This secondary analysis of data from the Sudden Cardiac Death in Heart Failure Trial examines the efficacy of implantable cardioverter defibrillator therapy in reducing risk of all-cause mortality and sudden cardiac death among patients with or without an improvement in ejection fraction to >35% during follow-up.
Key Points
Question
What is the efficacy of implantable cardioverter defibrillator (ICD) therapy in patients who had an improvement in ejection fraction (EF) to >35% during follow-up?
Findings
In this analysis of the Sudden Cardiac Death in Heart Failure Trial, during a median follow-up of 30 months, the all-cause mortality rate was lower in the ICD group than in the placebo group, both in patients whose EF remained ≤35% and in those whose EF improved to >35%. There was no interaction between ICD and repeated EF for predicting mortality.
Meaning
Patients who had an improvement in EF to >35% during follow-up accrued a similar mortality benefit with ICD as those whose EF remained at ≤35%.
Abstract
Importance
Improvement in left ventricular ejection fraction (EF) to >35% occurs in many patients with reduced EF at baseline. To our knowledge, whether implantable cardioverter defibrillator (ICD) therapy improves survival for these patients is unknown.
Objective
To examine the efficacy of ICD therapy in reducing risk of all-cause mortality and sudden cardiac death among patients with an EF ≤35% at baseline, with or without an improvement in EF to >35% during follow-up.
Design, Setting, and Participants
This retrospective analysis examined data collected in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), which randomly assigned 2521 patients to placebo, amiodarone, or ICD between 1997 and 2001. A subset of 1902 participants (75.4%) of the SCD-HeFT had a repeated assessment of EF a mean (SD) of 13.5 (6) months after randomization. We stratified these patients by EF ≤35% and >35% based on the first repeated EF measurement after randomization and compared all-cause mortality in 649 patients randomized to placebo vs 624 patients randomized to ICD. Follow-up started with the repeated EF assessment. Analysis was performed between January 2016 and July 2016.
Exposures
Implantable cardioverter-defibrillator therapy.
Main Outcomes and Measures
All-cause mortality and sudden cardiac death.
Results
Of the included 1273 patients, the mean (SD) age was 59 (12) years, and 977 (76.7%) were male and 1009 (79.3%) were white. Repeated EF was >35% in 186 participants (29.8%) randomized to ICD and 185 participants (28.5%) randomized to placebo. During a median follow-up of 30 months, the all-cause mortality rate was lower in the ICD vs placebo group, both in patients whose EF remained ≤35% (7.7 vs 10.7 per 100 person-year follow-up) and in those whose EF improved to >35% (2.6 vs 4.5 per 100 person-year follow-up). Compared with placebo, the adjusted hazard ratio for the effect of ICD on mortality was 0.64 (95% CI, 0.48-0.85) in patients with a repeated EF of ≤35% and 0.62 (95% CI, 0.29-1.30) in those with a repeated EF >35%. There was no interaction between treatment assignment and repeated EF for predicting mortality.
Conclusions and Relevance
Among participants in the SCD-HeFT who had a repeated EF assessment during the course of follow-up, those who had an improvement in EF to >35% accrued a similar relative reduction in mortality with ICD therapy as those whose EF remained ≤35%. Prospective randomized clinical trials are needed to test ICD efficacy in patients with an EF >35%.
Trial Registration
clinicaltrials.gov Identifier: NCT01114269
Introduction
Left ventricular ejection fraction (EF) is the cornerstone of the criteria used to select patients for implantable cardioverter defibrillator (ICD) implantation for primary prevention of sudden cardiac death (SCD). When added to optimal medical therapy, ICD therapy improves survival of many patients with heart failure with an EF ≤35%. This EF criterion is based on the results of pivotal randomized clinical trials, such as the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), that only enrolled patients with an EF ≤35% owing to the higher risk of SCD in this population. Thus, ICD implantation is recommended in patients with symptoms of heart failure whose EF remains ≤35% after 3 months of optimal medical therapy. However, to our knowledge, the risk of SCD as well as the efficacy of ICD for primary prevention of SCD has not been established in patients who have an improvement of EF to >35%.
Heart failure with improved (or recovered) EF describes a category of patients who have an improvement in EF, in part as a result of reverse remodeling. Previous work has shown that improvement in EF occurs in approximately 20% to 25% of patients with ischemic cardiomyopathy and 40% to 50% of patients with nonischemic cardiomyopathy. Previously, our group and others have shown that these patients have a lower, but not trivial, risk of receiving appropriate ICD shocks after EF improvement compared with patients whose EF remains less than 35%. However, data from these studies are insufficient to determine whether ICD therapy reduces all-cause mortality in patients with EF improvement to >35% because none of them included comparisons with a control group of patients without ICD.
The SCD-HeFT was a randomized clinical trial of patients with New York Heart Association (NYHA) class 2 or 3 symptoms and an EF ≤35% who were randomized to ICD, amiodarone, or placebo for primary prevention of SCD. After a median follow-up of 45.5 months, the mortality of patients randomized to ICD therapy was 22% vs 29% in patients randomized to placebo. A large subset of participants (1902 of 2521 [75.4%]) in the trial underwent a repeated measurement of EF during follow-up as part of their usual clinical care. The objective of the present study was to examine the efficacy of ICD in reducing risk of all-cause mortality and SCD in patients with a follow-up EF >35% compared with those whose EF remained ≤35%.
Methods
Study Patients
Per the SCD-HeFT protocol, 2521 participants were randomized to placebo (n = 847), amiodarone (n = 845), or ICD (n = 829) from 1997 to 2001. Eligible patients had to be 18 years or older with NYHA class 2 or 3 heart failure and a left ventricular EF ≤35% due to ischemic or nonischemic causes. To be enrolled, heart failure had to be present for at least 3 months prior to randomization and treated with a vasodilator. Less than ideal doses of angiotensin-converting enzyme inhibitors and β-blockers were approved as long as target doses were attained shortly after randomization.
During study follow-up, 1902 patients (75.4%) had a repeated measurement of EF a mean (SD) of 13.5 (6) months after randomization as part of their usual clinical care. We excluded 27 patients in whom the first repeated EF was measured after heart transplantation. The randomized treatment assignments in the remaining 1875 patients were placebo (n = 649), amiodarone (n = 602), and ICD (n = 624). In this post hoc analysis, we compared the 649 patients randomly assigned to placebo with the 624 randomly assigned to ICD (eFigure 1 in the Supplement). A similar proportion of patients who were randomly assigned to placebo (77.3%) and ICD (76.9%) had a repeated EF measurement. Patients with a repeated EF measurement were healthier and had lower mortality than those without a repeated EF measurement (eTable in the Supplement).
This study was approved by the Research and Development Committee of the Minneapolis Veterans Affairs Medical Center. Informed consent was waived because the database was deidentified and publicly available.
Assessment of EF
All participants underwent a baseline measurement of left ventricular EF within 3 months before enrollment into the SCD-HeFT. Studies of qualifying EF were performed and interpreted at the enrolling centers. A repeated measurement of EF was not required by the study protocol. However, if patients had a repeated EF assessment as part of their usual clinical care, the repeated EF and the study date were recorded. On page 7 of the study follow-up form, a question specifically asked about the patient’s follow-up EF and the date of the EF assessment. This form had to be filled at scheduled study follow-up visits at 12 and 30 months and at any unscheduled follow-up visits. Among patients with more than 1 follow-up EF measurement, we used the first EF after randomization to maximize the follow-up time.
ICD Specifics and Programming
Implantable cardioverter defibrillator therapy in the SCD-HeFT was limited to single-lead, shock-only devices; dual-chamber or biventricular devices were not permitted. The ICDs were programmed uniformly to have a detection rate of 188 or greater beats/min (tachycardia cycle length, ≤320 milliseconds). The number of intervals to detect ventricular arrhythmia was 18/24. Anti-tachycardia pacing was not allowed. Pacing for bradycardia was initiated only if the intrinsic rate decreased to less than 34 beats/min.
Outcome Variables
The primary outcome variable in this analysis was all-cause mortality after the first repeated EF measurement. Sudden cardiac death after the first repeated EF measurement was a secondary outcome. In the SCD-HeFT, all deaths were adjudicated by the independent events committee using the Hinkle-Thaler system. Cardiac events were subclassified as sudden death presumed to be due to ventricular tachyarrhythmia, bradyarrhythmia, heart failure, or other cardiac causes. An instantaneous or nearly instantaneous death was classified as being due to ventricular tachyarrhythmia in the absence of a clear indication of an alternative mode of death. Death during sleep was considered to be due to ventricular tachyarrhythmia if the event was unexpected and occurred in the absence of acceleration of heart failure symptoms. Deaths resulting from the consequences of a cardiac arrest or related to a device implantation (if within 30 days) were considered to be due to a ventricular tachyarrhythmia. The events committee reviewed clinical records, including histories, physical examination, hospitalization records, laboratory data, paramedic and emergency department records, electrocardiography rhythm strips at the onset of the event (or first available monitored rhythm after the onset of the event), autopsy reports, and a detailed narrative event summary provided by the physician investigator at the enrolling site. All information identifying randomized therapy assignment and ICD interrogation data were removed from the documents submitted to the events committee to maintain blinding.
Statistical Analysis
Continuous variables are presented as mean (SD) and categorical variables as No. (%). Highly skewed distributions are summarized as the median (25th and 75th quartiles). The first follow-up EF measurement after randomization in the SCD-HeFT was used to categorize patients into EF >35% and EF ≤35% strata. In this analysis, the follow-up period started with the date of the first EF measurement after randomization. Follow-up of patients who underwent heart transplantation after a repeated EF measurement was censored at the time of transplant, which occurred after a median follow-up of 38 months (minimum 13 months). We performed an intention-to-treat analysis.
Mortality is described as incidence rates per 100 person-years of follow-up. Probability of mortality was calculated with the formula 1 − e(−Rate × Time). The effect of ICD on all-cause mortality in each repeated EF strata was estimated using Cox proportional hazard regression analysis. We tested for an interaction between treatment assignment (ICD vs placebo) and repeated EF strata (>35% vs ≤35%) to determine whether there was evidence that the effect of ICD on mortality risk depended on repeated EF strata. The proportional hazards assumption was confirmed by examining the correlation between Schoenfeld residuals and follow-up time. Similarly, the Fine Gray competing risk model was used to estimate the effect of ICD on SCD in each repeated EF strata wherein non-SCD was considered a competing risk.
In multivariable analyses assessing the efficacy of ICD in each repeated EF strata, we adjusted for baseline variables that were previously found to be independent predictors of all-cause mortality in the SCD-HeFT, including age, sex, heart failure etiology and duration, NYHA class, baseline EF, systolic blood pressure, diabetes, mitral regurgitation, renal insufficiency, history of substance abuse, Duke Activity Status Index, the electrocardiographic QRS and QT intervals, and use of a β-blocker, angiotensin-converting enzyme inhibitor or receptor blocker, and digoxin. Six-minute walk test distance and the PR interval were excluded from the multivariable adjustment models owing to the amount of missing data. In addition, standardized differences between the ICD and placebo groups were examined to assess the balance on baseline variables within each nonrandomly assigned repeated EF strata. Any variable with a standardized difference greater than 10% that was not already included in the previously developed mortality model was added to the multivariable adjustment model if it was a known correlate of mortality. Thus, the final adjusted model included the covariates age, sex, heart failure etiology and duration, NYHA class, baseline EF, systolic blood pressure, diabetes, stroke, pulmonary disease, hypertension, hyperlipidemia, syncope, nonsustained ventricular tachycardia, atrial fibrillation, mitral regurgitation, renal insufficiency, current smoking, history of substance abuse, Duke Activity Status Index, serum potassium level, the electrocardiographic QRS and QT intervals, and use of aspirin, β-blocker, angiotensin-converting enzyme inhibitor or receptor blocker, statin, and digoxin. All data were analyzed using Stata version 12.1 SE (StataCorp).
Results
Repeated Ejection Fraction in the ICD and Placebo Groups
Of the 624 patients in the ICD group, 186 (29.8%) had a repeated EF >35%. Among these 186 patients, the mean (SD) EF increased from 26% (7%) to 46% (8%). Similarly, of the 649 patients in the placebo group, 185 (28.5%) had a repeated EF >35%, and among these 185 patients, the mean (SD) EF increased from 27% (6%) to 45% (7%). Among the remaining patients assigned to ICD (n = 438) or placebo (n = 464), there was no evidence that the mean EF substantially changed from baseline. Baseline and follow-up EF in each group is shown in eFigure 2 in the Supplement. Thus, in patients with an improvement in EF, the mean repeated EF was approximately 45%. Because of lack of power, we did not analyze the subgroup of patients with an EF >55%.
Baseline characteristics of the patients assigned to ICD vs placebo are shown in Table 1 within each repeated EF strata. The balance of key prognostic baseline variables between treatment groups was largely maintained (standardized difference <10%) in the larger group of participants who had a follow-up EF ≤35% but was less so maintained in the smaller group that had a follow-up EF >35%.
Table 1. Standardized Differences in Baseline Characteristics Between the ICD and Placebo Groups Within Each EF Category.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Follow-up EF ≤35% | Follow-up EF >35% | |||||
| Placebo (n = 464) | ICD (n = 438) | Standardized Difference, % | Placebo (n = 185) | ICD (n = 186) | Standardized Difference, % | |
| Age, mean (SD), y | 59 (12) | 59 (12) | 2.2 | 59 (12) | 59 (12) | 0.4 |
| Female sex | 100 (21.6) | 90 (20.5) | 2.5 | 57 (30.8) | 49 (26.3) | 9.9 |
| Nonwhite race | 104 (22.4) | 91 (20.8) | 4.0 | 33 (17.8) | 36 (19.4) | 3.9 |
| Length of HF diagnosis, mean (SD), mo | 42 (47) | 47 (52) | 10.5 | 33 (41) | 35 (45) | 5.0 |
| Nonischemic cardiomyopathy | 209 (45.0) | 200 (45.7) | 1.2 | 99 (53.5) | 107 (57.5) | 8.1 |
| NYHA class 3 | 131 (28.2) | 123 (28.1) | 0.3 | 56 (30.3) | 55 (29.6) | 1.5 |
| Baseline EF, mean (SD) | 23 (7) | 23 (7) | 0.9 | 27 (6) | 26 (7) | 13.3 |
| Pulmonary disease | 77 (16.6) | 84 (19.2) | 6.7 | 33 (17.8) | 43 (23.1) | 13.1 |
| Current smoker | 75 (16.2) | 76 (17.4) | 3.1 | 24 (13.0) | 36 (19.4) | 17.4 |
| Hyperlipidemia | 258 (55.6) | 244 (55.7) | 0.0 | 100 (54.1) | 88 (47.3) | 13.5 |
| Hypertension | 245 (52.8) | 228 (52.1) | 1.5 | 115 (62.2) | 106 (57.0) | 10.5 |
| Stroke | 35 (7.5) | 20 (4.6) | 12.5 | 13 (7.0) | 5 (2.7) | 20.2 |
| Diabetes | 138 (29.7) | 130 (29.7) | 0.1 | 66 (35.7) | 52 (28.0) | 16.6 |
| NSVT | 104 (22.4) | 112 (25.6) | 7.4 | 24 (13.0) | 45 (24.2) | 29.1 |
| Syncope | 28 (6.0) | 38 (8.7) | 10.1 | 17 (9.2) | 7 (3.8) | 22.1 |
| QRS >120 ms | 220 (47.4) | 207 (47.3) | 0.3 | 52 (28.1) | 47 (25.3) | 6.4 |
| AF on ECG | 26 (5.6) | 32 (7.3) | 6.9 | 11 (5.9) | 16 (8.6) | 10.2 |
| BP, mean (SD) | ||||||
| Systolic | 119 (18) | 118 (19) | 1.7 | 125 (20) | 123 (21) | 10.6 |
| Diastolic | 71 (11) | 70 (11) | 6.6 | 72 (12) | 72 (12) | 1.4 |
| Heart rate, mean (SD) | 75 (15) | 74 (14) | 7.4 | 73 (14) | 74 (13) | 3.0 |
| Sodium, mean (SD) | 139 (3) | 139 (3) | 2.3 | 139 (3) | 140 (3) | 3.5 |
| Potassium, mean (SD) | 4.4 (0.5) | 4.4 (0.5) | 11 | 4.4 (0.4) | 4.4 (0.5) | 16.7 |
| Creatinine, mean (SD) | 1.2 (0.4) | 1.2 (0.4) | 7.8 | 1.2 (0.4) | 1.2 (0.4) | 0.6 |
| eGFR, mean (SD) | 87 (36) | 88 (40) | 4.7 | 94 (43) | 94 (45) | 0.8 |
| ACEI/ARB | 457 (98.5) | 421 (96.1) | 14.7 | 180 (97.3) | 175 (94.1) | 15.8 |
| β-blocker | 316 (68.1) | 293 (66.9) | 2.6 | 138 (74.6) | 143 (76.9) | 5.3 |
| Digoxin | 323 (69.6) | 296 (67.6) | 4.4 | 120 (64.9) | 115 (61.8) | 6.3 |
| Diuretic | 381 (82.1) | 359 (82.0) | 0.4 | 146 (78.9) | 151 (81.2) | 5.7 |
| Spironolactone | 79 (17.0) | 77 (17.6) | 1.5 | 34 (18.4) | 39 (21.0) | 6.5 |
| Statin | 188 (40.5) | 178 (40.6) | 0.2 | 71 (38.4) | 60 (32.3) | 12.8 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BP, blood pressure; ECG, electrocardiogram; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association.
ICD Effect
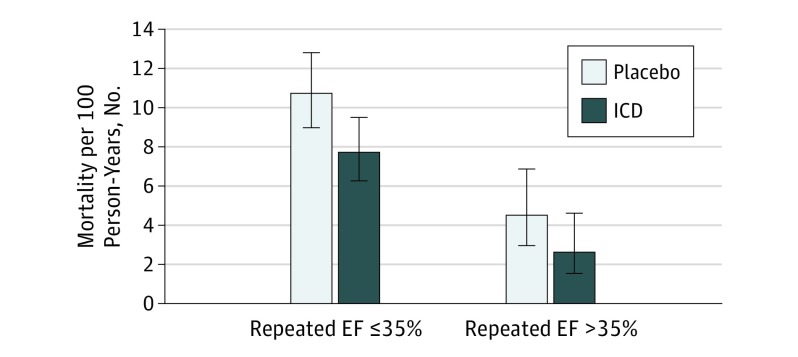
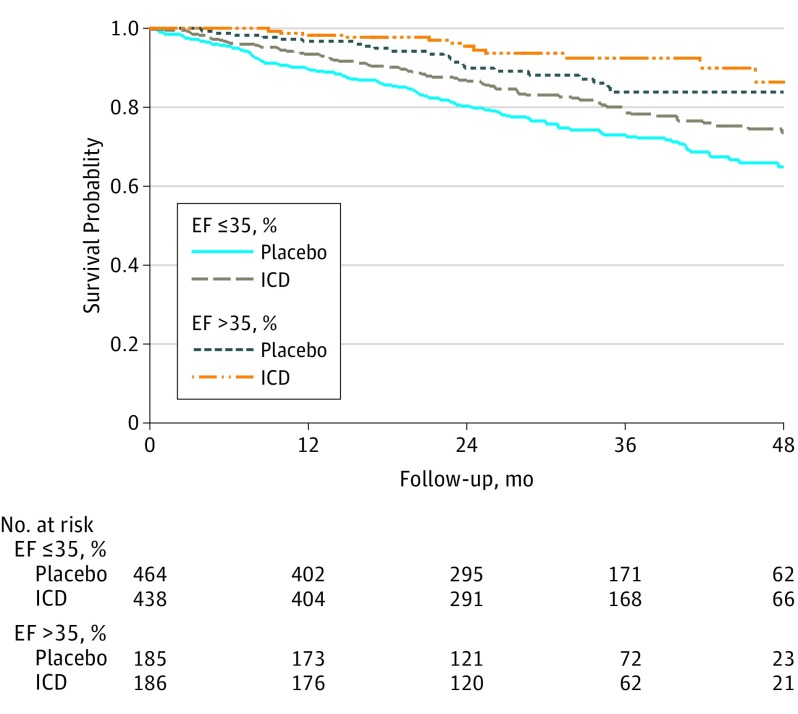
In the ICD and placebo groups combined, a total of 248 of 1273 patients (19.5%) died during a median (interquartile range) follow-up of 30 (20-42) months after the first repeated EF measurement. In both repeated EF strata, the mortality rate was lower in the ICD group compared with the placebo group (Figure 1) (Table 2). The unadjusted hazard ratio (HR) for effect of ICD on all-cause mortality was 0.72 (95% CI, 0.55-0.95) in the repeated EF ≤35% stratum and 0.58 (95% CI, 0.29-1.17) in the EF >35% stratum (Table 3). The HR for the effect of ICD on patients with an EF >35% relative to those with an EF ≤35% was 0.80 (95% CI, 0.38-1.70) and was not significantly different from 1.0 (interaction, P = .56) (Figure 2). Adjusting for variables in the previously developed SCD-HeFT mortality model and additional ones with a standardized difference greater than 10% in Table 1, the estimated effect of ICD was 0.64 (95% CI, 0.48-0.85) in patients with a repeated EF ≤35% and 0.62 (95% CI, 0.29-1.30) in those with a repeated EF >35% (Table 3). The adjusted interaction between the effect of ICD and repeated EF was not significant (interaction, P = .98), suggesting that there was no evidence that the effect of ICD on mortality risk depended on whether EF had improved to >35%. Sensitivity analysis using change in EF as a continuous variable also found no evidence of an interaction (HR, 1.00; 95% CI, 0.97 to 1.03; P = .88) in the fully adjusted model including the baseline EF as a covariate.
Figure 1. Incidence Rate of All-Cause Mortality of Patients Assigned to Implantable Cardioverter Defibrillator (ICD) vs Placebo.
Adjusted hazard ratios of all-cause mortality in the ICD vs the placebo groups were 0.64 (95% CI, 0.48-0.85) in patients with a repeated ejection fraction (EF) ≤35% and 0.62 (95% CI, 0.29-1.30) in those with an EF >35%.
Table 2. Incidence Rates of All-Cause Mortality and SCD in the ICD and Control Groups in Each EF Category.
| EF Group | No. | 100 Follow-up Person-years | Incidence Rate Mortality per 100 Person-year Follow-up (95% CI) | |
|---|---|---|---|---|
| Patients | Deaths | |||
| All-Cause Mortality | ||||
| EF ≤35% | ||||
| ICD | 438 | 89 | 11.5 | 7.7 (6.3-9.5) |
| Placebo | 464 | 125 | 11.7 | 10.7 (8.9-12.7) |
| EF >35% | ||||
| ICD | 186 | 12 | 4.6 | 2.6 (1.5-4.6) |
| Placebo | 185 | 22 | 4.9 | 4.5 (3.0-6.8) |
| SCD | ||||
| EF ≤35% | ||||
| ICD | 438 | 14 | 11.5 | 1.2 (0.7-2.0) |
| Placebo | 464 | 46 | 11.7 | 3.9 (2.9-5.2) |
| EF >35% | ||||
| ICD | 186 | 3 | 4.6 | 0.6 (0.2-2.0) |
| Placebo | 185 | 4 | 4.9 | 0.8 (0.3-2.2) |
Abbreviations: EF, ejection fraction; ICD, implantable cardioverter defibrillator; SCD, sudden cardiac death.
Table 3. Effect of ICD on All-Cause Mortality and SCD Compared With Placebo.
| EF Group | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|
| Unadjusted Analyses | P Value | Adjusted Analysesa | P Value | |
| All-Cause Mortality | ||||
| EF ≤35% | 0.72 (0.55-0.95) | .02 | 0.64 (0.48-0.85) | .002 |
| EF >35% | 0.58 (0.29-1.17) | .13 | 0.62 (0.29-1.30) | .20 |
| Test for interaction | 0.80 (0.38-1.70) | .56 | 0.97 (0.45-2.16) | .98 |
| SCD | ||||
| EF ≤35% | 0.31 (0.17-0.57) | <.001 | 0.30 (0.16-0.57) | <.001 |
| EF >35% | 0.80 (0.18-3.56) | .77 | 0.74 (0.19-2.93) | .67 |
| Test for interaction | 2.57 (0.51-12.9) | .25 | 2.84 (0.57-14.1) | .20 |
Abbreviations: EF, ejection fraction; ICD, implantable cardioverter defibrillator; SCD, sudden cardiac death.
The final adjusted model included the covariates age, sex, heart failure etiology and duration, New York Heart Association class, baseline EF, systolic blood pressure, diabetes, stroke, pulmonary disease, hypertension, hyperlipidemia, syncope, nonsustained ventricular tachycardia, atrial fibrillation, mitral regurgitation, renal insufficiency, current smoking, history of substance abuse, Duke Activity Status Index, serum potassium level, the electrocardiographic QRS and QT intervals, and use of aspirin, β-blocker, angiotensin-converting enzyme inhibitor or receptor blocker, statin, and digoxin.
Figure 2. Kaplan-Meier Survival Curves of Patients With or Without an Increase in Ejection Fraction (EF) to Greater Than 35% Assigned to Implantable Cardioverter Defibrillator (ICD) or Placebo.
Presence of an ICD was associated with an absolute reduction of 3.0 deaths per 100 person-year follow-up among patients with a follow-up EF ≤35% and 1.9 deaths per 100 person-year follow-up among patients with a follow-up EF >35% (Table 2). Thus, assuming a constant mortality rate over 5 years, 10.6 patients with a follow-up EF ≤35% vs 12.6 patients with a follow-up EF >35% needed to be treated to save 1 life.
Of the 248 deaths in the combined ICD and placebo groups, 67 (27.0%) were adjudicated as SCD. The incidence rate of SCD tended to be lower with ICD vs placebo within each EF strata (Table 2). The unadjusted and adjusted HR for ICD effects are summarized in Table 3. The estimated adjusted benefit of ICD on SCD among the larger repeated EF ≤35% stratum was significant (HR, 0.30; 95% CI, 0.16-0.57). Although there appeared to be a benefit—albeit smaller in magnitude—of ICD in the EF >35% stratum (HR, 0.74; 95% CI, 0.19-2.93), the confidence interval for this estimate was wide, given the smaller size of this stratum and the low number of SCD events. We did not find evidence that the effect of ICD on SCD risk varied by repeated EF strata (interaction, P = .20).
Discussion
This study showed that among patients with heart failure and reduced EF at baseline, those who had an improvement in EF to >35% during follow-up had a similar reduction of mortality with ICD therapy as those whose EF remained ≤35%. Although the risk of mortality and SCD was significantly lower among patients with improved EF, the benefit from ICD was largely preserved. To save 1 life over a 5-year period, 10.6 patients whose EF remained ≤35% vs 12.6 patients whose EF improved to >35% needed to be implanted with ICD. If confirmed in a prospective study, these results may have significant implications on risk stratification for SCD in patients with improvement of EF to >35% before or after ICD implantation.
To our knowledge, SCD in patients with an EF >35% is an important public health problem that has not been addressed in landmark ICD trials. In large, population-based series of individuals who had a cardiac arrest and died suddenly, 65% to 75% had an EF >35% on a prior echocardiogram, which would have rendered them ineligible for ICD therapy before the terminal event. However, to our knowledge, a randomized clinical trial of primary-prevention ICD implantation has not yet been completed in patients with an EF >35%. The results of the present analysis, suggesting that the benefit from ICD extends to patients whose EF improved to >35%, should be interpreted within this context. However, caution is necessary to refrain from extrapolating these results to patients with an EF between 35% and 50% but have never had an EF ≤35% in the past. These patients may represent a different population than the one studied in this investigation and are the focus of an ongoing large prospective cohort study to identify risk markers of SCD (clinicaltrials.gov identifier: NCT01114269).
Patients with improved EF differ from those with persistently reduced or preserved EF in substrate, pathophysiology, and risk for adverse outcomes. Previous studies have shown that patients with improved EF are younger and have less comorbidity and coronary heart disease than those with persistently reduced or preserved EF. Patients with improved EF also have less heart failure symptoms, lower risk of hospitalization, and lower risk of mortality. However, ongoing troponin release and elevated B-type natriuretic peptide levels detected in these patients suggest smoldering myocardial injury and continuing myocardial stretch, respectively. Cumulatively, this pathophysiology appears to create a substrate where the proportional risk of SCD exceeds the risk of death due to heart failure. Indeed, prior studies have shown that the risk of appropriate ICD shocks for ventricular tachycardia or ventricular fibrillation continues after improvement in EF to >35%. The results of the present investigation add to the existing knowledge by showing that ICD therapy reduces mortality compared with placebo in patients with an improvement in EF to >35%.
Although improvement in EF is a welcome development in patients with ICD and left ventricular dysfunction, this scenario creates a clinical dilemma when the ICD is nearing battery depletion. Is the risk of SCD still high enough to replace the device in such patients if they have not had a prior appropriate shock? The data presented in this investigation and prior observational data from patients with ICD might suggest yes. However, the lower event rate among patients who had improvement in EF should provide some reassurance to patients and clinicians who need to compare the reduced risk of an arrhythmic death with the potential adverse events and cost associated with a generator replacement. Further, there are not enough data to make a definitive recommendation in some very-low-risk subgroups, such as patients with normalized EF (>55%) and patients with nonischemic cardiomyopathy but no myocardial scar. Until more data become available, the value of ICD in such patients will need to be decided on a case-by-case basis.
The primary advantage of this study over previous observational studies limited only to patients with ICD is the presence of a control group of participants without ICD. Thus, the potential clinical implications of this analysis may extend beyond patients with existing ICDs who experience an improvement in EF to >35%. Improvement in EF is also observed in other clinical circumstances, such as after a myocardial infarction, after revascularization, after cardiac resynchronization therapy, and, most notably, with intensive medical therapy. If confirmed prospectively in a clinical trial, these data suggest that the benefit of ICD may extend to patients whose EF has improved to >35% regardless of the cause.
Limitations
Our analysis had some limitations. First, 77% of the patients enrolled in the SCD-HeFT had a repeated EF assessment during follow-up. Although these patients were healthier than those who did not have a repeated EF, treatment assignment does not appear to be a factor in the decision of who had a repeated EF assessment (eTable in the Supplement). A similar proportion of patients in each randomly assigned treatment group had a repeated EF, which suggests that a bias due to not having a follow-up echocardiogram is unlikely. Further, a similar proportion of patients randomized to ICD or placebo had an EF >35% during follow-up, which also argues against a bias affecting the ICD vs placebo comparison in this analysis. Although we adjusted for the differences in baseline characteristics between the ICD and placebo groups that were present in either repeated EF strata, we cannot exclude the possibility of residual confounding. Second, the definition of EF improvement in this analysis (EF >35%) was chosen based on the clinical relevance of this cutoff for ICD implantation. Although patients with a baseline EF of 30% to 35% can theoretically achieve this cutoff with minimal improvement or even with interreader variability, in reality, the mean increase in EF was much larger (approximately 19%) in these patients. Third, neither the original nor the repeated echocardiograms were analyzed in a core laboratory in the SCD-HeFT. Thus, the observed changes in EF may also be because of interreader variability, and misclassification of EF strata may have reduced our ability to detect differences in ICD efficacy. However, the mean absolute EF change of approximately 20% was similar to other cohorts without ICD and was much greater than would typically be expected from measurement error. Fourth, an eligibility requirement in the SCD-HeFT was to be on angiotensin-converting enzyme inhibitors before randomization. Although there was no requirement, almost 70% of the patients were taking β-blockers. Less than ideal doses of these medications were acceptable at study enrollment as long as target doses were attained shortly after randomization. Today, there is a greater emphasis on being on optimal medical therapy for heart failure, including β-blockers, before ICD implantation. Further, aldosterone blockers, which are known to reduce the incidence of SCD, have also become part of heart failure therapy since the publication of the SCD-HeFT. Despite these limitations, the proportion of patients with EF improvement in the SCD-HeFT was similar to more contemporary cohorts of patients with ICD.
Conclusions
Among patients from the SCD-HeFT who had a repeated EF assessment during the course of follow-up, the efficacy of ICD therapy on mortality reduction was similar in patients who had an improvement in EF to >35% compared with those with an EF persistently ≤35%. These data call into question the current risk stratification schemes for patients with heart failure who experience an improvement in EF with optimal medical therapy. A prospective randomized clinical trial is needed to assess ICD efficacy for primary prevention of SCD among patients with an EF >35%.
eTable. Comparison of the baseline characteristics and mortality of the SCD-HeFT participants who did or did not have a repeated assessment of EF after enrollment.
eFigure 1. Algorithm showing the selection of study patients.
eFigure 2. Baseline and follow-up EF among study patients stratified by EF improvement status.
References
- 1.Bardy GH, Lee KL, Mark DB, et al. ; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators . Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225-237. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, et al. ; Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877-883. [DOI] [PubMed] [Google Scholar]
- 3.Myerburg RJ, Reddy V, Castellanos A. Indications for implantable cardioverter-defibrillators based on evidence and judgment. J Am Coll Cardiol. 2009;54(9):747-763. [DOI] [PubMed] [Google Scholar]
- 4.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society . 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61(3):e6-e75. [DOI] [PubMed] [Google Scholar]
- 5.Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail. 2011;17(7):527-532. [DOI] [PubMed] [Google Scholar]
- 6.Basuray A, French B, Ky B, et al. . Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129(23):2380-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florea VG, Rector TS, Anand IS, Cohn JN. Heart failure with improved ejection fraction: clinical characteristics, correlates of recovery, and survival: results from the Valsartan Heart Failure Trial. Circ Heart Fail. 2016;9(7):9. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson LW. Heart failure with better ejection fraction: a modern diagnosis. Circulation. 2014;129(23):2364-2367. [DOI] [PubMed] [Google Scholar]
- 9.Naksuk N, Saab A, Li JM, et al. . Incidence of appropriate shock in implantable cardioverter-defibrillator patients with improved ejection fraction. J Card Fail. 2013;19(6):426-430. [DOI] [PubMed] [Google Scholar]
- 10.Steimle AE, Stevenson LW, Fonarow GC, Hamilton MA, Moriguchi JD. Prediction of improvement in recent onset cardiomyopathy after referral for heart transplantation. J Am Coll Cardiol. 1994;23(3):553-559. [DOI] [PubMed] [Google Scholar]
- 11.McNamara DM, Starling RC, Cooper LT, et al. ; IMAC Investigators . Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J Am Coll Cardiol. 2011;58(11):1112-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manfredi JA, Al-Khatib SM, Shaw LK, et al. . Association between left ventricular ejection fraction post-cardiac resynchronization treatment and subsequent implantable cardioverter defibrillator therapy for sustained ventricular tachyarrhythmias. Circ Arrhythm Electrophysiol. 2013;6(2):257-264. [DOI] [PubMed] [Google Scholar]
- 13.Madhavan M, Waks JW, Friedman PA, et al. . Outcomes after implantable cardioverter-defibrillator generator replacement for primary prevention of sudden cardiac death. Circ Arrhythm Electrophysiol. 2016;9(3):e003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schliamser JE, Kadish AH, Subacius H, et al. ; DEFINITE Investigators . Significance of follow-up left ventricular ejection fraction measurements in the Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation trial (DEFINITE). Heart Rhythm. 2013;10(6):838-846. [DOI] [PubMed] [Google Scholar]
- 15.Kini V, Soufi MK, Deo R, et al. . Appropriateness of primary prevention implantable cardioverter-defibrillators at the time of generator replacement: are indications still met? J Am Coll Cardiol. 2014;63(22):2388-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naksuk N, Adabag S. What to do about primary-prevention implantable cardiac defibrillators in patients with improved ejection fraction. Curr Heart Fail Rep. 2014;11(2):197-200. [DOI] [PubMed] [Google Scholar]
- 17.Merchant FM, Quest T, Leon AR, El-Chami MF. Implantable cardioverter-defibrillators at end of battery life: opportunities for risk (re)-stratification in ICD recipients. J Am Coll Cardiol. 2016;67(4):435-444. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Guallar E, Blasco-Colmenares E, et al. . Changes in follow-up left ventricular ejection fraction associated with outcomes in primary prevention implantable cardioverter-defibrillator and cardiac resynchronization therapy device recipients. J Am Coll Cardiol. 2015;66(5):524-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gula LJ, Klein GJ, Hellkamp AS, et al. . Ejection fraction assessment and survival: an analysis of the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J. 2008;156(6):1196-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Packer DL, Prutkin JM, Hellkamp AS, et al. . Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation. 2009;120(22):2170-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole JE, Johnson GW, Hellkamp AS, et al. . Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359(10):1009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228-1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 23.Buxton AE, Lee KL, Hafley GE, et al. ; MUSTT Investigators . Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50(12):1150-1157. [DOI] [PubMed] [Google Scholar]
- 24.Stecker EC, Vickers C, Waltz J, et al. . Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47(6):1161-1166. [DOI] [PubMed] [Google Scholar]
- 25.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest—the relevance of heart failure: the Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24(13):1204-1209. [DOI] [PubMed] [Google Scholar]
- 26.Kadish AH, Bello D, Finn JP, et al. . Rationale and design for the Defibrillators to Reduce Risk by Magnetic Resonance Imaging Evaluation (DETERMINE) trial. J Cardiovasc Electrophysiol. 2009;20(9):982-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perkiömäki J, Exner DV, Piira OP, et al. . Heart rate turbulence and T-wave alternans in patients with coronary artery disease: the influence of diabetes. Ann Noninvasive Electrocardiol. 2015;20(5):481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, et al. . Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol. 2016;1(5):510-518. [DOI] [PubMed] [Google Scholar]
- 29.Brenyo A, Barsheshet A, Kutyifa V, et al. . Predictors of spontaneous reverse remodeling in mild heart failure patients with left ventricular dysfunction. Circ Heart Fail. 2014;7(4):565-572. [DOI] [PubMed] [Google Scholar]
- 30.Shadman R, Poole JE, Dardas TF, et al. . A novel method to predict the proportional risk of sudden cardiac death in heart failure: derivation of the Seattle Proportional Risk Model. Heart Rhythm. 2015;12(10):2069-2077. [DOI] [PubMed] [Google Scholar]
- 31.Adabag S, Smith LG, Anand IS, Berger AK, Luepker RV. Sudden cardiac death in heart failure patients with preserved ejection fraction. J Card Fail. 2012;18(10):749-754. [DOI] [PubMed] [Google Scholar]
- 32.Adabag S, Rector TS, Anand IS, et al. . A prediction model for sudden cardiac death in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2014;16(11):1175-1182. [DOI] [PubMed] [Google Scholar]
- 33.Poole JE, Gleva MJ, Mela T, et al. ; REPLACE Registry Investigators . Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122(16):1553-1561. [DOI] [PubMed] [Google Scholar]
- 34.Kadish A, Dyer A, Daubert JP, et al. ; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators . Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151-2158. [DOI] [PubMed] [Google Scholar]
- 35.Ruwald MH, Solomon SD, Foster E, et al. . Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: results from the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT-CRT) trial. Circulation. 2014;130(25):2278-2286. [DOI] [PubMed] [Google Scholar]
- 36.Narayan MA, Vakil K, Reddy YNV, et al. . Efficacy of implantable cardioverter defibrillator therapy in patients with nonischemic cardiomyopathy: a systematic review and meta-analysis of randomized controlled trials. JACC Clin Electrophysiol. In press. [DOI] [PubMed] [Google Scholar]
- 37.Adabag AS, Therneau TM, Gersh BJ, Weston SA, Roger VL. Sudden death after myocardial infarction. JAMA. 2008;300(17):2022-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vakil K, Florea V, Koene R, Kealhofer JV, Anand I, Adabag S. Effect of coronary artery bypass grafting on left ventricular ejection fraction in men eligible for implantable cardioverter-defibrillator. Am J Cardiol. 2016;117(6):957-960. [DOI] [PubMed] [Google Scholar]
- 39.Adabag S, Roukoz H, Anand IS, Moss AJ. Cardiac resynchronization therapy in patients with minimal heart failure: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(9):935-941. [DOI] [PubMed] [Google Scholar]
- 40.Greenberg B, Quinones MA, Koilpillai C, et al. . Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction: results of the SOLVD echocardiography substudy. Circulation. 1995;91(10):2573-2581. [DOI] [PubMed] [Google Scholar]
- 41.Groenning BA, Nilsson JC, Sondergaard L, Fritz-Hansen T, Larsson HB, Hildebrandt PR. Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol. 2000;36(7):2072-2080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Comparison of the baseline characteristics and mortality of the SCD-HeFT participants who did or did not have a repeated assessment of EF after enrollment.
eFigure 1. Algorithm showing the selection of study patients.
eFigure 2. Baseline and follow-up EF among study patients stratified by EF improvement status.