Abstract
The Lin28/let-7 axis plays an important role in tumor initiation and developmental processes. Lin28B is upregulated in a variety of cancers, and its overexpression enhances cancer cell proliferation and radioresistance through the suppression of let-7 micro RNA expression. In this study, we investigated the role of the Lin28/let7 axis as a target for radiosensitization of melanoma cancer cells. The overexpression of Lin28B reduced mature let-7 microRNA expression in melanoma cell lines, and enhanced the sphere-forming ability of melanoma cell lines, which is a characteristic of cancer stem cell (CSC) populations. Interestingly, Lin28B-overexpressed melanoma cells were more resistant to X-ray irradiation than control cells, and Lin28B-induced radioresistance was abolished after carbon ion irradiation. Consistent with these results, Lin28B overexpression reduced the numbers of γH2A.X foci after X-ray irradiation, whereas carbon ion irradiation had no such effect. Our results suggest that a carbon ion beam is more effective than an X-ray beam in terms of killing cancer cells, possibly due to elimination of CSC populations.
Keywords: carbon-ion beam, Lin28/let-7 axis, cancer stem cell, radioresistance
INTRODUCTION
The cancer stem cell (CSC) hypothesis posits that tumors are maintained by a subpopulation of cancer cells that are able to proliferate and self-renew. Indeed, numerous studies have demonstrated that CSCs play a pivotal role in cancer initiation, progression and recurrence [1, 2]. Consequently, research has focused on the development of novel therapies that are cytotoxic to CSCs to achieve cures for various cancers. One of the major obstacles to cancer treatment is resistance to radiotherapy. It has been demonstrated that CSC populations are highly resistant to irradiation compared with non-stem cells [3–6]. Thus, targeting CSCs might enable improvement of the radiosensitivity of cancer cells.
Radiation therapy is one of the most common methods for cancer treatment. Most cancer patients receive radiation therapy, either alone or in combination with surgery and chemotherapy, to shrink the tumor mass or kill cancer cells [7]. Conventionally, X-ray beams are used for radiotherapy, but charged particles such as protons and carbon ions (C-ions) have attracted much interest because of their superior dose distribution and high biological effectiveness [8–10]. Although protons comprise the majority of cancer treatments involving charged particle therapy so far, the number of cancer patients treated with C-ion therapy has gradually increased since the National Institute of Radiological Sciences (NIRS) began clinical trials in 1994 [11, 12]. To date, >12 000 patients have been treated with C-ions [12], and the number of heavy ion facilities worldwide is constantly increasing [9].
MicroRNAs (miRNAs) are small non-coding RNAs that repress gene expression by binding to a 3’ untranslated region (UTR) of target messenger RNAs (mRNAs) [13]. One of these miRNAs, let-7, is well-known as a tumor suppressor. Members of the let-7 family are generally downregulated in various types of cancers, and play important roles in diverse biological processes, including cell proliferation, differentiation and apoptosis, through the regulation of multiple oncogenes such as Lin28, high-mobility group AT-hook 2 (HMGA2) and MYC [14, 15]. The RNA binding protein, Lin28B, is an inhibitor of let-7 microRNAs. This protein binds to the terminal loops of the precursors of let-7 and sabotages their post-transcriptional processing [16]. Several studies have suggested that the Lin28/let-7 axis plays a critical role in the regulation of embryonic and induced pluripotent stem cells [17, 18]. In addition, Cheng et al. suggested that Lin28B is a putative CSC marker for the recurrence of hepatocellular carcinoma [19].
Previously, our group suggested that the macroH2A1/Lin28B/let-7 regulatory network regulates the cancer stem–like properties of bladder cancer cells [20]. A previous study revealed that Lin28B-overexpressing bladder cancer cells are more resistant to γ-ray irradiation compared with control cells. Based on this finding, in this study we investigated the effect of X-ray or C-ion irradiation on the survival of control and Lin28B-overexpressing melanoma cells to clarify the role of the Lin28B/let-7 axis in radiosensitization.
MATERIALS AND METHODS
Cell culture
Human melanoma cells (G361, SK-MEL5, A375s2, A375 and A2058) were purchased from the American Type Culture Collection (Rockville, MD, USA) and cultured at 37°C under conditions of 20% O2 and 5% CO2 in Dulbecco's modified Eagle medium (Wako, Osaka, Japan) containing 10% (v/v) fetal bovine serum, 20 mM 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid, penicillin (50 units/ml) and streptomycin (50 μg/ml).
Western blot analysis
Cells were lysed by RIPA lysis buffer (Millipore, Billerica, MA, USA) containing phosphatase inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA). The protein concentrations were determined using Pierce 660 nm protein assay kit (Thermo Scientific, Rockford, IL, USA). The cell extracts were boiled in SDS sample buffer (Bio-Rad, Hercules, CA, USA) and equal amounts of the proteins were loaded onto SDS-PAGE and transferred to PVDF (Millipore). Protein bands were detected with ECL solution (Amersham, GE Healthcare, NJ, USA) and visualized using a ChemiDoc XRS+(Bio-Rad). The following primary antibodies were used: Lin28B (Abcam, Cambridge, MA, USA) and Actin (Santa Cruz, CA, USA).
Cell line generation
pCMV6-AC-GFP vectors with or without Lin28B ORF were purchased from ORIGENE (Rockville, MD, USA). Cells were transfected with these plasmids using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA). Transfected cells were selected with 500 μg/ml of Geneticin (Thermo Scientific).
Quantitative real-time PCR
Total RNA containing microRNA was extracted using a miRNeasy Mini Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer's protocol. The quantity of isolated RNA was measured using a NanoPhotometer (IMPLEN, München, Germany), and 1 μg of RNA was reverse-transcribed using a miScript II RT kit (QIAGEN). The miScript universal primer (QIAGEN) was used as the antisense primer. SNORD61 and SNORD68 (QIAGEN) were used as the internal controls. The following primers were used: hsa-let-7a-5p TGA GGT AGT AGG TTG TAT AGT T, hsa-let-7b-5p TGA GGT AGT AGG TTG TGT GGT T, hsa-let-7c-5p TGA GGT AGT AGG TTG TAT GGT T, hsa-let-7d-5p AGA GGT AGT AGG TTG CAT AGT T, hsa-let-7e-5p TGA GGT AGG AGG TTG TAT AGT T, hsa-let-7f-5p TGA GGT AGT AGA TTG TAT AGT T, hsa-let-7g-5p TGA GGT AGT AGT TTG TAC AGT T and hsa-let-7i-5p TGA GGT AGT AGT TTG TGC TGT T.
Sphere-forming assay
Cells were seeded on ultra-low attachment 24-well plates (Corning, Lowell, MA, USA) in serum-free Dulbecco's modified Eagle medium/nutrient mixture F-12 (Invitrogen) supplemented with B27 (Invitrogen), N2 (Invitrogen), epidermal growth factor (20 ng/ml; Peprotech, London, UK) and basic fibroblast growth factor (10 ng/ml; Peprotech). After 1 week, spheres were counted and visualized using a phase-contrast Olympus microscope (Tokyo, Japan).
Irradiations
X-rays were irradiated using the 200-kVp X-ray generator (MultiRad225, Faxitron Bioptics, LLC, Tucson, AZ, USA) with a total filtration of 0.5 mm aluminum and 0.5 mm copper. The dose rate of X-rays was 1.11 Gy/min. C-ion beam irradiation was performed at the Gunma University Heavy Ion Medical Center [21]. The conditions of irradiation were: energy of 290 MeV/nucleon and linear energy transfer of 50 keV/μm at the center of the 6 cm spread-out Bragg peak. The radiation dose (Gy) required for 10% survival rates (D10) were calculated using linear regression analysis.
Colony-forming assay
Cells were seeded into T-25 flasks and incubated at 37°C overnight. Ten days after irradiation, colonies were fixed with methanol and stained with 2% Giemsa solution. The colonies containing at least 50 cells were scored.
Immunocytochemistry
Cells were fixed with 3.7% formaldehyde for 15 min and permeabilized with 0.5% Triton X-100 for 30 min. Then, cells were blocked with PBS containing 0.1% Triton X-100 and 3% bovine serum albumin at room temperature for 30 min, and stained with γH2A.X antibody (Millipore). After staining, cells were incubated with Alexa Fluor 555 antibody (Life Technologies, Grand Island, NY, USA) at room temperature for 45 min in the dark. At least 100 cells in each group were counted and visualized using an Olympus microscope.
Statistical analysis
The data presented are representative of at least three independent experiments. Statistical analyses were performed using GraphPad Prism 7.0 Software (USACO Co., Tokyo, Japan). The Student's t test was used for statistical comparisons. P-values of <0.05 were considered statistically significant.
RESULTS
Repression of mature let-7 miRNA production by Lin28B overexpression
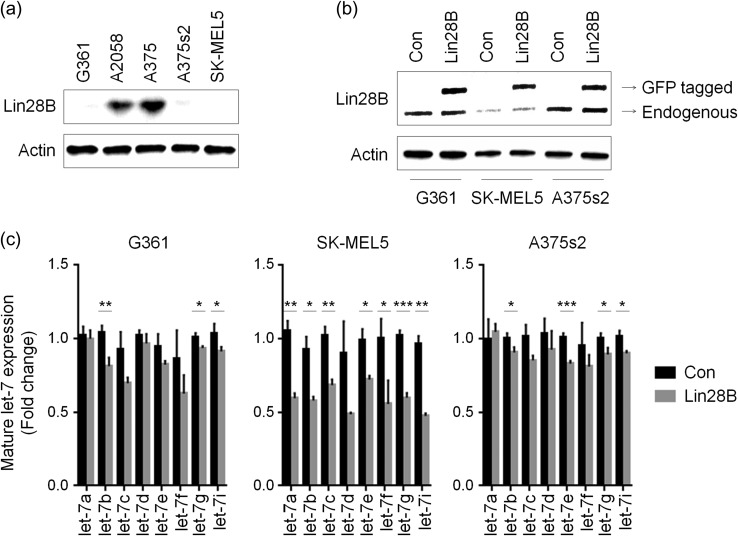
To establish Lin28B-overexpressing cells, we assessed the level of Lin28B expression in five melanoma cell lines. The G361, A375s2 and SK-MEL5 cell lines showed reduced Lin28B expression (Fig. 1a). Therefore, we generated Lin28B-overexpressing cells using these three cell lines (Fig. 1b). Since Lin28B is an inhibitor of let-7 miRNAs, we next determined whether its overexpression reduced the levels of mature let-7 miRNAs. As shown in Fig. 1c, the expression of mature let-7 miRNAs was reduced in Lin28B-overexpressing melanoma cell lines compared with in the control cells, even though differential rates of reductions were observed according to the cell line and the type of miRNA involved. Especially, all the let-7 miRNAs we tested were significantly reduced in Lin28B-overexpressed SK-MEL5 cells, while Lin28B-overexpressed G361 and A375s2 cells showed relatively less reduction of let-7 expression (Fig. 1c). Among the members of the let-7 family, let-7b, g and i miRNAs were significantly reduced by Lin28B overexpression in all three cell lines tested (Fig. 1c). These results indicate that the upregulation of Lin28B suppresses mature let-7 miRNA expression in melanoma cells in a cell line–dependent manner.
Fig. 1.
Overexpression of Lin28B suppresses let-7 miRNA expression. (a) The levels of Lin28B expression were determined using western blot analysis. (b) The expression of Lin28B in melanoma cells transfected with the pCMV6-AC-GFP (Con) or pCMV6-Lin28B-GFP (Lin28B) vectors was evaluated using western blot analysis. Actin was used as an internal control. (c) The levels of mature let-7 miRNAs in Con or Lin28B cells were assessed using quantitative real-time PCR (qPCR). P-values were calculated using Student's t test. *P < 0.05, **P < 0.01, ***P < 0.001.
Sphere-forming capability of control and Lin28B-overexpressing melanoma cells
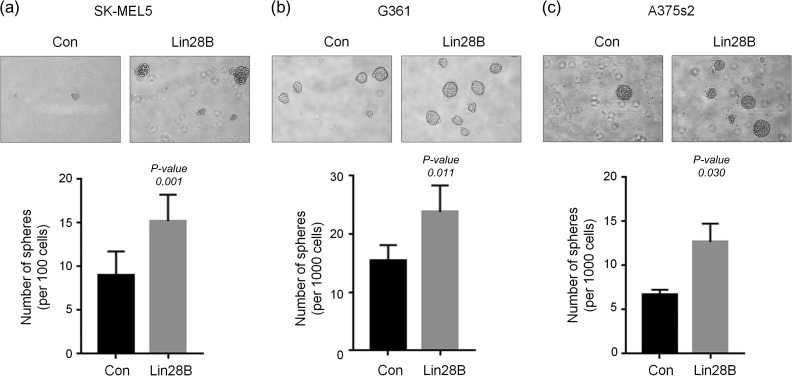
We next determined the sphere-forming capability of control and Lin28B-overexpressing melanoma cells to elucidate the role of Lin28B in CSC expansion. Control and Lin28B-overexpressing melanoma cells were seeded onto 24-well low-adhesion plates, and after 1 week, spheres were counted and visualized (Fig. 2). Overexpression of Lin28B significantly increased the sphere-forming capability of SK-MEL5 (Fig. 2a), G361 (Fig. 2b) and A375s2 (Fig. 2c) cells. These results suggest that Lin28B plays an important role in melanoma CSC regulation, possibly due to repression of mature let-7 miRNA production by Lin28B.
Fig. 2.
Overexpression of Lin28B enhances sphere-forming capability. (a–c) Con and Lin28B melanoma cells were incubated with DMEM/F12 supplemented with B27, N2, EGF and bFGF. The spheres in SK-MEL5 (a), G361 (b) and A375s2 (c) cells were counted and visualized after 1 week. P-values were calculated using Student's t test.
Survival of control and Lin28B-overexpressing melanoma cells after treatment with C-ion or X-ray beams
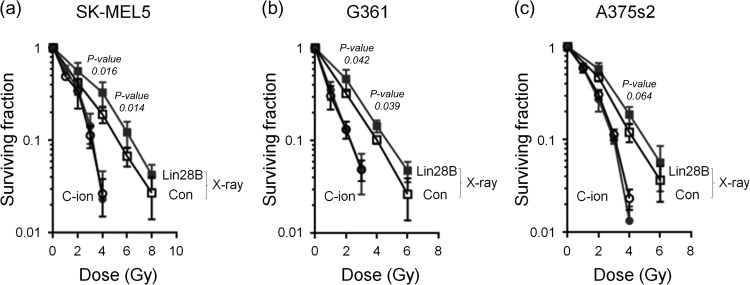
To determine the effect of Lin28B overexpression on the radiosensitivity of melanoma cells after X-ray or C-ion beam irradiation, clonogenic assays were performed. Control or Lin28B-overexpressing SK-MEL5, G361 and A375s2 cells (1 × 103) were seeded, and after 20 h, exponentially growing cells were exposed to X-ray or C-ion beams. Following X-ray irradiation, overexpression of Lin28B significantly increased radioresistance in all three of the cell lines we tested (Fig. 3). However, X-ray irradiation–induced radioresistance was completely abolished by irradiation with C-ion beams (Fig. 3). After X-ray irradiation, the D10 values of Lin28B-overexpressing SK-MEL5, G361 and A375s2 cells were greater than those of control cells (control: 5.40 ± 0.29, Lin28B: 6.10 ± 0.31, control: 4.49 ± 0.03, Lin28B: 5.02 ± 0.05 and control: 4.85 ± 0.09, Lin28B: 5.30 ± 0.35, respectively), but no significant changes were observed after C-ion irradiation (control: 3.30 ± 0.08, Lin28B: 3.40 ± 0.09, control: 2.44 ± 0.07, Lin28B: 2.44 ± 0.14 and control: 3.28 ± 0.05, Lin28B: 3.17 ± 0.09, respectively). These results suggest that a C-ion beam could eliminate melanoma cells that are resistant to X-ray irradiation.
Fig. 3.
The C-ion beam abolishes Lin28B-induced X-ray resistance. (a–c) Con and Lin28B melanoma cells were left untreated or were treated with X-ray (2–8 Gy) or C-ion (1–4 Gy) irradiation, and the survival rates of SK-MEL5 (a), G361 (b) and A375s2 (c) cells were determined using the colony-forming assay. Square: X-ray; circle: C-ion; open: Con; closed: Lin28B. P-values were calculated using Student's t test.
γH2A.X foci formation by control and Lin28B-overexpressing melanoma cells after irradiation with C-ion or X-ray beams
To further investigate the effect of Lin28B overexpression on DNA damage, we next examined γH2A.X foci formation by control and Lin28B-overexpressing SK-MEL5 and G361 cells after C-ion or X-ray irradiation. Consistent with the cell survival results, fewer γH2A.X foci were formed by Lin28B-overexpressing cells than in control cells after X-ray irradiation. However, the overexpression of Lin28B did not affect foci formation after C-ion beam irradiation (Fig. 4a and b). Moreover, at 24 h after X-ray irradiation, only 14% of γH2A.X foci remained in the Lin28B-overexpressing SK-MEL5 cells, compared with 23% in control cells (Fig. 4c). Furthermore, the number of foci remained higher in C-ion–irradiated Lin28B-overexpressing cells than X-ray–irradiated Lin28B-overexpressing cells. These results suggest that C-ion irradiation blocked the Lin28B-induced X-ray resistance through regulation of the DNA damage response.
Fig. 4.
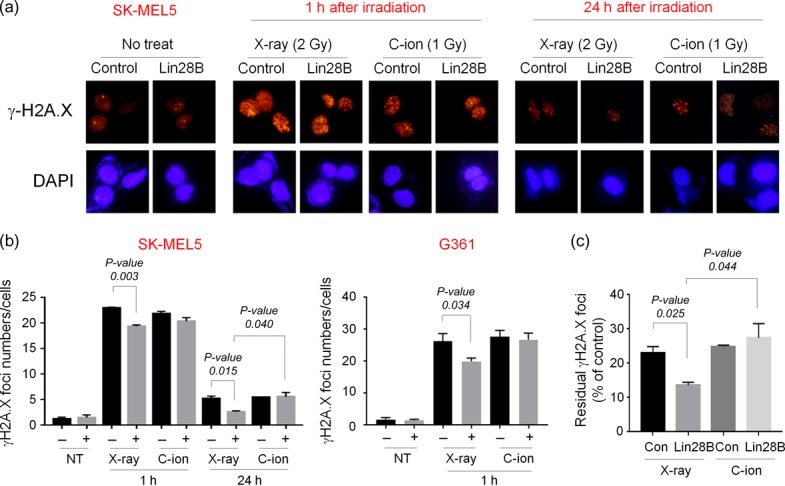
Lin28B overexpression decreases the number of γH2A.X foci after X-ray irradiation, but not after C-ion irradiation. (a–c) Con and Lin28B cells were left untreated or were treated with X-ray (2 Gy) or C-ion (1 Gy) irradiation, and γH2A.X foci formations were assessed using immunocytochemistry (ICC). (a) Representative images of ICC staining of γH2A.X in Con and Lin28B SK-MEL5 cells at different time points and conditions. (b) The average numbers of γH2A.X foci were evaluated. At least 100 cells were counted in each condition. (c) The rate of residual γH2A.X foci was calculated as follows. Number of foci after 24 h/number of foci after 1 h × 100. P-values were calculated using Student's t test.
DISCUSSION
Melanoma is considered a relatively radio-insensitive tumor type due to its poor prognosis and high recurrence rates after photon radiotherapy [22]. As such, surgery is generally preferred for the treatment of malignant melanoma, despite the high local recurrence rate and low overall survival rate [23, 24]. Nonetheless, recent studies have demonstrated that C-ion therapy has significant advantages over conventional radiotherapy in term of improving the overall survival of melanoma patients [25–28]. Recently, several studies have reported radiobiological benefits of a C-ion beam for CSC regulation over a photon beam in a variety of cancer cells. In these studies, the proportions of putative colon or pancreatic CSCs were increased after X-ray irradiation, but a few or no changes were observed after C-ion treatment [29, 30]. Furthermore, Sai et al. showed that a C-ion beam combined with cisplatin effectively disrupts triple negative breast CSCs [31]. Although C-ion irradiation could attenuate CSC-induced radioresistance, the effect of C-ion beam treatment on radioresistant melanoma cells, which could be regarded as CSCs, has not yet been fully elucidated. In the present study, we therefore aimed to evaluate the advantages of C-ion beam over conventional radiotherapy in terms of killing of melanoma stem-like cells.
The aberrant upregulation of Lin28A and its homologue Lin28B is present in a variety of cancers, and high levels of these proteins are associated with malignancy [20, 32–34]. The let-7 miRNAs are the main downstream targets of Lin28A and B, and their oncogenic roles are mediated mainly by suppression of mature let-7 production [35]. For example, knockdown of Lin28B in hepatocellular carcinoma cells (HCCs) inhibited proliferation in vitro and reduced in vivo tumor growth in SCID mice. In contrast, overexpression of Lin28B suppressed the expression of let-7 family members, hence enhancing the tumorigenicity of HCCs [36]. The Lin28/let-7 axis not only regulates cancer initiation and progression, but also has a role in regulating the radiosensitivity of cancer cells. Knockdown of Lin28B increased let-7g levels, which decreased the expression of K-RAS, resulting in enhanced radiosensitivity in lung cancer cells [37]. Similarly, the induction of Lin28B expression by knockdown of a histone variant, macroH2A1, leads to suppression of let-7 miRNAs, and enhanced radioresistance in bladder cancer cells [20]. In the present study, we also found that Lin28B-overexpressing melanoma cells exhibit suppression of the expression of mature let-7 miRNAs (Fig. 1C), supporting the notion that Lin28B is a key regulator of let-7 miRNAs.
Although limited data on the role of Lin28B in radiosensitivity have been reported, the importance of the Lin28/let-7 axis in CSC regulation is established in various types of cancer [16, 35]. In this study, we have shown that Lin28B overexpression significantly enhances the sphere-forming capability of melanoma cells (Fig. 2). Sphere-forming assays have been widely used to identify CSCs in vitro. Many studies suggest that the subpopulation of cancer cells that comprise spheres exhibits increased potential for self-renewal and tumorigenicity [38, 39]. Thus, our results suggest that the enhanced expression of Lin28B in melanoma cells may contribute to CSC expansion. A large body of evidence suggests the importance of CSCs in radioresistance [40]. In the present study, we have shown that Lin28B-overexpressing melanoma cells are more resistant to X-ray irradiation compared with control cells, and that Lin28B-induced X-ray resistance was completely abolished by C-ion irradiation, suggesting that the expansion of stem-like melanoma cells due to Lin28B overexpression could be prevented by C-ion treatment (Fig. 3).
The phosphorylation of histone H2A.X on Ser-139 (which is termed γH2A.X) is a marker for the DNA double-strand breaks and occurs rapidly (within minutes) after γ-ray irradiation, and dephosphorylation occurs after the repair of DNA [40–42]. Because of its characteristics, the ability to form γH2A.X foci after ionizing radiation has been used to evaluate the radiosensitivity of cancer cells. In this study, we demonstrated that the overexpression of Lin28B significantly reduced γH2A.X foci formation in SK-MEL5 and G361 cells after 2 Gy of X-ray irradiation, indicating that overexpression of Lin28B attenuates the DNA damage of melanoma cells (Fig. 4). Since the relative biological effectiveness values of the C-ion beam were ~2 in both SK-MEL5 and G361 cells (1.83 and 1.88, respectively), we used 1 Gy of C-ion irradiation to evaluate the effect of Lin28B overexpression on the DNA damage. Interestingly, we found that Lin28B overexpression did not affect the formation of γH2A.X foci after C-ion irradiation. One possible explanation for this observation is that elimination of CSCs is enhanced by Lin28B overexpression after C-ion beam irradiation. However, precise downstream mechanisms should be determined to clarify this possibility. In conclusion, the results of the present study suggest that the Lin28B/let-7 axis may play an important role in melanoma CSC expansion, thereby regulating X-ray sensitivity in melanoma cells. Moreover, we provide evidence that C-ion beams could be used to eliminate X-ray–resistant melanoma cells, supporting the notion that C-ion therapy has an advantage over conventional radiotherapy in terms of improving the survival of cancer patients.
ACKNOWLEDGEMENTS
This work was performed as part of the Research Project with heavy ions at the Gunma University Heavy Ion Medical Center (GHMC,Gunma University), the Program for Cultivating Global Leaders in Heavy Ion Therapeutics and Engineering granted to Gunma University, and Gunma University Initiative for Advanced Research (GIAR).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), JSPS KAKENHI (Grant Number JP16K10341), and the International Association for the Sensitization of Cancer Treatment (IASCT).
REFERENCES
- 1. Das S, Srikanth M, Kessler JA. Cancer stem cells and glioma. Nat Clin Pract Neurol 2008;4:427–35. [DOI] [PubMed] [Google Scholar]
- 2. Nguyen LV, Vanner R, Dirks P, et al. Cancer stem cells: an evolving concept. Nat Rev Cancer 2012;12:133–43. [DOI] [PubMed] [Google Scholar]
- 3. Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756–60. [DOI] [PubMed] [Google Scholar]
- 4. Blazek ER, Foutch JL, Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133– cells, and the CD133+ sector is enlarged by hypoxia. Int J Radiat Oncol Biol Phys 2007;67:1–5. [DOI] [PubMed] [Google Scholar]
- 5. Phillips TM, McBride WH, Pajonk F. The response of CD24(–/low)/CD44+ breast cancer–initiating cells to radiation. J Natl Cancer Inst 2006;98:1777–85. [DOI] [PubMed] [Google Scholar]
- 6. Woodward WA, Chen MS, Behbod F, et al. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci U S A 2007;104:618–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thariat J, Hannoun-Levi JM, Sun Myint A, et al. Past, present, and future of radiotherapy for the benefit of patients. Nat Rev Clin Oncol 2013;10:52–60. [DOI] [PubMed] [Google Scholar]
- 8. Durante M, Loeffler JS. Charged particles in radiation oncology. Nat Rev Clin Oncol 2010;7:37–43. [DOI] [PubMed] [Google Scholar]
- 9. Loeffler JS, Durante M. Charged particle therapy—optimization, challenges and future directions. Nat Rev Clin Oncol 2013;10:411–24. [DOI] [PubMed] [Google Scholar]
- 10. Ohno T. Particle radiotherapy with carbon ion beams. EPMA J 2013;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamada T, Tsujii H, Blakely EA, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol 2015;16:e93–100. [DOI] [PubMed] [Google Scholar]
- 12. Marx V. Cancer treatment: sharp shooters. Nature 2014;508:133–8. [DOI] [PubMed] [Google Scholar]
- 13. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bussing I, Slack FJ, Grosshans H. Let-7 microRNAs in development, stem cells and cancer. Trends Mol Med 2008;14:400–9. [DOI] [PubMed] [Google Scholar]
- 15. Barh D, Malhotra R, Ravi B, et al. MicroRNA let-7: an emerging next-generation cancer therapeutic. Curr Oncol 2010;17:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shyh-Chang N, Daley GQ. Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell 2013;12:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moss EG, Tang L. Conservation of the heterochronic regulator Lin-28, its developmental expression and microRNA complementary sites. Dev Biol 2003;258:432–42. [DOI] [PubMed] [Google Scholar]
- 18. Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–20. [DOI] [PubMed] [Google Scholar]
- 19. Cheng SW, Tsai HW, Lin YJ, et al. Lin28B is an oncofetal circulating cancer stem cell-like marker associated with recurrence of hepatocellular carcinoma. PLoS One 2013;8:e80053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park SJ, Shim JW, Park HS, et al. MacroH2A1 downregulation enhances the stem-like properties of bladder cancer cells by transactivation of Lin28B. Oncogene 2016;35:1292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohno T, Kanai T, Yamada S, et al. Carbon ion radiotherapy at the Gunma University Heavy Ion Medical Center: new facility set-up. Cancers 2011;3:4046–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khan N, Khan MK, Almasan A, et al. The evolving role of radiation therapy in the management of malignant melanoma. Int J Radiat Oncol Biol Phys 2011;80:645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lund VJ, Howard DJ, Harding L, et al. Management options and survival in malignant melanoma of the sinonasal mucosa. Laryngoscope 1999;109:208–11. [DOI] [PubMed] [Google Scholar]
- 24. Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck 2002;24:247–57. [DOI] [PubMed] [Google Scholar]
- 25. Jingu K, Kishimoto R, Mizoe JE, et al. Malignant mucosal melanoma treated with carbon ion radiotherapy with concurrent chemotherapy: prognostic value of pretreatment apparent diffusion coefficient (ADC). Radiother Oncol 2011;98:68–73. [DOI] [PubMed] [Google Scholar]
- 26. Zhang H, Li S, Wang XH, et al. Results of carbon ion radiotherapy for skin carcinomas in 45 patients. Br J Dermatol 2012;166:1100–6. [DOI] [PubMed] [Google Scholar]
- 27. Toyama S, Tsuji H, Mizoguchi N, et al. Long-term results of carbon ion radiation therapy for locally advanced or unfavorably located choroidal melanoma: usefulness of CT-based 2-port orthogonal therapy for reducing the incidence of neovascular glaucoma. Int J Radiat Oncol Biol Phys 2013;86:270–6. [DOI] [PubMed] [Google Scholar]
- 28. Karasawa K, Wakatsuki M, Kato S, et al. Working Group for Gynecological Tumors. Clinical trial of carbon ion radiotherapy for gynecological melanoma. J Radiat Res 2014;55:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cui X, Oonishi K, Tsujii H, et al. Effects of carbon ion beam on putative colon cancer stem cells and its comparison with X-rays. Cancer Res 2011;71:3676–87. [DOI] [PubMed] [Google Scholar]
- 30. Oonishi K, Cui X, Hirakawa H, et al. Different effects of carbon ion beams and X-rays on clonogenic survival and DNA repair in human pancreatic cancer stem-like cells. Radiother Oncol 2012;105:258–65. [DOI] [PubMed] [Google Scholar]
- 31. Sai S, Vares G, Kim EH, et al. Carbon ion beam combined with cisplatin effectively disrupts triple negative breast cancer stem-like cells in vitro. Mol Cancer 2015;14:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. West JA, Viswanathan SR, Yabuuchi A, et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 2009;460:909–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xue D, Peng Y, Wang F, et al. RNA-binding protein LIN28 is a sensitive marker of ovarian primitive germ cell tumours. Histopathology 2011;59:452–9. [DOI] [PubMed] [Google Scholar]
- 34. Lu L, Katsaros D, Mayne ST, et al. Functional study of risk loci of stem cell–associated gene lin-28B and associations with disease survival outcomes in epithelial ovarian cancer. Carcinogenesis 2012;33:2119–25. [DOI] [PubMed] [Google Scholar]
- 35. Viswanathan SR, Daley GQ. Lin28: a microRNA regulator with a macro role. Cell 2010;140:445–9. [DOI] [PubMed] [Google Scholar]
- 36. Wang YC, Chen YL, Yuan RH, et al. Lin-28B expression promotes transformation and invasion in human hepatocellular carcinoma. Carcinogenesis 2010;31:1516–22. [DOI] [PubMed] [Google Scholar]
- 37. Jeong SH, Wu HG, Park WY. LIN28B confers radio-resistance through the posttranscriptional control of KRAS. Exp Mol Med 2009;41:912–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cao L, Zhou Y, Zhai B, et al. Sphere-forming cell subpopulations with cancer stem cell properties in human hepatoma cell lines. BMC Gastroenterol 2011;11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res 2005;65:9328–37. [DOI] [PubMed] [Google Scholar]
- 40. Celeste A, Fernandez-Capetillo O, Kruhlak MJ, et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol 2003;5:675–9. [DOI] [PubMed] [Google Scholar]
- 41. Takahashi A, Ohnishi T. Does γH2AX foci formation depend on the presence of DNA double strand breaks? Cancer Lett 2005;229:171–9. [DOI] [PubMed] [Google Scholar]
- 42. Kuo LJ, Yang LX. Gamma-H2AX—a novel biomarker for DNA double-strand breaks. In Vivo 2008;22:305–9. [PubMed] [Google Scholar]