Abstract
Radiation may affect essential functions and behaviors such as locomotion, feeding, learning and memory. Although whole-body irradiation has been shown to reduce motility in the nematode Caenorhabditis elegans, the detailed mechanism responsible for this effect remains unknown. Targeted irradiation of the nerve ring responsible for sensory integration and information processing would allow us to determine whether the reduction of motility following whole-body irradiation reflects effects on the central nervous system or on the muscle cells themselves. We therefore addressed this issue using a collimating microbeam system. However, radiation targeting requires the animal to be immobilized, and previous studies have anesthetized animals to prevent their movement, thus making it impossible to assess their locomotion immediately after irradiation. We developed a method in which the animal was enclosed in a straight, microfluidic channel in a polydimethylsiloxane chip to inhibit free motion during irradiation, thus allowing locomotion to be observed immediately after irradiation. The head region (including the central nervous system), mid region around the intestine and uterus, and tail region were targeted independently. Each region was irradiated with 12 000 carbon ions (12C; 18.3 MeV/u; linear energy transfer = 106.4 keV/μm), corresponding to 500 Gy at a φ20 μm region. Motility was significantly decreased by whole-body irradiation, but not by irradiation of any of the individual regions, including the central nervous system. This suggests that radiation inhibits locomotion by a whole-body mechanism, potentially involving motoneurons and/or body-wall muscle cells, rather than affecting motor control via the central nervous system and the stimulation response.
Keywords: region-specific irradiation, microbeam, carbon ions, behavior chip, motility, C. elegans
INTRODUCTION
Animals are exposed to radiation from natural and artificial sources, cosmic rays, and nuclear accidents. Radiation may affect essential functions and behaviors such as locomotion, feeding, learning and memory, and it is therefore necessary to understand the effects of radiation exposure on these aspects. Locomotory behavior is vital for securing food and escaping from harmful environments. Previous experiments showed that motility (locomotion) in the nematode Caenorhabditis elegans was reduced by whole-body irradiation with either gamma rays or carbon ions, in a dose-dependent manner [1–3]. Furthermore, hydrogen peroxide, which is a reactive oxygen species (ROS) produced by radiation exposure, was shown to be potentially involved in this reduced motility [2]. However, whether the reduction in motility was induced by irradiation of a specific tissue/region remains unknown.
C. elegans is a nematode, ~1 mm long, comprising 959 cells, including 302 neurons, in adult hermaphrodites [4]. Locomotion in C. elegans is accomplished by the body-wall muscles located from the head to the tail, which produce snake-like crawling involving forward and backward motion and turns, controlled by motoneurons in the ventral nerve cord [4–6]. In contrast, most of the sensory integration takes place in a nerve ring wrapped round the pharynx in the head [6]. Targeted irradiation of the nerve ring alone, which is responsible for sensory integration and information processing, would allow us to determine whether the reduction in motility following whole-body irradiation reflects its effect on the central nervous system.
In the present study, we aimed to address this issue using a collimating microbeam system [7], which has previously been used as a powerful tool for investigating the effects of localized irradiation in various biological targets [8–14]. Using this irradiation system, we previously succeeded in irradiating a limited area in C. elegans, such as the tip region of the gonad, and found that region-specific irradiation induced DNA damage, which in turn led to cell cycle arrest and apoptosis [15]. Microbeam irradiation of individual C. elegans or embryos has subsequently been conducted at several facilities [16–19]. However, in these previous studies, C. elegans individuals were immobilized by anesthesia prior to irradiation, making it impossible to observe any immediate effects of region-specific microbeam irradiation on motility, owing to the nerve-calming effects of the anesthesia. There is currently no evidence for the effects of radiation on specific tissues, including the nervous system, in active C. elegans individuals.
We therefore established a novel method of delivering microbeam irradiation to individual C. elegans without anesthesia, in which the animal's neural activity was not constrained. We focused on the use of a polydimethylsiloxane (PDMS) microfluidic chip, a so-called behavior chip [20, 21], developed for behavioral assays and neural activity imaging. This system has the advantage of not requiring the animal to be anesthetized, because it is effectively stationary. We therefore inhibited free motion during irradiation by enclosing an individual C. elegans in a straight channel on a PDMS microfluidic chip in buffer solution. The proposed method of microbeam irradiation enabled us to irradiate a specific region of the body, and to analyze the effects of that irradiation immediately, in an active animal. The results of this study provide the first evidence for the involvement of specific tissues, especially the central nervous system, in the radiation-induced reduction of motility in adult hermaphrodite C. elegans. These results have important implications for research into the individual, cellular and molecular effects of radiation, and the novel techniques developed will be essential for future studies of the effects of radiation on vital functions and behaviors in C. elegans.
MATERIALS AND METHODS
Strains and culture
C. elegans strain wild-type N2 and Escherichia coli strain HB101 were obtained from the Caenorhabditis Genetics Center. C. elegans hermaphrodites were grown at 20°C on a non-treated petri plate (IWAKI 60 mm/non-treated dish, AGC Techno Glass Co., Ltd, Shizuoka, Japan) containing 10 ml of nematode growth medium (NGM) spread with E. coli [22] as food. Well-fed adult animals (~3 days after hatching) were used in all experiments.
Sample preparation immediately before irradiation
Before irradiation, an individual C. elegans was collected from the culture plate using a platina picker (WormStuff Worm Pick, Genesee Scientific Corporation, San Diego, CA, USA) and washed twice in drops of S basal buffer solution [22] on a non-treated petri plate (Fig. 1A). Free motion of C. elegans during irradiation was inhibited using a PDMS microfluidic chip [21] (Fig. 1B). The surface of the chip included five straight microfluidic channels (depth, 40 μm; width, 40, 50, 60, 70 or 80 μm). The chip was located on a polystyrene cover (Cover Film, TaKaRa Slide Seal for in situ PCR; Takara Bio Inc., Shiga, Japan) and placed on an aluminum frame (Fig. 1C) custom-made for the microbeam-irradiation facility. A washed animal was picked up using a platina picker and transferred to a drop of S basal buffer solution placed on the surface of a chip (Fig. 1C). A polystyrene cover was placed carefully over the chip and pressed gently over the channel from one end of the chip to the other end, to maintain humidity. An animal was thus placed in a straight, close-fitting microfluidic channel in the PDMS chip, allowing it to be immobilized during irradiation without the need for anesthesia (Fig. 1D).
Fig. 1.
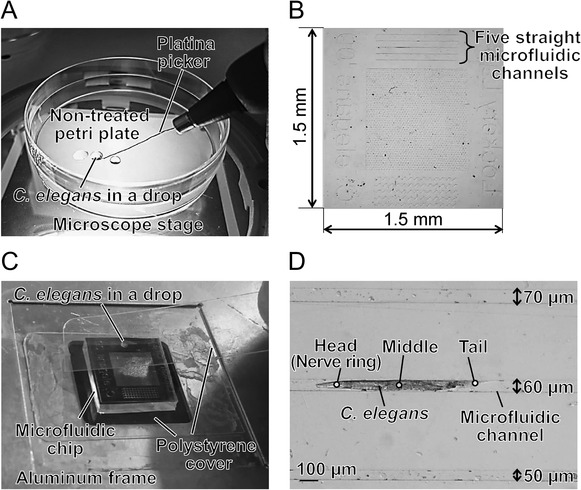
Preparation of region-specific microbeam irradiation. (A) Washing of C. elegans. An animal was collected from the culture plate using a platina picker and washed twice in drops of S basal buffer solution on a non-treated petri plate under the microscope. (B) Schematic of PDMS microfluidic chip with five straight microfluidic channels at the surface, 40 μm deep and 40, 50, 60, 70 or 80 μm wide. (C) A washed individual C. elegans was placed in a drop of S basal buffer solution on the surface of a chip, located on an aluminum frame custom-made for the microbeam-irradiation facility. A polystyrene cover was placed carefully over the chip to maintain humidity. The cover was pressed gently over the channels from one end of the chip to the other, and an individual C. elegans was enclosed in the channel. (D) C. elegans enclosed in a PDMS microfluidic chip before irradiation. Circles 20 μm in diameter indicate each targeting area for microbeam irradiation.
Region-specific microbeam or whole-body broad-beam irradiation with high-linear energy transfer carbon ions
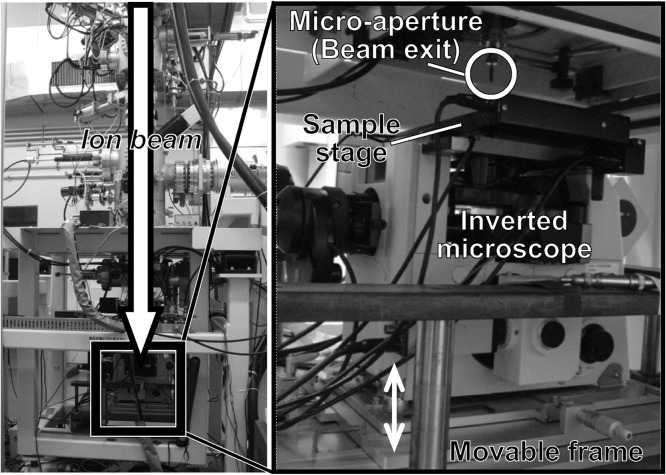
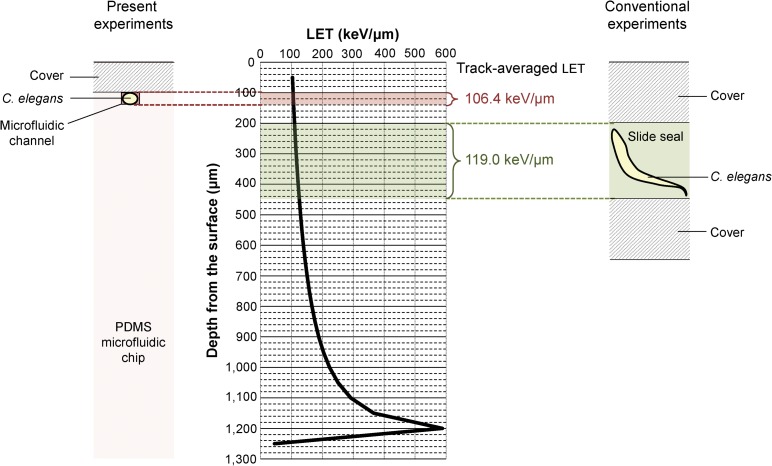
We investigated the effects of region-specific microbeam irradiation using carbon ion (12C5+) particles accelerated by an azimuthally varying field cyclotron installed at the Takasaki Ion Accelerators for Advanced Radiation Application (TIARA) facility of QST-Takasaki. We delivered region-specific microbeam irradiation using a collimating microbeam system [7] (Fig. 2). The microfluidic chip enclosing an individual C. elegans placed on a custom-made aluminum frame (Fig. 1C) was located on the sample stage of the collimating microbeam system (Fig. 2). The animal was then moved up to immediately under the micro-aperture (beam exit) using the movable frame, and irradiated with a carbon-ion microbeam from the micro-aperture. The incident energy of the carbon ions delivered was 18.3 MeV/u. The theoretical energy loss of carbon ion particles and the linear energy transfer (LET) in C. elegans were calculated using the Energy Loss Modify (ELOSSM) code, part of the Induced Radioactivity Analysis Code System (IRACM) [23]. The LETs of the carbon-ion microbeam passing through the C. elegans were 105.5 keV/μm at the surface of the microfluidic chip (depth from the surface of a sample = 100 μm) and 107.4 keV/μm at the bottom of the microfluidic channel (depth from the surface of a sample = 140 μm) (Fig. 3). We used a track-averaged LET of 106.4 keV/μm in the animal in a microfluidic channel, and converted particle fluence to dose in Gy using the following relationship: Dose [Gy] = 1.6 × 10−9 × LET [keV/μm] × Fluence [particle/cm2] [15]. Because the thickness of the microfluidic chip enclosing the animal was thicker than the range of the carbon ions (~1 mm), the ions could not pass through it and therefore did not reach the ion counter located under the sample stage. We therefore evaluated the distribution and fluence rate of the collimated carbon-ion beam in advance by irradiating an ion-track detector CR-39 (Solid State Nuclear Track Detector (TNF-1), Fukuvi Chemical Industry Co., Ltd, Fukui, Japan), as described previously [7], with slight modifications. As described previously [15], ~75% of the particles hit within a diameter of φ20 μm, and most of the remainder hit within a diameter of φ20–φ50 μm. The head region, including the nerve ring as the central nervous system, the mid region around the intestine and uterus, and the tail region were targeted independently and irradiated with 12 000 carbon ions, corresponding to a dose of 500 Gy at φ20 μm micro-aperture region (Fig. 1D). The irradiation time with 12 000 ions was set based on the fluence rate, and was ~2 s. Five animals were independently irradiated in each region (head, mid and tail).
Fig. 2.
Schematic of collimating microbeam system at the TIARA facility of QST-Takasaki. Left panel indicates entire system and right panel is enlarged view of the irradiation area. The custom-made aluminum frame shown in Fig. 1C with a microfluidic chip enclosing an individual C. elegans was located on the sample stage under the micro-aperture (beam exit). An animal was observed using the inverted microscope and a limited area was targeted. Before irradiation, the sample stage was moved up to immediately under the micro-aperture by raising the movable frame.
Fig. 3.
The sectional view of the sample enclosing a C. elegans for microbeam irradiation and the LET corresponding to sample depth. Left panel indicates schematic of a PDMS microfluidic chip enclosing a C. elegans in the present study and right panel is schematic of a thick device (Slide Seal) enclosing a C. elegans in the previous reported study [15]. Middle panel indicates the LET in the case of microbeam irradiation.
For comparison, we also conducted whole-body broad-beam irradiation. The track-averaged LET of the carbon-ion broad beam passing through the C. elegans was 111.5 keV/μm. The microfluidic chip enclosing the animal was located on a 6-cm non-treated petri plate, and the entire plate was irradiated with a scan beam at a dose of 500 Gy. It took ~50 s to irradiate a plate. For all irradiation experiments, non-irradiated control animals were handled in parallel with irradiated animals, except in terms of carbon-ion irradiation.
Locomotion assay
We evaluated motility using a locomotion assay, as described previously [2], except for use of a digital camera video-recorder. Immediately after irradiation, we removed the polystyrene cover from the microfluidic chip and added a drop of S basal buffer solution to an individual C. elegans. The animal swimming in the drop was then picked up using a platina picker and placed on a freshly prepared NGM [22] petri plate, without bacteria (food). Locomotion was recorded ~5 min after transfer, using a digital camera video-recorder (EX-F1, Casio Computer Co., Ltd, Tokyo, Japan) mounted on a stereomicroscope (SZX7, Olympus Corporation, Tokyo, Japan). ‘Body bends’ [24], defined as the number of bends in the anterior body region at 20-s intervals, were counted manually in videos of the five animals for each irradiated region and averaged, respectively.
Statistical analysis
Statistical analysis was performed based on the method described previously [2]. The mean number of body bends in the five animals irradiated in each region was normalized to the mean number in five non-irradiated control animals. These normalized values from seven independent experiments, conducted on distinct days, were averaged and used to evaluate the effects of irradiation. Numerical values are presented as values with standard errors of the mean (SEM). Data were analyzed by one-way analysis of variance (ANOVA). All statistical analyses were conducted using Microsoft Excel software (Microsoft Co. Ltd, Redmond, WA, USA) at the 0.01 and 0.05 significance levels.
RESULTS AND DISCUSSION
Establishment of method for region-specific irradiation of an animal without anesthesia
To examine the immediate effects of region-specific irradiation on locomotion in C. elegans, we developed a method that inhibited free motion only during irradiation, without the need for anesthesia. To achieve this, we used a behavior chip (PDMS microfluidic chip) to immobilize an animal, by placing it in a straight, close-fitting microfluidic channel in a chip (Fig. 1D). This allowed accurate targeting of region-specific irradiation to a limited area of the body, and observation of locomotion immediately after irradiation. In addition, by enclosing the animal in a 40-μm microfluidic channel, the LET of carbon ions passing thorough the animal was controlled within a very limited range, i.e. track-averaged LET ± ~1.0 (left panel in Fig. 3). In comparison, the conventional device used in a previous study [15] was 250 μm in depth, and the difference in LET between the surface and bottom of the device was ≥10.0 (track-averaged LET ± 5.0 or more) (right panel in Fig. 3). Our novel method thus reduced the effects of differences in LET on the radiation response. Furthermore, our proposed method expanded the application of the behavior chips from conventional uses, such as behavioral assays and neural activity imaging [20, 21], to their use for achieving region-specific irradiation. However, the current method had some residual problems. The method meant that it was not possible to irradiate a sufficiently large number of individuals for statistical analysis (at least five individuals) simultaneously, because we could not enclose multiple animals in a microfluidic chip at the same time because of limited capacity. Further improvements in the irradiation method are therefore needed to allow irradiation experiments to be conducted more efficiently, including the development of a microfluidic chip specialized for microbeam irradiation.
Region-specific irradiation had no significant effect on motility
We determined whether the observed radiation-induced reduction in motility was caused by irradiation to a specific region, e.g. the nerve ring responsible for information processing, or by irradiation of the muscle cells themselves.
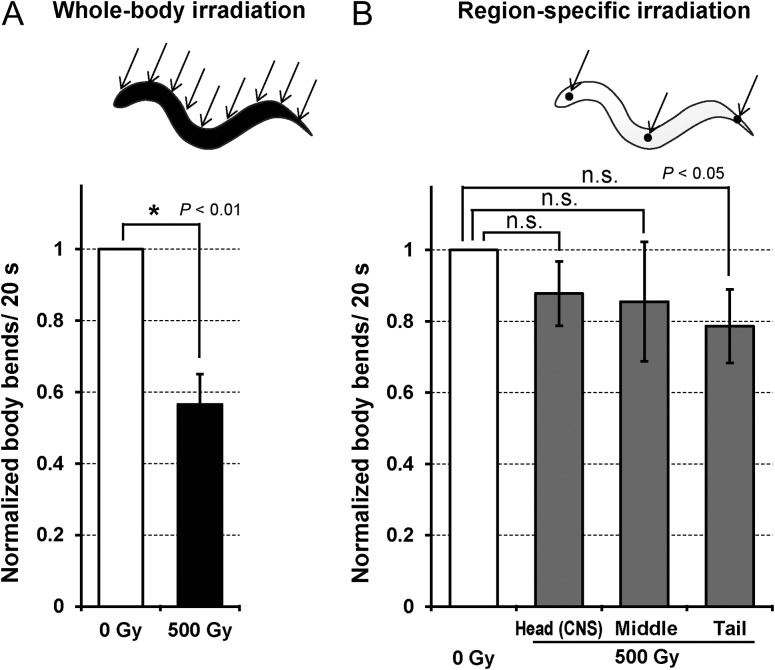
As a control experiment, we examined the effects of whole-body irradiation on C. elegans restrained in a microfluidic chip, and found that motility was significantly decreased immediately after whole-body irradiation with 500 Gy of carbon-ion broad beam (Fig. 4A). This result was consistent with the results of previous experiments [3]. In contrast, there was no significant reduction in motility in animals irradiated in the head region alone with 12 000 carbon ions, corresponding to 500 Gy, even though this region included the nerve ring (central nervous system), which plays an important role in the response to external stimulation and in information processing (Fig. 4B). This suggests that the reduction in motility induced by whole-body irradiation was not caused by an effect on the central nervous system. Furthermore, this result suggests that the reduction in motility could not be attributed to a lack of motor control due to temporal damage or agitation of the nervous system, and/or suppression of motor control in responses to internal and/or external stimuli. We previously found that hydrogen peroxide, an ROS produced by radiation exposure, might be involved in radiation-induced reduced motility [2]. However, the current results following head-only irradiation suggest that the ROS-related mechanism responsible for reduced motility does not involve simple sensory responses to ROS and radiation-induced molecules. In addition to head-region irradiation, microbeam irradiation of the mid region around the uterus, and of the tail region including the tail ganglia, also failed to induce any significant reduction in motility (Fig. 4B), indicating that no specific region responded to radiation stimuli. These results indicated that the effects of whole-body broad-beam irradiation were greater than those of region-specific microbeam irradiation at the same dose. This could be explained in relation to motor innervation of the body-wall muscles via motoneurons in the nerve cord, which is located from the head through to the tail. Whole-body irradiation might thus affect the motoneurons and/or body-wall muscle cells of the whole body equally, leading to reduced motility.
Fig. 4.
Motility of C. elegans immediately after carbon-ion irradiation with a dose of 500 Gy. (A) Normalized numbers of body bends in whole-body irradiated animals. (B) Normalized numbers of body bends in region-specific irradiated animals. For each experiment (whole body–, head (CNS)-, middle-, and tail-irradiated animals), numbers of body bends were averaged from five animals in each group of irradiation. The value for each group of irradiation was normalized according to the ‘standard body bends’ (the mean number of body bends in non-irradiated control animals tested at the same time). Finally, data for seven independent irradiation experiments were averaged for each group. Error bars represent SEM of seven independent experiments. All data were analyzed using one-way ANOVA at the 0.01 (for the whole-body irradiation) and 0.05 significance levels (region-specific irradiation). *Indicates significant difference.
For reference, the mean numbers of the standard body bends in each irradiation group (averaged from seven distinct experiments in each group) were 10.8 for whole-body irradiation, 12.1 for head irradiation, 12.1 for middle-body irradiation, and 11.1 for tail irradiation, respectively.
Interestingly, we previously observed DNA damage–induced cell cycle arrest and apoptosis after microbeam irradiation of C. elegans larvae at L4 stage (within two days after hatching) with only 1500 carbon ions [15], whereas microbeam irradiation of adult animals with 12 000 carbon ions was not enough to reduce motility in the present experiments. These results indicate that the effects of region-specific microbeam irradiation differ according to each evaluation end-point and/or developmental stage, even in the same species. In addition, anesthesia-dependent irradiation effects may occur, and the involvement of anesthesia-induced nerve-calming effects on the responses of individual C. elegans to irradiation remain unknown. However, previous studies of local irradiation of individual C. elegans using anesthesia [15, 17, 19] reported local irradiation-induced effects such as cell death. Given that nervous system activity during irradiation may play an role in the radiation response, including radioresistance, it is essential to examine the effects of radiation in the absence of anesthesia, as proposed in the current study.
In conclusion, our study provides the first evidence showing that region-specific carbon-ion irradiation has no significant effect on motility in C. elegans. Further studies comparing the long-term effects of whole-body and partial irradiation, focusing on homeostatic mechanisms and life span, will be indispensable for understanding this phenomenon. However, further studies are needed to clarify several important issues. These include determining the irradiation area–dependent effects, which require further technical developments in beam collimation to allow irradiation of specifically shaped tissues. The dose-dependent effect of region-specific irradiation also remains to be established, which requires the production of an ion-penetrable ultra-thin microfluidic chip permitting careful control of the number of ions (dose) applied.
ACKNOWLEDGEMENTS
The authors thank Dr Shawn R. Lockery for kindly providing microfluidic chips and detailed information; Dr Katsuyoshi Takano for technical advice on treatment of microfluidic chips; and the Caenorhabditis Genetic Center at the University of Minnesota for providing strains of C. elegans and E. coli. We thank Dr Yasuko Mutou-Yoshihara, Dr Hiroko Ikeda, and the crew of the cyclotron of TIARA at QST-Takasaki for their kind assistance with the experiments. Some of the results of this research have been presented at the 11th International Workshop on Microbeam Probes of Cellular Radiation Response, the 15th International Congress of Radiation Research, and the 12th International Workshop on Microbeam Probes of Cellular Radiation Response.
FUNDING
This study was supported in part by MEXT KAKENHI Grant Number JP20115010 to M.S. and JSPS KAKENHI Grant Numbers JP24620013, JP15K11921, JP15H03950 to M.S.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Sakashita T, Hamada N, Ikeda DD, et al. Locomotion-learning behavior relationship in Caenorhabditis elegans following gamma-ray irradiation. J Radiat Res 2008;49:285–91. [DOI] [PubMed] [Google Scholar]
- 2. Suzuki M, Sakashita T, Yanase S, et al. Effects of ionizing radiation on locomotory behavior and mechanosensation in Caenorhabditis elegans. J Radiat Res 2009;50:119–25. [DOI] [PubMed] [Google Scholar]
- 3. Sakashita T, Suzuki M, Hamada N, et al. Behavioral resistance of Caenorhabditis elegans against high-LET radiation exposure. Biol Sci Space 2012;26:7–11. [Google Scholar]
- 4. White JG, Southgate E, Thomson JN, et al. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 1986;314:1–340. [DOI] [PubMed] [Google Scholar]
- 5. Ware RW, Clark D, Crossland K, et al. The nerve ring of the nematode Caenorhabditis elegans: sensory input and motor output. J Comp Neurol 1975;162:71–110. [Google Scholar]
- 6. White JG, Southgate E, Thomson JN, et al. The structure of the ventral nerve cord of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 1976;275:327–48. [DOI] [PubMed] [Google Scholar]
- 7. Funayama T, Wada S, Yokota Y, et al. Heavy-ion microbeam system at JAEA-Takasaki for microbeam biology. J Radiat Res 2008;49:71–82. [DOI] [PubMed] [Google Scholar]
- 8. Yokota Y, Funayama T, Kobayashi Y, et al. Development of an ion microbeam system for irradiating single plant cell[s]. Biol Sci Space 2003;17:298–301. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi Y, Funayama T, Wada S, et al. Microbeams of heavy charged particles. Biol Sci Space 2004;18:235–40. [DOI] [PubMed] [Google Scholar]
- 10. Miyazawa Y, Sakashita T, Funayama T, et al. Effects of locally targeted heavy-ion and laser microbeam on root hydrotropism in Arabidopsis thaliana. J Radiat Res 2008;49:373–9. [DOI] [PubMed] [Google Scholar]
- 11. Fukamoto K, Shirai K, Sakata T, et al. Development of the irradiation method for the first instar silkworm larvae using locally targeted heavy-ion microbeam. J Radiat Res 2007;48:247–53. [DOI] [PubMed] [Google Scholar]
- 12. Hino M, Wada S, Tajika Y, et al. Heavy ion microbeam irradiation induces ultrastructural changes in isolated single fibers of skeletal muscle. Cell Struct Funct 2007;32:51–6. [DOI] [PubMed] [Google Scholar]
- 13. Funayama T, Hamada N, Sakashita T, et al. Heavy-ion microbeams—development and applications in biological studies. IEEE Trans Plasma Sci 2008;99:1431–40. [Google Scholar]
- 14. Tomita M, Matsumoto H, Funayama T, et al. Nitric oxide–mediated bystander signal transduction induced by heavy-ion microbeam irradiation. Life Sci Space Res 2015;6:36–43. [DOI] [PubMed] [Google Scholar]
- 15. Sugimoto T, Dazai K, Sakashita T, et al. Cell cycle arrest and apoptosis in Caenorhabditis elegans germline cells following heavy-ion microbeam irradiation. Int J Radiat Biol 2006;82:31–8. [DOI] [PubMed] [Google Scholar]
- 16. Bertucci A, Pocock RDJ, Randers-Pehrson G, et al. Microbeam irradiation of the C. elegans nematode. J Radiat Res 2009;50:A49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo X, Sun J, Bian P, et al. Radiation-induced bystander signaling from somatic cells to germ cells in Caenorhabditis elegans. Radiat Res 2013;180:268–75. [DOI] [PubMed] [Google Scholar]
- 18. Le Trequesser Q, Saez G, Devès G, et al. In situ titanium dioxide nanoparticles quantitative microscopy in cells and in C. elegans using nuclear microprobe analysis. Nucl Instr Meth Phys Res B 2014;341:58–64. [Google Scholar]
- 19. Tang H, Chen L, Chen L, et al. Interaction between radioadaptive response and radiation-induced bystander effect in Caenorhabditis elegans: a unique role of the DNA damage checkpoint. Radiat Res 2016;186:662–8. [DOI] [PubMed] [Google Scholar]
- 20. Chronis N, Zimmer M, Bargmann CI. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods 2007;4:727–31. [DOI] [PubMed] [Google Scholar]
- 21. Lockery SR, Lawton KJ, Doll JC, et al. Artificial dirt: microfluidic substrates for nematode neurobiology and behavior. J Neurophysiol 2008;99:3136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brenner S. The genetics of Caenorhabditis elegans. Genetics 1974;77:71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanaka S, Fukuda M, Nishimura K, et al. The IRAC code system to calculate activation and transmutation in the TIARA facility. J Nucl Sci Technol 2000;37:840–4. [Google Scholar]
- 24. Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 2000;26:619–31. [DOI] [PubMed] [Google Scholar]