Abstract
Importance
Higher body mass index (BMI) is a risk factor for cardiometabolic disease; however, the underlying causal associations remain unclear.
Objectives
To use UK Biobank data to report causal estimates of the association between BMI and cardiometabolic disease outcomes and traits, such as pulse rate, using mendelian randomization.
Design, Setting, and Participants
Cross-sectional baseline data from a population-based cohort study including 119 859 UK Biobank participants with complete phenotypic (medical and sociodemographic) and genetic data. Participants attended 1 of 22 assessment centers across the United Kingdom between 2006 and 2010. The present study was conducted from May 1 to July 11, 2016.
Main Outcomes and Measures
Prevalence of hypertension, coronary heart disease, and type 2 diabetes were determined at assessment, based on self-report. Blood pressure was measured clinically. Participants self-reported sociodemographic information pertaining to relevant confounders. A polygenic risk score comprising 93 single-nucleotide polymorphisms associated with BMI from previous genome-wide association studies was constructed, and the genetic risk score was applied to derive causal estimates using a mendelian randomization approach.
Results
Of the 119 859 individuals included in the study, 56 816 (47.4%) were men; mean (SD) age was 56.87 (7.93) years. Mendelian randomization analysis showed significant positive associations between genetically instrumented higher BMI and risk of hypertension (odds ratio [OR] per 1-SD higher BMI, 1.64; 95% CI, 1.48-1.83; P = 1.1 × 10−19), coronary heart disease (OR, 1.35; 95% CI, 1.09-1.69; P = .007) and type 2 diabetes (OR, 2.53; 95% CI, 2.04-3.13; P = 1.5 × 10−17), as well as systolic blood pressure (β = 1.65 mm Hg; 95% CI, 0.78-2.52 mm Hg; P = 2.0 × 10−04) and diastolic blood pressure (β = 1.37 mm Hg; 95% CI, 0.88-1.85 mm Hg; P = 3.6 × 10−08). These associations were independent of age, sex, Townsend deprivation scores, alcohol intake, and smoking history.
Conclusions and Relevance
The results of this study add to the burgeoning evidence of an association between higher BMI and increased risk of cardiometabolic diseases. This finding has relevance for public health policies in many countries with increasing obesity levels.
Key Points
Question
Based on estimates derived using mendelian randomization, what is the association between body mass index and cardiometabolic traits?
Findings
In this population-based cohort study of approximately 120 000 individuals, associations were identified between body mass index and risk of hypertension, coronary heart disease, type 2 diabetes, and elevated systolic and diastolic blood pressures. No associations between body mass index and stroke or pulse rate were observed.
Meaning
Body mass index represents an important modifiable factor for ameliorating the risk of cardiometabolic disease in the general population.
This population-based cohort study uses UK Biobank data to examine the association between body mass index and cardiometabolic outcomes in individuals with phenotypic information available in a databank.
Introduction
Obesity is a major public health concern in terms of economic costs and effect on quality of life and health. A total of 40% of adults worldwide are overweight, with a body mass index (BMI) of 25 or more (calculated as weight in kilograms divided by height in meters squared), and 13% are obese (BMI 30). Higher BMI is a risk factor for outcomes associated with lower quality of life and functional impairment, including cardiometabolic diseases such as hypertension, coronary heart disease (CHD), type 2 diabetes, stroke, and cognitive impairment. Although lifestyle factors are considerable drivers of excess adiposity, BMI has a significant genetic component: approximately 34% of BMI variation can be attributed to common genetic loci.
Correlations between higher BMI and cardiometabolic disease risk usually arise from observational studies that are unable to fully account for confounding by shared risk factors, such as socioeconomic deprivation, or reverse causality whereby the presence of disease may influence BMI. Mendelian randomization (MR) is an approach that partially overcomes these limitations. In instances where genetic risk factors for a trait (eg, BMI) have been reported, these can be used to proxy lifetime variation of the trait and facilitate analysis of whether the trait plays a causal role in disease risk through instrumental variable analysis.
Studies by Nordestgaard et al (N = 75 627), Holmes et al (N = 34 548), and Hägg et al (N = 22 193) compared observational with causal estimates of the association of BMI with CHD, stroke, and type 2 diabetes. The investigators reported discrepant results first in terms of observational vs causal estimate odds ratios (ORs) and showed inconsistency in terms of finding significant associations with CHD and stroke.
The conflicting evidence as to whether a true association exists between BMI and cardiometabolic diseases and, if so, the magnitude of that association lends support to adopting an MR approach to investigating whether BMI has a causal relationship with a range of important phenotypes related to health in a large biobank. The present study used UK Biobank (n = 119 859 with usable data). Our report advances the field in 3 important ways: (1) by including a relatively large sample size in a single study with detailed measurement of covariates (eg, smoking history); (2) by using a 93 single-nucleotide polymorphism (SNP) polygenic risk (PGR) score based on a genome-wide association study of 339 224 individuals, with 56 recent discoveries; and (3) by conducting MR of BMI on a range of outcomes, including CHD, type 2 diabetes, hypertension, and stroke as well as blood pressure (BP) and pulse rate, the latter two being easily and commonly assessed noninvasive variables that are prognostic for cardiovascular outcomes.
Methods
Study Design and Participants
The UK Biobank cohort is a large prospective cohort of 502 628 participants with phenotypic information. In the present analysis, we report on cross-sectional data at baseline. All participants attended 1 of 22 assessment centers from 2006 to 2010 where they completed a series of physical, sociodemographic, and medical assessments. In total, 152 729 participants currently have genetic data. This study was conducted from May 1 to July 11, 2016, under generic approval from the UK National Health Service National Research Ethics Service. Participants provided written informed consent.
Exclusions
Of 152 729 UK Biobank participants who were genotyped, we first excluded nonwhite European participants (based on self-report), leaving 137 178 individuals. We then removed participants who had a relatedness coefficient above 0.0442 (eg, first cousins), mismatch between reported and genetic sex, or failed UK Biobank quality controlling and were recommended exclusions (by UK Biobank), resulting in a final sample of 119 859 individuals.
Physical Traits
Participants were asked during the baseline assessment about any previous or current cardiometabolic conditions that had been diagnosed by their physician. Specifically, participants were asked whether their physician had diagnosed myocardial infarction, angina, hypertension, or stroke. We defined CHD as either myocardial infarction or angina (the results were similar when we analyzed myocardial infarction individually). We excluded participants who stated only “prefer not to answer” (170 [0.14%] of the total to this multiple-choice question). Participants were separately asked whether their physician had diagnosed diabetes. A minority may have had type 1 rather than type 2 diabetes. We removed participants with missing data for this question (259 [0.22%]). Because UK Biobank is nationally representative of the United Kingdom, we would expect most strokes to be ischemic.
Height was measured (seca 202 stadiometer; seca) and weight was measured to the nearest 0.1 kg (BC-418 MA body composition analyzer; Tanita Corp). Body mass index was derived from weight in kilograms divided by height in meters squared. Participants self-reported their sex and age; stated their smoking status as never, previous, or current; and reported their alcohol intake as never, special occasions only, 1 to 3 times a month, once or twice a week, 3 or 4 times a week, and daily or almost daily. Alcohol intake and smoking status were treated as ordinal variables after removing participants who preferred not to answer and for whom their alcohol/smoking status was therefore ambiguous; the low missing numbers of 77 (0.1%) for alcohol and 320 (0.3%) for smoking are unlikely to bias the results meaningfully. Blood pressure and pulse rate were assessed using digital blood pressure monitors (HEM-7015IT; Omron Healthcare Inc). We used the first reading because it had the largest sample size. There was a significant reduction in systolic BP from the first to second measurement (mean [SD], 139.9 [19.69] and 136.0 [18.65] mm Hg; P < .001; Cohen d = 0.20, which represents a small effect size), but not for diastolic BP (82.2 [10.7] and 82.2 [10.2] mm Hg; P = .81; Cohen d = <0.01). Both diastolic and systolic BPs first and second readings correlated at r = 0.9 (P < .001). The results were unchanged when we ran analyses on the subset of individuals with available measurements from second readings only or a mean of first and second readings. Participants self-reported estimated physical activity, which was summated to create an overall physical activity score.
Participants were asked whether they were receiving any antihypertensive medication during an on-screen assessment with response options of yes, no, or don’t know. Those who did not know were removed from analysis (242 [0.20%]), and we adjusted for yes/no status of self-reported antihypertensive medication use in all BP and pulse rate analyses as an additional covariate. Participants self-reported their ethnicity and we recoded this as a white European vs other ethnicity variable. A Townsend deprivation score (minimum, −6.26; maximum, 10.78; mean [SD], −1.47 [3.00]) for all participants was derived. Higher Townsend scores equate to higher levels of area-based socioeconomic deprivation.
Genetic Data
UK Biobank genotyping was conducted by Affymetrix using a bespoke BiLEVE Axiom array for 49 979 participants and on a further updated bespoke Affymetrix Axiom array for the remaining 102 750 individuals in this study, based on the first array. The 2 arrays are very similar, sharing more than 95% marker content. We controlled for array type as a covariate. Further information on the genotyping process is available on the UK Biobank website (http://www.ukbiobank.ac.uk/scientists-3/genetic-data), which includes detailed technical documentation. There were 33 genetic batches: 11 BiLEVE and 22 UK Biobank. Approximately 4000 to 5000 participants were in each batch, and they are described in the above-referenced documentation.(p20) We followed UK Biobank recommendations on which participants to exclude from analysis based on whether the sample failed quality control and had significant missing data or heterozygosity. We used 10 UK Biobank–provided genetic principal components to account for population stratification.
Results were similar when we ran analyses unadjusted for these 10 principal components. All SNPs had a missingness rate lower than 1%, except for rs10733682 (8%), although the results were unchanged with or without inclusion of this SNP. We therefore report results with this SNP included.
The PGR score was derived from 97 SNPs associated with BMI at genome-wide significance in a previous study of more than 339 224 participants of European descent (eTable 1 in the Supplement). Of these, 95 SNPs were directly genotyped in UK Biobank. Two SNPs failed in Hardy-Weinberg equilibrium (rs9925964 and rs17001654; P < 1 × 10−6) as assessed with PLINK, and were excluded, leaving 93 SNPs for the BMI genetic instrument. We constructed an externally weighted PGR score for each participant, weighted by the effect estimates reported in GIANT (β per 1-SD unit of BMI).
Statistical Analysis
For all analyses, in the partially adjusted model we adjusted for assessment center, age, sex, genetic batch effects, array, and stratification with the 10 genetic principal components. In the fully adjusted model, Townsend deprivation scores, smoking status, and alcohol intake were added as covariates. All statistical tests were 2-sided and P < .01 denoted nominal significance. A conservative P value was chosen as we had multiple outcomes; formal correction for type I error can be overly conservative when testing intercorrelated outcomes. Stata, version 13, was used for all analyses.
Observational Analysis
For the observational analyses of BMI association with various binary disease traits, we report logistic regression ORs and 95% CIs. Continuous traits are reported as unstandardized β values with linear regression.
MR Analysis
We used 2 MR approaches. First, we ran conventional MR using individual-level data to conduct 2-stage least squares analysis for continuous trait data (using Stata command ivreg2) and control function estimator for binary traits. We used robust SE variances throughout and MR-Egger for the second MR method. MR-Egger is a linear regression of estimated SNP effects for the risk allele on exposure against the corresponding estimates of SNPs on outcome weighted by the inverse variance of the SNP on outcome effect estimates. The regression line in MR-Egger is not forced through the origin, and the presence of unbalanced horizontal pleiotropy (ie, where the SNPs are associated with alternative pathways to the exposure that have independent effects on the outcome) can be inferred from an intercept that differs from zero. While the inclusion of pleiotropic SNPs can bias causal estimates and increase the rate of false positives (ie, type I errors) or introduce bias to the null, MR-Egger can bypass this by showing evidence of pleiotropy in the instrument (the PGR score) and providing a causal estimate (making certain assumptions) that is robust to such pleiotropy. For MR-Egger, we used the same covariate models as the previous analyses. We conducted checks to support the validity of the MR analysis using multiple genetic variants (ie, the 93-SNP PGR score) as follows. Possible pleiotropy for each of the 93 SNPs can be assessed visually by a scatterplot, where each point should be roughly visually compatible with a linear effect of genetic associations with the outcome vs genetic associations with the risk factor. This linearity ideally reflects homogeneity of causal estimates from different genetic variants; the eFigure in the Supplement shows a scatterplot of these variants for each outcome (eg, CHD) vs genetic associations with BMI, adjusted for age and sex. There was 1 outlier each for type 2 diabetes (rs7903146), systolic BP (rs1191560), and diastolic BP (rs13107325); the results were unchanged when these outliers were removed and, for simplicity, we report estimates derived using all 93 SNPs.
Results
Descriptives
Of the 119 859 participants who satisfied the inclusion criteria (eg, passed quality control as described in the Genetic Data section), the mean (SD) age was 56.87 (7.93) years, and 56 816 (47.4%) of the population were men. The mean (SD) BMI in this 119 859 subset of the UK Biobank was 27.53 (4.83), which was similar to the BMI for the entire cohort (N = 449 472 with BMI data mean = 27.43, SD = 4.80). Descriptive statistics are reported in Table 1.
Table 1. Baseline Characteristics of Participants From UK Biobank Used in the Analysis.
| Variable | Participants (n = 119 859) |
|---|---|
| Age, mean (SD), y | 56.87 (7.93) |
| Male, No. (%) | 56 816 (47.4) |
| BMI, mean (SD) | 27.53 (4.83) |
| Smoking history, No. (%) | |
| Never | 63 840 (53.26) |
| Past | 41 026 (34.23) |
| Current | 14 673 (12.24) |
| Missing | 320 (0.27) |
| Alcohol intake, No. (%) | |
| Never | 25 727 (21.46) |
| Special occasions only | 28 074 (23.42) |
| 1-3 Times/mo | 31 249 (26.07) |
| 1-2 Times/wk | 13 420 (11.20) |
| 3 or 4 Times/wk | 13 160 (10.98) |
| Daily or almost daily | 8152 (6.80) |
| Missing | 77 (0.06) |
| Disease prevalence rates, No. (%) | |
| Stroke | 1958 (1.6) |
| Coronary heart disease | 5760 (4.8) |
| Hypertension | 32 874 (27.5) |
| Type 2 diabetes | 6290 (5.3) |
| Assessed physical traits, mean (SD) | |
| Systolic BP, mm Hg | 140.28 (19.70) |
| Diastolic BP, mm Hg | 82.31 (10.64) |
| Pulse rate, beats/min | 69.68 (11.77) |
| Receiving BP-related medication, No. (%) | 11 167 (9.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure.
Observational Analysis BMI and Outcome Variables
In the fully adjusted models (Table 2), an SD increase in BMI (4.83 kg/m2) was associated with the risk of stroke (OR, 1.26; 95% CI, 1.21-1.31; P = 2.0 × 10−29), CHD (OR, 1.43; 95% CI, 1.40-1.46; P = 2.0 × 10−184), hypertension (OR, 1.75; 95% CI, 1.73-1.78; P < 4.51 × 10−308), and type 2 diabetes (OR, 1.97; 95% CI, 1.93-2.02; P < 4.51 × 10−308). In addition, BMI was associated with higher systolic BP (β = 3.02 mm Hg; 95% CI, 2.91-3.13 mm Hg; P < 4.5 × 10−308), diastolic BP (β = 2.82 mm Hg; 95% CI, 2.76-2.89 mm Hg; P < 4.5 × 10−308), and pulse rate (β = 1.86 beats/min; 95% CI, 1.79-1.94 beats/min; P < 4.5 × 10−308).
Table 2. Observational Associations Between BMI and All Outcome Variablesa.
| Variable | No. (%) | Partially Adjusted Model, OR (95% CI)b | P Value | Fully Adjusted Model, OR (95% CI)c | P Value |
|---|---|---|---|---|---|
| Stroke | 1958 (2) | 1.33 (1.29-1.39) | 3.5 × 10−47 | 1.26 (1.21-1.31) | 2.0 × 10−29 |
| CHD | 5760 (5) | 1.50 (1.47-1.54) | 4.0 × 10−244 | 1.43 (1.40-1.46) | 2.0 × 10−184 |
| Hypertension | 32 874 (27) | 1.76 (1.74-1.79) | 4.5 × 10−308 | 1.75 (1.73-1.78) | 4.5 × 10−308 |
| Type 2 diabetes | 6290 (5) | 2.09 (2.04-2.14) | 4.5 × 10−308 | 1.97 (1.93-2.02) | 4.5 × 10−308 |
| Blood pressured | |||||
| Systolic | 111 637 (93) | β = 3.11 (3.01-3.22) | 4.5 × 10−308 | β = 3.02 (2.91-3.13) | 4.5 × 10−308 |
| Diastolic | 111 638 (93) | β = 2.75 (2.69-2.81) | 4.5 × 10−308 | β = 2.82 (2.76-2.89) | 4.5 × 10−308 |
| Pulse rated | 111 638 (93) | β = 1.97 (1.90-2.05) | 4.5 × 10−308 | β = 1.86 (1.79-1.94) | 4.5 × 10−308 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHD, coronary heart disease; OR, odds ratio.
Values represent a per-SD increase in BMI (4.83 kg/m2).
Adjusted for assessment center, genetic batch effects, array, stratification, age, and sex. All significant at P < .01.
Additionally adjusted for smoking history, alcohol intake, and Townsend scores. All significant at P < .01.
Additionally corrected for self-reported use of antihypertensive medication; reported as unstandardized β coefficient.
MR Assumptions
The first MR assumption is that the polygenic score is associated with the exposure under investigation (BMI), and this is supported by the data (Pearson r = 0.14, P < 4.51 × 10−308; r2 = 2% variance in BMI explained; F statistic = 2175). The second MR assumption is that SNPs associate with disease risk only through the instrumented variable (BMI here), and the third MR assumption is that the genetic variants do not show association with traits that can confound the BMI to disease relationships.
The second and third MR assumptions may be violated when the SNPs employed in the genetic instrument show associations with variables (eg, smoking) which could influence the SNP to outcome association. Such so-called pleiotropy can be either vertical (ie, reflecting an association of SNPs with traits on the same pathway from exposure through to outcome, eg, in this case an association of BMI SNPs with systolic BP on the pathway to CHD), or horizontal (ie, on pathways that are distinct to the one from exposure through to outcome). Directional (also termed unbalanced) horizontal pleiotropy can lead to biased causal estimates from MR (see Figure 1 in White et al for a recent pictorial description of vertical and balanced/unbalanced horizontal pleiotropy). eTable 2 in the Supplement shows correlations between the BMI PGR score with smoking status (Pearson r = 0.02; P < .001), Townsend score (r = 0.01; P = .02), and alcohol intake (r = 0.03; P < .001). It is unclear whether these results reflect vertical (ie, downstream of BMI) or rather horizontal (ie, genetic associations with traits that are on separate pathways to BMI) pleiotropy. If these associations with smoking, deprivation, and alcohol were to lead to directional horizontal pleiotropy, the intercept from MR-Egger would be expected to differ from zero; in such cases that we found evidence that the intercept was different to zero, we interpreted the coefficient from MR-Egger as being the more valid causal estimate. Conversely, in the absence of statistical evidence for horizontal pleiotropy from the intercept on MR-Egger, we used the conventional MR analysis as it retains greater power.
MR Analysis of BMI and Outcomes
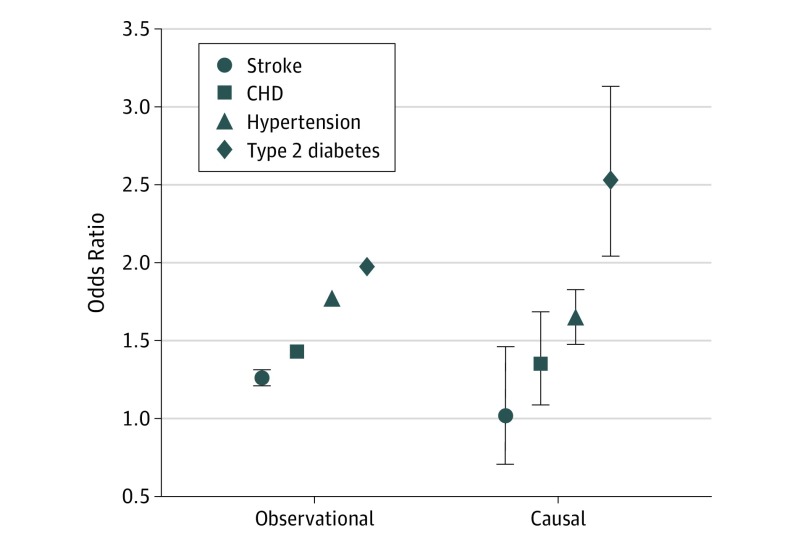
Mendelian randomization analyses (Table 3) using individual participant data showed generally similar effect sizes compared with observational analyses for hypertension (OR, 1.64; 95% CI, 1.48-1.83; P = 1.1 × 10−19) and CHD (OR, 1.35; 95% CI, 1.09-1.69; P = .007). The association with type 2 diabetes increased in magnitude from the association noted on observational analysis (OR, 1.97; 95% CI, 1.93-2.02; to OR, 2.53; 95% CI, 2.04-3.13). There was no association of BMI with stroke on MR analysis (P = .93). The Figure shows the causal estimate ORs vs the observational estimates for disease outcomes. Use of MR-Egger analyses did not identify evidence of unbalanced horizontal pleiotropy (eTables 3 and 4 in the Supplement). Associations with systolic/diastolic BP remained significant, but pulse rate did not. Results were similar in the partially vs fully adjusted models.
Table 3. Causal Estimates Derived From Instrumental Variable Analysis for an SD Increase in BMI and Risk of Cardiometabolic Traits Using Individual Participant Data.
| Variable | No. (%) | Partially Adjusted Model, OR (95% CI)a | P Value | Fully Adjusted Model, OR (95% CI)b | P Value |
|---|---|---|---|---|---|
| Stroke | 1958 (1.6) | 1.15 (0.81 to 1.63) | .43 | 1.02 (0.71 to 1.46) | .93 |
| CHD | 5760 (4.8) | 1.49 (1.20 to 1.83)c | 2.4 × 10−04 | 1.35 (1.09 to 1.69)c | .007 |
| Hypertension | 32 874 (27.5) | 1.65 (1.49 to 1.83)c | 1.6 × 10−21 | 1.64 (1.48 to 1.83)c | 1.1 × 10−19 |
| Type 2 diabetes | 6290 (5.3) | 2.75 (2.24 to 3.37)c | 3.9 × 10−22 | 2.53 (2.04 to 3.13)c | 1.5 × 10−17 |
| Blood pressured | |||||
| Systolic | 111 637 (93.1) | β = 1.53 (0.69 to 2.36)c | 3.3 × 10−04 | β = 1.65 (0.78 to 2.52)c | 2.0 × 10−04 |
| Diastolic | 111 638 (93.1) | β = 1.23 (0.76 to 1.69)c | 2.5 × 10−07 | β = 1.37 (0.88 to 1.85)c | 3.6 × 10−08 |
| Pulse rated | 111 638 (93.1) | β = −0.21 (−0.75 to 0.32) | .44 | β = −0.46 (−1.02 to 0.10) | .12 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHD, coronary heart disease; OR, odds ratios.
Adjusted for age and sex plus 10 genetic principal components, assessment center, and batch effects.
Additionally adjusted for smoking history, alcohol intake, and Townsend scores.
Significant at P < .01.
Additionally corrected for use of antihypertensive medication; reported as unstandardized β coefficient.
Figure. Observational Odds Ratios and Causal Estimates (Derived From Mendelian Randomization) for the Association Between Body Mass Index per SD and Cardiovascular and Metabolic Disease Outcomes .
CHD indicates coronary heart disease; error bars, 95% CI. Causal estimate is based on control function estimator mendelian randomization model. Plotted estimates are based on the fully adjusted models.
Additional Analyses
As a check, we also repeated the analysis of type 2 diabetes, CHD, and hypertension results after removing participants who reported their age at diagnosis as unknown, chose not to answer, or age at diagnosis 10 years or younger (cases removed: 238 for type 2 diabetes, 229 for CHD, and 3155 for hypertension); findings were unchanged. Including physical activity in the final models made no difference to the final results.
Discussion
Using the UK Biobank cohort, we examined the causal effects of BMI, using a genetic risk score comprising 93 SNPs associated with BMI, on important cardiometabolic traits and outcomes. The main advantage of an MR approach is that certain types of study bias can be minimized. Because DNA is stable and randomly inherited, which helps to mitigate errors from reverse causality and confounding, genetic variation can be used as a proxy for lifetime BMI to overcome limitations such as reverse causality and confounding, a process that hampers observational analyses of obesity and its consequences.
Using MR in the UK Biobank, we found that higher BMI was associated with a range of deleterious outcomes based on causal MR estimates: increased risk of CHD (causal estimate OR, 1.35; 95% CI, 1.09-1.69; per-SD [4.8-unit] increase in BMI), hypertension (OR, 1.64; 95% CI, 1.48-1.83), and type 2 diabetes (OR, 2.53; 95% CI, 2.04-3.13), as well as increased blood pressure (β = 1.65 mm Hg; 95% CI, 0.78-2.52 mm Hg systolic; and β = 1.37; 95% CI, 0.88-1.85 mm Hg diastolic). However, there were no causal effects on pulse rate or stroke identified. Associations were independent of Townsend deprivation scores, alcohol intake, smoking status, age, sex, and antihypertensive medication.
Three previous studies have provided causal estimates of BMI and cardiometabolic diseases based on polygenic instruments. Studies have reported their ORs and effect sizes in different scales, eg, per 1- unit of BMI (Holmes et al), per 4-units of BMI (Nordestgaard et al), and per 4.8 (this study; equivalent to one standard deviation). In terms of CHD: Nordestgaard et al reported an OR of 1.28 per 4 units of BMI on risk of CHD (N = 75 627) and Holmes et al reported an OR of 1.04 (in a meta-analysis of 219 423 participants) per 1-unit of BMI (an OR of 1.17 when scaled to a 4-unit increase in BMI as per Nordestgaard et al, or OR of 1.21 in our study where 1 SD equaled 4.8 units of BMI). Hägg et al reported no association for incident CHD (hazard ratio [HR], 1.13; P = .62), per 4.5 units of BMI. We report an OR of 1.35 per SD of BMI on the risk of CHD, similar to that of Nordestgaard et al. In terms of type 2 diabetes: Holmes et al reported an association with type 2 diabetes (OR, 1.29 per unit of BMI or 2.77 extrapolated to 4 units of BMI as per Nordestgaard et al), which is also similar to our results (OR, 2.53). The BMI and type 2 diabetes association estimates were larger for the causal MR analyses (OR in fully adjusted models, 2.53; 95% CI, 2.04-3.13), vs observational (OR, 1.97; 95% CI, 1.93-2.02). This is often the case in MR analyses since the genetic instrument represents a lifelong exposure to a risk factor and minimizes regression dilution bias. It is not clear why Hägg et al found a significant association with stroke (HR, 1.83; P = .03) in their study, and we did not in UK Biobank.
Studies by Fall et al (N = 198 502) and Timpson et al (N = 37 027, specifically investigating BP/hypertension) showed similar results using a single SNP in the FTO locus as an instrumental variable. In contrast to previous studies, we used a 93-SNP PGR score and corrected for population stratification with 10 principal components: these factors may contribute to slight differences between studies, but, on the whole, findings are generally concordant. Our PGR score used many more SNPs (appropriate given that BMI is not encoded for by any single variant in isolation) compared with the 2 previous studies, meaning that our genetic instrument explained a greater proportion of variance of BMI, which increased the precision of and power to detect the causal estimates that we report. Holmes et al summarized 4 large studies that conducted MR on BMI (N = 219 423), where the instrument r2 range was 0.4% to 0.8%; our instrument explained 1.79% of the variance. Taken together, our data provide strong evidence for a causal role of higher BMI and risk of type 2 diabetes and hypertension, and evidence that BMI increases risk of CHD.
Limitations
There are some limitations to the present report. There may be a degree of selection bias in the sense that less physically and cognitively impaired individuals responded to the UK Biobank invitation and attended the assessment. The UK Biobank cohort is representative of the UK population in terms of some characteristics, such as age, sex, ethnicity, and deprivation, but not necessarily all (eg, some participants are less likely to smoke and drink alcohol regularly). That said, the disease rates were similar to those reported in the British Heart Foundation 2015 report for hypertension (31% for men and 26% for women vs 27.5% in the present study), type 2 diabetes (6.9% for men and 5.6% for women vs 5.3%), and in the Health Survey for England 2014 report on data for CHD (5% vs 4.8%) and stroke (2% vs 1.6%). We found a small but significant correlation between the PGR score and higher rates of smoking, alcohol intake, and Townsend deprivation scores and thus made additional adjustments for these factors in our analyses, which did not alter the estimates we obtained. As described above, whether these associations represent vertical or horizontal pleiotropy remains unknown. The present sample also lacks data on a complete repertoire of potential mediators, such as lipid traits and glucose levels; these data will be available in the full UK Biobank in the next few years (along with genetic data on the total sample of 502 628 individuals in 2017). Because the MR-Egger analyses did not provide evidence to suggest presence of unbalanced horizontal pleiotropy, we have discussed the results in terms of conventional MR analytical approaches. It should be noted, however, that evidence for a causal BMI association with CHD and systolic/diastolic BP would be significantly weakened based on the MR-Egger estimates (to P > .05). Because MR-Egger is inherently more conservative, potentially meaningful causal associations might not be identified (type II error). Furthermore, this approach may be inadequately powered to detect pleiotropy when it is present.
Our analysis focused on prevalent disease. Although this should not result in major bias, the UK Biobank data set includes linkage to National Health Service records and hospital admissions data. During 4.9 years of follow-up from baseline, 3433 incident cardiovascular events occurred (including CHD). This finding was based on the full baseline data set of 502 649 participants, whereas genetic data are available on only 119 859 individuals, resulting in approximately 1000 incident events in the current data set. We will investigate genetically estimated BMI and cardiovascular events (eg, myocardial infarction and onset of hypertension, diabetes, and stroke) when genetic data are available on the full data set in 2017.
In terms of mechanisms, Holmes et al reported that a BMI PGR score based on 14 SNPs was associated with known cardiovascular risk factors, including glycemic traits, inflammation, and higher systolic and diastolic BPs. It seems a reasonable hypothesis that BMI would increase the risk of diseases through these established causal risk factors for CHD; the UK Biobank serum biomarker data are not yet available; however, we will investigate the role of these potentially mediating variables in the future. In terms of public health, with 39% and 13% of adults overweight or obese, respectively, and cardiovascular disease remaining the leading cause of mortality globally, our data emphasize the role of BMI, a modifiable risk factor, in cardiometabolic disease and the importance of reducing BMI at a population level to lower the incidence of cardiometabolic disease.
Conclusions
We report that BMI increases the risk of CHD, hypertension, type 2 diabetes, and diastolic and systolic BPs based on causal MR estimates. Body mass index represents an important modifiable risk factor for ameliorating the risk of cardiometabolic disease in the general population.
eTable 1. List of BMI-Associated SNPs as Reported by Locke et al
eTable 2. Intercorrelations Among PGR Score, BMI, and Potential Confounders
eTable 3. Constant Terms From MR-Egger Regression Analyses
eTable 4. Causal Estimates Derived From MR-Egger Analysis for a One Standard Deviation Increase in BMI, and Cardiometabolic Traits
eFigure. Scatter Plots of Genetic Associations With the Outcome Against Genetic Associations With BMI
References
- 1.De Schutter A, Lavie CJ, Milani RV. The impact of obesity on risk factors and prevalence and prognosis of coronary heart disease—the obesity paradox. Prog Cardiovasc Dis. 2014;56(4):401-408. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/. Updated June 2016. Accessed May 12, 2016.
- 3.Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckel RH, Kahn SE, Ferrannini E, et al. ; Endocrine Society; American Diabetes Association; European Association for the Study of Diabetes . Obesity and type 2 diabetes: what can be unified and what needs to be individualized? Diabetes Care. 2011;34(6):1424-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G; Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration (BMI Mediated Effects) . Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet. 2014;383(9921):970-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benito-León J, Mitchell AJ, Hernández-Gallego J, Bermejo-Pareja F. Obesity and impaired cognitive functioning in the elderly: a population-based cross-sectional study (NEDICES). Eur J Neurol. 2013;20(6):899-906, e76-e77. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Manolio TA, Pasquale LR, et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat Genet. 2011;43(6):519-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89-R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GD, Ebrahim S. Mendelian randomization: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1-22. [DOI] [PubMed] [Google Scholar]
- 10.Nordestgaard BG, Palmer TM, Benn M, et al. The effect of elevated body mass index on ischemic heart disease risk: causal estimates from a mendelian randomisation approach. PLoS Med. 2012;9(5):e1001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes MV, Lange LA, Palmer T, et al. Causal effects of body mass index on cardiometabolic traits and events: a mendelian randomization analysis. Am J Hum Genet. 2014;94(2):198-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hägg S, Fall T, Ploner A, et al. ; European Network for Genetic and Genomic Epidemiology Consortium . Adiposity as a cause of cardiovascular disease: a mendelian randomization study. Int J Epidemiol. 2015;44(2):578-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locke AE, Kahali B, Berndt SI, et al. ; LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium . Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Böhm M, Reil J-C, Deedwania P, Kim JB, Borer JS. Resting heart rate: risk indicator and emerging risk factor in cardiovascular disease. Am J Med. 2015;128(3):219-228. [DOI] [PubMed] [Google Scholar]
- 15.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen N, Sudlow C, Downey P, et al. UK Biobank: current status and what it means for epidemiology. Health Policy Technol. 2012;1:123-126. [Google Scholar]
- 17.Bhatnagar P, Scarborough P, Smeeton NC, Allender S. The incidence of all stroke and stroke subtype in the United Kingdom, 1985 to 2008: a systematic review. BMC Public Health. 2010;10:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Celis-Morales CA, Perez-Bravo F, Ibañez L, Salas C, Bailey MES, Gill JMR. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One. 2012;7(5):e36345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend P. Deprivation. J Soc Policy. 1987;16(2):125–146. [Google Scholar]
- 20.Genotyping and quality control of UK Biobank, a large-scale, extensive phenotyped prospective resource. US Biobank. http://www.ukbiobank.ac.uk/wp-content/uploads/2014/04/UKBiobank_genotyping_QC_documentation-web.pdf. Published 2015. Accessed January 31, 2017.
- 21.Rodriguez S, Gaunt TR, Day INM. Hardy-Weinberg equilibrium testing of biological ascertainment for mendelian randomization studies. Am J Epidemiol. 2009;169(4):505-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp Inc; 2013.
- 23.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams SM, Haines JL. Correcting away the hidden heritability. Ann Hum Genet. 2011;75(3):348-350. [DOI] [PubMed] [Google Scholar]
- 25.Baum CF, Schaffer ME, Stillman S IVREG2: Stata module for extended instrumental variables/2SLS and GMM estimation. http://ideas.repec.org/c/boc/bocode/s425401.html. Published 2016. Accessed February 11, 2016.
- 26.Burgess S, Thompson SG; CRP CHD Genetics Collaboration . Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol. 2011;40(3):755-764. [DOI] [PubMed] [Google Scholar]
- 27.Palmer TM, Sterne JAC, Harbord RM, et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in mendelian randomization analyses. Am J Epidemiol. 2011;173(12):1392-1403. [DOI] [PubMed] [Google Scholar]
- 28.White J, Swerdlow DI, Preiss D, et al. Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol. 2016;1(6):692-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fall T, Hägg S, Mägi R, et al. ; European Network for Genetic and Genomic Epidemiology (ENGAGE) Consortium . The role of adiposity in cardiometabolic traits: a mendelian randomization analysis. PLoS Med. 2013;10(6):e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timpson NJ, Harbord R, Davey Smith G, Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. Does greater adiposity increase blood pressure and hypertension risk? mendelian randomization using the FTO/MC4R genotype. Hypertension. 2009;54(1):84-90. [DOI] [PubMed] [Google Scholar]
- 32.Lyall DM, Cullen B, Allerhand M, et al. Cognitive test scores in UK Biobank: data reduction in 480,416 participants and longitudinal stability in 20,346 participants. PLoS One. 2016;11(4):e0154222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins R. What makes UK Biobank special? Lancet. 2012;379(9822):1173-1174. [DOI] [PubMed] [Google Scholar]
- 34.Bhatnagar P, Wickramasinghe K, Williams J, Rayner M, Townsend N. The epidemiology of cardiovascular disease in the UK 2014. Heart. 2015;101(15):1182-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Health Service Health survey for England, 2014: trend tables. http://content.digital.nhs.uk/catalogue/PUB19297. Published December 16, 2015. Accessed September 7, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. List of BMI-Associated SNPs as Reported by Locke et al
eTable 2. Intercorrelations Among PGR Score, BMI, and Potential Confounders
eTable 3. Constant Terms From MR-Egger Regression Analyses
eTable 4. Causal Estimates Derived From MR-Egger Analysis for a One Standard Deviation Increase in BMI, and Cardiometabolic Traits
eFigure. Scatter Plots of Genetic Associations With the Outcome Against Genetic Associations With BMI