This longitudinal study investigates whether positive parenting behaviors moderate the effects of socioeconomic disadvantage on brain development and adaptive functioning in adolescents.
Key Points
Question
Does positive parenting buffer the effects of neighborhood-level and family-level socioeconomic disadvantage on adolescent brain development?
Findings
In this longitudinal study of 166 adolescents, positive parenting moderated the effect of socioeconomic disadvantage on the development of the amygdala and prefrontal cortex. Prefrontal cortex development was in turn associated with school completion.
Meaning
While socioeconomic disadvantage has established negative mental health and other outcomes, the family environment may serve to mitigate some of these effects via effects on the developing brain.
Abstract
Importance
The negative effects of socioeconomic disadvantage on lifelong functioning are pronounced, with some evidence suggesting that these effects are mediated by changes in brain development. To our knowledge, no research has investigated whether parenting might buffer these negative effects.
Objective
To establish whether positive parenting behaviors moderate the effects of socioeconomic disadvantage on brain development and adaptive functioning in adolescents.
Design, Setting, and Participants
In this longitudinal study of adolescents from schools in Melbourne, Australia, data were collected at 3 assessments between 2004 and 2012. Data were analyzed between August 2016 and April 2017.
Exposures
Both family (parental income-to-needs, occupation, and education level) and neighborhood measures of socioeconomic disadvantage were assessed. Positive maternal parenting behaviors were observed during interactions in early adolescence.
Main Outcomes and Measures
Structural magnetic resonance imaging scans at 3 times (early, middle, and late adolescence) from ages 11 to 20 years. Global and academic functioning was assessed during late adolescence. We used linear mixed models to examine the effect of family and neighborhood socioeconomic disadvantage as well as the moderating effect of positive parenting on adolescent brain development. We used mediation models to examine whether brain developmental trajectories predicted functional outcomes during late adolescence.
Results
Of the included 166 adolescents, 86 (51.8%) were male. We found that neighborhood, but not family, socioeconomic disadvantage was associated with altered brain development from early (mean [SD] age, 12.79 [0.425] years) to late (mean [SD] age, 19.08 [0.460] years) adolescence, predominantly in the temporal lobes (temporal cortex: random field theory corrected; left amygdala: B, −0.237; P < .001; right amygdala: B, −0.209; P = .008). Additionally, positive parenting moderated the effects of neighborhood disadvantage on the development of dorsal frontal and lateral orbitofrontal cortices as well as the effects of family disadvantage on the development of the amygdala (occupation: B, 0.382; P = .004; income-to-needs: B, 27.741; P = .004), with some male-specific findings. The pattern of dorsal frontal cortical development in males from disadvantaged neighborhoods exposed to low maternal positivity predicted increased rates of school noncompletion (indirect effect, −0.018; SE, 0.01; 95% CI, −0.053 to −0.001).
Conclusions and Relevance
Our findings highlight the importance of neighborhood disadvantage in influencing brain developmental trajectories. Further, to our knowledge, we present the first evidence that positive maternal parenting might ameliorate the negative effects of socioeconomic disadvantage on frontal lobe development (with implications for functioning) during adolescence. Results have relevance for designing interventions for children from socioeconomically disadvantaged backgrounds.
Introduction
The effects of socioeconomic disadvantage on lifelong functioning are pronounced, with negative effects thought to begin in childhood or adolescence. A number of studies have sought to identify neurobiological factors that may mediate these effects and have documented associations between socioeconomic disadvantage (particularly poverty) and brain structure, with widespread alterations identified in the subcortex and frontal, parietal, and temporal cortices.
Numerous processes might affect the association between socioeconomic disadvantage and neurobiology. Socioeconomic disadvantage may affect parenting quality and, in turn, child brain development. Alternatively, caregiving behaviors may serve to protect children from the effects of disadvantageous experiences. Little is known about the buffering effects of caregivers on neurobiology in adolescence, a critical period of development that sets the stage for functioning across the lifespan. Also, the effect of socioeconomic disadvantage on structural brain development across adolescence has received little attention (eAppendix in the Supplement), and to our knowledge, no research has investigated whether positive parenting buffers these effects. Given the large numbers of children affected by socioeconomic disadvantage, such investigations are crucial for identifying modifiable targets for intervention.
The primary aim of this study was to investigate the potential buffering role of positive parenting on the effects of socioeconomic disadvantage on adolescent brain development. Our previous work demonstrated that positive parental behavior protects against psychopathology and promotes cognitive function and optimal brain developmental trajectories. We hypothesized that positive parenting would moderate the effects of socioeconomic disadvantage on brain development and that these brain developmental trajectories would, in turn, be associated with aspects of adolescent functioning relevant to trajectories of health and well-being.
Despite evidence that family-level vs neighborhood-level socioeconomic measures have different effects on neurobiology and other outcomes in adults, to our knowledge, no previous studies in adolescents have investigated their differential effects. A secondary aim of this study was to assess the neurodevelopmental effects of family (parental income-to-needs, occupation, and education level) and neighborhood socioeconomic disadvantage. Finally, given evidence that environmental factors may have sex-specific effects on brain development, we investigated sex differences in all analyses.
Methods
Participants and Recruitment
The sample was derived from a larger Australian longitudinal cohort of 2453 adolescents. Briefly, 177 adolescents from the general community in Melbourne, Australia, completed magnetic resonance imaging (MRI) scans at 1 to 3 times (at average ages of 13, 17, and 19 years). At the baseline assessment, adolescents also participated in mother-child interaction tasks. Based on visual inspection of processed MRI data, 9 participants were excluded owing to poor MRI image quality and parcellation. In addition, 2 participants with full-scale IQ scores lower than 70 were excluded from analyses. Following exclusions, 166 participants aged 11 to 20 years were available for analysis. Of these, 73 participants (44.0%) had 3 scans, 55 (33.1%) had 2 scans, and 38 (22.9%) had 1 scan (Table). The research was approved by the human research ethics committee at The University of Melbourne, and written informed consent was obtained from each child and a parent/guardian.
Table. Sample Demographic Characteristics.
| Characteristic | Mean (SD) | ||
|---|---|---|---|
| Sex | Total (N = 166) | ||
| Male (n = 86) | Female (n = 86) | ||
| T1 age, y | 12.83 (0.452) | 12.77 (0.394) | 12.79 (0.425) |
| T2 age, y | 16.70 (0.559) | 16.71 (0.480) | 16.70 (0.518) |
| T3 age, y | 19.10 (0.507) | 19.05 (0.413) | 19.08 (0.460) |
| Delay time from T1 to T2, y | 3.80 (0.158) | 3.87 (0.237) | 3.83 (0.204) |
| Delay time from T2 to T3, y | 2.40 (0.177) | 2.35 (0.251) | 2.38 (0.219) |
| Estimated FSIQ | 107.96 (15.51) | 107.75 (15.80) | 107.86 (15.60) |
| ANU parent occupation | 58.14 (20.42) | 58.01 (21.36) | 58.08 (20.80) |
| ANU parent educationa | 50.27 (14.55) | 49.86 (15.94) | 50.07 (15.18) |
| INRb | 1.18 (0.76) | 1.57 (1.15) | 1.35 (0.97) |
| IRSDc | 38.52 (26.31) | 35.54 (27.16) | 37.07 (26.69) |
| Maternal positivity during PSI | 1.75 (0.60) | 1.68 (0.61) | 1.71 (0.62) |
| Maternal positivity during EPI | 2.28 (0.451) | 2.29 (0.450) | 2.29 (0.449) |
| CGAS | 74.27 (12.698) | 75.46 (13.94) | 74.86 (13.289) |
| ATAR | 74.59 (16.975) | 78.15 (16.213) | 76.43 (16.590) |
| DNCYr12, No./total No. (%) | 13/64 (20) | 8/63 (13) | 21/127 (16.5) |
Abbreviations: ANU, Australian National University Four; ATAR, Australian Tertiary Admission Rank; CGAS, Child Global Assessment Scale; DNCYr12, did not complete year 12/senior year school; EPI, event-planning interaction; FSIQ, full-scale IQ; INR, income-to-needs ratio; IRSD, Index of Relative Socioeconomic Disadvantage (ie, neighborhood disadvantage; note, higher scores = higher disadvantage); PSI, problem-solving interaction; T1, time 1; T2, time 2; T3, time 3.
Thirty-seven fathers (22.3%) and 46 mothers (27.7%) of 166 total participants did not complete high school.
Significant sex difference (T, −2.110; df, 164; P = .04).
IRSD range was 1 to 100. Eight of 166 participants (4.8%) fell into the top IRSD decile (ie, most disadvantaged) and 23 (13.9%) fell into the bottom decile (ie, least disadvantaged) (eFigure 1 in the Supplement).
Socioeconomic Disadvantage
Four measures of socioeconomic disadvantage were used. First, parental occupation status was assessed based on the Australian National University Four scale, which draws on national census data (compulsory for all Australian households) and classifies occupations by skill level and occupation type. Second, parental education level was also assessed using the Australian National University Four scale. Note that for 2-parent families, the highest ranked occupation and education level was used. Third, income-to-needs was measured based on reported family income relative to the relevant Australian poverty line for household size. Finally, we measured neighborhood socioeconomic disadvantage based on the 2006 Socio-Economic Indexes for Areas Index of Relative Socioeconomic Disadvantage, a region-based socioeconomic index that summarizes a range of information from national census data about the economic and social conditions of people and households within small geographical areas (approximately 250 homes). Percentiles were used for analyses, and high-percentile scores indicate relatively greater neighborhood disadvantage (eMethods 1 and eTable 1 in the Supplement).
Family Interaction Assessment and Measures
Adolescents and mothers completed laboratory-based interactions at the first assessment. Mother-adolescent dyads completed two 20-minute interaction tasks that were video recorded for subsequent coding. An event-planning interaction was completed first, followed by a problem-solving interaction (PSI). The Living in Family Environments coding system was used to code verbal and nonverbal maternal behavior from the video-recorded interactions. A positive behavior construct was developed that included all behaviors with happy or caring affect, as well as approving, validating, affectionate, or humorous comments made with neutral affect. Measures of frequency of maternal positive behavior during the PSI and event-planning interaction were used separately in analyses (eMethods 2 in the Supplement).
MRI Acquisition and Processing
Note that different scanners were used at the first (Signa Horizon LX Human; General Electric Company) vs second and third (MAGNETOM Trio, A Tim System; Siemens) imaging times. An interscanner reliability assessment showed no interscanner bias (eFigure 2 in the Supplement). Cortical reconstruction was performed using the longitudinal stream of FreeSurfer version 5.3. Cortical thickness values were automatically quantified within FreeSurfer on a vertex-wise basis. Subcortical volumes were estimated using an automated subcortical segmentation procedure (eMethods 3 in the Supplement).
Outcome Measures
The Children’s Global Assessment Scale was administered via interview during late adolescence to assess current global functioning (eMethods 4 in the Supplement). During late adolescence, information was also collected pertaining to academic functioning via interview, including 12th grade completion and Australian Tertiary Admission Rank scores (a percentile ranking of high school graduates’ final assessment performance).
Statistical Analysis
For cortical thickness, vertex-wise analyses were conducted using SurfStat, a statistical toolbox for MATLAB (http://www.math.mcgill.ca/keith/surfstat/). Linear mixed models were used to assess associations between socioeconomic disadvantage measures and cortical thickness development with age. Mixed-effects models permit the use of all available data in unbalanced data sets, thus improving the statistical power to detect effects. For each vertex of the cortical reconstruction, we fitted full analytic models to investigate the interaction between socioeconomic disadvantage and age. Subsequent analysis investigated whether there were sex differences in the interactive effect of social disadvantage and age on cortical thickness (eMethods 5 in the Supplement). Although estimated full-scale IQ (assessed at baseline using a short form of the Wechsler Intelligence Scale for Children) was associated with indices of social disadvantage, it was not included as a covariate in analyses owing to methodological and theoretical issues with doing so. Cortical analyses were corrected for multiple comparisons at a whole-brain level using a random field theory at 0.01 (to account for the 4 types of disadvantage) and a cluster-forming vertex-wise threshold of P < .001.
For subcortical volumes (left and right amygdala, hippocampus, caudate, putamen, pallidum, thalamus, and nucleus accumbens), analyses were conducted in R version 3.3.1 (The R Foundation), and results were considered significant at P < .01 (corrected for multiple comparisons [14 regions × 4 disadvantage variables] using a false discovery rate). Similar linear mixed models as described for cortical thickness were used to analyze the data, with separate models used for each region.
Moderating Effect of Positive Maternal Behavior
For each vertex of the cortical reconstruction and each subcortical region of interest, we fitted full analytic (linear-mixed) models to investigate the interaction between socioeconomic disadvantage, positive maternal behavior, and age. Separate models were run for positive behavior during the PSI and event-planning interaction. Subsequent analysis investigated possible sex differences. Analyses were corrected for multiple comparisons.
All models were run with mean-centered variables. For all models yielding significant results, models were rerun controlling for the other socioeconomic disadvantage variables (and their interactions with age, sex, and maternal positive behavior where relevant).
Outcomes
For any brain regions where development was predicted by socioeconomic disadvantage or its interaction with positive parenting, mediation or moderated mediation analyses were used to test whether brain development in turn predicted late adolescent outcomes (Children’s Global Assessment Scale score, school completion, and Australian Tertiary Admission Rank score). A bootstrapping method was used to test the significance of indirect (mediation) effects. Five thousand resamples were taken, and 95% CIs were used. Significant mediation is indicated if the CIs do not contain zero.
Results
Demographic characteristics are shown in the Table. Associations between demographic and other variables of interest are shown in eTable 2 in the Supplement.
Effects of Socioeconomic Disadvantage on Brain Development
Parental Education, Occupation, and Income-to-Needs
There were no significant main effects or age-moderated or sex-moderated effects of parental education, occupation, or income-to-needs on cortical thickness or subcortical volumes.
Neighborhood Disadvantage
Greater neighborhood disadvantage was associated with increased thickness in the right middle temporal gyrus and temporal pole across age (eFigure 3, eTable 3, and eTable 4 in the Supplement). However, longitudinal effects were considerably more pronounced. In regions including the bilateral middle and inferior temporal gyri, temporal pole, fusiform gyrus, and right parahippocampal gyrus, where we have previously reported normative thickening longitudinally, greater neighborhood disadvantage was associated with relatively increased cortical thickening (Figure 1 and Figure 2). Greater neighborhood disadvantage was also associated with greater increases in left and right amygdala volume longitudinally (left: B, −0.237; SE, 0.062; P < .001; right: B, −0.209; SE, 0.078; P = .008). Sex moderated the association between neighborhood disadvantage and right amygdala development (B, 0.455; SE, 0.154; P = .005). Follow-up analyses showed that this effect (found for the whole sample) was significant in males (B, −0.457; SE, 0.122; P < .001) but not females (P > .01) (Figure 2). Note that controlling for family disadvantage (and its interaction with age and sex) did not change results.
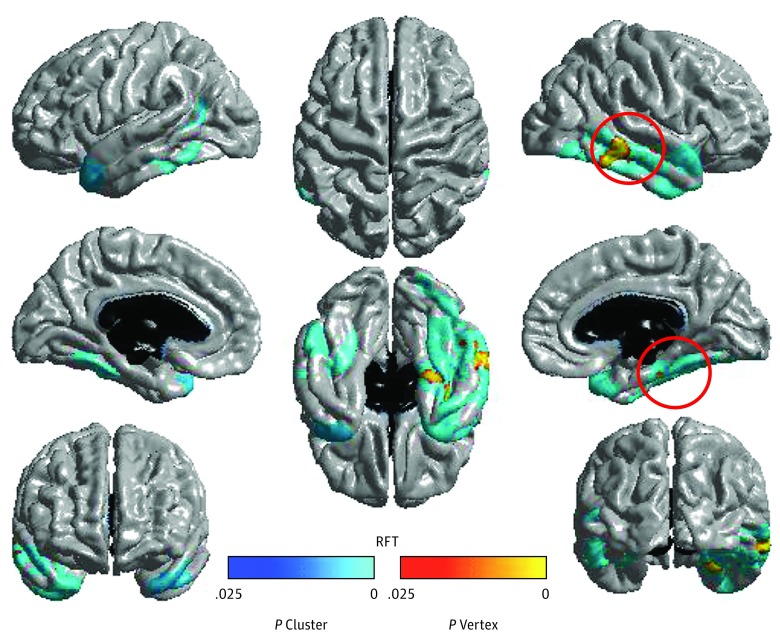
Figure 1. Clusters With Significant Age × Neighborhood Disadvantage Interactions in Cortical Thickness.
Clusters with significant age × neighborhood disadvantage interactions in cortical thickness (random field theory [RFT] corrected, P < .01). Relatively greater neighborhood disadvantage was associated with increased thickening in the temporal lobes with age. Plots of the interactions for the clusters that are circled can be found in Figure 2.
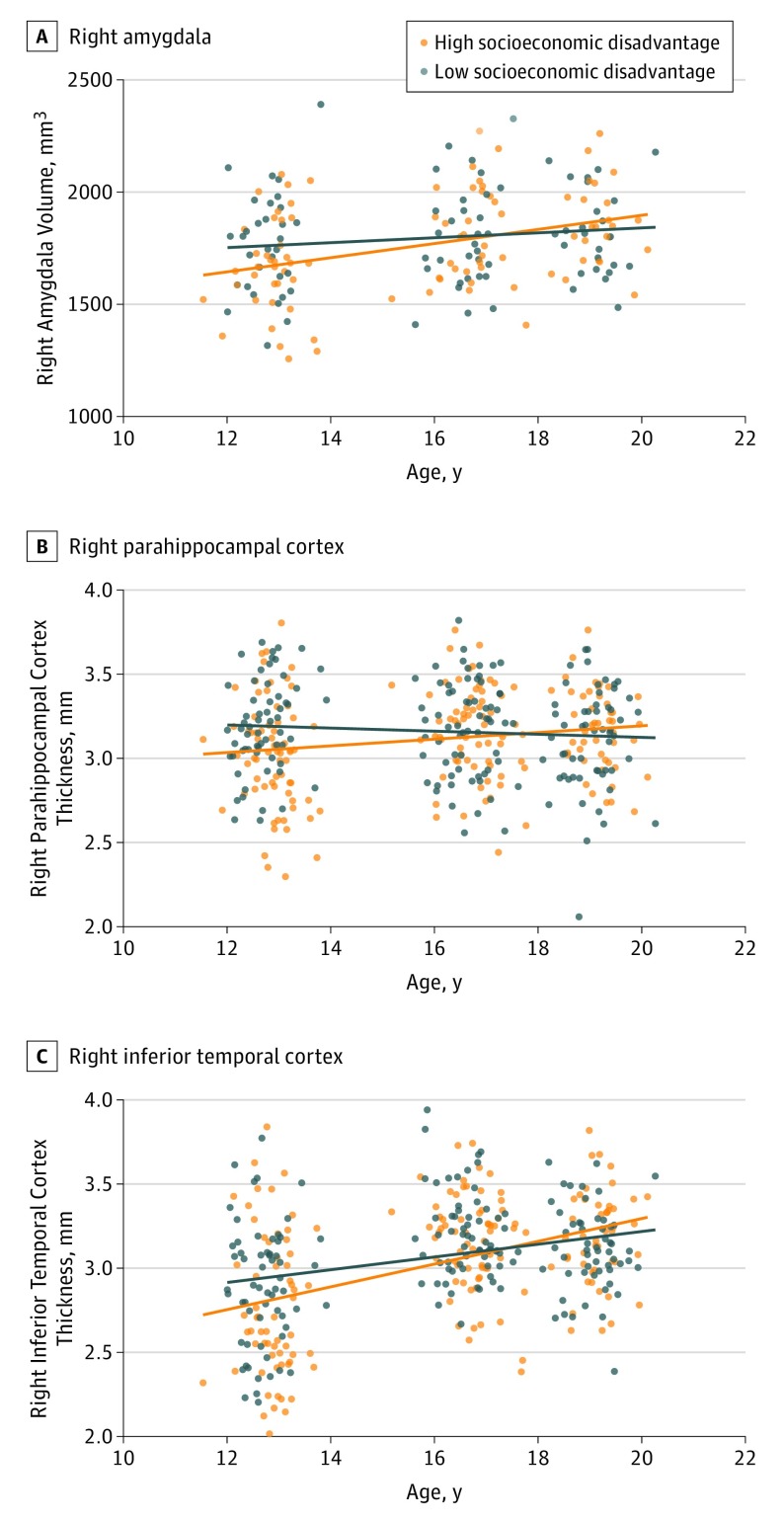
Figure 2. Associations Between Neighborhood Disadvantage and Age-Related Structural Brain Change.
Brain developmental trajectories are depicted for right amygdala volume (A), right parahippocampal cortex thickness (B), and right inferior temporal cortex thickness (C) for adolescents with relatively high and low neighborhood disadvantage. The slopes represent the average trajectories for high and low groups based on a median split of the Index of Relative Socioeconomic Disadvantage. Note that the amygdala finding is specific to males. We found normative thickening of temporal cortical regions in the sample; while this finding is inconsistent with some previous reports, it is consistent with others. Individual developmental trajectories are depicted in eFigure 4 in the Supplement.
Moderating Effect of Positive Maternal Behavior on Association Between Disadvantage and Brain Development
Parental Education, Occupation, and Income-to-Needs
Positive maternal behavior during the PSI moderated the effect of parental occupation on development of the left amygdala (B, 0.382; SE, 0.132; P = .004). In adolescents with parents with relatively low-status occupations (B, −7.631; SE, 3.738; P = .04), higher (relative to lower) levels of positive maternal behavior were associated with attenuated left amygdala growth. In adolescents with parents with high-status occupations (B, 7.948; SE, 3.919; P = .05), higher (relative to lower) levels of positive maternal behavior were associated with accelerated left amygdala growth (Figure 3). Positive maternal behavior during the PSI also moderated the effect of parental income-to-needs on development of the right amygdala in a sex-dependent manner (B, 27.741; SE, 9.544; P = .004). Separate analyses by sex showed that the effect was significant in males (B, −21.685; SE, 8.484; P = .01) but not females (B, 6.208; SE, 4.920; P = .21). In boys with relatively low income-to-needs, higher (relative to lower) levels of positive maternal behavior were associated with relatively increased amygdala volume longitudinally (Figure 3). Note that controlling for neighborhood disadvantage (and its interaction with age and positive maternal behavior) did not change results.
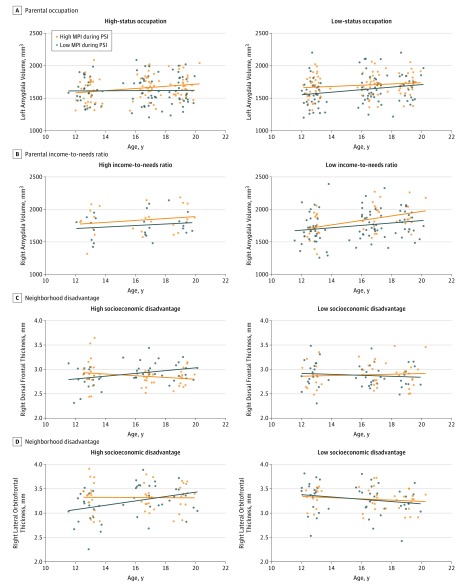
Figure 3. Effects of Positive Maternal Behavior on Associations Between Social Disadvantage and Age-Related Structural Brain Change.
Effect of positive maternal behavior on brain developmental trajectories in the left amygdala for adolescents with relatively high-status and low-status parental occupations (A), right amygdala for adolescent males with relatively high and low parental income-to-needs ratio (B), and right dorsal frontal thickness (C) and right lateral orbitofrontal thickness (D) in males with relatively high and low neighborhood disadvantage by the Index of Relative Socioeconomic Disadvantage. Slopes represent average trajectories for relatively high and low groups based on a median split of the data. MPI indicates maternal positive interpersonal behavior; PSI, problem-solving interaction. Individual developmental trajectories are depicted in eFigure 5 in the Supplement.
Neighborhood Disadvantage
There were significant sex differences in the association between maternal positive behavior during the PSI and its moderating effect in attenuating the effect of neighborhood disadvantage on brain development. Specifically, this was associated with development of thickness in the right lateral orbitofrontal cortex (lOFC) and dorsal frontal cortex, regions for which we have previously reported normative thinning longitudinally (Figure 4). Similar effects were found for maternal positive behavior during the event-planning interaction (eFigure 6 in the Supplement). Note that controlling for family disadvantage (and its interaction with age, sex, and positive maternal behavior) did not change results. There were no effects for subcortical volumes.
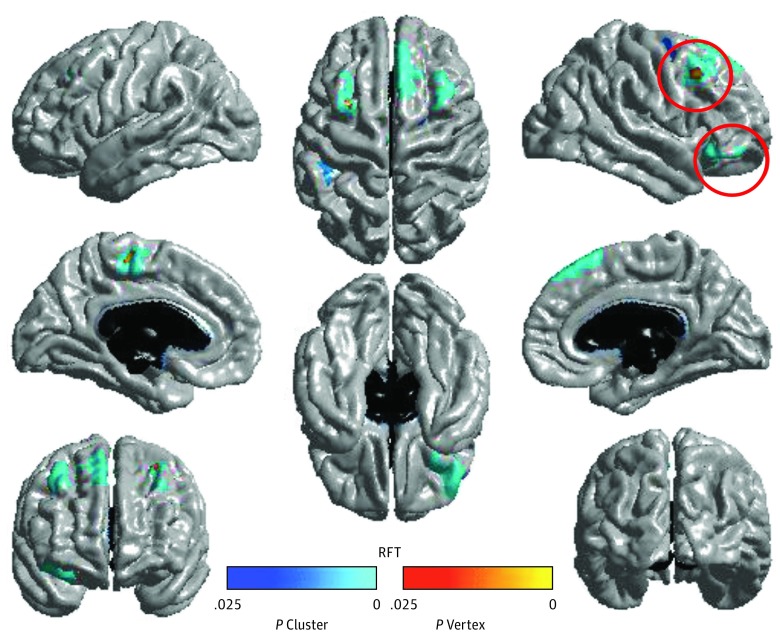
Figure 4. Clusters With Significant Age × Sex × Positive Maternal Behavior × Neighborhood Disadvantage Interactions in Cortical Thickness.
Clusters with significant age × sex × positive maternal behavior × neighborhood disadvantage interactions in cortical thickness (random field theory [RFT] corrected, P < .01). Positive maternal behavior (during the problem-solving task) moderated the association between neighborhood disadvantage and development of cortical thickness in males. Plots of the interactions for the clusters that are circled can be found in Figure 3.
After extracting average thickness from the right lOFC and dorsal frontal clusters, follow-up analyses showed that effects were significant in males (lOFC: B, 0.001; SE, 0.0004; P = .005; dorsal frontal: B, 0.001; SE, 0.0004; P = .002) but not females (lOFC: B, −0.0005; SE, 0.0003; P = .08; dorsal frontal: B, −0.0007; SE, 0.0004; P = .07). In males, for both lOFC and dorsal frontal cortex, the effect of positive maternal behavior was more pronounced in those with higher levels of neighborhood disadvantage (lOFC: B, −0.032; SE, 0.014; P = .03; dorsal frontal: B, −0.046; SE, 0.012; P < .001) compared with those with lower levels of neighborhood disadvantage (lOFC: B, 0.013; SE, 0.012; P = .22; dorsal frontal: B, 0.028; SE, 0.013; P = .03) (Figure 3). Specifically, positive maternal behavior was associated with greater relative thinning with age in male adolescents within the context of higher neighborhood disadvantage (such that trajectories more closely resembled those with low levels of disadvantage).
Predicting Late Adolescent Outcomes
A moderated mediation model run in males indicated that development of the right dorsal frontal cortex significantly mediated the association between neighborhood disadvantage, positive maternal behavior during the PSI, and school noncompletion (Figure 3C and Figure 4). Specifically, in adolescent males with relatively high social disadvantage and low positive maternal parenting, change in cortical thickness with age was associated with school noncompletion (indirect effect for low maternal positive behavior, −0.018; SE, 0.014; 95% CI, −0.053 to −0.001). No other brain development trajectories associated with socioeconomic disadvantage or its interaction with positive maternal behavior significantly indirectly predicted other late adolescent outcomes (ie, Children’s Global Assessment Scale score or Australian Tertiary Admission Rank score).
Discussion
In this longitudinal study of adolescents aged 11 to 20 years, we found that neighborhood but not family socioeconomic disadvantage was associated with brain development, predominantly in the temporal lobes. Further, positive parenting moderated the effects of socioeconomic disadvantage on adolescent development of the amygdala and frontal cortex, particularly in males. In the context of relatively high socioeconomic disadvantage, positive parenting predicted developmental trajectories of the dorsal frontal and lateral orbitofrontal cortices and amygdala that resembled those in adolescents with lower levels of socioeconomic disadvantage. The pattern of dorsal frontal cortical development in adolescent males with high social disadvantage and low maternal positivity, which was characterized by reduced cortical thinning, in turn predicted increased rates of school noncompletion.
Neighborhood socioeconomic disadvantage was found to have more prominent effects on brain cortical development than did family indicators of disadvantage. This is consistent with some research that has found associations between neighborhood socioeconomic disadvantage and cognitive outcomes independent of family indices of disadvantage.
High neighborhood disadvantage was also associated with relatively increased volume of the amygdala with age and increased thickening of the temporal cortex with age, including the middle and inferior temporal gyri and parahippocampal and fusiform gyri. These temporal lobe findings are consistent with previous reports linking socioeconomic disadvantage and brain structure in children and adolescents (including, to our knowledge, the only other study to date to investigate the association between socioeconomic disadvantage and cortical thickness development) and with other research showing early stress to affect temporal lobe structure. We have previously reported normative thickening of the temporal cortex in this sample; while consistent with some research, this finding is at odds with much research showing normative temporal lobe thinning across adolescence.
Given the current inconsistencies in the literature and the fact that the development of these temporal regions was not associated with late adolescent outcomes in our analyses, the functional significance of our findings are unclear. Nevertheless, given the known functions of these brain regions, findings suggest that relatively high neighborhood disadvantage may affect responsivity to stress/threat processing, memory, and/or language. Indeed, localized findings within the temporal lobe are consistent with reports linking socioeconomic disadvantage to functions ascribed to these brain regions. Socioeconomic disadvantage has been associated with poorer language development, believed to result from reduced environmental exposure to language. Thus, our findings identify a plausible neural mechanism that could partially explain the enduring nature of the effects of socioeconomic disadvantage on stress responsivity, language deficits, and other temporal lobe–mediated functions.
While other research suggests that socioeconomic disadvantage may exert its effects on brain development via parenting behavior, we found that socioeconomic disadvantage was not associated with our measure of positive parenting but rather that positive maternal parenting had a moderating effect on the association between family disadvantage and amygdala development and a buffering effect on the effect of neighborhood disadvantage on cortical development in males. While amygdala trajectories did not predict any of the functional outcomes examined in this study, prior work suggests that the pattern of amygdala development observed here in adolescents with relatively high occupational disadvantage and low levels of maternal positive behavior (ie, increased volume with age) may be associated with emotion dysregulation. The pattern was reversed in males in relation to income-to-needs.
Regarding our parenting findings, the frontal cortical regions implicated continue to show maturational change during adolescence (normative thinning across this age period is a consistent finding both in our work and in other samples) and are important for emotion processing and executive functioning. Much research supports a negative effect of socioeconomic disadvantage on executive functions and on emotion regulation processes. Our findings suggest that positive parenting practices might protect male children who have grown up in relatively socioeconomically disadvantaged neighborhoods from experiencing such environmentally related neural changes. The results of mediation analyses supported an interpretation of accelerated frontal cortical thinning as being one neural mechanism by which positive parenting may buffer the effects of high neighborhood disadvantage on poor functional outcomes (eg, school noncompletion). Of note, we and others have found accelerated cortical thinning during adolescence to be associated with more adaptive individual characteristics and have suggested that accelerated cortical thinning may be due to dynamic synaptic reorganization and/or continued intracortical myelination.
It is unclear why some of our findings were male-specific. One possibility is that the male frontal cortex may be more susceptible to positive environmental influence during adolescence owing to its relative immaturity compared with females. Further, given evidence that deficits in frontal lobe–mediated executive functions are associated with behavioral problems, which is in turn associated with school dropout in males, it is possible that our findings reflect a sociobiological pathway specific to males.
Limitations
Our study had limitations, including single assessments of parenting and socioeconomic disadvantage measures, our inability to assess effects earlier than adolescence, and the use of 2 MRI scanners.
Conclusions
Despite the limitations of this work, to our knowledge, this study presents the first evidence that positive parenting practices might buffer the negative effects of socioeconomic disadvantage on brain development and specific aspects of adaptive functioning during adolescence. Our findings also highlight the importance of disadvantage at the neighborhood level in influencing brain development trajectories. Importantly, in this study, socioeconomic disadvantage was not associated with our measures of positive parenting. As such, we have identified a type of parenting behavior that is not intrinsically tied to disadvantage and thus represents a modifiable target for intervention to scaffold optimal brain development within the context of socioeconomic disadvantage.
eAppendix. Summary of literature on the effect of socioeconomic disadvantage on structural brain development across adolescence.
eMethods 1. Measures of socioeconomic disadvantage.
eMethods 2. Family interaction assessment and measures.
eMethods 3. MRI acquisition and analysis and interscanner reliability.
eMethods 4. Children’s Global Assessment Scale.
eMethods 5. Statistical analysis.
eTable 1. Index of Relative Socioeconomic Disadvantage (IRSD) variable loadings, weights, and descriptions (SEIFA 2006).
eTable 2. Pearson bivariate correlations between variables.
eTable 3. Clusters where cortical thickness and its development was significantly associated with neighborhood disadvantage (random field theory cluster corrected, P < .013).
eTable 4. Linear mixed effects models where there were significant effects of disadvantage (main or in interaction with age, sex, and/or maternal positive behavior).
eFigure 1. Histogram of Index of Relative Socioeconomic Disadvantage (IRSD) scores in the sample (higher scores indicate greater disadvantage).
eFigure 2. Proportion of participants for whom ROI thickness increased (light gray), decreased (dark gray), or did not change (mid gray), based on interscanner reliability analysis.
eFigure 3. Neighborhood disadvantage associated with increased right middle temporal lobe thickness across age.
eFigure 4. Individual developmental trajectories of the right amygdala, parahippocampal, and inferior temporal in adolescents with relatively high and low neighborhood disadvantage.
eFigure 5. Individual development trajectories of regions associated with an interaction between positive maternal behavior and different measures of socioeconomic disadvantage.
eFigure 6. Sex differences in the moderating effect of positive maternal behavior (during the event-planning interaction) on the association between neighborhood disadvantage and development of cortical thickness.
eReferences.
References
- 1.Johnson SB, Riis JL, Noble KG. State of the art review: poverty and the developing brain. Pediatrics. 2016;137(4). doi: 10.1542/peds.2015-3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luby J, Belden A, Botteron K, et al. . The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 2013;167(12):1135-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blair C, Raver CC. Poverty, stress, and brain development: new directions for prevention and intervention. Acad Pediatr. 2016;16(3, suppl):S30-S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9(2):69-74. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz O, Simmons JG, Whittle S, et al. . Affective parenting behaviors, adolescent depression, and brain development: a review of findings from the Orygen Adolescent Development Study [published online November 16, 2016]. Child Dev Perspect. doi: 10.1111/cdep.12215 [DOI] [Google Scholar]
- 6.Levin H, Belfield C, Muennig P, Rouse C. The Costs and Benefits of an Excellent Education for All of America’s Children. New York, NY: Teachers College, Columbia University; 2007. [Google Scholar]
- 7.Wight RG, Aneshensel CS, Miller-Martinez D, et al. . Urban neighborhood context, educational attainment, and cognitive function among older adults. Am J Epidemiol. 2006;163(12):1071-1078. [DOI] [PubMed] [Google Scholar]
- 8.Whittle S, Simmons JG, Dennison M, et al. . Positive parenting predicts the development of adolescent brain structure: a longitudinal study. Dev Cogn Neurosci. 2014;8:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yap MB, Allen NB, O’Shea M, di Parsia P, Simmons JG, Sheeber L. Early adolescents’ temperament, emotion regulation during mother-child interactions, and depressive symptomatology. Dev Psychopathol. 2011;23(1):267-282. [DOI] [PubMed] [Google Scholar]
- 10.Jones FL, McMillan J. Scoring occupational categories for social research: a review of current practice, with Australian examples. Work Employ Soc. 2001;15(3):539-563. doi: 10.1177/09500170122119147 [DOI] [Google Scholar]
- 11.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaffer D, Gould MS, Brasic J, et al. . A children’s global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40(11):1228-1231. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons RD, Hedeker D, DuToit S. Advances in analysis of longitudinal data. Annu Rev Clin Psychol. 2010;6:79-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wechsler D. Wechsler Intelligence Scale for Children. 4th ed San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 15.Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009;15(3):331-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165-1188. doi: 10.1214/aos/1013699998 [DOI] [Google Scholar]
- 17.Vijayakumar N, Allen NB, Youssef G, et al. . Brain development during adolescence: a mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp. 2016;37(6):2027-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandekar SN, Shinohara RT, Raznahan A, et al. . Topologically dissociable patterns of development of the human cerebral cortex. J Neurosci. 2015;35(2):599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hair NL, Hanson JL, Wolfe BL, Pollak SD. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 2015;169(9):822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jednoróg K, Altarelli I, Monzalvo K, et al. . The influence of socioeconomic status on children’s brain structure. PLoS One. 2012;7(8):e42486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccolo LR, Merz EC, He X, Sowell ER, Noble KG; Pediatric Imaging, Neurocognition, Genetics Study . Age-related differences in cortical thickness vary by socioeconomic status. PLoS One. 2016;11(9):e0162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Brito SA, Viding E, Sebastian CL, et al. . Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J Child Psychol Psychiatry. 2013;54(1):105-112. [DOI] [PubMed] [Google Scholar]
- 23.Mutlu AK, Schneider M, Debbané M, Badoud D, Eliez S, Schaer M. Sex differences in thickness, and folding developments throughout the cortex. Neuroimage. 2013;82:200-207. [DOI] [PubMed] [Google Scholar]
- 24.Tamnes CK, Walhovd KB, Dale AM, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Brain development and aging: overlapping and unique patterns of change. Neuroimage. 2013;68:63-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120-126. [DOI] [PubMed] [Google Scholar]
- 26.Acheson DJ, Hagoort P. Stimulating the brain’s language network: syntactic ambiguity resolution after TMS to the inferior frontal gyrus and middle temporal gyrus. J Cogn Neurosci. 2013;25(10):1664-1677. [DOI] [PubMed] [Google Scholar]
- 27.Creem SH, Proffitt DR. Defining the cortical visual systems: “what”, “where”, and “how.” Acta Psychol (Amst). 2001;107(1-3):43-68. [DOI] [PubMed] [Google Scholar]
- 28.Hoff E. How social contexts support and shape language development. Dev Rev. 2006;26(1):55-88. doi: 10.1016/j.dr.2005.11.002 [DOI] [Google Scholar]
- 29.Chen BK, Sassi R, Axelson D, et al. . Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry. 2004;56(6):399-405. [DOI] [PubMed] [Google Scholar]
- 30.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47(3-4):296-312. [DOI] [PubMed] [Google Scholar]
- 31.Ursache A, Noble KG. Neurocognitive development in socioeconomic context: multiple mechanisms and implications for measuring socioeconomic status. Psychophysiology. 2016;53(1):71-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Front Hum Neurosci. 2012;6:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ducharme S, Albaugh MD, Hudziak JJ, et al. ; Brain Development Cooperative Group . Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb Cortex. 2014;24(11):2941-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ensminger ME, Slusarcick AL. Paths to high school graduation or dropout: a longitudinal study of a first-grade cohort. Sociol Educ. 1992;65(2):95-113. doi: 10.2307/2112677 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Summary of literature on the effect of socioeconomic disadvantage on structural brain development across adolescence.
eMethods 1. Measures of socioeconomic disadvantage.
eMethods 2. Family interaction assessment and measures.
eMethods 3. MRI acquisition and analysis and interscanner reliability.
eMethods 4. Children’s Global Assessment Scale.
eMethods 5. Statistical analysis.
eTable 1. Index of Relative Socioeconomic Disadvantage (IRSD) variable loadings, weights, and descriptions (SEIFA 2006).
eTable 2. Pearson bivariate correlations between variables.
eTable 3. Clusters where cortical thickness and its development was significantly associated with neighborhood disadvantage (random field theory cluster corrected, P < .013).
eTable 4. Linear mixed effects models where there were significant effects of disadvantage (main or in interaction with age, sex, and/or maternal positive behavior).
eFigure 1. Histogram of Index of Relative Socioeconomic Disadvantage (IRSD) scores in the sample (higher scores indicate greater disadvantage).
eFigure 2. Proportion of participants for whom ROI thickness increased (light gray), decreased (dark gray), or did not change (mid gray), based on interscanner reliability analysis.
eFigure 3. Neighborhood disadvantage associated with increased right middle temporal lobe thickness across age.
eFigure 4. Individual developmental trajectories of the right amygdala, parahippocampal, and inferior temporal in adolescents with relatively high and low neighborhood disadvantage.
eFigure 5. Individual development trajectories of regions associated with an interaction between positive maternal behavior and different measures of socioeconomic disadvantage.
eFigure 6. Sex differences in the moderating effect of positive maternal behavior (during the event-planning interaction) on the association between neighborhood disadvantage and development of cortical thickness.
eReferences.