Key Points
Question
Do changes in personality traits occur before the onset of mild cognitive impairment or clinical dementia?
Findings
In a cohort study that followed up 2046 older adults for as long as 36 years, no evidence of significant change in self-rated personality was found before the onset of mild cognitive impairment or clinical dementia.
Meaning
No personality changes that could be characterized as an early sign of dementia were found.
Abstract
Importance
Changes in behavior and personality are 1 criterion for the diagnosis of dementia. It is unclear, however, whether such changes begin before the clinical onset of the disease.
Objective
To determine whether increases in neuroticism, declines in conscientiousness, and changes in other personality traits occur before the onset of mild cognitive impairment or dementia.
Design, Setting, and Participants
A cohort of 2046 community-dwelling older adults who volunteered to participate in the Baltimore Longitudinal Study of Aging were included. The study examined personality and clinical assessments obtained between 1980 and July 13, 2016, from participants with no cognitive impairment at first assessment who were followed up for as long as 36 years (mean [SD], 12.05 [9.54] years). The self-report personality scales were not considered during consensus diagnostic conferences.
Main Outcomes and Measures
Change in self-rated personality traits assessed in the preclinical phase of Alzheimer disease and other dementias with the Revised NEO Personality Inventory, a 240-item questionnaire that assesses 30 facets, 6 for each of the 5 major dimensions: neuroticism, extraversion, openness, agreeableness, and conscientiousness.
Results
Of the 2046 participants, 931 [45.5%] were women; mean (SD) age at first assessment was 62.56 (14.63) years. During 24 569 person-years, mild cognitive impairment was diagnosed in 104 (5.1%) individuals, and all-cause dementia was diagnosed in 255 (12.5%) participants, including 194 (9.5%) with Alzheimer disease. Multilevel modeling that accounted for age, sex, race, and educational level found significant differences on the intercept of several traits: individuals who developed dementia scored higher on neuroticism (β = 2.83; 95% CI, 1.44 to 4.22; P < .001) and lower on conscientiousness (β = −3.34; 95% CI, −4.93 to −1.75; P < .001) and extraversion (β = −1.74; 95% CI, −3.23 to −0.25; P = .02). Change in personality (ie, slope), however, was not significantly different between the nonimpaired and the Alzheimer disease groups (eg, neuroticism: β = 0.00; 95% CI, −0.08 to 0.08; P = .91; conscientiousness: β = −0.06; 95% CI, −0.16 to 0.04; P = .24). Slopes for individuals who developed mild cognitive impairment (eg, neuroticism: β = 0.00; 95% CI, −0.12 to 0.12; P = .98; conscientiousness: β = −0.09; 95% CI, −0.23 to 0.05; P = .18) and all-cause dementia (eg, neuroticism: β = 0.02; 95% CI, −0.06 to 0.10; P = .49; conscientiousness: β = −0.08; 95% CI, −0.16 to 0.00; P = .07) were also similar to those for nonimpaired participants.
Conclusions and Relevance
No evidence for preclinical change in personality before the onset of mild cognitive impairment or dementia was identified. These findings provide evidence against the reverse causality hypothesis and strengthen evidence for personality traits as a risk factor for dementia.
This cohort study examines whether changes in personality traits develop before the onset of mild cognitive impairment or dementia in individuals with no initial impairment.
Introduction
Changes in personality and behavior are one clinical criterion for the diagnosis of dementia. Little is known, however, as to whether personality changes before the onset of clinical dementia. The neuropathologic processes that underlie Alzheimer disease (AD) and related dementias start years before onset of clinical dementia. It is, therefore, possible that changes in personality (eg, loss of motivation or increased irritability) might be an early sign of AD that precedes the onset of cognitive and functional impairment.
Consistent with diagnostic criteria, change in personality traits is commonly observed by caregivers after the onset of clinical dementia. Several studies have asked family members to rate the care recipient’s personality before and after the onset of dementia and have consistently found large changes on the 5 major dimensions of personality, especially for neuroticism and conscientiousness. These informant-based retrospective studies, however, cannot determine the timing of personality change, and the changes may have occurred after the onset of cognitive impairment. A few studies suggest that personality might change even before the onset of clinical dementia. Previous prospective studies have either not assessed all 5 major dimensions of personality or the assessments were close to the diagnosis of dementia (within 2 years or at the time), which blurs the distinction between personality change that occurs before the onset of cognitive impairment with changes during the dementia prodrome or at the time of diagnosis.
The present study examined personality change before the onset of AD, mild cognitive impairment (MCI), or all-cause dementia in an ongoing longitudinal study of older adults who have completed a 5-factor model personality questionnaire since 1980. Using data up to 3 decades before onset of the disease, we tested the hypothesis that individuals later diagnosed with AD would show greater changes in personality before symptom onset compared with unaffected individuals (Figure 1). In particular, we expected increases in neuroticism and declines in conscientiousness as early signs of the underlying neurodegeneration before the onset of clinical symptoms of dementia. We focused on AD because it is the most common cause of dementia but also examined change before the onset of MCI and all-cause dementia. In addition to the 5 major traits, we examined changes in 30 facets of personality to provide a detailed account of personality change.
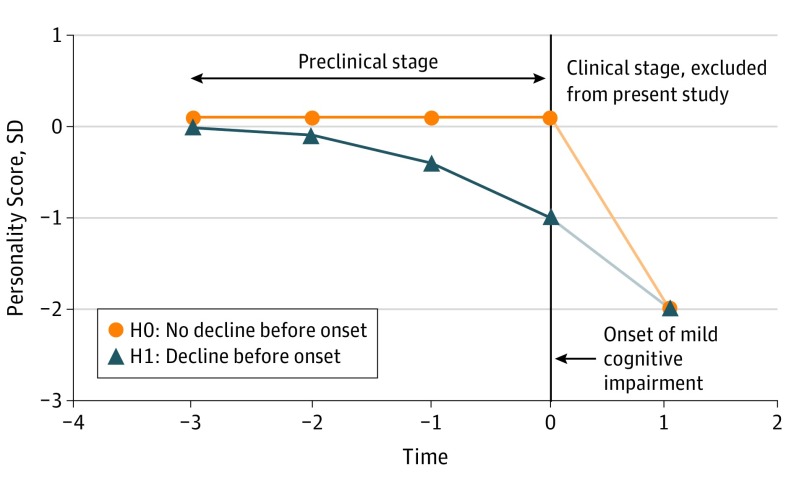
Figure 1. Hypothetical Personality Changes in Preclinical Alzheimer Disease.
The H1 line represents the hypothesis that personality (eg, conscientiousness) changes before the onset of clinical dementia compared with no preclinical change (H0).
This research advances knowledge on the preclinical course of a psychological feature that is altered by AD. It also provides a test of whether reverse causality may explain the association between personality traits and risk of AD and dementia found in prospective studies. That is, if there is evidence that personality traits change before the onset of dementia, it could indicate that the personality traits associated with AD are a consequence of the neuropathologic process rather than independent risk factors for AD.
Methods
Participants
The Baltimore Longitudinal Study of Aging (BLSA) began to study physical and cognitive changes associated with aging in 1958. The BLSA is carried out by the Intramural Research Program of the National Institute on Aging. Healthy individuals of different ages are continuously enrolled in the study and assessed with regular follow-up visits. Current follow-up care occurs annually for individuals aged 80 years or older, biennially for those aged 60 to 79 years, and every 4 years for younger participants. Eligibility criteria have been reported. The BLSA participants receive an in-depth physical and cognitive evaluation and complete a detailed personality assessment. Participants provide written informed consent approved by the National Institutes of Health Institutional Review Board. The analyses in this article were also approved by the Florida State University Institutional Review Board. Participants do not receive financial compensation.
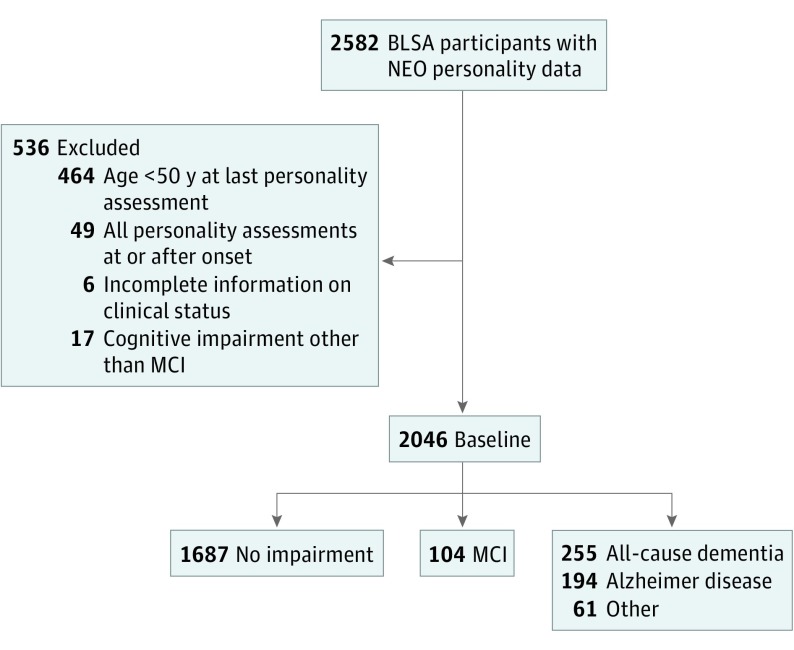
We excluded 536 individuals from an initial sample of 2582 individuals with personality data (Figure 2). Participants were excluded mainly because they were younger than 50 years at the last personality assessment or because they completed the personality questionnaire only after the onset of cognitive impairment (ie, MCI or dementia). Because we were interested in changes in the preclinical phase of the disease, we also excluded all personality assessments that occurred in the year of the disease onset or after. We replaced missing education information with the sample mean for 6 nonimpaired individuals. As of July 13, 2016, 784 (38.3%) participants had died, and 289 (14.1%) were not active for a variety of reasons, including loss to follow-up. To evaluate the link between personality and attrition, we compared the baseline personality scores of the active and nonactive participants, adjusting for age, sex, race, and educational level in a multivariate analysis of covariance. The multivariate test was significant (F1238 = 3.33; P = .005), and the nonactive participants scored approximately 0.2 SD lower on extraversion compared with the active cohort (P = .006); there were no significant differences in the other traits (all P > .05).
Figure 2. Sample Selection at Baseline and Cognitive Outcomes at Follow-up.
BLSA indicates Baltimore Longitudinal Study of Aging; MCI, mild cognitive impairment.
Personality Assessment
Participants completed the self-report version of the Revised NEO Personality Inventory (NEO-PI-R) or an earlier version in 1980 and 1986 (eMethods in the Supplement). The NEO-PI-R is a 240-item questionnaire that assesses 30 facets, 6 for each of the 5 major dimensions: neuroticism, extraversion, openness, agreeableness, and conscientiousness. Raw scores were standardized to T scores (mean [SD], 50 [10]) using combined sex norms reported in the manual. In the BLSA sample, the NEO-PI-R factor structure shows high congruence with the normative structure (Tucker φ = 0.97-0.99), the Cronbach α internal consistencies for the 5 dimensions ranged from 0.87 to 0.92, and the test-retest correlations for the 5 dimensions ranged from 0.78 to 0.85 over a mean interval of 10 years.
Clinical and Neuropsychological Evaluations
The BLSA participants were evaluated at enrollment for history of neurologic or cerebrovascular disease and for impairment of cognitive or behavioral functioning. Follow-up evaluations included a neuropsychological battery and clinical examination, including an informant and participant structured interview. The latter was based on the Clinical Dementia Rating (CDR) scale after 1998 and the Dementia Questionnaire before 1998. The CDR was administered at each visit to participants enrolled in the autopsy and positron emission tomography amyloid imaging studies; the remaining participants received the CDR if they scored 4 or more on the Blessed Information Memory Concentration test. All participants were reviewed at a diagnostic consensus conference if they screened positive (score ≥4) on the Blessed Information Memory Concentration, if their informant or participant CDR score was 0.5 or higher, or if the results of their Dementia Questionnaire were abnormal. In addition, all participants were evaluated by case conference on death or withdrawal. All neuropsychological diagnostic tests and clinical data were available for review at the diagnostic conference. The NEO personality scores were not available or considered during consensus conferences. Mild cognitive impairment was based on the criteria of Petersen et al. Diagnosis of dementia was based on Diagnostic and Statistical Manual of Mental Disorders (Third Edition Revised) criteria, which did not include personality change as a criterion, and diagnosis of AD was based on the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer Disease and Related Disorders Association criteria.
Statistical Analysis
The primary aim was to examine the course of personality change before the onset of symptoms of MCI or dementia. We used multilevel modeling (ie, hierarchical linear modeling [HLM]), also known as mixed models or growth curve analysis, to estimate the trajectory of each trait. In HLM, the number and spacing of measurement observations may vary across individuals given that the time-series observations for each person are used to estimate the individual’s trajectory (level 1), and those individual parameters are the basis of group estimates (level 2). Using HLM, we examined the association between cognitive status and the intercept and slope of each personality trait, with primary interest in the slope (ie, the rate of change). For individuals who developed MCI or dementia, time was coded as time to onset of MCI, with the year of onset coded as 0. For individuals who remained nonimpaired, time was set as 0 for the date of the most recent personality assessment. With this coding, the intercept parameters refer to the most recent assessment and not the usual baseline. The nonimpaired cohort was the reference group in all analyses. The primary analyses examined the effect of AD on the intercept and slope of each trait; MCI and dementia other than AD were excluded from this analysis. Then we examined a broader model that included all-cause dementia and MCI. To examine whether changes from the NEO-PI (1980-1986) to NEO-PI-R (1989-2016) had an effect on the results, we excluded the 1980-1986 assessments in sensitivity analyses. We included participants with only 1 personality assessment in the HLM models because such information can contribute to more robust HLM estimates. In sensitivity analyses, we examined whether the results were similar when excluding cases with only 1 assessment. All models included age, age squared sex, race, and educational level as covariates for the intercept and slope because these demographic variables have been associated with both personality and risk of dementia. Age in decade and educational level were grand-mean centered.
Because we expected the changes in personality to accelerate in the last few years before the onset of cognitive impairment (Figure 1), we repeated the analyses by estimating models with curvilinear slopes (ie, time squared). We also plotted personality trajectories based on the last 2 assessments. Using these last 2 assessments, we evaluated rank-order stability to test whether it declines in the preclinical phase.
Results
Descriptive statistics for the overall sample and by cognitive status are presented in Table 1. The sample included 931 (45.5%) women, and race/ethnicity was 1582 (77.3%) white, 374 (18.3%) black, 55 (2.7%) Asian or Pacific Islander, and 35 (1.7%) other participants. The 2046 participants completed 8665 personality assessments between 1980 and July 13, 2016 (range, 1-18; mean [SD], 4.24 [3.06]). The time elapsed from the first personality assessment ranged from 1 to 36 years (12.05 [9.54]). During the study (24 569 person-years), 104 (5.1%) participants developed MCI and 255 (12.5%) participants developed all-cause dementia, including 194 (9.5%) who developed AD. Comparison of the unadjusted baseline means indicate that the group that later developed AD or all-cause dementia scored higher on neuroticism and lower on extraversion, openness, and conscientiousness compared with the nonimpaired participants.
Table 1. Baseline Characteristics.
| Characteristic | Mean (SD) | ||||
|---|---|---|---|---|---|
| Total | Not Impaired | AD | All-Cause Dementia | MCI | |
| No. (%) | 2046 (100) | 1687 (82.5) | 194 (9.5) | 255 (12.5) | 104 (5.1) |
| Female, No. (%) | 931 (45.5) | 782 (46.4) | 94 (48.5) | 116 (45.5) | 33 (31.7) |
| Age, ya | 62.56 (14.63) | 60.70 (14.74) | 72.07 (10.18) | 71.51 (10.58) | 70.93 (9.24) |
| Education, y | 17.01 (2.66) | 17.03 (2.66) | 16.99 (2.79) | 16.98 (2.69) | 16.74 (2.72) |
| Follow-up time, y | 12.05 (9.54) | 12.12 (9.87) | 11.74 (7.94) | 11.82 (7.91) | 11.60 (7.66) |
| Personality assessments, No. | 4.24 (3.06) | 4.39 (3.05) | 3.52 (2.81) | 3.58 (2.92) | 3.33 (3.17) |
| Personalitya | |||||
| Neuroticism | 46.96 (9.40) | 46.68 (9.34) | 49.02 (10.13)b | 49.10 (10.02)b | 46.31 (8.19) |
| Extraversion | 49.56 (10.12) | 50.20 (10.11) | 46.69 (9.10)b | 46.41 (9.11)b | 46.89 (10.88)b |
| Openness | 51.93 (10.52) | 52.41 (10.57) | 50.09 (10.00)b | 50.21 (9.81)b | 48.33 (10.41)b |
| Agreeableness | 50.73 (8.85) | 50.78 (9.10) | 50.48 (7.36) | 50.65 (7.39) | 50.16 (7.76) |
| Conscientiousness | 51.08 (9.42) | 51.46 (9.48) | 48.70 (7.64)b | 48.13 (8.44)b | 51.78 (9.39) |
Abbreviations: AD, Alzheimer disease; MCI, mild cognitive impairment.
Measured at first assessment. Raw scores were standardized to T scores (mean [SD] 50 [10]).
Personality differences compared with individuals who were not impaired, P < .05.
Domain-Level Multilevel Modeling Analysis
Table 2 reports estimates from the HLM analysis. The basic parameters for the slope indicated that, in the reference group, there were small declines in neuroticism, extraversion, and openness and small increases in agreeableness and conscientiousness. These patterns are consistent with previous reports. We tested our primary hypothesis by comparing the personality trajectory of the nonimpaired group with the trajectories of the AD group. Contrary to our hypothesis, there were no significant differences in the rates of change between the nonimpaired and AD groups for neuroticism (β = 0.00; 95% CI, −0.08 to 0.08; P = .91), conscientiousness (β = −0.06; 95% CI, −0.16 to 0.04; P = .24), and the other personality traits. Although the trajectories were similar, there were significant mean-level differences on the intercept. The AD cohort scored higher on neuroticism (β = 2.83; 95% CI, 1.44 to 4.22; P < .001) and lower on conscientiousness (β = −3.34; 95% CI, −4.93 to −1.75; P < .001) and extraversion (β = −1.74; 95% CI, −3.23 to −0.25; P = .02) than the nonimpaired group. Further data are reported in Table 2.
Table 2. Estimates From Hierarchical Linear Modeling Analyses.
| Characteristic | Neuroticism | Extraversion | Openness | Agreeableness | Conscientiousness | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | |
| Intercepta | 45.61 (0.34) | <.001 | 48.84 (0.37) | <.001 | 48.83 (0.37) | <.001 | 49.50 (0.36) | <.001 | 52.09 (0.38) | <.001 |
| Female | 0.67 (0.41) | .10 | 1.20 (0.44) | .007 | 4.02 (0.45) | <.001 | 5.45 (0.43) | <.001 | −0.36 (0.45) | .43 |
| Ageb | −0.14 (0.21) | .49 | −1.70 (0.23) | <.001 | −1.84 (0.23) | <.001 | 0.63 (0.22) | .005 | −0.78 (0.23) | .001 |
| Age squared | 0.01 (0.02) | .46 | −0.02 (0.02) | .18 | 0.02 (0.02) | .18 | 0.00 (0.02) | .97 | −0.05 (0.02) | .005 |
| Educationb | −0.38 (0.08) | <.001 | 0.46 (0.08) | <.001 | 1.09 (0.08) | <.001 | −0.09 (0.08) | .27 | 0.49 (0.08) | <.001 |
| African American | −0.90 (0.52) | .09 | 1.44 (0.57) | .01 | 0.41 (0.58) | .48 | 0.67 (0.54) | .21 | 2.03 (0.57) | .001 |
| Alzheimer disease | 2.83 (0.71) | <.001 | −1.74 (0.76) | .02 | −1.43 (0.78) | .07 | −0.98 (0.77) | .20 | −3.34 (0.81) | <.001 |
| Slope | ||||||||||
| Time | −0.07 (0.02) | <.001 | −0.06 (0.02) | .001 | −0.11 (0.02) | <.001 | 0.09 (0.02) | <.001 | 0.05 (0.02) | .01 |
| Female | −0.04 (0.02) | .03 | −0.03 (0.02) | .17 | −0.04 (0.02) | .05 | 0.08 (0.02) | <.001 | −0.02 (0.02) | .34 |
| Ageb | 0.07 (0.01) | <.001 | −0.04 (0.01) | .001 | −0.02 (0.01) | .09 | −0.04 (0.01) | <.001 | −0.08 (0.01) | <.001 |
| Age squared | 0.00 (0.00) | .003 | 0.00 (0.00) | .12 | 0.00 (0.00) | .002 | 0.00 (0.00) | .78 | 0.00 (0.00) | .04 |
| Educationb | 0.00 (0.00) | .36 | 0.00 (0.00) | .42 | 0.00 (0.00) | .20 | 0.00 (0.00) | .90 | 0.00 (0.00) | .28 |
| African American | 0.10 (0.03) | <.001 | −0.05 (0.02) | .05 | 0.05 (0.03) | .05 | −0.05 (0.03) | .07 | −0.01 (0.03) | .65 |
| Alzheimer disease | 0.00 (0.04) | .91 | 0.06 (0.04) | .10 | −0.04 (0.04) | .29 | 0.01 (0.05) | .75 | −0.06 (0.05) | .24 |
Intercept refers to last assessment: n = 1881 (nonimpaired, 1687; AD, 194).
Group mean centering was used for age (in decade) and education.
In a model that included quadratic in addition to linear slopes, we found no evidence of accelerated change in personality in the AD group compared with the nonimpaired group (eTable 1 in the Supplement). We also found similar results in sensitivity analyses that excluded assessments in 1980 and 1986 based on the NEO-PI (eTable 2 in the Supplement) or when participants with only 1 personality assessment were excluded (eTable 3 in the Supplement).
We repeated the analysis by comparing the nonimpaired with all-cause dementia and MCI groups (eTable 4 in the Supplement). The dementia group scored higher on the intercept of neuroticism (β = 2.87; 95% CI, 1.64 to 4.10; P < .001) and lower on conscientiousness (β = −4.02; 95% CI, −5.43 to −2.61; P < .001), extraversion (β = −2.38; 95% CI, −3.71 to −1.05; P = .001), and openness (β = −1.65; 95% CI, −2.98 to −0.32; P = .02). The MCI group scored lower on the intercept of openness (β = −2.95; 95% CI, −4.97 to −0.93; P = .005). Similar to the AD analysis, the rate of change in conscientiousness (β = −0.08; 95% CI, −0.16 to 0.00; P = .07) and neuroticism (β = 0.02; 95% CI, −0.06 to 0.10; P = .49) were not significantly different between the all-cause dementia and the nonimpaired groups. Individuals who developed all-cause dementia had a steeper decline in openness compared with those who were not impaired (β = −0.07, 95% CI, −0.13 to −0.01; P = .03). The slopes of the MCI were not significantly different from the slopes of the nonimpaired group (eg, neuroticism: β = 0.00; 95% CI, −0.12 to 0.12; P = .98; conscientiousness: β = −0.09; 95% CI, −0.23 to 0.05; P = .18). The results were similar in a model that compared nonimpaired individuals with those with MCI, AD, and other dementias (eTable 5 in the Supplement).
Facets-Level Multilevel Modeling Analysis
We applied the same analytic model to the 30 facets. As for the 5 factors, there were significant differences on intercept of facet between the AD and nonimpaired groups (eTables 6-10 in the Supplement). In particular, compared with the nonimpaired group, the AD group scored significantly higher on most neuroticism facets (anxiety, angry hostility, depression, and vulnerability to stress) and scored lower on most conscientiousness facets (competence, order, dutifulness, and self-discipline). Of primary interest, the rate of change for the facets of neuroticism and conscientiousness did not differ significantly between the AD and the non-impaired groups.
Personality Change Approaching Disease Onset
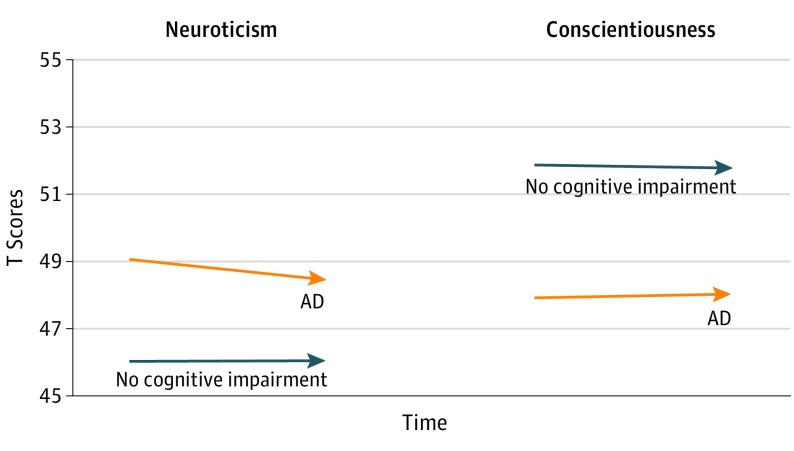
It is possible that change in the slope would occur mainly within the years nearest to the onset of the disease. To test this possibility, we selected the last 2 assessments for each participant with available data. For the AD group, the last 2 assessments were means (SDs) of 6.44 (4.32) and 3.47 (3.09) years before the onset of dementia (eTable 11 in the Supplement). Consistent with the HLM results and the broader literature, the AD group scored higher on neuroticism and lower on conscientiousness. Contrary to expectations, the AD group did not increase in neuroticism or decline in conscientiousness (Figure 3).
Figure 3. Personality Trajectories Over the Last 2 Assessments Before the Onset of AD Compared With Those With No Cognitive Impairment.
Changes in neuroticism and conscientiousness for 131 individuals who later developed Alzheimer disease (AD) and 1374 individuals with no cognitive impairment. The AD group was tested an average of 6.44 (time −2) and 3.47 (time −1) years before the onset of the disease. At time −2, the mean (SD) age was 77.58 (7.95) years. The AD group scored higher on neuroticism and lower on conscientiousness before the onset of the disease. Contrary to expectations, the AD group did not increase in neuroticism or decline in conscientiousness in the preclinical phase of the disease.
Rank-Order Stability
There is marked variability in cognitive performance in the preclinical phase of AD, and personality stability is significantly lower in individuals with cognitive impairment or dementia. Rank-order stability, however, remained high for personality in the preclinical phase. Using the last 2 assessments before the onset of the disease, we found no evidence of lower rank-order stability in the AD group (eTable 11 in the Supplement).
Discussion
Our analyses of longitudinal data that spanned up to 36 years found no evidence of personality change in the preclinical phase of dementia. The trajectory of personality traits and facets for individuals who were later diagnosed with MCI or dementia did not differ significantly from that of nonimpaired older adults. We further found that personality remained stable even within the last few years before the onset of the disease, and the high rank-order correlations supported the reliability of the data. However, we found differences of approximately 0.3 SD between the AD and nonimpaired groups on the intercept of neuroticism and conscientiousness, which were not an emerging prodromal sign of the disease and were consistent with results from other prospective studies. These findings provide evidence against the reverse causality hypothesis as an explanation for the association between personality and risk of incident dementia. From a clinical perspective, these findings suggest that tracking change in self-rated personality as an early indicator of dementia is unlikely to be fruitful, while a single assessment provides reliable information on the personality traits that increase resilience (eg, conscientiousness) or vulnerability (eg, neuroticism) to clinical dementia.
This research has relevance to the question of reverse causality for the association between personality and risk of incident AD. That is, if personality changed in response to increasing neuropathology in the brain in the preclinical phase, the association between personality and AD could have been due to the disease process rather than personality as an independent risk factor. We did not, however, find any evidence that neuroticism and conscientiousness changed significantly as the onset of the disease approached. Thus, rather than an effect of AD neuropathology, these traits appear to confer risk for the development of the disease.
Our findings are also relevant for delineating the natural history of personality change in relation to incident dementia and should be interpreted in the context of previous studies that have tracked the course of clinical and psychological traits before the onset of dementia. Weight loss and cognitive decline, for example, precede the onset of clinical dementia, but evidence for changes in depressive symptoms in the prodromal phase of dementia is more mixed. For personality traits, 2 previous studies reported that personality change preceded the clinical diagnosis. These studies, however, were based on items from the Blessed Dementia Scale or the Cambridge Examination for Mental Disorders of the Elderly. These instruments are not standardized measures of major personality traits, and both instruments include questions about changes in personality that ask informants to make retrospective evaluations. It is unclear whether such retrospective evaluations are accurate. In a large study of healthy adults, for example, perception of personality change was mostly unrelated to actual change measured with prospective assessments.
Another important difference across personality studies is the use of self-report vs informant ratings. Self-rated personality provides participants’ perspectives of themselves—the most common method of personality assessment. Informant reports are another important source of information, especially when the individual to be evaluated is cognitively impaired. Individuals with AD could be anosognosic to change in their psychological traits and functioning. Self-reported personality might remain stable and reflect premorbid functioning more than current traits. Contrary to this idea, however, there is evidence that self-report personality traits change with the onset of cognitive impairment and dementia: a large study has found that rank-order stability declines to rtt = 0.43 with the onset of dementia. The present study extends knowledge by indicating that such changes do not begin in the preclinical phase of AD (rtt>0.80).
Strengths and Limitations
Among the strengths of the study are the prospective design, the long-term follow-up, the relatively large sample size, and the in-depth personality and clinical assessments. Among the limitations of the study is the use of a selective sample with a higher educational level. The findings from the BLSA, however, are generally consistent with those from other samples, as in the case of the association between personality and risk of dementia. Another limitation is the relatively younger age of nonimpaired participants, some of whom may develop dementia. In addition, more research is needed on personality and AD biomarkers and how personality may increase resilience against neuropathology and forestall the emergence of clinical dementia.
Conclusions
This study found that change in personality does not initiate before the onset of MCI or AD. The findings are contrary to the reverse causality hypothesis and provide further support that personality traits are a risk factor for the development of dementia.
eMethods. NEO-PI Administration
eTable 1. Estimates From Hierarchical Linear Modeling (HLM) Analyses That Compared AD to Non-Impaired and Testing Linear and Quadratic Slope
eTable 2. Estimates From Hierarchical Linear Modeling (HLM) Analyses That Compared AD to Non-Impaired Excluding NEO-PI (1980-1986) Assessments
eTable 3. Estimates From Hierarchical Linear Modeling (HLM) Analyses That Compared AD to Non-Impaired Among Individuals With 2 or More Personality Assessment (Excluding Individuals With Only One Personality Assessment)
eTable 4. Estimates From Hierarchical Linear Modeling (HLM) Analyses That Compared All-Cause Dementia and MCI to Non-Impaired
eTable 5. Estimates From Hierarchical Linear Modeling (HLM) Analyses That Compared AD, Other Dementias, and MCI to Non-Impaired
eTable 6. Estimates From Hierarchical Linear Modeling (HLM) Analyses for Neuroticism Facets
eTable 7. Estimates From Hierarchical Linear Modeling (HLM) Analyses for Extraversion Facets
eTable 8. Estimates From Hierarchical Linear Modeling (HLM) Analyses for Openness Facets
eTable 9. Estimates From Hierarchical Linear Modeling (HLM) Analyses for Agreeableness Facets
eTable 10. Estimates From Hierarchical Linear Modeling (HLM) Analyses for Conscientiousness Facets
eTable 11. Mean-Level and Rank-Order Stability Over Last Two Personality Assessments
References
- 1.McKhann GM, Knopman DS, Chertkow H, et al. . The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR Jr, Knopman DS, Jagust WJ, et al. . Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resnick SM, Bilgel M, Moghekar A, et al. . Changes in Aβ biomarkers and associations with APOE genotype in 2 longitudinal cohorts. Neurobiol Aging. 2015;36(8):2333-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry. 2012;69(1):98-106. [DOI] [PubMed] [Google Scholar]
- 5.Pocnet C, Rossier J, Antonietti JP, von Gunten A. Personality changes in patients with beginning Alzheimer disease. Can J Psychiatry. 2011;56(7):408-417. [DOI] [PubMed] [Google Scholar]
- 6.Dawson DV, Welsh-Bohmer KA, Siegler IC. Premorbid personality predicts level of rated personality change in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14(1):11-19. [DOI] [PubMed] [Google Scholar]
- 7.Siegler IC, Dawson DV, Welsh KA. Caregiver ratings of personality change in Alzheimer’s disease patients: a replication. Psychol Aging. 1994;9(3):464-466. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee A, Strauss ME, Smyth KA, Whitehouse PJ. Personality changes in Alzheimer’s disease. Arch Neurol. 1992;49(5):486-491. [DOI] [PubMed] [Google Scholar]
- 9.Robins Wahlin TB, Byrne GJ. Personality changes in Alzheimer’s disease: a systematic review. Int J Geriatr Psychiatry. 2011;26(10):1019-1029. [DOI] [PubMed] [Google Scholar]
- 10.Balsis S, Carpenter BD, Storandt M. Personality change precedes clinical diagnosis of dementia of the Alzheimer type. J Gerontol B Psychol Sci Soc Sci. 2005;60(2):P98-P101. [DOI] [PubMed] [Google Scholar]
- 11.Smith-Gamble V, Baiyewu O, Perkins AJ, et al. . Informant reports of changes in personality predict dementia in a population-based study of elderly African Americans and Yoruba. Am J Geriatr Psychiatry. 2002;10(6):724-732. [PubMed] [Google Scholar]
- 12.Yoneda T, Rush J, Berg AI, Johansson B, Piccinin AM. Trajectories of personality traits preceding dementia diagnosis. J Gerontol B Psychol Sci Soc Sci. Published online March 4, 2016;pii: gbw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waggel SE, Lipnicki DM, Delbaere K, et al. . Neuroticism scores increase with late-life cognitive decline. Int J Geriatr Psychiatry. 2015;30(9):985-993. [DOI] [PubMed] [Google Scholar]
- 14.Costa PT Jr, McCrae RR. Revised NEO Personality Inventory (NEO-PI-R) and NEO Five-Factor Inventory (NEO-FFI) Professional Manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- 15.Terracciano A, Sutin AR, An Y, et al. . Personality and risk of Alzheimer’s disease: new data and meta-analysis. Alzheimers Dement. 2014;10(2):179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terracciano A, Stephan Y, Luchetti M, Albanese E, Sutin AR. Personality traits and risk of cognitive impairment and dementia. J Psychiatr Res. 2017;89:22-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson L, Guo X, Duberstein PR, et al. . Midlife personality and risk of Alzheimer disease and distress: a 38-year follow-up. Neurology. 2014;83(17):1538-1544. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RS, Begeny CT, Boyle PA, Schneider JA, Bennett DA. Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry. 2011;19(4):327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson RS, Schneider JA, Arnold SE, Bienias JL, Bennett DA. Conscientiousness and the incidence of Alzheimer disease and mild cognitive impairment. Arch Gen Psychiatry. 2007;64(10):1204-1212. [DOI] [PubMed] [Google Scholar]
- 20.Duberstein PR, Chapman BP, Tindle HA, et al. . Personality and risk for Alzheimer’s disease in adults 72 years of age and older: a 6-year follow-up. Psychol Aging. 2011;26(2):351-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shock NW, Greulich RC, Andres R, et al. . Normal Human Aging: The Baltimore Longitudinal Study of Aging (NIH Publication No. 84-2450). Bethesda, MD: National Institutes of Health; 1984. [Google Scholar]
- 22.clinicaltrials.gov. Baltimore Longitudinal Study of Aging. NCT00233272. https://clinicaltrials.gov/ct2/show/NCT00233272. Accessed August 3, 2017.
- 23.Terracciano A, Costa PT Jr, McCrae RR. Personality plasticity after age 30. Pers Soc Psychol Bull. 2006;32(8):999-1009.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16861305&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(suppl 1):173-176. [DOI] [PubMed] [Google Scholar]
- 25.Kawas C, Segal J, Stewart WF, Corrada M, Thal LJ. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51(9):901-906. [DOI] [PubMed] [Google Scholar]
- 26.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797-811. [DOI] [PubMed] [Google Scholar]
- 27.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303-308. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 3rd ed, revised. Washington, DC: American Psychiatric Association ; 1987. [Google Scholar]
- 29.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. [DOI] [PubMed] [Google Scholar]
- 30.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed Thousand Oaks, California: Sage Publications; 2002. [Google Scholar]
- 31.Terracciano A, McCrae RR, Brant LJ, Costa PT Jr. Hierarchical linear modeling analyses of the NEO-PI-R scales in the Baltimore Longitudinal Study of Aging. Psychol Aging. 2005;20(3):493-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gamaldo AA, An Y, Allaire JC, Kitner-Triolo MH, Zonderman AB. Variability in performance: identifying early signs of future cognitive impairment. Neuropsychology. 2012;26(4):534-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terracciano A, Stephan Y, Luchetti M, Sutin AR. Cognitive impairment, dementia, and personality stability among older adults. Assessment. 2017. http://journals.sagepub.com/doi/abs/10.1177/1073191117691844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69(8):739-746. [DOI] [PubMed] [Google Scholar]
- 35.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63(9):1312-1317. [DOI] [PubMed] [Google Scholar]
- 36.Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68(3):351-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amieva H, Le Goff M, Millet X, et al. . Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64(5):492-498. [DOI] [PubMed] [Google Scholar]
- 38.Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry. 2008;65(4):439-445. [DOI] [PubMed] [Google Scholar]
- 39.Singh-Manoux A, Dugravot A, Fournier A, et al. . Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry. 2017;74(7):712-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strauss ME, Pasupathi M. Primary caregivers’ descriptions of Alzheimer patients’ personality traits: temporal stability and sensitivity to change. Alzheimer Dis Assoc Disord. 1994;8(3):166-176. [DOI] [PubMed] [Google Scholar]
- 41.Herbst JH, McCrae RR, Costa PT Jr, Feaganes JR, Siegler IC. Self-perceptions of stability and change in personality at midlife: the UNC Alumni Heart Study. Assessment. 2000;7(4):379-388. [DOI] [PubMed] [Google Scholar]
- 42.McCrae RR, Terracciano A; Personality Profiles of Cultures Project . Universal features of personality traits from the observer’s perspective: data from 50 cultures. J Pers Soc Psychol. 2005;88(3):547-561. [DOI] [PubMed] [Google Scholar]
- 43.Gross AL, Hassenstab JJ, Johnson SC, et al. . A classification algorithm for predicting progression from normal cognition to mild cognitive impairment across five cohorts: The preclinical AD consortium. Alzheimers Dement (Amst). 2017;8:147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terracciano A, Iacono D, O’Brien RJ, et al. . Personality and resilience to Alzheimer’s disease neuropathology: a prospective autopsy study. Neurobiol Aging. 2013;34(4):1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tautvydaitė D, Antonietti JP, Henry H, von Gunten A, Popp J. Relations between personality changes and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology. J Psychiatr Res. 2017;90:12-20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. NEO-PI Administration
eTable 1. Estimates From Hierarchical Linear Modeling (HLM) Analyses That Compared AD to Non-Impaired and Testing Linear and Quadratic Slope
eTable 2. Estimates From Hierarchical Linear Modeling (HLM) Analyses That Compared AD to Non-Impaired Excluding NEO-PI (1980-1986) Assessments
eTable 3. Estimates From Hierarchical Linear Modeling (HLM) Analyses That Compared AD to Non-Impaired Among Individuals With 2 or More Personality Assessment (Excluding Individuals With Only One Personality Assessment)
eTable 4. Estimates From Hierarchical Linear Modeling (HLM) Analyses That Compared All-Cause Dementia and MCI to Non-Impaired
eTable 5. Estimates From Hierarchical Linear Modeling (HLM) Analyses That Compared AD, Other Dementias, and MCI to Non-Impaired
eTable 6. Estimates From Hierarchical Linear Modeling (HLM) Analyses for Neuroticism Facets
eTable 7. Estimates From Hierarchical Linear Modeling (HLM) Analyses for Extraversion Facets
eTable 8. Estimates From Hierarchical Linear Modeling (HLM) Analyses for Openness Facets
eTable 9. Estimates From Hierarchical Linear Modeling (HLM) Analyses for Agreeableness Facets
eTable 10. Estimates From Hierarchical Linear Modeling (HLM) Analyses for Conscientiousness Facets
eTable 11. Mean-Level and Rank-Order Stability Over Last Two Personality Assessments