Abstract
Importance
Intracranial pressure (ICP) monitoring is a mainstay of therapy for children with traumatic brain injury (TBI), but its overall association with patient outcome is unclear.
Objective
To test the hypothesis that ICP monitoring is associated with improved functional survival of children with severe TBI.
Design, Setting, and Participants
A propensity-weighted effectiveness analysis was conducted using 2 linked national databases with data from 30 US children’s hospitals from January 1, 2007, to December 31, 2012, on 3084 children with severe TBI. Clinical events including neurosurgical procedures were identified using validated computable phenotypes. Data analysis was conducted from September 1, 2016, to March 1, 2017.
Exposure
Placement of an ICP monitor.
Main Outcomes and Measures
A composite of hospital mortality, discharge to hospice, or survival with placement of new tracheostomy and gastrostomy tubes.
Results
Of the 3084 children in the study (1128 girls and 1956 boys; mean [SD] age, 7.03 [5.44] years), 1002 (32.4%) underwent ICP monitoring, with substantial hospital variation (6% to 50% by hospital). Overall, 484 children (15.7%) experienced the primary composite outcome. A propensity approach using matching weights generated good covariate balance between those who did and those who did not undergo ICP monitoring. Using a propensity-weighted logistic regression model clustered by hospital, no statistically significant difference was found in functional survival between monitored and unmonitored patients (odds ratio of poor outcome among those who underwent ICP monitoring, 1.31; 95% CI, 0.99-1.74). In a prespecified secondary analysis, no difference in mortality was found (odds ratio, 1.16; 95% CI, 0.89-1.50). Prespecified subgroup analyses of children younger and older than 2 years of age and among those with unintentional and inflicted (intentional) injuries also showed no difference in outcome with ICP monitoring.
Conclusions and Relevance
With the use of linked national data and validated computable phenotypes, no evidence was found of a benefit from ICP monitoring on functional survival of children with severe TBI. Intracranial pressure monitoring is a widely but inconsistently used technology with incompletely demonstrated effectiveness. A large prospective cohort study or randomized trial is needed.
This propensity-weighted analysis uses data from 30 US children’s hospitals to test the hypothesis that intracranial pressure monitoring is associated with improved functional survival of children with severe traumatic brain injury.
Key Points
Question
Does intracranial pressure monitoring improve the functional survival of children with severe traumatic brain injury?
Findings
In a propensity-weighted effectiveness analysis using 2 linked national databases, no statistically significant difference was found in functional survival between children who underwent intracranial pressure monitoring and those who did not.
Meaning
Because intracranial pressure monitoring is a widely but inconsistently used technology with incompletely demonstrated effectiveness, a large prospective cohort study or randomized trial is needed.
Introduction
Traumatic brain injury (TBI) causes approximately 2200 deaths and 35 000 hospitalizations among US children annually.1 Children who survive severe TBI frequently have new motor, communication, and/or behavioral morbidities.2,3 Elevated intracranial pressure (ICP) often results from severe TBI and worsens patient outcome by causing additional brain injury.4,5 Intracranial pressure monitoring is used to detect elevated ICP and to guide treatment of severe TBI.
The overall association of ICP monitoring with patient outcome is unclear. Therapies to reduce ICP are mainstays of treatment for severe TBI, and the treatment of elevated ICP is associated with the best reported outcomes.4,6,7,8 However, because of the relatively low quality of evidence, the current guidelines for the medical care of children with severe TBI state only that ICP monitoring “may be considered” for children with severe TBI.4(pS11) Perhaps because of the weak evidence but despite the current guidelines, studies have shown that hospitals use ICP monitoring for children with severe TBI at variable rates.9,10,11,12 Studies of ICP monitoring are complicated by this existing expert recommendation, and sufficient equipoise for a randomized clinical trial is unlikely.
Without the necessary equipoise to conduct a clinical trial, several multicenter observational studies addressing this question have been published. Some of those studies support ICP monitoring,8,9 whereas others report mixed results or no association of ICP monitoring with patient outcome.12,13 All have been limited by 1 or more issues: small sample size, inadequate severity and confounder adjustment due to missing variables, database codes with unknown accuracy, mortality as the only outcome, or lack of consideration of clustering of patient outcomes by hospital.8 To overcome the limitations of small sample size and missing variables, we probabilistically linked 2 large, overlapping databases that each contain a portion of the necessary information: the Pediatric Health Information System (PHIS) database and the National Trauma Data Bank (NTDB).14 The PHIS database contains rich clinical information, particularly regarding treatments such as medications and nursing interventions. The NTDB is a standardized collection of hospital trauma registries that contains the necessary injury variables but does not contain detailed treatment data. To address the uncertain accuracy of database codes, we developed and prospectively validated computable phenotypes (sets of data elements and logical expressions that identify a clinical condition or characteristic)15 to identify ICP monitoring, other key neurosurgical and critical care interventions, and a proxy functional outcome.16,17 We then conducted this propensity-weighted comparative effectiveness analysis testing the hypothesis that ICP monitoring is associated with improved functional survival of children with severe TBI.
Methods
Data Sources and Cohort Design
The PHIS-NTDB linkage that generated this data set has been described in detail.14,18 Additional information is available in the eAppendix in the Supplement. This study was approved by the University of Utah Institutional Review Board and the Colorado Multiple Institutional Review Board, and written permission was obtained from both the Children’s Hospital Association (PHIS owner) and the American College of Surgeons (NTDB owner). Patient and parental consent was waived by both institutional review boards.
Inclusion and Exclusion Criteria
We defined TBI using the International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes used by the Centers for Disease Control and Prevention (eTable 1 in the Supplement).1 Study participants were children younger than 18 years of age admitted from January 1, 2007, to December 31, 2012, to the 30 hospitals that participated in both the PHIS and the NTDB during 2007-2012. Other inclusion criteria were severe TBI (Glasgow Coma Scale [GCS] score ≤8 in the emergency department [ED]), hospital length of stay greater than 24 hours, and nonmissing disposition. We excluded children who were transferred to another acute care hospital, left against medical advice, had been admitted previously for TBI at the same hospital, or had a diagnosis code for late effects of TBI (907.0).
Variable Definitions
Bennett et al17 applied machine learning techniques to develop and validate highly accurate computable phenotypes using PHIS and NTDB data for ICP monitoring (98% accurate), craniotomy (94% accurate), gastrostomy tube (GT) placement (100% accurate), and new tracheostomy (98% accurate). We used the most accurate and parsimonious phenotype for each clinical event in this study (eAppendix and eTable 1 in the Supplement).
Primary Exposure and Outcome
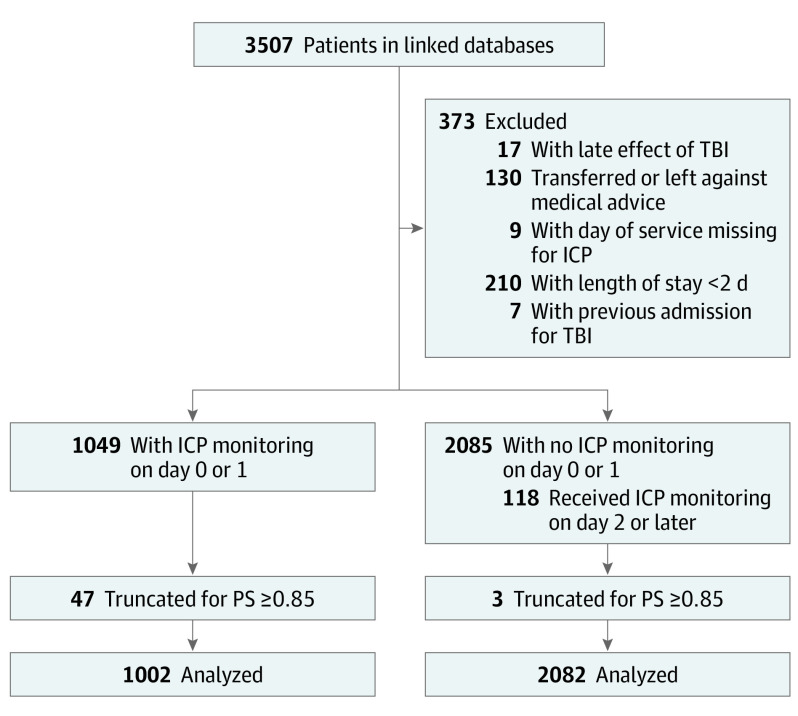
The primary exposure was ICP monitoring, defined using the phenotype developed and validated by Bennett et al17 (eTable 1 in the Supplement).17 To avoid including children who died prior to the opportunity for ICP placement (“immortal time” bias19,20) in the primary analysis, we defined an exposure period (24 hours after admission) and an observation period (after the first 24 hours of admission). Children who died or were discharged before 24 hours had elapsed were excluded from the study population,19 and children who underwent ICP monitors after 24 hours were placed in the untreated group (Figure 1) for all analyses.
Figure 1. CONSORT Diagram for Patient Eligibility and Flow.
ICP indicates intracranial pressure; PS, propensity score; and TBI, traumatic brain injury.
The primary outcome was a composite of mortality, discharge to hospice,21 or poor functional survival, defined as survival with placement of both a new tracheostomy and a new GT. Bennett et al16 demonstrated that, among all children who survive TBI, those who receive both a new tracheostomy and GT during the acute hospitalization have a significantly poorer functional outcome as measured by the Functional Status Scale.
Statistical Analysis
General
Statistical analysis was conducted from September 1, 2016, to March 1, 2017. Mean values are reported with SD, and median values are reported with interquartile range (IQR). Durations (eg, ventilator-days) were compared using the Wilcoxon rank sum test. Unadjusted differences between patients who underwent ICP monitoring and those who did not are shown as standardized mean differences (SMDs).22 The SMD, which compares differences in mean values using the unit of the pooled SD, is the criterion standard for assessment of covariate balance in propensity analyses.23 This approach is less sensitive to sample size than typical inferential testing. Standardized mean differences greater than 10% are generally considered meaningful.
Propensity Model
Propensity methods are based on the estimation of a single variable that defines the likelihood of having received the treatment in question as a function of the background differences.24 They are particularly useful when the outcome occurs less often than the treatment, as is the case in this study. The goal of the propensity model is covariate balance between the treated (those who underwent ICP monitoring) and untreated groups. Good covariate balance is considered to be present if all SMDs in a propensity model are less than 10%.23
We used a machine learning approach, generalized boosted regression, to achieve optimal covariate balance.25 To do so, we calculated each patient’s probability (propensity) of ICP monitoring as implemented by the twang package in R.26 We targeted the smallest maximum SMD as the stopping rule for the generalized boosted regression process. No variable had an SMD greater than 5% in the final propensity model (using matching weights; eFigure 1 in the Supplement).
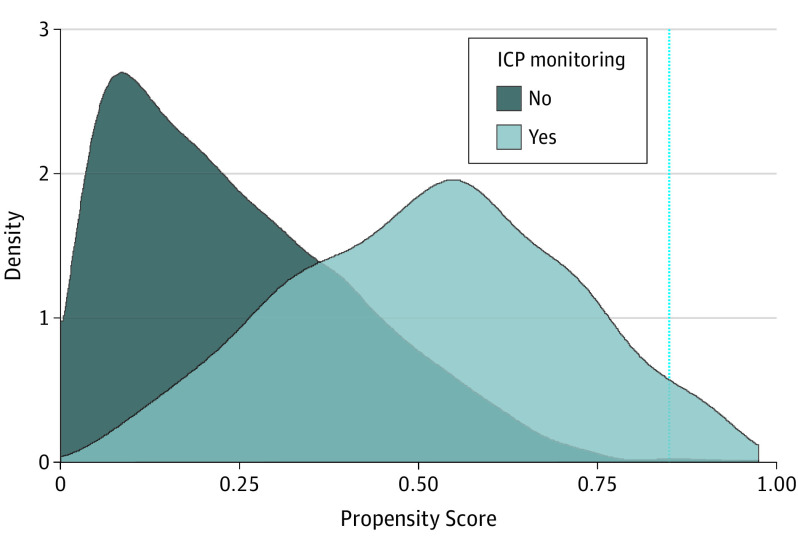
Covariates in the propensity model include demographic characteristic, clinical events, types of injury, neurologic examinations, and hospital capability variables (eTable 2 in the Supplement). After we built the propensity model, we examined the distribution of propensity scores stratified by actual receipt of ICP monitoring (Figure 2). Three of 50 children with a propensity score greater than 0.85 did not undergo ICP monitoring. To restrict the outcome analysis to the area of common support27 (where a child might receive or not receive an ICP monitor), we excluded the 50 children with propensity scores greater than 0.85.
Figure 2. Density of Propensity Scores by Actual Receipt of Intracranial Pressure (ICP) Monitoring.
Overlap between the 2 density regions indicates “common support” and the potential for clinical equipoise. Patients with a propensity score greater than 0.85 (vertical dashed line) were excluded from the analyses because nearly all (47 of 50) received ICP monitoring.
Primary Outcome Model
To preserve the statistical power of the outcome analysis and avoid multiple testing, we finalized the propensity model and prespecified the design of the outcome model before any outcome assessment. After estimating the propensity score, we applied weighted logistic regression with clustering by hospital28 to estimate the association of ICP monitoring with mortality (which included discharge to hospice) or poor functional survival (yes or no). No covariates were included in the primary outcome model. The analysis achieved covariate balance between those with and those without ICP monitoring through application of propensity matching weights.29 Using matching weights leads to a weighted mean estimate that assigns more emphasis to individuals with propensity scores close to 0.50 than to those whose propensity scores close to 0 or 1. It represents an analog to 1-to-1 pair matching.
Secondary Outcome and Subgroup Analyses
Using identical methods, we also tested the association of ICP monitoring with mortality alone, with mortality or tracheostomy, and with mortality or GT placement. We assessed the association of our choice of matching weights by fitting outcome models using the 4 other most commonly used weighting estimates. As prespecified subgroup analyses, we fit separate propensity models for children with unintentional injuries, inflicted (intentional) injuries, age younger than 2 years, and age of 2 years or older. We then fit outcome models using the subgroup propensity scores as already described for the primary outcome. Post hoc, we performed subgroup analyses restricting the cohort to children with Abbreviated Injury Scale scores of 3 to 5 to compare our results with those of other observational studies, and we performed subgroup analyses using an exposure period of 48 hours instead of 24 hours.
Sensitivity Analyses
We measured the intensity of medical and surgical therapy typically directed at intracranial hypertension among children who did or did not undergo ICP monitoring. This measurement provides an indirect assessment of how clinicians responded to measured ICP and/or differences between groups that may have become apparent after the first day of admission and, therefore, would not have been included in the propensity model that balanced the groups. We also evaluated the properties that an unmeasured confounder would need to have to affect our results (eAppendix in the Supplement).30
Data analysis was conducted in R, version 3.4.0.31 The code to generate the analysis was written using rmarkdown,32 compiled using knitr,33 and is entirely reproducible. The figures were generated using the ggplot234 package. P < .05 was considered statistically significant.
Results
Patient, Hospital, and Injury Characteristics
After exclusions, the study cohort included 3084 patients at 30 hospitals (Figure 1). Hospital enrollment varied from 8 to 310 patients. eTables 3 and 4 in the Supplement show the demographic, hospital, and injury characteristics for the cohort. A total of 675 patients (21.9%) were injured by known or suspected child abuse. At the time of GCS assessment in the ED, 2236 patients (72.5%) were intubated, and approximately half (1569 [50.9%]) had received sedating medications.
ICP Monitoring
Overall, 1002 patients (32.5%) underwent ICP monitoring. Rates of ICP monitoring varied widely by hospital (6%-50%; eFigure 2 in the Supplement). Modest differences were seen between those who underwent ICP monitoring and those who did not by admission year, insurance payer, and hospital trauma certification by the American College of Surgeons. Those who underwent ICP monitoring were more likely to be injured in motor vehicle incidents (477 [47.6%] vs 859 [41.3%]); had poorer mean (SD) Injury Severity Scores (27 [11] vs 20 [12]), head Abbreviated Injury Scale scores (score of 4 or 5: 876 [87.4%] vs 1346 [64.6%]), GCS scores from the ED (score of 3: 670 [66.9%] vs 1300 [62.4%]), and GCS motor scores from the ED (score of 1: 699 [69.8%] vs 1357 [65.2%]); and were more likely to have subdural (258 [25.7%] vs 410 [19.7%]) and intraventricular or subarachnoid hemorrhages (172 [17.2%] vs 279 [13.4%]) (eTables 3 and 4 in the Supplement). Substantial clinical equipoise appeared to be present for ICP monitoring because many children with propensities from approximately 0.1 to 0.6 either underwent or did not undergo ICP monitoring (Figure 2).
Hospital Outcomes and Complications
Hospital mortality was 12.4% overall (n = 382) and 484 patients (15.7%) had the primary outcome of mortality, discharge to hospice, or poor functional survival (Table 1). Both mortality rates (185 [18.5%] vs 197 [9.5%]) and poor functional survival rates (55 [5.5%] vs 43 [2.1%]) were higher among those who underwent ICP monitoring. No between-group differences were seen in rates of complications such as prehospital or ED hypotension (95 [9.5%] ICP monitoring vs 150 [7.2%] no ICP monitoring; SMD, 0.78%) or cardiac arrest (40 [4.0%] ICP monitoring vs 83 [4.0%] no ICP monitoring; SMD, 1.72%).
Table 1. Hospital Outcomesa.
| Variable | Children, No. (%) | |
|---|---|---|
| No ICP (n = 2082) |
ICP (n = 1002) |
|
| Discharge outcome | ||
| Mortality | 197 (9.5) | 185 (18.5) |
| Hospice | 1 (0.05) | 3 (0.3) |
| Primary outcomeb | 241 (11.6) | 243 (24.3) |
| New technology dependence | ||
| New GT | 272 (13.1) | 288 (28.7) |
| New tracheostomy | 55 (2.6) | 66 (6.6) |
| Day of tracheostomy, median (IQR) | 13 (7-22) | 14 (6-22) |
| Tracheostomy and GT | 43 (2.1) | 55 (5.5) |
| Complications | ||
| Hypotensionc | 150 (7.2) | 95 (9.5) |
| Cardiac arrest | 83 (4.0) | 40 (4.0) |
| Seizure | 454 (21.8) | 219 (21.9) |
Abbreviations: GT, gastrostomy tube; ICP, intracranial pressure; IQR, interquartile range.
Data are unweighted.
In-hospital mortality, discharge to hospice, or survival with a new tracheostomy and GT placement during the acute hospitalization.
Hypotension occurring either during prehospital care or in the Emergency Department.
Primary and Secondary Outcomes and Subgroup Analyses
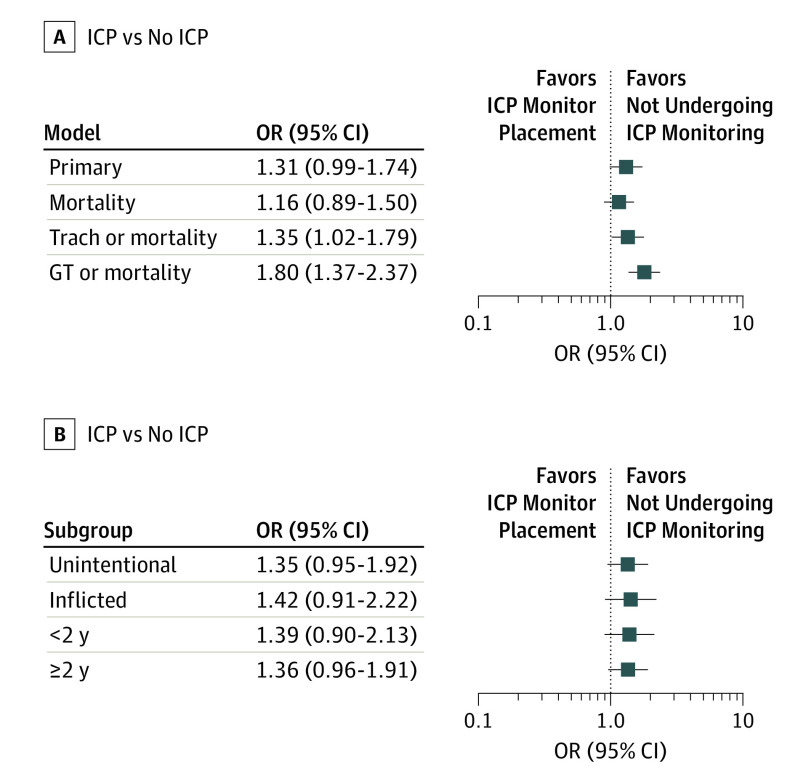
Using propensity matching weights to adjust for patient-level differences and clustering by hospital, we found no statistically significant difference in functional survival between those who underwent ICP monitoring and those who did not (odds ratio [OR], 1.31; 95% CI, 0.99-1.74) (Figure 3). The point estimate was contrary to our hypothesis and reflected poorer outcomes among children who underwent ICP monitoring. In prespecified secondary analyses (also performed using matching weights), ICP monitoring was not significantly associated with hospital mortality (OR, 1.16; 95% CI, 0.89-1.50) but was associated with a higher likelihood of mortality, discharge to hospice, or either tracheostomy or GT placement. (Figure 3). Our choice of matching weights for propensity score weighting did not affect the results; otherwise, identical analyses using the 4 other most common propensity weighting strategies had similar overall results (eFigure 3 in the Supplement).
Figure 3. Association of Intracranial Pressure (ICP) Monitoring With Mortality or Poor Functional Survival.
A, Primary outcome (hospital mortality, discharge to hospice, or both of new tracheostomy and new gastrostomy tube [GT]) and prespecified secondary models. B, Prespecified subgroups. An odds ratio (OR) greater than 1.0 reflects poorer outcomes with ICP monitoring. GT or mortality indicates hospital mortality, discharge to hospice, or new GT; Mortality, hospital mortality or discharge to hospice; Trach, tracheostomy; and Trach or mortality, hospital mortality, discharge to hospice, or new tracheostomy.
In prespecified subgroup analyses, we found no significant difference in functional survival between those who did and those who did not undergo ICP monitoring among children with unintentional injuries (OR, 1.35; 95% CI, 0.95-1.92), inflicted injuries (OR, 1.42; 95% CI, 0.91-2.22), and those 2 years of age or older (OR, 1.36; 95% CI, 0.96-1.91) or younger than 2 years of age (OR, 1.39; 95% CI, 0.90-2.13) (Figure 3). Results of post hoc subgroup analyses are in the eAppendix in the Supplement.
Sensitivity Analyses
Children who underwent ICP monitoring had longer lengths of hospital stay (median [IQR], 19 [10-34] days vs 6 [3-14] days; P < .001) and mechanical ventilation (median [IQR], 7 [4-13] days vs 2 [1-4] days; P < .001), more days of osmolar therapy (median [IQR], 4 [2-8] days vs 2 [1-5] days; P < .001), more days of inotropes or pressors (median [IQR], 3 [2-5] days vs 2 [1-3] days; P < .001), and more days of pentobarbital (median [IQR], 3 [2-5] days vs 1 [1-3] days; P < .001) (Table 2). Differences in pentobarbital use were not explained by use as an antiepileptic rather than an anesthetic because more children without ICP monitoring (37 of 121 [30.6%]) than with ICP monitoring (61 of 252 [24.2%]) had seizures (SMD = 14%). Children who underwent ICP monitoring were also more likely to undergo a craniotomy or craniectomy (312 [31.1%] vs 153 [7.3%]; SMD = 57%).
Table 2. Therapeutic Intensitya.
| Variable | Children, No. (%) | |
|---|---|---|
| No ICP (n = 2082) |
ICP (n = 1002) |
|
| Hospital stay | ||
| Mechanical ventilation | 1692 (81.3) | 893 (89.1) |
| Ventilation, median (IQR), d | 2 (1-4) | 7 (4-13) |
| Length of stay, median (IQR), d | 6 (3-14) | 19 (10-34) |
| Osmolar therapy | ||
| Any osmolar therapy | 527 (25.3) | 628 (62.7) |
| Osmolar therapy, median (IQR), d | 2 (1-5) | 4 (2-8) |
| Any hypertonic saline | 449 (21.6) | 590 (58.9) |
| Hypertonic saline, median (IQR), d | 2 (1-5) | 4 (2-8) |
| Any mannitol | 234 (11.2) | 381 (38.0) |
| Mannitol, median (IQR), d | 1 (1-2) | 1 (1-3) |
| Inotropes or pressors | ||
| Any pressor | 380 (18.3) | 504 (50.3) |
| Pressor therapy, median (IQR), d | 2 (1-3) | 3 (2-5) |
| Pentobarbital | ||
| Any pentobarbital | 121 (5.8) | 252 (25.1) |
| Pentobarbital therapy, median (IQR), d | 1 (1-3) | 3 (2-5) |
| Craniotomy or craniectomy | ||
| Any craniotomy or craniectomy | 153 (7.3) | 312 (31.1) |
| Day of first craniotomy or craniectomy, median (IQR) | 0 (0-1) | 0 (0-1) |
Abbreviations: ICP, intracranial pressure; IQR, interquartile range.
Median days of ventilation or medications are among those who received any. Data are unweighted.
The following sensitivity analyses reflect, in general, the ways in which an unmeasured confounder could change the results of our study. With the current approach, a meaningful difference in the hypothesized direction is unlikely (eFigure 4 [upper panel] in the Supplement). Instead of no association, our study would show significant benefit from ICP monitoring only if both (1) the probability of having the confounder, given covariates, was at least 0.80 among those who underwent ICP monitoring and (2) the poor outcome, given ICP monitoring and covariates, was at least twice as likely among those with the confounder compared with those without the confounder.30 Our study would instead show significant harm (eFigure 4 [lower panel] in the Supplement) from ICP monitoring if poor outcome, given ICP monitoring and covariates, was more likely among those without the confounder compared with those with the confounder.
Discussion
In this large, multicenter, propensity-weighted analysis of children with severe TBI, we found no evidence of an association of ICP monitoring with functional survival. Mortality, a prespecified secondary outcome, also did not differ significantly between treatment groups. Children who underwent ICP monitoring had longer hospital stays and received more therapies directed at intracranial hypertension.
This result is consistent with the results of a randomized trial of ICP monitoring of adults,35 which found no difference in 6-month outcomes between care directed by ICP monitoring vs imaging and clinical examination. Although that trial has been criticized for its overall high mortality and the limited prehospital and postacute care available to the trial participants, it currently provides the best available evidence.36 To our knowledge, no randomized trial of ICP monitoring has been conducted for children.
Strengths and Limitations
The present study has methodological advantages over several other observational studies of the outcome of ICP monitoring, including a robust sample size, validation of key database codes, appropriate confounder adjustment, consideration of clustering of patient outcomes by center, and a functional outcome measure more granular than mortality. In observational studies, selection bias in the distribution of ICP monitoring is highly likely unless propensity techniques are used to achieve covariate balance. To our knowledge, only one other observational study of ICP monitoring of children was both large and used propensity techniques.8 That study showed ICP monitoring to be associated with improved survival but did not examine functional outcome.
One strength of the present study is the use of a primary outcome that captured not only hospital mortality but also survival with new and severe functional impairment requiring technological dependence at discharge. Although this outcome was not a nuanced functional or quality-of-life outcome assessment at 6 or 12 months after injury, it captures outcomes important to families and clinicians. One possible explanation for the lack of association is that some survivors received aggressive treatment, including ICP monitoring, but were ultimately left with severe impairment and new technological dependence. However, results of a secondary analysis with mortality as the outcome did not differ between the 2 groups. It is unlikely that our use of a broader outcome explains the difference between our study and that of Alali et al.8
The present study was conducted using richer covariate data than previous observational studies of ICP monitoring. Because of the database linkage14 and code validation17 studies that we performed, the present study includes variables missing from some previous studies, such as injury mechanism, disposition in the ED, medications, and length of mechanical ventilation. All observational studies to date, including this one, have lacked information about computed tomography results and the progression of neurologic examination findings through the early hospital course. Those variables may be important to a decision about whether or not to place an ICP monitor and will be critical in any future prospective study of ICP monitoring. An additional limitation of the present study is that we used accurate phenotypes to identify clinical events, but the medical decision making that led to those events was not available in the databases we used.
In that light, our sensitivity analyses raise questions about between-group differences despite the seemingly excellent covariate balance we achieved. Children who underwent ICP monitoring had longer periods of mechanical ventilation and hospital stay and received more therapy directed at intracranial hypertension. It is possible that measured ICP led clinicians to make these choices, but it is a limitation that unmeasured differences (perhaps in computed tomography results or progression of GCS score) between those who received ICP monitors and those who did not may have contributed to subsequent treatment intensity. Given the possibility of unmeasured confounding, the data in this article should be interpreted as provocative, but we should not change the standard of care.36
One criticism of studies of ICP monitoring is that ICP monitoring is a diagnostic and surveillance technique and not a treatment per se. Because ICP monitoring is believed to be a low-risk procedure,37 it is unlikely to have a large direct benefit or cause harm. Any contributions to overall outcome are likely to operate through the benefits and risks of treatments ordered by clinicians because of the measured ICP. The present study does not allow for the separation of direct and indirect outcomes, but it can be viewed as estimating the overall association, incorporating both direct and indirect pathways.30
Conclusions
In this propensity-weighted analysis, we found no statistically significant association of ICP monitoring with functional survival of children with severe TBI. The 2 largest and most carefully analyzed retrospective studies of this important question have generated conflicting results. Because ICP monitoring is a widely but inconsistently used technology with incompletely demonstrated effectiveness, a large prospective cohort study or randomized trial is needed.
eAppendix. Methods
eFigure 1. Standardized Differences for Propensity Model Covariates, Before and After Propensity Weighting
eFigure 2. ICP Monitoring Rates by Hospital
eFigure 3. Choice of Matching Weights Did Not Affect Primary Analysis
eFigure 4. Sensitivity Analyses for Robustness to Unmeasured Confounding
eTable 1. Variable Definitions
eTable 2. Propensity Model Covariates
eTable 3. Demographic and Hospital Characteristics
eTable 4. Injury Characteristics
eReferences.
References
- 1.Faul M, Likang X, Wald M, Coronado V. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. Atlanta, GA: National Center for Injury Prevention, Centers for Disease Control and Prevention, US Department of Health and Human Services; 2010.
- 2.Frieden TR, Houry D, Baldwin G. Report to Congress: Traumatic Brain injury in the United States: Epidemiology and Rehabilitation. Atlanta, GA: Division of Unintentional Injury Prevention, National Center for Injury Prevention, Centers for Disease Control and Prevention, US Department of Health and Human Services; 2014. [Google Scholar]
- 3.Anderson VA, Catroppa C, Haritou F, Morse S, Rosenfeld JV. Identifying factors contributing to child and family outcome 30 months after traumatic brain injury in children. J Neurol Neurosurg Psychiatry. 2005;76(3):401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochanek PM, Carney N, Adelson PD, et al. ; American Academy of Pediatrics-Section on Neurological Surgery; American Association of Neurological Surgeons/Congress of Neurological Surgeons; Child Neurology Society; European Society of Pediatric and Neonatal Intensive Care; Neurocritical Care Society; Pediatric Neurocritical Care Research Group; Society of Critical Care Medicine; Paediatric Intensive Care Society UK; Society for Neuroscience in Anesthesiology and Critical Care; World Federation of Pediatric Intensive and Critical Care Societies . Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents—second edition. Pediatr Crit Care Med. 2012;13(suppl 1):S1-S82. [DOI] [PubMed] [Google Scholar]
- 5.Stocchetti N, Maas AIR. Traumatic intracranial hypertension. N Engl J Med. 2014;370(22):2121-2130. [DOI] [PubMed] [Google Scholar]
- 6.Alberico AM, Ward JD, Choi SC, Marmarou A, Young HF. Outcome after severe head injury: relationship to mass lesions, diffuse injury, and ICP course in pediatric and adult patients. J Neurosurg. 1987;67(5):648-656. [DOI] [PubMed] [Google Scholar]
- 7.Jagannathan J, Okonkwo DO, Yeoh HK, et al. Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J Neurosurg Pediatr. 2008;2(4):240-249. [DOI] [PubMed] [Google Scholar]
- 8.Alali AS, Gomez D, Sathya C, et al. Intracranial pressure monitoring among children with severe traumatic brain injury. J Neurosurg Pediatr. 2015;16(5):1-10. [DOI] [PubMed] [Google Scholar]
- 9.Bennett TD, Riva-Cambrin J, Keenan HT, Korgenski EK, Bratton SL. Variation in intracranial pressure monitoring and outcomes in pediatric traumatic brain injury. Arch Pediatr Adolesc Med. 2012;166(7):641-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Cleve W, Kernic MA, Ellenbogen RG, et al. ; PEGASUS (Pediatric Guideline Adherence and Outcomes) Project . National variability in intracranial pressure monitoring and craniotomy for children with moderate to severe traumatic brain injury [published correction appears in Neurosurgery. 2014;74(1):E156]. Neurosurgery. 2013;73(5):746-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris KP, Forsyth RJ, Parslow RC, Tasker RC, Hawley CA; UK Paediatric Traumatic Brain Injury Study Group; Paediatric Intensive Care Society Study Group . Intracranial pressure complicating severe traumatic brain injury in children: monitoring and management. Intensive Care Med. 2006;32(10):1606-1612. [DOI] [PubMed] [Google Scholar]
- 12.Tilford JM, Simpson PM, Yeh TS, et al. Variation in therapy and outcome for pediatric head trauma patients. Crit Care Med. 2001;29(5):1056-1061. [DOI] [PubMed] [Google Scholar]
- 13.Alkhoury F, Kyriakides TC. Intracranial pressure monitoring in children with severe traumatic brain injury: National trauma data bank–based review of outcomes. JAMA Surg. 2014;149(6):544-548. [DOI] [PubMed] [Google Scholar]
- 14.Bennett TD, Dean JM, Keenan HT, McGlincy MH, Thomas AM, Cook LJ. Linked records of children with traumatic brain injury: probabilistic linkage without use of protected health information. Methods Inf Med. 2015;54(4):328-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rethinking Clinical Trials. Electronic health records–based phenotyping. https://sites.duke.edu/rethinkingclinicaltrials/ehr-phenotyping/. Accessed February 17, 2017.
- 16.Bennett TD, Dixon RR, Kartchner C, et al. Functional Status Scale in children with traumatic brain injury: a prospective cohort study. Pediatr Crit Care Med. 2016;17(12):1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett TD, DeWitt PE, Dixon RR, et al. Development and prospective validation of tools to accurately identify neurosurgical and critical care events in children with traumatic brain injury. Pediatr Crit Care Med. 2017;18(5):442-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett KS, DeWitt PE, Harlaar N, Bennett TD. Seizures in children with severe traumatic brain injury. Pediatr Crit Care Med. 2017;18(1):54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. 2005;162(10):1016-1023. [DOI] [PubMed] [Google Scholar]
- 20.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492-499. [DOI] [PubMed] [Google Scholar]
- 21.Kozar RA, Holcomb JB, Xiong W, Nathens AB. Are all deaths recorded equally? the impact of hospice care on risk-adjusted mortality. J Trauma Acute Care Surg. 2014;76(3):634-639. [DOI] [PubMed] [Google Scholar]
- 22.Flury BK, Riedwyl H. Standard distance in univariate and multivariate analysis. Am Stat. 1986;40(3):249-251. doi: 10.2307/2684560 [DOI] [Google Scholar]
- 23.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55. [Google Scholar]
- 25.McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403-425. [DOI] [PubMed] [Google Scholar]
- 26.Ridgeway G, McCaffrey D, Morral A, Griffin BA, Burgette L. Twang: toolkit for weighting and analysis of nonequivalent groups. https://cran.r-project.org/web/packages/twang/index.html. Accessed October 1, 2016.
- 27.Stürmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution—a simulation study. Am J Epidemiol. 2010;172(7):843-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lumley T. The ‘survey’ R package: analysis of complex survey samples. Version 3.31-5. https://cran.r-project.org/web/packages/survey/index.html. Accessed January 15, 2017.
- 29.Li L, Greene T. A weighting analogue to pair matching in propensity score analysis. Int J Biostat. 2013;9(2):215-234. [DOI] [PubMed] [Google Scholar]
- 30.VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. New York, NY: Oxford University Press; 2015. [Google Scholar]
- 31.R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. http://www.R-project.org/. Accessed October 17, 2016.
- 32.Allaire J, Cheng J, Xie Y, et al. rmarkdown: Dynamic documents for R. R package version 1.3. http://cran.r-project.org/web/packages/rmarkdown/index.html. Accessed January 21, 2017.
- 33.Xie Y. knitr: A general-purpose package for dynamic report generation in R. R package version 1.15.1. https://cran.r-project.org/web/packages/knitr/index.html. Accessed January 22, 2017.
- 34.Wickham H, Chang W. ggplot2: Create elegant data visualisations using the grammar of graphics. RStudio. R package version 2.2.1. https://cran.r-project.org/web/packages/ggplot2/index.html. Accessed February 12, 2017.
- 35.Chesnut RM, Temkin N, Carney N, et al. ; Global Neurotrauma Research Group . A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367(26):2471-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chesnut RM, Bleck TP, Citerio G, et al. A consensus-based interpretation of the benchmark evidence from South American trials: treatment of intracranial pressure trial. J Neurotrauma. 2015;32(22):1722-1724. [DOI] [PubMed] [Google Scholar]
- 37.Jensen RL, Hahn YS, Ciro E. Risk factors of intracranial pressure monitoring in children with fiberoptic devices: a critical review. Surg Neurol. 1997;47(1):16-22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods
eFigure 1. Standardized Differences for Propensity Model Covariates, Before and After Propensity Weighting
eFigure 2. ICP Monitoring Rates by Hospital
eFigure 3. Choice of Matching Weights Did Not Affect Primary Analysis
eFigure 4. Sensitivity Analyses for Robustness to Unmeasured Confounding
eTable 1. Variable Definitions
eTable 2. Propensity Model Covariates
eTable 3. Demographic and Hospital Characteristics
eTable 4. Injury Characteristics
eReferences.