Abstract
Although radon inhalation increases superoxide dismutase (SOD) activities in mouse organs, the mechanisms and pathways have not yet been fully clarified. The aim of this study was to determine the details of SOD activation in mouse brain tissue following the inhalation of radon at concentrations of 500 or 2000 Bq/m3 for 24 h. After inhalation, brains were removed quickly for analysis. Radon inhalation increased the manganese (Mn)-SOD level and mitochondrial SOD activity. However, the differences were not significant. There were no changes in the Cu/Zn-SOD level or cytosolic SOD activity. Radon inhalation increased the brain nuclear factor (NF)-κB content, which regulates the induction of Mn-SOD, in the nuclear and cytosolic compartments. The level of inhibitor of nuclear factor κB kinase subunit β (IKK-β), which activates NF-κB, was slightly increased by radon inhalation. The expression of cytoplasmic ataxia-telangiectasia mutated kinase in mice inhaling radon at 500 Bq/m3 was 50% higher than in control mice. In addition, NF-κB–inducing kinase was slightly increased after inhaling radon at 2000 Bq/m3. These findings suggest that radon inhalation might induce Mn-SOD protein via NF-κB activation that occurs in response to DNA damage and oxidative stress.
Keywords: radon, Mn-SOD, NF-κB, ataxia-telangiectasia mutated kinase, NF-κB–inducing kinase
INTRODUCTION
Superoxide dismutase (SOD) is an antioxidant enzyme that catalyzes the conversion of superoxide into hydrogen peroxide. SOD exists in multiple forms, including Mn-SOD, Cu/Zn-SOD and extracellular SOD, which are located in mitochondria, cytoplasm or extracellularly, respectively.
We have reported that radon inhalation increases SOD activity in many organs of mice, including the brain, heart, lung, liver, pancreas, kidney and small intestine [1]. These increases inhibit several kinds of oxidative stress–induced damage such as transient global cerebral ischemia in gerbils [2] and carrageenan-induced inflammatory paw edema in mice [3]. However, we have not yet demonstrated which kinds of SODs are increased by radon inhalation. Local X-ray irradiation to mice increases Mn-SOD but not Cu/Zn-SOD activity in heart [4]. Other reports suggest that moderate exercise resulting in oxidative stress increases the expression of antioxidant enzymes, at least Mn-SOD, in humans and rodents [5, 6].
In general, changes in SOD activity are controlled by various transcription factors such as NF-κB that are activated by oxidative stress. It is assumed that specific regions of the SOD gene recognize NF-κB, and Mn-SOD is thus highly expressed via NF-κB, which in turn is activated by hyperoxia-mediated oxidative stress. Increased expression of Mn-SOD by exercise has been reported to be via nuclear factor (NF)-κB [7]. A similar result was reported in alveolar type II cells, where exposure to hyperoxia induced Mn-SOD activity [8].
The mechanisms and pathways mediating increases in SOD activity following radon inhalation have not been fully clarified. The absorbed radiation doses due to radon inhalation, which we have reported previously [9], are much smaller than those of X-irradiation. The aim of this study was to closely examine increases in SOD activity following radon inhalation. We focused on SODs in mouse brain, because our previous report demonstrated the possibility of inhibiting reactive oxygen species (ROS)-induced brain damage by increasing SOD activity in this tissue [1]. We examined various biochemical parameters and upregulation of several transcription factors, including NF-κB, NF-κB–inducing kinase (NIK), inhibitor of κB kinase-β (IKK-β) and ataxia-telangiectasia mutated kinase (ATM). We also measured total SOD, Mn-SOD and Cu/Zn-SOD activities and protein levels.
MATERIALS AND METHODS
Animals
Male BALB/c mice, 8 weeks of age, weighing 24–28 g, were obtained from Charles River Laboratories Japan (Yokohama, Japan). Animals were housed in standard environmental conditions: temperature 22 ± 2°C and a preset light–dark cycle (12:12 h). Mice had free access to tap water and a standard diet (Oriental MF diet; Oriental Yeast Tokyo, Japan). Ethics approval was obtained from the animal experimental committee of Okayama University.
Experimental procedures
After acclimation, experimental mice were divided randomly into three groups of seven animals each. The control group received a sham inhalation only. The radon group was treated with radon inhalation at concentrations of 500 or 2000 Bq/m3 for 24 h. The concentrations of radon were selected on the basis of our preliminary studies [1]. Radon concentrations in the cages were measured using a radon monitor (CMR-510; femto-Tech, Franklin, OH, USA). Mice were killed by deep diethyl ether inhalation. After euthanasia, brains were removed quickly and washed with 10 mM phosphate-buffered saline (PBS; pH 7.4). Brain samples were used to assess SOD activities, protein content, and for western blotting. Samples were analyzed immediately or stored at −80°C until biochemical analysis.
Biochemical assays
To assay total SOD activity, mouse brain tissue was homogenized in 10 mM PBS on ice. The homogenate was centrifuged at 12 000 × g for 45 min at 4°C and the supernatant was used for the assay. Mitochondrial and cytosolic fractions were isolated by using a Mitochondria/Cytosol Fractionation Kit (#K256–25; BioVision, Milpitas, CA, USA) according to the manufacture's protocols. Briefly, brains were washed with ice-cold 10 mM PBS, suspended in 1 × cytosol extraction buffer mix containing protease inhibitor cocktail and dithiothreitol, and then incubated for 10 min on ice. Brains were then homogenized by 10 strokes of a Dounce homogenizer to yield a crude extract, transferred to a microcentrifuge tube, and centrifuged at 700 × g for 10 min at 4°C. The resulting supernatant was removed and centrifuged at 10 000 × g for 30 min at 4°C. This supernatant was collected as the cytosolic fraction and used to measure cytosolic SOD activity. The final pellet was vortexed with 1 × mitochondrial extraction buffer mix containing protease inhibitor cocktail and dithiothreitol, and saved as the mitochondrial fraction. This fraction was used to assay the activity of mitochondrial SOD.
All SOD activities were measured by the nitroblue tetrazolium (NBT) reduction method using the Wako SOD test kit (Wako Pure Chemical Industry, Osaka, Japan) [10]. Briefly, the extent of inhibition of NBT reduction was measured at 560 nm using a spectrophotometer. One unit of enzyme activity was defined as 50% inhibition of NBT reduction. SOD activities are expressed as units per mg of protein. The protein content of each fraction was estimated by the Bradford method using the Protein Quantification Kit-Rapid (Dojindo Molecular Technologies, Kumamoto, Japan) [11].
Determination of SOD and transcription factors by enzyme-linked immunosorbent assays
For the quantitative determination of total and Mn-SOD in brain, protein concentrations of these enzymes were measured using a commercial enzyme-linked immunoassay kit (Cusabio Biotech Hubei, China). Briefly, brain tissue was homogenized in 10 mM PBS on ice. Two freeze–thaw cycles were performed to break the cell membranes. Then, the homogenates were centrifuged for 5 min at 5000 × g, 4°C. The supernatant was used for the assays according to the manufacture's protocols.
Determination of SOD and transcription factors by western blotting
Western blot analyses with mouse monoclonal antibodies against Cu/Zn-SOD, NF-κB, ATM, NIK and IKK-β were performed. Protein extracts from whole cell lysates were obtained by gently homogenizing brain tissue (1 g) in 3 ml radioimmunoprecipitation assay lysis buffer with added protease inhibitor cocktail and phenylmethylsulfonyl fluoride (sc-24 948; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The homogenates were incubated on ice for 30 min and clarified by centrifugation at 10 000 × g for 10 min at 4°C. The supernatants were again centrifuged at 10 000 × g for 10 min, then stored at −80°C until analysis. The supernatant was used as the whole cell protein extract. Protein concentrations were estimated by using a detergent compatible Dc protein assay kit (Bio-Rad Laboratories, Tokyo, Japan).
Nuclear and cytoplasmic extracts were isolated using the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (#78 833; Pierce Biotechnology, Rockford, IL, USA) according to the manufacture's protocols. Briefly, cells were centrifuged to obtain a packed cell volume and lysed in ice cold CER I with protease inhibitors. Following vortexing at the highest setting and incubation on ice for 10 min, ice-cold CER II was added and samples centrifuged at 10 000 × g for 10 min. The supernatant was used as the cytoplasmic extract. The pellet was resuspended in ice-cold NER with protease inhibitors and incubated on ice for 40 min with vortexing for 15 s every 10 min, then centrifuged at 10 000 × g for 10 min to obtain the supernatant containing nuclear proteins.
These protein extracts were mixed with Laemmli's sample buffer containing (final concentrations) 50 mM Tris (pH 6.8), 1% sodium dodecyl sulfate, 5% 2-mercaptoethanol, and bromophenol blue as a marker. This mixture was then stirred thoroughly and boiled for 3 min to prepare samples for western blotting by the method of Laemmli. Volumes containing 25 μg of total protein per sample were loaded onto polyacrylamide gels (0.75 mm thick) and electrophoresed at 40 V for 1 h, then 160 V for 30 min. The separating gel was 7.5 or 12% (w/v), and the stacking gel was 4% (w/v). Blots were transferred onto polyvinylidene fluoride membranes using a semi-dry transblotting apparatus (Bio-Rad Laboratories). Membranes were blocked with 3% bovine serum albumin in TBS-T (50 mM Tris–HCl-buffered saline, pH 7.5, with 0.1% Tween 20) for 3 h at room temperature. After blocking, membranes were incubated with the antibodies (1:1000) in Can Get Signal Immunoreaction Enhancer Solution A (Toyobo, Osaka, Japan) overnight at 4°C. Membranes were washed three times with TBS-T, then incubated with a horseradish peroxidase-conjugated goat anti-mouse antibody at 1:3000 in Can Get Signal Immunoreaction Enhancer Solution B (Toyobo) for 1 h at room temperature. Membranes were washed with TBS-T buffer and incubated with Amersham enhanced chemiluminescence Prime reagents (GE Healthcare, Pittsburgh, PA, USA). The bands were scanned and quantitated with the Chemi Doc XRS Plus System (Bio-Rad Laboratories) according to the manufacturer's recommendations. Band intensities were normalized to β-actin or lamin to account for loading variations between lanes.
Statistical analyses
Data are presented as means ± standard error of the mean (SEM). Statistical significance of the biochemical assay and western blotting results was determined using analysis of variance with Dunnett's post-hoc test to make multiple comparisons. Differences with P < 0.05 were considered to be statistically significant.
RESULTS AND DISCUSSION
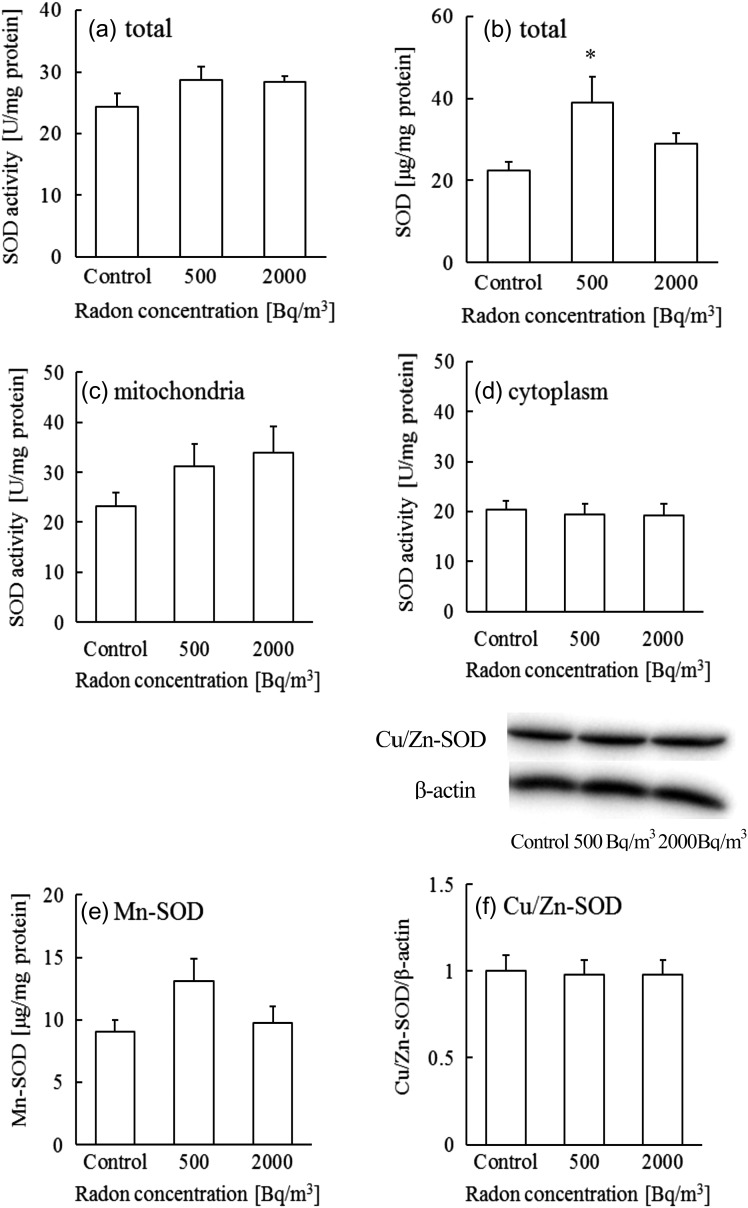
We reported previously that radon inhalation increased antioxidant substances such as SOD [1]. However, the mechanisms of the activation were not clear. Therefore, we first examined the activities of total, mitochondrial and cytosolic SOD in brain. Because Mn-SOD and Cu/Zn-SOD show different responses against X-irradiation [4], we focused on 24 h inhalation because our previous report suggested that peak activation of SOD activities was observed at this time. The results showed that radon inhalation at a concentration of 500 Bq/m3 increased SOD protein levels (Fig. 1b). However, although radon inhalation at this concentration increased SOD activity by ~20%, the difference was not significant (Fig. 1a).
Fig. 1.
Changes in (a) total SOD activity, (b) total SOD protein levels, (c) mitochondrial SOD activity, (d) cytosolic SOD activity, (e) Mn-SOD levels and (f) Cu/Zn-SOD levels in mouse brain after exposure to control, 500 or 2000 Bq/m3 radon. Data are presented as means ± SEM. Seven to eight mice were included for each experimental point. *P < 0.05, control vs 500 Bq/m3.
To clarify the activation in detail, we examined Mn-SOD and Cu/Zn-SOD, which are located in mitochondria and the cytoplasm, respectively. Although radon inhalation did not change the Cu/Zn-SOD protein level or cytoplasmic SOD activity (Fig. 1d and f), the Mn-SOD protein level and mitochondrial SOD activity following exposure to 500 Bq/m3 appeared elevated, although not significantly (Fig. 1c and e). These results indicate that SOD activation by radon inhalation at a dose of 500 Bq/m3 may be attributed to the induction of Mn-SOD. On the other hand, both the protein levels of total-SOD and Mn-SOD, and the cytosolic SOD activity and Cu/Zn-SOD protein levels in the 2000 Bq/m3 group, were the same as in controls (Fig. 1b). A possible reason why mitochondrial SOD activity in the 2000 Bq/m3 group increased despite Mn-SOD protein being unchanged is that radon inhalation at a concentration of 2000 Bq/m3 may increase the enzymatic activity, but not the protein levels. Further studies are needed to clarify the increase in enzymatic activity.
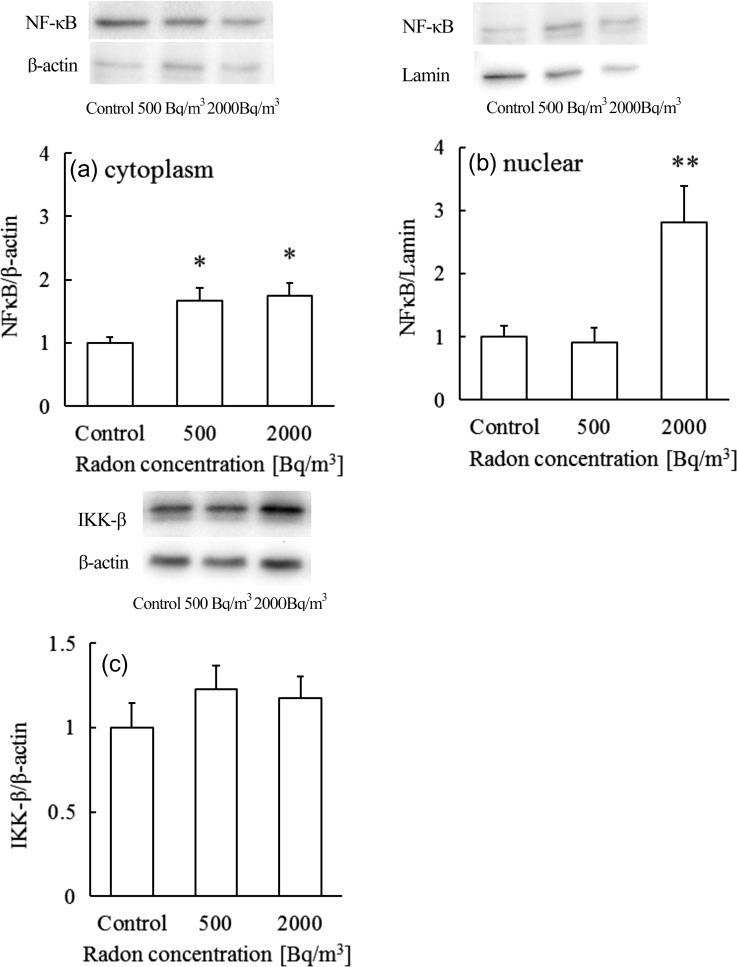
Induction of Mn-SOD is regulated by NF-κB [12], and the binding site is located in the SOD2 gene [13–15]. Therefore, we next examined NF-κB in the cytoplasmic and nuclear fractions. The results shown in Fig. 2a demonstrated that the content of NF-κB in brain cytoplasm from mice that inhaled radon at concentrations of 500 and 2000 Bq/m3 was significantly higher than in control mice. In addition, the content of nuclear NF-κB in mice treated with radon at 2000 Bq/m3 was higher than in control mice (Fig. 2b). These findings suggest that the increased NF-κB might contribute to the induction of Mn-SOD. Although NF-κB in the nuclear fraction of the 2000 Bq/m3 group increased significantly, the Mn-SOD protein level was unchanged from the control. Therefore, Mn-SOD protein might be induced soon afterward.
Fig. 2.
Changes in (a) cytoplasmic NF-κB, (b) nuclear NF-κB, and (c) IKK-β levels in mouse brain after exposure to control, 500 or 2000 Bq/m3 radon. Data are presented as means ± SEM. Seven to eight mice were included for each experimental point. *P < 0.05, control vs 500 Bq/m3, 2000 Bq/m3. **P < 0.01, control vs 2000 Bq/m3.
NF-κB can be activated via two different pathways: classical and alternative. In the classical pathway, NF-κB can be activated via an IKK-β–dependent mechanism responsible for the rapid degradation of IκB-α, -β and -ε [12]. The IKK-α–dependent pathway, leading to processing of p100 and activation of p52/RelB, is defined as the alternative pathway [16].
NF-κB activation typically occurs via the classical pathway. Therefore, we examined IKK-β in the brain after radon inhalation. The results showed that the levels of IKK-β were increased by 22 and 17% after radon inhalation at 500 and 2000 Bq/m3, respectively. However, these differences were not significant (Fig. 2c). These findings suggest that NF-κB activation following radon inhalation may occur via regulation of IKK-β activity.
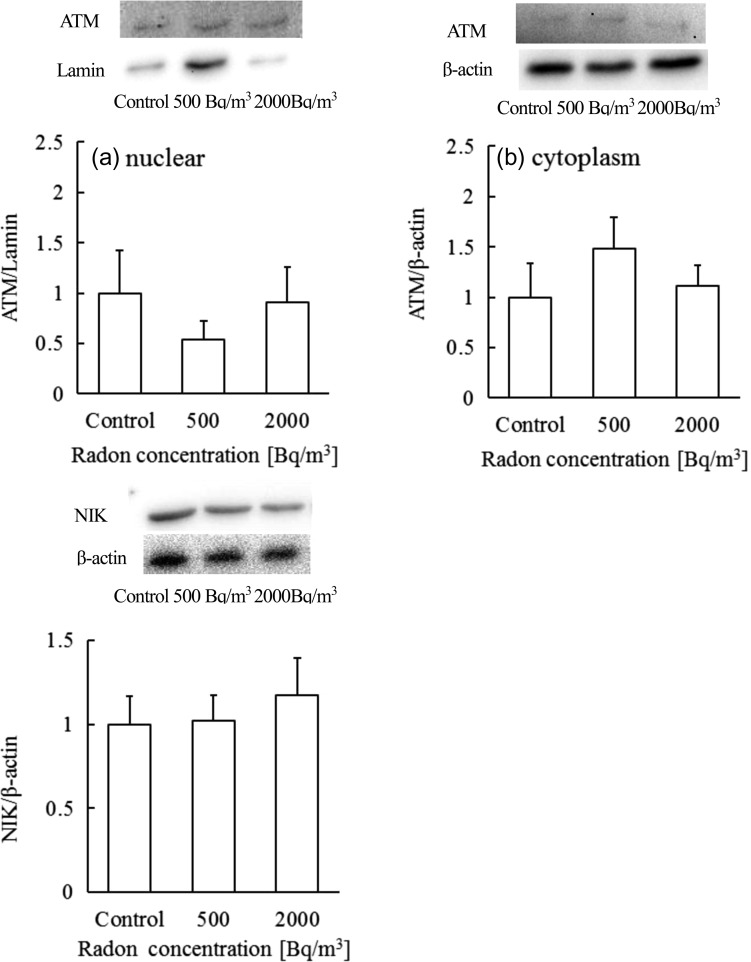
ATM plays an important role in NF-κB activation following DNA damage, including IKK activation, IκB degradation, and induction of NF-κB DNA binding activity [17]. Specifically, damaged DNA activates nuclear ATM, which in turn translocates to the cytoplasm to activate IKK. Therefore, investigation of ATM (both nuclear and cytoplasmic) is important. The results shown in Fig. 3a demonstrated that the nuclear content of ATM in brains from mice treated with radon at a concentration of 500 Bq/m3 appeared to be lower, and the cytoplasmic content appeared to be higher, than in control mice. However, these differences were not significant. These findings suggest that radon inhalation does not induce severe DNA damage, but at a concentration of 500 Bq/m3 may contribute to the activation of IKK-β. A limitation of this study was the absence of an assessment of DNA damage induced by radon inhalation.
Fig. 3.
Changes in (a) nuclear ataxia-telangiectasia mutated kinase (ATM), (b) cytoplasmic ATM, and (c) NF-κB inducing kinase (NIK) levels in mouse brain after exposure to control, 500 or 2000 Bq/m3 radon. Data are presented as means ± SEM. Six to eight mice were included for each experimental point.
ROS induced by irradiation may play an important role in tissue responses to this exposure. As described above, there are two different NF-κB activation pathways. NIK is an integral component of the alternative pathway [18] and plays an important role in cytokine-induced NF-κB activation. To clarify whether ROS contributed to NF-κB activation, NIK in brain tissue was assayed. No large changes were seen with the expression of NIK in brains from mice treated with 500 Bq/m3 radon. NIK was slightly increased (about 18%) after radon inhalation at 2000 Bq/m3 (Fig. 3c), but the change was not significant. These findings suggest that radon inhalation at a concentration of 2000 Bq/m3 may contribute to the activation of IKK-β activity. However, from the IKK-β, ATM and NIK results, the activation effect of IKK-β may be mild. One possible reason is that the absorbed radiation dose due to radon inhalation is very low.
In conclusion, radon inhalation induces SOD proteins. The increase in total-SOD may be attributed to the induction of Mn-SOD, because cytosolic SOD activity and Cu/Zn-SOD are unchanged from the control levels. Mn-SOD is probably induced via NF-κB activation, which is stimulated by DNA damage and oxidative stress. In spite of the different types of radiation (radon emits α particles), the results of our study are similar to those using X-irradiation. However, the absorbed radiation dose due to radon inhalation, which has been reported [9], is much smaller than that using X-irradiation. In this study, we could not determine why there is such a difference between radon and X-irradiation. Further studies are needed to clarify the effects of radon on the induction of antioxidant enzymes.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
This work was supported by Okayama University and Japan Atomic Energy Agency.
ACKNOWLEDGEMENTS
The authors thank the staff members of the Department of Animal Resources and Radiation Research, Shikata Laboratory Advanced Science Research Center, Okayama University, for their technical support in this work.
REFERENCES
- 1. Kataoka T, Sakoda A, Ishimori Y et al. Study of the response of superoxide dismutase in mouse organs to radon using a new large-scale facility for exposing small animals to radon. J Radiat Res 2011;52:775–81. [DOI] [PubMed] [Google Scholar]
- 2. Kataoka T, Etani R, Takata Y et al. Radon inhalation protects against transient global cerebral ischemic injury in gerbils. Inflammation 2014;37:1675–82. [DOI] [PubMed] [Google Scholar]
- 3. Kataoka T, Teraoka J, Sakoda A et al. Protective effects of radon inhalation on carrageenan-induced inflammatory paw edema in mice. Inflammation 2012;35:713–22. [DOI] [PubMed] [Google Scholar]
- 4. Oberley LW, St Clair DK, Autor AP et al. Increase in manganese superoxide dismutase activity in the mouse heart after X-irradiation. Arch Biochem Biophys 1987;254:69–78. [DOI] [PubMed] [Google Scholar]
- 5. Higuchi M, Cartier LJ, Chen M et al. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol 1985;40:281–6. [DOI] [PubMed] [Google Scholar]
- 6. Ohno H, Yamashita H, Ookawara T et al. Training effects on concentrations of immunoreactive superoxide dismutase iso-enzymes in human plasma. Acta Physiol Scand 1992;146:291–2. [DOI] [PubMed] [Google Scholar]
- 7. Ji LL, Gomez-Cabrera MC, Steinhafel N et al. Acute exercise activates nuclear factor (NF)-κB signaling pathway in rat skeletal muscle. FASEB J 2004:18;1499–506. [DOI] [PubMed] [Google Scholar]
- 8. Freeman BA, Mason RJ, Williams MC et al. Antioxidant enzyme activity in alveolar type II cells after exposure of rats to hyperoxia. Exp Lung Res 1986;10:203–22. [DOI] [PubMed] [Google Scholar]
- 9. Sakoda A, Ishimori Y, Kawabe A et al. Physiologically based pharmacokinetic modeling of inhaled radon to calculate absorbed doses in mice, rats, and humans. J Nucl Sci Technol 2010:47;731–38. [Google Scholar]
- 10. Baehner R, Murrmann S, Davis J et al. The role of superoxide anion and hydrogen peroxide in phagocytosis-associated oxidative metabolic reactions. J Clin Investig 1975;56:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54. [DOI] [PubMed] [Google Scholar]
- 12. Ahmed KM, Li JJ. NF-κB–mediated adaptive resistance to ionizing radiation. Free Radic Biol Med 2008:44;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan XS, Devalaraja MN, St Clair DK. Molecular structure and organization of the human manganese superoxide dismutase gene. DNA Cell Biol 1994;13:1127–36. [DOI] [PubMed] [Google Scholar]
- 14. Duttaroy A, Parkes T, Emtage P, et al. The manganese superoxide dismutase gene of Drosophila: structure, expression, and evidence for regulation by MAP kinase. DNA Cell Biol 1997:16;391–9. [DOI] [PubMed] [Google Scholar]
- 15. Xu Y, Kiningham KK, Devalaraja MN et al. An intronic NF-κB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-α and interleukin-1β. DNA Cell Biol 1999:18;709–22. [DOI] [PubMed] [Google Scholar]
- 16. Senftleben U, Cao Y, Xiao G et al. Activation by IKKα of a second, evolutionarily conserved, NF-κ B signaling pathway. Science 2001:293;1495–9. [DOI] [PubMed] [Google Scholar]
- 17. Li N, Banin S, Ouyang H et al. ATM is required for IκB kinase (IKK) activation in response to DNA double strand breaks. J Biol Chem 2001:276;8898–903. [DOI] [PubMed] [Google Scholar]
- 18. Ling L, Cao Z, Goeddel DV. NF-κB–inducing kinase activates IKK-α by phosphorylation of Ser-176. Proc Natl Acad Sci U S A 1998:95;3792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]