, chronic pulmonary disease, or cancer), all effect estimates were lower for NOAC agents compared with warfarin, while only treatment with rivaroxaban reached a statistically significant lower death rate (HR, 0.39; 95% CI, 0.17-0.91).
Excluding approximately 3084 patients (22%) with a conjectured high mortality risk resulted in a pronounced reduction in the absolute number of deaths (from 345 to 112) across treatment groups (eFigure 1 in the Supplement). The results of the supplementary analyses generally align with the main analysis, but should be interpreted with caution in light of the sensitivity analysis on the group with low mortality rates.
Bleeding Events
A total of 157 events (90% primary diagnoses) of the combined end point of “any bleeding” were observed during the first year of follow-up. The weighted event rates ranged between 0.57 (apixaban) to 1.53 (warfarin).
When compared with warfarin, apixaban and dabigatran were associated with lower bleeding events (HR, 0.35; 95% CI, 0.17-0.72; and HR, 0.48; 95% CI, 0.30-0.77, respectively) (Figure 1). This was statistically nonsignificant for the comparison of rivaroxaban vs warfarin (HR, 0.84; 95% CI, 0.49-1.44).
In sensitivity analyses restricted to patients with AF with an AF hospital discharge code or to the cohort with low mortality rates, the rates were overall lower in the latter cohort, although altogether the direction of associations from the main analysis was generally maintained. For the supplementary subgroup analyses, the smaller sample sizes led to more uncertain effect estimates, although they were aligned with the main analyses (eTables 3-8 and eFigures 6 and 7 in the Supplement).
After analyzing the outcomes of intracranial (including traumatically induced) bleeding and gastrointestinal bleeding, few events were observed among the populations treated with NOACs (ie, no more than 6 events in each treatment group) (Table 2). Fifty five bleeding events (35%) were observed. When compared with warfarin, each NOAC exhibited a lower relative risk of intracranial bleeding with estimates (ie, HRs) ranging from 0.11 (95% CI, 0.01-0.78) to 0.53 (95% CI, 0.15-1.87). The low event numbers resulted in wide confidence intervals, which preclude any statistically significant conclusions.
Results for a follow-up of 2.5 years (eFigure 8 in the Supplement) aligned with these results, as did the trimmed IPTW analyses when compared with the adjusted Cox regression on the unweighted population (data not shown).
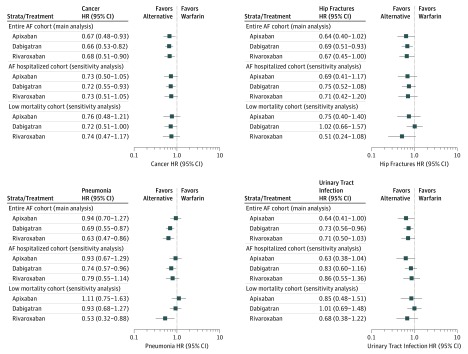
Falsification Analysis
For the falsification outcomes analyses on the associated treatment-exposure relationship, the a priori null-hypothesis of neutral associations was generally rejected, indicating a possible persistent bias. In the subgroup analysis of the cohort hospitalized with AF and the cohort with low mortality rates, the associations between exposure and studied falsification outcomes were neutral, indicating a low likelihood of persistent bias (Figure 2, eFigure 8, and eTable 9 in the Supplement).
Figure 2. Inverse Probability of Treatment Weighted Cox Hazard Ratios for 1-Year Follow-up for Nonvitamin K Antagonist Oral Anticoagulants Compared With Warfarin for Falsification Analysis.
AF indicates atrial fibrillation.
Discussion
In this study of patients with AF with 1 nonsex-related CHA2DS2-VASc risk factor, our principal findings were as follows: (1) for ischemic stroke/systemic embolism, no significant differences of the NOACs compared with warfarin were evident; (2) death was significantly lower for all 3 NOACs compared with warfarin, but this appears mainly driven by intrinsic selective prescription patterns that warrant cautious interpretation; and (3) any bleeding was significantly lower for apixaban and dabigatran compared with warfarin and showed nonsignificant differences between rivaroxaban and warfarin. Nonetheless, the falsification end points generally did not falsify, indicating some residual confounding across these comparisons that was presumably related to selective prescribing and unobserved comorbidities.
In patients with a CHADS2 score of 0 to 1, ancillary analyses from the RELY, ARISTOTLE, and AVERROES clinical trials show that there was no significant interaction for the efficacy and safety data on NOACs (ie, dabigatran or apixaban) compared with warfarin or aspirin. However, the randomized clinical trials for rivaroxaban (ROCKET-AF) or edoxaban (ENGAGE-AF) did not include patients with 1 stroke risk factor; for example, the inclusion criteria for ROCKET-AF was confined to patients with a CHADS2 score of 2 or more or having experienced a prior stroke, and even those with a CHADS2 score of 2 were capped at 10%. Hence, the relative effectiveness and safety of these 2 NOAC agents (ie, rivaroxaban or edoxaban, compared with warfarin) can benefit from observational cohort studies. As mentioned, the lack of clinical trial data has led to some guidelines that express a preference for treatment with dabigatran or apixaban for patients with 1 stroke risk factor, with the other NOACs or warfarin as “alternatives.” However, the current analysis cannot firmly support these preferences, and prescribing physicians are also encouraged to evaluate each patient with AF individually to select the preferred agent.
Many guidelines have expressed a preference for NOACs over warfarin for treating patients with AF with 1 or more stroke risk factors. The current threshold for OAC for stroke prevention has been quoted as a stroke rate of 1.7%year for warfarin, and 0.9%/year for an NOAC, reflecting the change in tipping point for treatment with the availability of NOACs. Even this treatment threshold of 1.7%/year with warfarin could be lowered with good-quality anticoagulation control with a time in therapeutic range of more than 70%. Given the lack of specific randomized clinical trial data in an exclusive patient population with 1 stroke risk factor, guidelines have suggested that OACs should be considered (grade IIa recommendation) for those with 1 risk factor (CHA2DS2-VASc score of 1 in men or 2 in women) whereas OACs are recommended (grade I recommendation) for patients with 2 or more stroke risk factors.
Nevertheless, there has been controversy regarding the actual stroke event rates with 1 stroke risk factor, given the likely differences of risk associated with different risk factors. Also, different study settings (eg, hospitalized vs community), races/ethnicities (Asian vs non-Asian) and methods of analyses may be relevant factors. Indeed, some studies putatively reporting low event rates excluded patients who ever took OACs, hence conditioning analyses on the future, and artificially biasing results toward low event rates. Even 1 stroke risk factor has a positive net clinical benefit for OAC treatment compared with aspirin or no treatment. Also, stroke risk among patients with AF not a static phenomenon, and clinical scores like the CHA2DS2-VASc scores are reductionist and a simplification.
The observation on the lower risk of death among patients who received NOAC treatment compared with warfarin requires attention. This difference in conclusion is likely because of selective prescribing by physicians; these analyses could suggest that rivaroxaban may be preferred for frailer patients compared with the other treatment alternatives. Causes of death available in real-world data analyses are in general not adjudicated and either cerebral imaging or post mortems are not mandated. It is evident that some deaths could be because of fatal strokes, as there is an increased risk of stroke in AF. Nonetheless, both stroke and death has been shown to be significantly reduced by treatment with warfarin vs placebos/controls. For mortality rates, there is even an added 10% reduction with treatment with NOACs over warfarin.
Our falsification end points generally did not falsify, indicating the presence of residual confounding across these comparisons. Indeed, there are multiple considerations that indicate confounding. For example, there are important variables that are known to influence treatment selection that are not present in the data set, such as body mass index (calculated as weight in kilograms divided by height in meters squared), actual blood pressures, heart rate, smoking status, estimated glomerular filtration rate, hematocrit, New York Heart Association class, left ventricle ejection fraction, and type of AF. For the results to be totally unconfounded, these factors would need to be unrelated to the treatment assignment. Successful falsification does not prove a lack of confounding, as the sources of confounding may be different for different outcomes. However, the failure to falsify (as in our study) raises the likelihood that unmeasured confounders exist, and may well be relevant to our outcomes of interest and, perhaps add bias toward NOACs.
Indeed, our analyses indicate that some selective prescribing may contribute to an important bias of the rates, and caution concerning conclusions on effects associated with death is warranted. Indeed, the analyses on falsification outcomes could not provide a reassurance of unbiased estimates, recognizing the findings of positive associations in the 4 investigations of false outcomes. However, when investigating falsified outcomes among the cohort with low mortality rates, we observed neutral associations when contrasting exposure groups, while the associations on treatment exposure and effectiveness or safety outcomes were generally maintained. While these (sensitivity) analyses do not guarantee that our analyses are completely unbiased, it does provide some reassurance and indicates that the observed associations may be “true” associations.
Concisely, observational studies are inherently subject to confounding and may lead to biased estimates. However, we systematically and analytically investigated confounding and biases in sensitivity analyses and falsification outcomes, and in general, our approaches pointed toward causal associations between treatment exposure and effectiveness and safety outcomes.
The main analysis of the end point of “any bleeding” indicated that it was significantly lower for apixaban and dabigatran compared with warfarin but was nonsignificant for the comparison of rivaroxaban vs warfarin. Again, this is broadly consistent with prior analyses. Ancillary analyses of randomized clinical trial cohorts for the minority of patients with a CHADS2 score of 0 to 1 show consistency between the effect sizes of NOAC vs warfarin and the main trial observations. There was broad consistency across most subgroups in our sensitivity analyses and whether 1- or 2.5-year follow-up periods were analyzed.
Limitations
The main limitation of this study relates to the observational nature of the data, and residual or unmeasured confounding probably persists. A propensity weighing approach to account for baseline differences were used; however, the full extent and effect of different prescribing behavior may not be fully captured and may have caused biased effect estimates. Indeed, because we investigated rare outcomes, even a few erroneously coded outcomes could shift the direction of associations. Nevertheless, the outcomes ascertained have been previously validated with a high-positive predictive value (eg, 97%-100% for ischemic stroke).
The presented sensitivity analysis on the cohort with low mortality rates suggests selective prescribing and warrants caution on the conclusions drawn on mortality rates. Indeed, end points were not adjudicated (unlike among a clinical trial cohort) and postmortems were not mandated, which leaves the possibility that some deaths could be because of fatal strokes uncertain. Information on the time in therapeutic range among people treated with warfarin was not available, nor did we have detailed information on laboratory (eg, serum creatinine), anthropometric, or socioeconomic data. Thus, our confounder adjustment was partially determined by the information available in the registries. Patient adherence to the NOACs and prescribing practices was not considered, but may be a potential driver of the differences between drugs, and differences in a patient’s medical experience may influence his or her adherence to treatment. Our data apply to a predominantly white European population, and differential efficacy and safety benefits may be evident among Asian and non-Asian people. Indeed, these limitations could nullify the possible benefits of NOACs, notwithstanding that our data show that at the very minimum, the NOACs have a similar effectiveness to warfarin with trends toward a better safety profile.
Conclusions
In this Danish observational cohort study of patients with AF and a single stroke risk factor, there was no difference between NOACs compared with warfarin in the risk of patients experiencing an ischemic stroke/systemic embolism. For “any bleeding,” this was lower for apixaban and dabigatran compared with warfarin. These data do not allow for a definitive statement regarding the comparative effectiveness or safety of NOACs because of possible residual confounding unmasked with falsification outcomes.
eMethods. Extended Methods.
eReferences.
eTable 1. Definitions on Comorbidity and Concomitant Medication According to ICD-10 Codes and ATC-Codes.
eTable 2. Risk Score Definitions
eTable 3. Number of Events, Crude and Weighted Event Rates According to Treatment. AF Hospitalized Cohort—Sensitivity Analysis.
eTable 4. Number of Events, Crude and Weighted Event Rates According to Treatment. Low Mortality Cohort—Sensitivity Analysis.
eTable 5. Number of Events, Crude and Weighted Event Rates According to Treatment. Patients in Age Between 65 and 75 years—Supplementary Analysis.
eTable 6. Number of Events, Crude and Weighted Event Rates According to Treatment. Patients With Hypertension—Supplementary Analysis.
eTable 7. Number of Events, Crude and Weighted Event Rates According to Treatment. Male Patients—Supplementary Analysis.
eTable 8. Number of Events, Crude and Weighted Event Rates According to Treatment. Female Patients—Supplementary analysis.
eTable 9. Number of Events, Crude and Weighted Event Rates According to Treatment. Falsification Analysis\
eFigure 1. Flowchart Diagram of Study Population Selection.
eFigure 2. Plot of Maximum of Pairwise Standardized Differences for Cohort Baseline Characteristics Before and After Applying Propensity Weights (IPTW).
eFigure 3. Propensity Score Densities for the Treatment Options. Main Analysis.
eFigure 4. Propensity Score Densities for the Treatment Options. AF Hospitalized Cohort.
eFigure 5. Propensity Score Densities for the Treatment Options. Low Mortality Cohort.
eFigure 6. Plot of Maximum of Pairwise Standardized Differences for Cohort Baseline Characteristics Before and After Applying Propensity Weights (IPTW). AF-Hospitalised Cohort.
eFigure 7. Plot of Maximum of Pairwise Standardized Differences for Cohort Baseline Characteristics Before and After Applying Propensity Weights (IPTW). Low Mortality Cohort.
eFigure 8. Propensity Weighted (IPTW) Cox Hazard Ratios for 2.5 Years Follow-Up for NOACs Compared With Warfarin for Stroke and Death End Points.
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Eikelboom J, Joyner C, et al. ; AVERROES Steering Committee and Investigators . Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806-817. [DOI] [PubMed] [Google Scholar]
- 3.Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7)(suppl):38-41. [DOI] [PubMed] [Google Scholar]
- 4.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7)(suppl):30-33. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7)(suppl):22-25. [DOI] [PubMed] [Google Scholar]
- 6.Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26(5):546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen TB, Gorst-Rasmussen A, Rasmussen LH, Skjøth F, Rosenzweig M, Lip GYH. Bleeding events among new starters and switchers to dabigatran compared with warfarin in atrial fibrillation. Am J Med. 2014;127(7):650-656.e5. [DOI] [PubMed] [Google Scholar]
- 8.Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ETTL. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145(1):105-112.e15. [DOI] [PubMed] [Google Scholar]
- 9.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093-1100. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt M, Schmidt SA, Sandegaard JLJ, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550-560. [DOI] [PubMed] [Google Scholar]
- 12.Stürmer T, Wyss R, Glynn RJ, Brookhart MA. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J Intern Med. 2014;275(6):570-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51(1):171-184. [DOI] [PubMed] [Google Scholar]
- 14.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopes RD, Al-Khatib SM, Wallentin L, et al. . Efficacy and safety of apixaban compared with warfarin according to patient risk of stroke and of bleeding in atrial fibrillation: a secondary analysis of a randomised controlled trial. Lancet. 2012;380(9855):1749-1758. [DOI] [PubMed] [Google Scholar]
- 16.Lip GYH, Connolly S, Yusuf S, et al. ; ERROES Investigators . Modification of outcomes with aspirin or apixaban in relation to CHADS(2) and CHA(2)DS(2)-VASc scores in patients with atrial fibrillation: a secondary analysis of the AVERROES study. Circ Arrhythm Electrophysiol. 2013;6(1):31-38. [DOI] [PubMed] [Google Scholar]
- 17.Oldgren J, Alings M, Darius H, et al. ; RE-LY Investigators . Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE-LY trial. Ann Intern Med. 2011;155(10):660-667. [DOI] [PubMed] [Google Scholar]
- 18.Patel MR, Mahaffey KW, Garg J, et al. ; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891. [DOI] [PubMed] [Google Scholar]
- 19.Giugliano RP, Ruff CT, Braunwald E, et al. ; ENGAGE AF-TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa S, Aonuma K, Tse H-F, et al. . The APHRS’s 2013 statement on antithrombotic therapy of patients with nonvalvular atrial fibrillation. J Arrhythmia. 2013;29(3):190-200. doi: 10.1016/j.joa.2013.03.002 [DOI] [Google Scholar]
- 21.JCS Joint Working Group Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013). Circ J. 2014;78(8):1997-2021. [DOI] [PubMed] [Google Scholar]
- 22.Shields AM, Lip GYH. Choosing the right drug to fit the patient when selecting oral anticoagulation for stroke prevention in atrial fibrillation. J Intern Med. 2015;278(1):1-18. [DOI] [PubMed] [Google Scholar]
- 23.Eckman MH, Singer DE, Rosand J, Greenberg SM. Moving the tipping point: the decision to anticoagulate patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2011;4(1):14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proietti M, Lip GYH. Major outcomes in atrial fibrillation patients with one risk factor: impact of time in therapeutic range observations from the SPORTIF trials. Am J Med. 2016;129(10):1110-1116. [DOI] [PubMed] [Google Scholar]
- 25.Kirchhof P, Benussi S, Kotecha D, et al. . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen PB, Larsen TB, Skjøth F, Overvad TF, Lip GYH. Stroke and thromboembolic event rates in atrial fibrillation according to different guideline treatment thresholds: a nationwide cohort study. Sci Rep. 2016;6:27410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lip GYH, Nielsen PB. Should patients with atrial fibrillation and 1 stroke risk factor (CHA2DS2-VASc score 1 in men, 2 in women) be anticoagulated? yes: even 1 stroke risk factor confers a real risk of stroke. Circulation. 2016;133(15):1498-1503. [DOI] [PubMed] [Google Scholar]
- 28.Friberg L, Skeppholm M, Terént A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2-VASc score of 1. J Am Coll Cardiol. 2015;65(3):225-232. [DOI] [PubMed] [Google Scholar]
- 29.Aspberg S, Chang Y, Atterman A, Bottai M, Go AS, Singer DE. Comparison of the ATRIA, CHADS2, and CHA2DS2-VASc stroke risk scores in predicting ischaemic stroke in a large Swedish cohort of patients with atrial fibrillation. Eur Heart J. 2016;37(42):3203-3210. [DOI] [PubMed] [Google Scholar]
- 30.Lip GYH, Skjøth F, Rasmussen LH, Nielsen PB, Larsen TB. Net clinical benefit for oral anticoagulation, aspirin, or no therapy in nonvalvular atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex). J Am Coll Cardiol. 2015;66(4):488-490. [DOI] [PubMed] [Google Scholar]
- 31.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857-867. [DOI] [PubMed] [Google Scholar]
- 32.Ruff CT, Giugliano RP, Braunwald E, et al. . Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955-962. [DOI] [PubMed] [Google Scholar]
- 33.Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GYH. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lip GYH, Keshishian A, Kamble S, et al. . Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin. a propensity score matched analysis. Thromb Haemost. 2016;116(5):975-986. [DOI] [PubMed] [Google Scholar]
- 35.Lozano-Velasco E, Hernández-Torres F, Daimi H, et al. . Pitx2 impairs calcium handling in a dose-dependent manner by modulating Wnt signalling. Cardiovasc Res. 2016;109(1):55-66. [DOI] [PubMed] [Google Scholar]
- 36.Krarup L-H, Boysen G, Janjua H, Prescott E, Truelsen T. Validity of stroke diagnoses in a national register of patients. Neuroepidemiology. 2007;28(3):150-154. [DOI] [PubMed] [Google Scholar]
- 37.Wang K-L, Lip GYH, Lin S-J, Chiang C-E, Non–Vitamin K. Non-vitamin k antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: meta-analysis. Stroke. 2015;46(9):2555-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiang C-E, Wang K-L, Lip GYH. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost. 2014;111(5):789-797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Extended Methods.
eReferences.
eTable 1. Definitions on Comorbidity and Concomitant Medication According to ICD-10 Codes and ATC-Codes.
eTable 2. Risk Score Definitions
eTable 3. Number of Events, Crude and Weighted Event Rates According to Treatment. AF Hospitalized Cohort—Sensitivity Analysis.
eTable 4. Number of Events, Crude and Weighted Event Rates According to Treatment. Low Mortality Cohort—Sensitivity Analysis.
eTable 5. Number of Events, Crude and Weighted Event Rates According to Treatment. Patients in Age Between 65 and 75 years—Supplementary Analysis.
eTable 6. Number of Events, Crude and Weighted Event Rates According to Treatment. Patients With Hypertension—Supplementary Analysis.
eTable 7. Number of Events, Crude and Weighted Event Rates According to Treatment. Male Patients—Supplementary Analysis.
eTable 8. Number of Events, Crude and Weighted Event Rates According to Treatment. Female Patients—Supplementary analysis.
eTable 9. Number of Events, Crude and Weighted Event Rates According to Treatment. Falsification Analysis\
eFigure 1. Flowchart Diagram of Study Population Selection.
eFigure 2. Plot of Maximum of Pairwise Standardized Differences for Cohort Baseline Characteristics Before and After Applying Propensity Weights (IPTW).
eFigure 3. Propensity Score Densities for the Treatment Options. Main Analysis.
eFigure 4. Propensity Score Densities for the Treatment Options. AF Hospitalized Cohort.
eFigure 5. Propensity Score Densities for the Treatment Options. Low Mortality Cohort.
eFigure 6. Plot of Maximum of Pairwise Standardized Differences for Cohort Baseline Characteristics Before and After Applying Propensity Weights (IPTW). AF-Hospitalised Cohort.
eFigure 7. Plot of Maximum of Pairwise Standardized Differences for Cohort Baseline Characteristics Before and After Applying Propensity Weights (IPTW). Low Mortality Cohort.
eFigure 8. Propensity Weighted (IPTW) Cox Hazard Ratios for 2.5 Years Follow-Up for NOACs Compared With Warfarin for Stroke and Death End Points.