Abstract
We evaluated the reproducibility and predictive value of texture parameters and existing parameters of 18F-FDG PET/CT images in Stage I non-small-cell lung cancer (NSCLC) patients treated with stereotactic body radiotherapy (SBRT). Twenty-six patients with Stage I NSCLC (T1-2N0M0) were retrospectively analyzed. All of the patients underwent an 18F-FDG PET/CT scan before treatment and were treated with SBRT. Each tumor was delineated using PET Edge (MIM Software Inc., Cleveland, OH), and texture parameters were calculated using open-source code CGITA. From 18F-FDG PET/CT images, three conventional parameters, including maximum standardized uptake value (SUV), metabolic tumor volume (MTV) and total lesion glycolysis (TLG), and four texture parameters, including entropy and dissimilarity (derived from a co-occurrence matrix) and high-intensity large-area emphasis (HILAE) and zone percentage (derived from a size-zone matrix) were analyzed. Reproducibility was evaluated using two independent delineations conducted by two observers. The ability to predict local control (LC), progression-free survival (PFS) and overall survival (OS) was tested for each parameter. All of the seven parameters except zone percentage showed good reproducibility, with intraclass correlation coefficient values >0.8. In univariate analysis, only HILAE was a significant predictor for LC. Histology, dose fractionation, and maximum SUV were associated with PFS, and histology and dose fractionation were associated with OS. We showed that texture parameters derived from 18F-FDG PET/CT were reproducible and potentially beneficial for predicting LC in Stage I lung cancer patients treated with SBRT.
Keywords: lung cancer, stereotactic body radiotherapy (SBRT), 18F-FDG PET/CT, texture analysis, radiomics, prognostic factor
INTRODUCTION
Of all malignancies, lung cancer is the leading cause of death, worldwide. In 2012, 1.8 million people in the world were affected by lung cancer, and 1.6 million people died from it [1]. Stereotactic body radiotherapy (SBRT) is a potentially curative treatment for early-stage lung cancer, in both operable and inoperable patients [2, 3]. Although there has been no completed randomized controlled trial (RCT) in which surgery and SBRT for early-stage lung cancer were compared, a pooled analysis of the results of two uncompleted RCTs (the STARS trial [NCT00840749] and the ROSEL trial [NCT00687986]) was conducted [4]. In the pooled analysis, it was suggested that SBRT for operable stage I non-small-cell lung cancer (NSCLC) might lead to better overall survival (OS) than surgery. However, optimal indication and the optimal dose fractionation of SBRT have not yet been established. To identify patients who can be cured with SBRT or who need dose escalation or surgical resection, it would be useful to establish predictive factors for risk of recurrence or prognosis at the beginning of the treatment course.
In patients who receive SBRT for lung cancer, 18F-FDG PET/CT is used for initial staging and for diagnosing recurrence or metastases. The predictive value of PET images for clinical prognosis has been investigated. For analyzing PET images, histogram-based parameters [such as maximum standardized uptake value (SUV) and mean SUV] and volume-based parameters [such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG)] have been investigated [5–9]. Recently, texture analysis of 18F-FDG PET/CT images has been shown to have prognostic value in local control (LC), distant metastases and survival of patients with early-stage lung cancer treated with SBRT [10–12].
Some problems have been demonstrated in texture analysis of 18F-FDG PET/CT images. One problem is the difficulty in identifying the useful and robust parameters from the many parameters calculated in the analysis. Some parameters do not have sufficient reproducibility, and others have strong correlations with each other [13, 14]. Hatt et al. chose 4 texture parameters which were considered to have enough reproducibility, independency, and clinical usefulness and showed that one parameter which shows image heterogeneity may be useful for predicting local control [15]. Another problem is reproducibility of delineating the volume of interest (VOI) for textual analysis. To overcome this problem, automated delineation algorithms have been used in some studies [15, 16]. PET Edge (MIM Software Inc., Cleveland, OH) is a semi-automated gradient-based delineation algorithm for PET images that can be operated easily and produces robust delineation, and its clinical usefulness has been reported [17, 18]. In the present study, we used the parameters described above and two graphical user interface software programs (PET Edge and CGITA) for texture analysis of 18F-FDG PET/CT images of lung cancer patients treated with SBRT; we evaluated the reproducibility and predictive value of texture parameters.
MATERIALS AND METHODS
All procedures performed in studies involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Declaration of Helsinki and its later amendments, or comparable ethical standards. This study was approved by the Tohoku University Hospital Institutional Review Board (2017-1-39).
Patients
Twenty-eight patients satisfied the following inclusion criteria: (i) clinically diagnosed as having NSCLC staged to be T1–2N0M0 (according to UICC TNM classification 7th edition), (ii) treated with SBRT in a single institution in the period between March 2005 and December 2011 and (iii) having available 18F-FDG PET/CT images acquired by a single PET/CT scanner within the 90 days before starting treatment. Two patients were excluded because no positive FDG uptake was recognized; thus, 26 patients were analyzed. Diagnosis was made clinically, by considering the X-ray photograph, CT, PET/CT, blood tumor markers, endoscopic biopsy and/or CT-guided needle biopsy. In this study, pathological diagnosis by a bronchoscopy was not available in some of the patients because of peripheral small lesion or respiratory comorbidities. In these patients, clinical diagnosis of NSCLC was made based on clinical and radiological features, including increasing size of tumor in CT scans, FDG uptake, and/or tumor markers. Clinical diagnosis and treatment strategy were decided by an experienced pulmonologist, thoracic surgeon, diagnostic radiologist and radiation oncologist. FDG uptake was evaluated visually, and when the tumor was located nearby a structure other than a lung, FDG uptake higher than that in the mediastinum was considered to be a positive indicator. Clinical information (including information on sex, age, location of the tumor, clinical staging, histopathological diagnosis and treatment) was acquired from the hospital information system.
Treatment
All of the patients were treated with SBRT. The SBRT technique used in our institution has been reported previously [19, 20]. In principle, 10–12 Gy was prescribed in each fraction, and the total dose was 40–48 Gy. For regions near the mediastinum or other organs at risk, a smaller dose (4–7.5 Gy per fraction and a total dose of 50–60 Gy) was used. The prescription point was set on the isocenter until May 2009. From June 2009, dose prescription for D95 was adopted. SBRT was delivered with a linear accelerator (Clinac 23EX, Varian Medical Systems Inc., Palo Alto, CA), using 6 MV X-ray beams with five to seven non-coplanar static ports. An abdominal pressure belt was used for patients with large tumor motion.
Image acquisition
All of the patients in this study underwent an 18F-FDG PET/CT scan (Biograph DUO, Siemens Medical Solutions, Erlangen, Germany). After a 4-hour fast, patients were injected with 3.7 MBq 18F-FDG/kg body weight. After ~1 h, a spiral CT scan with ~25 effective mAs, 130 kVp and a 5-mm slice thickness was made, followed by a PET emission scan from the distal femur to the top of the skull. The PET scanning time was 2 min per bed position, with increments of 16.2 cm (3D mode), and all of the patients were scanned in eight bed positions. PET images were reconstructed using iterative algorithms (ordered-subset expectation maximization, 6 iterations, 8 subsets) to a final pixel size of 5.3 × 5.3 × 2.5 mm. A 6-mm full-width at half maximum Gaussian filter was applied after the reconstruction.
Image analysis
PET Edge, which is a delineation tool running on MIM (MIM Software Inc., Cleveland, OH), was used for delineation of the tumor on an 18F-FDG PET/CT image. First, two observers (a nuclear medicine specialist with 15 years of experience and a senior resident with 2 years of experience in the radiation oncology course) delineated the primary tumor of the lung independently, using the graphical user interface PET Edge. These delineations were used for testing the reproducibility of texture parameters. In the latter part of the study, a single delineation (drawn using PET Edge by the nuclear medicine specialist with 15 years of experience) was used for analysis.
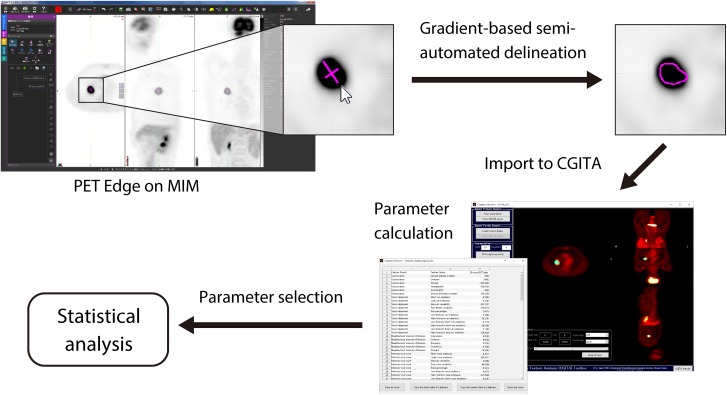
The 18F-FDG PET/CT image and delineation data were imported to CGITA [21], which is an open-source software code with a graphical user interface for texture analysis running on MATLAB (version 2014a, MathWorks Inc., Natick, MA). We calculated seven parameters using CGITA. Of the histogram-based parameters, maximum SUV, MTV and total lesion glycolysis (TLG) were used, as they have been reported to be useful for predicting prognosis [8, 9]. From textual parameters, four parameters, including two parameters derived from a co-occurrence matrix (entropy and dissimilarity) and two parameters derived from a size-zone matrix [high-intensity large-area emphasis (HILAE) and zone percentage], were selected for analysis based on a study by Hatt et al. [15]. These texture parameters have been shown to be clinically useful in past studies and were selected with consideration of reproducibility and confounding between the factors [10, 14, 15, 22]. For texture analysis, each 18F-FDG PET/CT image was rescaled into a 64-level gray-scale (scaling from minimum to maximum SUV values inside the contoured lesion) image. A flowchart of texture analysis is shown in Fig. 1.
Fig. 1.
A flowchart of texture analysis. A semi-automated delineation tool PET Edge (MIM Software Inc., Cleveland, OH) was used for tumor delineation. For parameter calculation, CGITA, developed by Fang et al., was used.
Follow-up of patients
Information on patients’ prognosis was obtained from the medical records. The latest follow-up was conducted in July 2016. Each patient was followed up by a medical interview, chest X-ray, CT and/or 18F-FDG PET/CT. Local relapse was diagnosed mainly by regrowth of the tumor and/or increasing accumulation of 18F-FDG at the tumor site to a maximum SUV of >5.0.
Statistical analysis
To evaluate the reproducibility of textural parameters, texture parameters calculated on the basis of two different delineations conducted independently by two observers were compared using the intraclass correlation coefficients (ICCs) method. LC, progression-free survival (PFS) and OS were estimated from the day when radiation therapy was started, using the Kaplan–Meier method. A log-rank test was used to evaluate the predictive value of each parameter, and the cut-off value of each PET parameter was set as the median. The Kaplan–Meier method was used to draw a survival curve. Receiver operating characteristic (ROC) analysis was used to evaluate the predictive value of a parameter. A P value of <0.05 was defined as significant in all tests. JMP version 12.2.0 (SAS Institute, Cary, NA) was used for the statistical analysis.
RESULTS
Patients’ characteristics
The patients’ characteristics are shown in Table 1. Twenty-four patients (92%) were aged 70 years or older. In 19 (73%) of the patients, a pathological diagnosis of NSCLC was obtained, whereas in the other patients a clinical diagnosis was made. In 13 (50%) of the patients, radiation therapy was selected because of lack of surgical indication due to age and/or low respiratory function, and the others received radiation therapy because they refused surgery. Seventeen patients (65%) were treated with ≥100 Gy in biological equivalent dose (BED), calculated by a linear quadratic (LQ) model with an α/β ratio of 10 Gy.
Table 1.
Patient characteristics
| Number of patients | ||
|---|---|---|
| Sex | Female | 6 (23%) |
| Male | 20 (77%) | |
| Age | 78.5 (48–88)a | |
| Location | Upper lobe | 19 (73%) |
| Middle lobe | 1 (4%) | |
| Lower lobe | 6 (23%) | |
| Tumor diameter | 24 (10–39)a | |
| Histology | Adeno | 10 (38%) |
| SCC | 7 (27%) | |
| NSCLC, NOS | 2 (8%) | |
| Unknown | 7 (27%) | |
| Performance status | 0 | 10 (38%) |
| 1 | 13 (50%) | |
| 2 | 2 (8%) | |
| 3 | 1 (4%) | |
| Operability | Operable | 13 (50%) |
| Inoperable | 13 (50%) | |
| Dose fractionation | 4 | 13 (50%) |
| 8 | 10 (38%) | |
| 15 | 3 (12%) | |
| BED10 | ≥100 | 17 (65%) |
| <100 | 9 (35%) |
aShown as median with ranges. SCC = squamous cell carcinoma, Adeno = adenocarcinoma, BED10 = biological equivalent dose calculated with an α/β ratio = 10.
Reproducibility of delineation
In this study, seven parameters (based on tumor delineation using PET Edge) were used for analysis. The results of the ICC analysis of the texture parameters, based on two different delineations generated by two observers, are shown in Table 2. For all of the parameters except zone percentage (ICC value of 0.65), the values of ICC were between 0.81 and 1.00.
Table 2.
Intraclass correlation analysis between two different observers
| ICC | |
|---|---|
| Maximum SUV | 1.00 |
| Metabolic tumor volume | 0.92 |
| Total lesion glycolysis | 0.99 |
| Entropy | 0.81 |
| Dissimilarity | 0.95 |
| HILAE | 0.94 |
| Zone percentage | 0.66 |
ICC = intraclass correlation coefficient, SUV = standard uptake value, HILAE = high intensity large area emphasis.
Clinical outcomes and prognostic factors
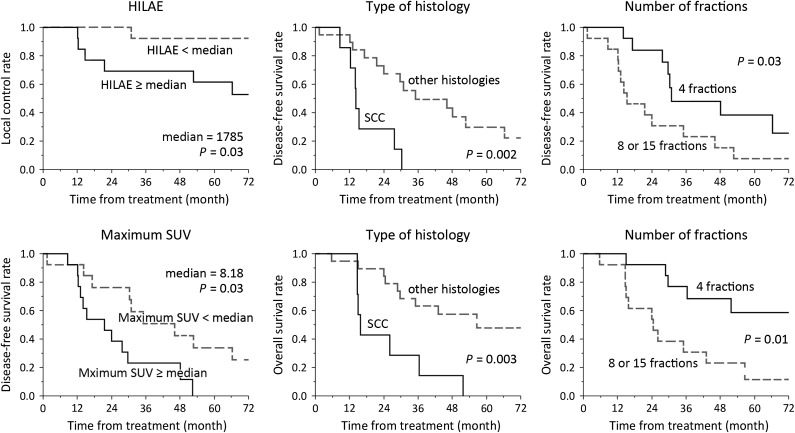
The median follow-up period was 36.0 months in all patients and 57.8 months in living patients. Seven patients (25%) experienced local relapse and 10 patients (36%) were alive at the latest follow-up. The results of the univariate analysis are shown in Table 3. For parameters considered to be significant in univariate analysis, Kaplan–Meier plots are shown in Fig. 2. Among texture parameters, HILAE was a significant predictor for LC but not for PFS or OS. In contrast, maximum SUV was a significant predictor for PFS, but not for LC or OS. None of the PET-derived parameters were significant predictors for OS. ROC analysis was conducted to evaluate the predictive value of HILAE for LC, and the area under the ROC curve (AUC) was 0.72 (Fig. 3).
Table 3.
Univariate analysis for local control, progression-free survival and overall survival with a log-rank test
| LC | PFS | OS | ||
|---|---|---|---|---|
| P value | P value | P value | ||
| Sex | Female vs male | 0.50 | 0.46 | 0.06 |
| Age | ≥78.5 vs <78.5† | 0.58 | 0.21 | 0.19 |
| Tumor diameter | ≥30 mm vs <30 mm | 0.31 | 0.07 | 0.35 |
| Location of the tumor | Lower vs upper/middle lobe | 0.62 | 0.87 | 0.81 |
| Histology | SCC vs others | 0.43 | 0.002* | 0.003* |
| Performance status | ≥2 vs <2 | 0.89 | 0.47 | 0.78 |
| Operability | Inoperable vs operable | 0.68 | 0.72 | 0.45 |
| Number of fractions | 4 vs 8 or 15 | 0.15 | 0.03* | 0.01* |
| BED10 | ≥100 Gy vs <100 Gy | 0.52 | 0.07 | 0.62 |
| Maximum SUV | ≥8.18 vs <8.18† | 0.78 | 0.03* | 0.08 |
| Metabolic tumor volume | ≥5.99 vs <5.99† | 0.55 | 0.41 | 0.87 |
| Total lesion glycolysis | ≥23.4 vs <23.4† | 0.81 | 0.14 | 0.31 |
| Entropy | ≥–58.1 vs <–58.1† | 0.55 | 0.39 | 0.72 |
| Dissimilarity | ≥2393 vs <2393† | 0.81 | 0.22 | 0.36 |
| HILAE | ≥1785 vs <1785† | 0.03* | 0.16 | 0.31 |
| Zone percentage | ≥0.41 vs <0.41† | 0.51 | 0.60 | 0.64 |
Asterisk (*) shows significance with P value <0.05 (shown in bold), and dagger (†) represents a median. LC = local control, PFS = progression-free survival, OS = overall survival, SCC = squamous cell carcinoma, Adeno = adenocarcinoma, BED10 = biological equivalent dose calculated with an α/β ratio = 10, SUV = standard uptake value, HILAE = high-intensity large-area emphasis.
Fig. 2.
Kaplan–Meyer curves for parameters considered to be significant in log-rank test. HILAE = high-intensity large-area emphasis, SCC = squamous cell carcinoma, SUV = standard uptake value.
Fig. 3.
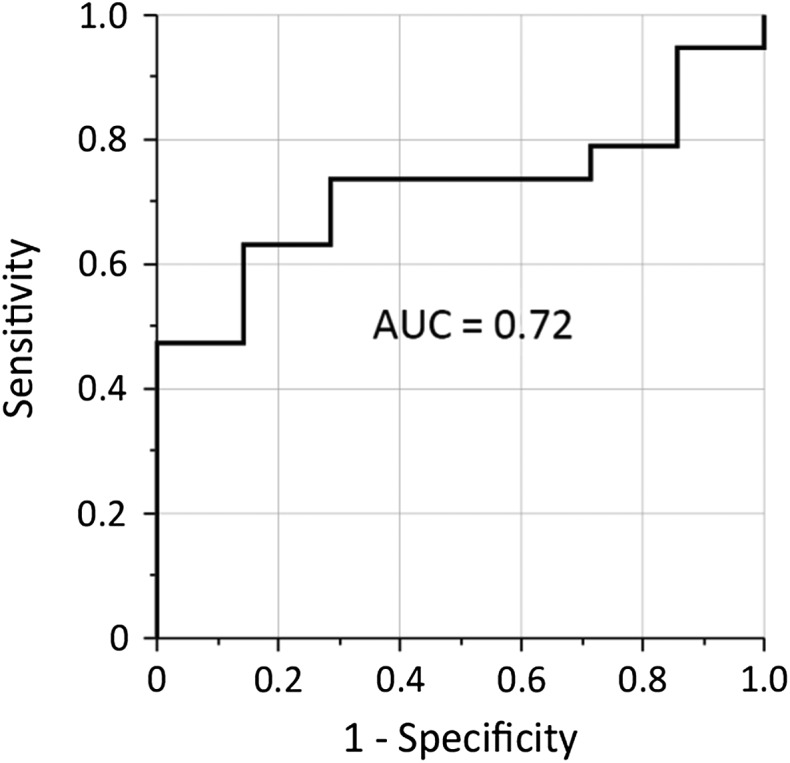
Evaluation of the predictive value of high-intensity large-area emphasis (HILAE) for local control by Receiver operating characteristic (ROC) analysis. AUC = area under the ROC curve.
DISCUSSION
In the present study, we evaluated the clinical utility of texture and other conventional parameters based on 18F-FDG PET/CT images in lung cancer patients treated with SBRT. The usefulness of 18F-FDG PET/CT for predicting prognosis and treatment response has been investigated in patients with various types of cancer. The predictive value of maximum SUV has been investigated in many studies. In lung cancer patients, some studies showed maximum SUV to be a prognostic factor [5, 6], but other studies showed that it was not a predictor [23, 24]. Maximum SUV is easy to use and is a robust and reproducible parameter, but it does not fully reflect tumor size or tumor heterogeneity. For this reason, volume-based parameters, including metabolic tumor volume (MTV) and total lesion glycolysis (TLG), have been studied. We previously showed that MTV and TLG might be better prognostic factors than maximum SUV in lung cancer patients treated with SBRT [9]. MTV is the volume of voxels with higher 18F-FDG accumulation than the cut-off value, and TLG is calculated by multiplying MTV by mean SUV within the lesion, and they therefore reflect both tumor metabolism and tumor size. However, heterogeneity inside the tumor is not considered in MTV or TLG. Texture analysis of 18F-FDG PET/CT images was first reported in 2009 by Naqa et al. for patients with cervical cancer and patients with head and neck cancer [25]. Texture analysis can reflect heterogeneity inside the tumor and is considered to be a method that is better than or complementary to known tumor features. Texture parameters have been shown to have predictive value in various types of cancer [16, 26–28].
Recently, a few studies have shown the prognostic value of texture parameters in disease control and survival in Stage I NSCLC patients treated with SBRT. Pyka et al. reported that entropy, correlation and busyness were significant predictors for LC and/or disease-specific survival [10], and Lovinfosse et al. reported contrast (based on co-occurrence matrix), entropy, dissimilarity, coarseness and contrast (based on neighborhood intensity-difference matrix) as significant predictors [11]. Wu et al. reported that the combination of Gauss cluster shade and other known parameters improved the prediction for distant metastasis in 101 lung cancer patients [12]. In the present study, one texture parameter, HILAE, was shown to be a significant predictor for LC. Although interpreting the biological meaning of texture parameters of PET image has a lot of difficulties, HILAE might show some of heterogeneity inside the tumor that reflects the biological and metabolic characteristics of the tumor. In the various studies, different parameters have been shown to have a predictive value with respect to patient prognosis. This might be because of variation in the image acquisition and reconstruction methods. If these variables are considered and managed appropriately, using texture parameters might improve estimation of the clinical outcome after SBRT, which may lead to personalized treatment. For instance, dose escalation for patients with a higher risk of local recurrence might be possible.
Among conventional histogram parameters, maximum SUV showed significant correlation with PFS and OS, but not with LC. Combining multiple parameters from histogram, volume-based and texture parameters might improve the predictive value of 18F-FDG PET/CT images.
Although texture analysis might be clinically useful, there are some problems regarding its reproducibility. Some texture parameters are known to depend on small differences in such as image reconstruction parameters [13], and reproducibility of delineation is also an important problem. To conduct texture analysis in the present study, we used PET images acquired by a single scanner with a single reconstruction method, and used a commercially available software (PET Edge) for tumor delineation. PET Edge uses a gradient-based tumor delineation method, and ICC analysis showed good reproducibility for ICC values over 0.8 for all of the parameters except zone percentage. These results indicate that PET Edge has good reliability for texture analysis. Other delineation algorithms, including the fuzzy locally adaptive Bayesian method and, threshold method and manual delineation were used in previous studies [11, 16]. Compared with those methods, PET Edge seems to have an advantage because it is a combination of manual and automated delineation methods. In PET Edge, an observer draws the tumor diameter on the console; the software makes a reproducible delineation based on a gradient-based algorithm, considering the tumor diameter drawn by the observer. This allows easy and adjustable delineation, with good reproducibility, that is suitable for each clinical situation.
CGITA developed by Fang et al. was used for calculating texture parameters in the present study [21]. In many past studies, home-made codes were used for texture analysis. The code used for analysis was disclosed in only a few reports, and this might have led to differences in the calculation methods used in studies. The use of an open source code such as CGITA may have an advantage in making the calculating process clear, as well as making texture analysis easier for researchers and clinicians.
The present study has some limitations. One is the number of cases being too small to evaluate independent prognostic factors by multivariate analysis. This problem is common in studying texture parameters because many parameters are calculated from a single image set. To overcome this problem, some studies have used machine-learning methods such as neural networks [29] and the least absolute shrinkage and selection operator (LASSO) method [12]. Such methods do not require as many cases as conventional regression analysis does, and may be effective in texture analysis. In the future, a larger and prospective validation trial will be needed. In a larger study, a method will be needed to correct for differences in images due to the images being acquired by different PET scanners.
A further limitation is that 27% of the patients lacked histological information. The possibility arises that patients with a benign tumor or small-cell lung cancer could have been included, which could have affected treatment response and prognosis. Verstegen et al. investigated 591 patients with Stage I non-small-cell lung cancer treated with SBRT, among whom 209 patients (36%) had lesions with diagnostic pathologic findings and 382 (66%) patients did not [30]. They reported that there were no differences between the two groups with respect to OS, LC, regional control or distant control. This suggests that appropriate clinical consideration allows a reasonable clinical diagnosis of NSCLC for a decision of therapeutic indication. Similarly, our present study showed no statistical difference in OS, PFS or LC between the two groups (data not shown), although the number of the patients was too small to draw a conclusion. However, there is a possibility that the predictive value of texture parameters could vary between different types of histology. To investigate this, further studies with complete pathological information will be needed.
CONCLUSION
Texture parameters of 18F-FDG PET/CT images calculated using a gradient-based delineation method have sufficient reproducibility; one of these texture parameters, HILAE, might be a better predictor than maximum SUV for LC in patients with early-stage lung cancer treated with SBRT.
ACKNOWLEDGEMENTS
Results from this study were presented at ESTRO 36 (e-poster session).
CONFLICT OF INTEREST
The authors have no conflict of interest regarding this manuscript.
FUNDING
None.
REFERENCES
- 1. Elisabeth B, William DT. Lung cancer In: Stewart BW, Wild CP (eds). World Cancer Report 2014. Lyon: the International Agency for Research on Cancer, 2014, 350–61. [Google Scholar]
- 2. Lagerwaard F, Verstegen N, Haasbeek C et al. . Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348–53. [DOI] [PubMed] [Google Scholar]
- 3. Timmerman R, Paulus R, Galvin J et al. . Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang J, Senan S, Paul M et al. . Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takeda A, Sanuki N, Fujii H et al. . Maximum standardized uptake value on FDG-PET is a strong predictor of overall and disease-free survival for non–small-cell lung cancer patients after stereotactic body radiotherapy. J Thorac Oncol 2014;9:65–73. [DOI] [PubMed] [Google Scholar]
- 6. Hamamoto Y, Sugawara Y, Inoue T et al. . Relationship between pretreatment FDG uptake and local control after stereotactic body radiotherapy in stage I non-small-cell lung cancer: the preliminary results. Jpn J Clin Oncol 2011;41:543–7. [DOI] [PubMed] [Google Scholar]
- 7. Abelson J, Murphy J, Trakul N et al. . Metabolic imaging metrics correlate with survival in early stage lung cancer treated with stereotactic ablative radiotherapy. Lung Cancer 2012;78:219–24. [DOI] [PubMed] [Google Scholar]
- 8. Satoh Y, Onishi H, Nambu A et al. . Volume-based parameters measured by using FDG PET/CT in patients with stage I NSCLC treated with stereotactic body radiation therapy: prognostic value. Radiology 2014;270:275–81. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi N, Yamamoto T, Matsushita H et al. . Metabolic tumor volume on FDG-PET/CT is a possible prognostic factor for Stage I lung cancer patients treated with stereotactic body radiation therapy: a retrospective clinical study. J Radiat Res 2016;57:655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pyka T, Bundschuh RA, Andratschke N et al. . Textural features in pre-treatment [F18]-FDG-PET/CT are correlated with risk of local recurrence and disease-specific survival in early stage NSCLC patients receiving primary stereotactic radiation therapy. Radiat Oncol 2015;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lovinfosse P, Janvary ZL, Coucke P et al. . FDG PET/CT texture analysis for predicting the outcome of lung cancer treated by stereotactic body radiation therapy. Eur J Nucl Med Mol Imaging 2016;43:1453–60. [DOI] [PubMed] [Google Scholar]
- 12. Wu J, Aguilera T, Shultz D et al. . Early-stage non-small cell lung cancer: quantitative imaging characteristics of 18F fluorodeoxyglucose PET/CT allow prediction of distant metastasis. Radiology 2016;281:270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galavis PE, Hollensen C, Jallow N et al. . Variability of textural features in FDG PET images due to different acquisition modes and reconstruction parameters. Acta Oncol 2010;49:1012–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hatt M, Tixier F, Cheze Le Rest C et al. . Robustness of intratumour 18F-FDG PET uptake heterogeneity quantification for therapy response prediction in oesophageal carcinoma. Eur J Nucl Med Mol Imaging 2013;40:1662–71. [DOI] [PubMed] [Google Scholar]
- 15. Hatt M, Majdoub M, Vallières M et al. . 18F-FDG PET uptake characterization through texture analysis: investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J Nucl Med 2015;56:38–44. [DOI] [PubMed] [Google Scholar]
- 16. Nakajo M, Jinguji M, Nakabeppu Y et al. . Texture analysis of 18F-FDG PET/CT to predict tumour response and prognosis of patients with esophageal cancer treated by chemoradiotherapy. Eur J Nucl Med Mol Imaging 2017;44:206–14. [DOI] [PubMed] [Google Scholar]
- 17. Dibble EH, Alvarez AC, Truong MT et al. . 18F-FDG metabolic tumor volume and total glycolytic activity of oral cavity and oropharyngeal squamous cell cancer: adding value to clinical staging. J Nucl Med 2012;53:709–15. [DOI] [PubMed] [Google Scholar]
- 18. Liao S, Penney B, Wroblewski K et al. . Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2012;39:27–38. [DOI] [PubMed] [Google Scholar]
- 19. Shirata Y, Jingu K, Koto M et al. . Prognostic factors for local control of stage I non-small cell lung cancer in stereotactic radiotherapy: a retrospective analysis. Radiat Oncol 2012;7:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamamoto T, Jingu K, Shirata Y et al. . Outcomes after stereotactic body radiotherapy for lung tumors, with emphasis on comparison of primary lung cancer and metastatic lung tumors. BMC Cancer 2014;14:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fang YH, Lin CY, Shih MJ et al. . Development and evaluation of an open-source software package ‘CGITA’ for quantifying tumor heterogeneity with molecular images. Biomed Res Int 2014;2014:248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tixier F, Hatt M, Rest C et al. . Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18F-FDG PET. J Nucl Med 2012;53:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoopes D, Tann M, Fletcher J et al. . FDG-PET and stereotactic body radiotherapy (SBRT) for stage I non-small-cell lung cancer. Lung Cancer 2007;56:229–34. [DOI] [PubMed] [Google Scholar]
- 24. Burdick M, Stephans K, Reddy C et al. . Maximum standardized uptake value from staging FDG-PET/CT does not predict treatment outcome for early-stage non-small-cell lung cancer treated with stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:1033–9. [DOI] [PubMed] [Google Scholar]
- 25. El Naqa I, Grigsby P, Apte A et al. . Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit 2009;42:1162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng NM, Fang YH, Lee LY et al. . Zone-size nonuniformity of 18F-FDG PET regional textural features predicts survival in patients with oropharyngeal cancer. Eur J Nucl Med Mol Imaging 2015;42:419–28. [DOI] [PubMed] [Google Scholar]
- 27. Hyun S, Kim H, Choi S et al. . Intratumoral heterogeneity of 18F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging 2016;43:1461–8. [DOI] [PubMed] [Google Scholar]
- 28. Cook G, Yip C, Siddique M et al. . Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med 2013;54:19–26. [DOI] [PubMed] [Google Scholar]
- 29. Ypsilantis PP, Siddique M, Sohn HM et al. . Predicting response to neoadjuvant chemotherapy with PET imaging using convolutional neural networks. PLoS One 2015;10:e0137036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verstegen N, Lagerwaard F, Haasbeek C et al. . Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol 2011;101:250–4. [DOI] [PubMed] [Google Scholar]