Introduction
At the beginning of 2016, an increase in paediatric haemolytic uremic syndrome (HUS) cases was observed in Romania. The microbiological investigations allowed isolation of Shiga toxin-producing Escherichia coli (STEC) O26 as the causative agent from most cases. Methods: An enhanced national surveillance of HUS and severe diarrhoea was established across the country following the identification of the first cases and was carried out until August 2016. A total of 15 strains were isolated from 10 HUS and five diarrhoea cases. Strains were characterised by virulence markers (i.e. stx type/subtype, eae, ehxA genes), phylogroup, genetic relatedness and clonality using PCR-based assays, PFGE and multilocus sequence typing (MLST). The first six strains were further characterised by whole genome sequencing (WGS). Results: Five PCR-defined genotypes were distinguished. All strains from HUS cases harboured stx2a and eae, with or without stx1a, while strains from diarrhoea cases carried exclusively stx1a and eae genes. PFGE resolved strains into multiple pulsotypes, compatible with a certain geographic segregation of the cases, and strains were assigned to phylogroup B1 and sequence type (ST) 21. WGS confirmed the results of conventional molecular methods, brought evidence of O26:H11 serotype, and complemented the virulence profiles. Discussion/conclusion: This first description of STEC O26 strains from cases in Romania showed that the isolates belonged to a diverse population. The virulence content of most strains highlighted a high risk for severe outcome in infected patients. Improving the national surveillance strategy for STEC infections in Romania needs to be further considered.
Keywords: Shiga toxin-producing E. coli, STEC, STEC O26, laboratory surveillance, molecular methods, food-borne infections, haemolytic uremic syndrome
Introduction
The role of Shiga toxin-producing Escherichia coli (STEC) in outbreaks of food-borne illnesses is well recognised [1]. According to surveillance studies, STEC of serotype O157:H7 are those most often associated with epidemics of STEC infections worldwide [2-4] and most likely to cause severe infections with systemic complications such as haemolytic uremic syndrome (HUS) [5]. Nonetheless, STEC belonging to non-O157 serogroups raised public health concern in many countries by displaying the same pathogenic potential as O157:H7 [6-8]. Among them, STEC O26 poses a significant threat to human health in terms of illness severity and the risk of causing outbreaks. This serogroup is considered emerging in Europe and in the last decade, it was reported as the most frequent non-O157 STEC serogroup in human sporadic cases of infection, including those developing HUS [9]. Severe outbreaks of STEC O26 infection have been reported both in community [6] and childcare settings [10]. In 2013, the emergence of a highly virulent clone of STEC O26 strongly associated with HUS in Europe was described in the scientific literature [11].
At the beginning of 2016, Romania alerted the European Centre for Disease Prevention and Control (ECDC) of an unprecedented rise in haemolytic uremic syndrome (HUS) cases in the paediatric population. It reported on 15 children aged between 5 and 38 months who were diagnosed with HUS, some of whom progressed to HUS after bloody or non-bloody diarrhoea. Cases were linked to STEC O26 infection by serology and most resided in one of the southern districts [12]. Food consumption history of cases suggested that dairy products from a local producer were among the foodstuff potentially implicated in the outbreak as the source or vehicle of infection, but this suspicion was not confirmed microbiologically. Soon after the description of the first cases in Romania, Italy reported one HUS case with epidemiological link to Romania through the Early Warning and Response System (EWRS) of the European Union, suggesting that a multi-country outbreak of STEC infections was ongoing [13].
Microbiological characterisation and typing were carried out on STEC strains isolated from children with HUS, bloody or non-bloody diarrhoea identified through the ad hoc enhanced surveillance established in Romania following the identification of the outbreak. The aim was to provide insight into the epidemiology of STEC O26 infection. The goal of the presented microbiological investigation was to provide evidence for the national public health authorities of the relevance of adopting typing/subtyping strategies to support surveillance of STEC infection in humans.
Methods
Strain collection
Fifteen STEC O26 strains that were recovered from the stool samples of 15 children hospitalised in Romania for HUS (10 cases), bloody diarrhoea (one case) or non-bloody diarrhoea (four cases) were characterised in this study. The children had a median age of 12 months (range 6–24 months) and the male to female ratio was 1.14. They resided in several districts across the country (Figure 1). The strains were given identification numbers from 1 to 15 in accordance with the chronological order of isolation (Table 1).
Figure 1.
Geographic distribution of the children with culture-confirmed Shiga toxin-producing Escherichia coli O26 HUS, bloody or non-bloody diarrhoea included in the study, Romania, 2016 (n = 5)
AG: Arges; B: Bucharest; BC: Bacau; CV: Covasna; HUS: haemolytic uremic syndrome; IL: Ialomita; IS: Iasi; SB: Sibiu; SV: Suceava.
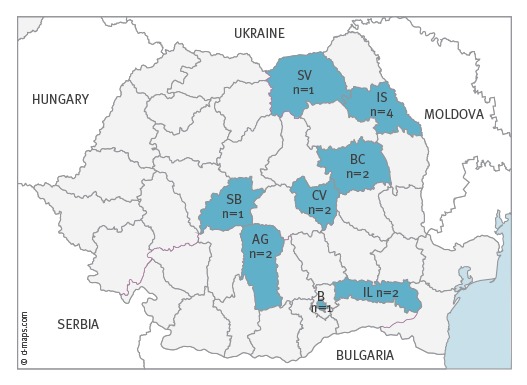
Table 1. Clinical and epidemiological information associated with the Shiga toxin-producing Escherichia coli O26 strains isolated from the stool samples of children, Romania, 2016 (n = 15).
| Strain identification numbera | Month of isolation | Clinical diagnostic | District of residence |
|---|---|---|---|
| 1b | February | HUS | Bacau |
| 2b | February | HUS | Arges |
| 3b | February | BD | Sibiu |
| 4b | February | HUS | Bucharest |
| 5b | March | HUS | Ialomita |
| 6b | March | HUS | Ialomita |
| 7 | April | D | Suceava |
| 8 | April | HUS | Iasi |
| 9 | April | D | Iasi |
| 10 | April | HUS | Iasi |
| 11 | May | HUS | Arges |
| 12 | May | HUS | Bacau |
| 13 | May | HUS | Iasi |
| 14 | May | D | Covasna |
| 15 | June | D | Covasna |
BD: bloody diarrhoea; D: diarrhoea; HUS: haemolytic uremic syndrome.
a The strains were given identification numbers from 1 to 15 in accordance with the chronological order of isolation.
b Strain subjected to whole genome sequencing.
Shiga toxin-producing Escherichia coli O26 characterisation
The strains, biochemically confirmed as E. coli, were typed as O26 using slide agglutination with commercially available OK O pool and single antisera (SSI Diagnostica, Hillerød, Denmark). Phenotypical test for H antigen identification was not performed. The presence of genes considered as hallmarks for the STEC pathotype was assessed using a multiplex PCR-based commercial kit (DEC Primer Mix, SSI Diagnostica, Hillerød, Denmark). The kit contained a mix of primers directed towards stx1 (Shiga toxin 1), stx2 (Shiga toxin 2), eae (intimin), eltA (heat-labile enterotoxin), estA (heat-stable enterotoxin, human and porcine variants), and ipaH (invasive plasmid antigen) genes, suited for the distinction between STEC, enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC) and enteroinvasive E. coli (EIEC) strains. Additional commercially available primers (EAEC PCR kit, SSI Diagnostica, Hillerød, Denmark) were used to detect enteroaggregative E. coli (EAEC)-associated genes attA, aggR, aap, and aaiC. Enterohaemolysin-encoding gene ehxA was identified using primers described by Schmidt et al. [14].
stx gene subtype detection by conventional PCR
The stx1 and stx2 genes were subtyped using published primers and protocols [15]. The complete set of strains harbouring the genes encoding all stx subtypes, which were used as PCR positive controls, were kindly provided by the World Health Organization (WHO) Collaborating Centre for Reference and Research on Escherichia and Klebsiella from Statens Serum Institute, Denmark.
Phylogenetic group typing, PFGE, and multilocus sequence typing
Phylogenetic group assignment of the STEC O26 strains was performed according to the method described by Clermont et al. [16].
PFGE was performed using the protocol approved by ECDC [17] and the cluster analysis was performed with BioNumerics software (Version 6.6, Applied Maths, Sint-Martens-Latem, Belgium). DNA fragments smaller than 33 kb were not included in the analysis. The dendrogram of similarity was generated using the band-based Dice similarity coefficient (with 1.5% band position and 1.5% optimisation tolerance), and the unweighted pair group method with arithmetic mean (UPGMA). Clusters were defined as a group of profiles sharing ≥ 93% similarity, corresponding to a ca 3-bands difference [18].
All study strains were subjected to standard multilocus sequence typing (MLST) with primers and protocols specified at the E. coli MLST website [19].
Whole genome sequencing and bioinformatic analysis
An Ion Torrent Personal Genome Machine (Thermo Fisher Scientific, Waltham, United States (US)) was used for the whole genome sequencing (WGS) of six STEC O26 strains obtained from the first culture-confirmed cases of infection. Five of the sequenced strains (i.e. strains 1, 2, 4–6) were isolated from stool specimens of children with HUS and one (i.e. strain 3) was isolated from the faecal sample of a child hospitalised with gastrointestinal symptoms.
The DNA was extracted using PureLink Genomic DNA Mini Kit (Invitrogen, Thermo Fisher Scientific, Waltham, US), according to the manufacturer’s protocol. Enzymatic shearing of genomic DNA samples and generation of barcoded libraries were carried out using the Ion Xpress Plus Fragment Library Kit and Ion Xpress Barcode Adapters Kit (Thermo Fisher Scientific, Waltham, US). Libraries were size-selected by electrophoresis using E-Gel SizeSelect 2% gels (Thermo Fisher Scientific, Waltham, US) and templates were prepared using the Ion PGM Hi-Q OT2 Kit and the Ion OneTouch 2 System. Sequencing was conducted on an Ion 318 chip Kit using the Ion PGM Hi-Q 400bp Sequencing Kit (Thermo Fisher Scientific, Waltham, US). The sequence raw data were analysed using the tools present in the Advanced Research Infrastructure for Experimentation in genomics (ARIES) public Galaxy server [20].
The raw reads were trimmed to remove the adaptors and to accept 25 as the lowest Phred value. The detection of the virulence genes was performed through the virulotyper pipeline, which performs mapping of sequencing reads on the database of sequences of E. coli virulence genes [21] using the Bowtie2 tool [22]. Typing of the intimin-coding genes was performed in silico by comparing the sequence of the eae allele identified in the database against the National Center for Biotechnology Information (NCBI) Reference Sequence Database (RefSeq).
The trimmed reads were subjected to de novo assembly using the tool SPADES with default parameters (k-mers length set at 21, 33 and 55) [23]. The obtained contigs were filtered to accept a minimum length of 1,000 bp and used 10 as coverage cut-off. The NCBI Basic Local Alignment Search Tool (BLAST) + blastn tool was used to determine the serotype through alignment of the assembled contigs with the database containing the sequences of serotype-associated genes of pathogenic E. coli [24]. In silico MLST was performed by interrogating the database downloaded from the MLST website [19] using the SRST2 software [25].
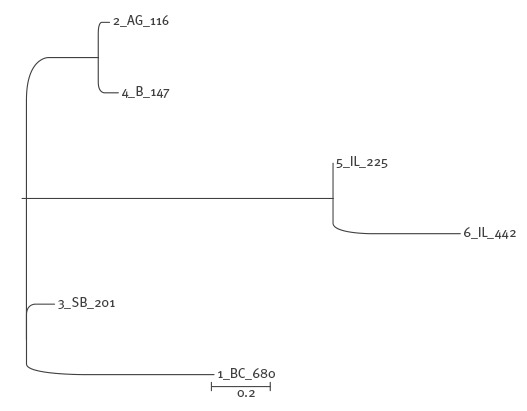
Whole genome single nucleotide polymorphisms (WG SNP) analysis was performed using the kSNP3 pipeline [26] and 19 as kmer size. The optimum value for the kmer size was selected as that producing the highest number of unique kmers of the median length in all the genomes of the dataset, and was calculated by using the kchooser tool included in the kSNP3 pipeline [26]. The dendrogram was obtained by using the maximum likelihood clustering algorithm [27] (Figure 2).
Figure 2.
Phylogenetic analysis of six Shiga toxin-producing Escherichia coli O26 strains part of the outbreak according to whole genome single nucleotide polymorphisms (WG SNP) analysis, Romania, 2016
The tree was obtained by using the kSNP3 tool to identify the total SNPs in the set of sequences analysed. The phylogenetic tree was estimated based upon those SNPs using the Maximum Likelihood clustering algorithm. The names of the strains include the strain identification number, the abbreviation for the district of residence of the child from whom the strain was isolated and the strain specific alleles count, each separated by an underscore. The scale of 0.2 indicates the branch lengths, expressed in terms of changes per number of SNPs.
The sequencing reads and the corresponding assembled contigs have been deposited in the European Molecular Biology Laboratory (EMBL)-European Nucleotide Archive (ENA) sequence database (Accession number PRJEB19376).
Results
PCR-based virulotyping of Shiga toxin-producing Escherichia coli O26 strains
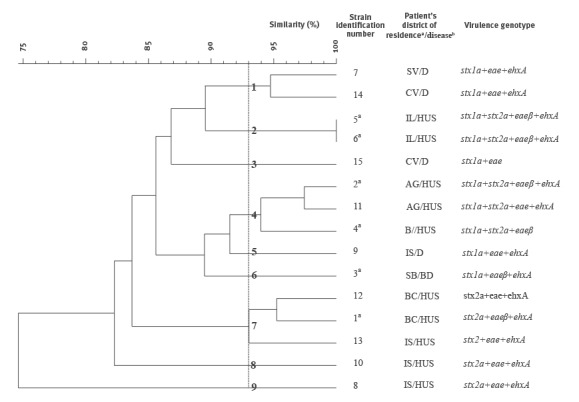
The study collection included 15 STEC O26 strains isolated from cases with HUS (n = 10), bloody diarrhoea (n = 1) or non-bloody diarrhoea (n = 4) reported across Romania between February and June 2016. PCR assays showed that 10 strains were positive for stx2 gene, either alone (n = 5) or in combination with stx1 (n = 5), while the remaining five were positive for stx1 gene alone. Subtyping of the stx genes showed that the stx1-positive strains carried stx1a subtype and the stx2-positive strains harboured stx2a subtype. The eae gene was found in all the strains and ehxA in all but two of them (Figure 3). While eae and ehxA genes were detected irrespective of the strain origin, a difference in the distribution of the Shiga toxin-coding genes was observed. In fact, stx2 gene alone or associated with stx1 was found only in HUS-associated strains while stx1 as sole stx gene was detected only in the strains recovered from children with diarrhoea. No other pathogenic E. coli virulence genes were identified.
Figure 3.
Dendrogram of XbaI PFGE profiles showing the genetic relatedness of Shiga toxin-producing Escherichia coli O26 strains and associated bacterial virulence genes, Romania, 2016
AG: Arges; B: Bucharest; BC: Bacau; BD, bloody diarrhoea; CV: Covasna; D: diarrhoea; HUS: haemolytic uremic syndrome; IL: Ialomita; IS: Iasi; SB: Sibiu; SV: Suceava.
a Strain subjected to whole genome sequencing.
Phylogenetic group typing, PFGE and multilocus sequence typing
The STEC O26 strains assayed in this study were classified into phylogenetic group B1 and sequence type ST21. All the strains yielded interpretable XbaI PFGE profiles that clustered on the dendrogram with 74.5% overall similarity (Figure 3). The strains were assigned to 14 different pulsotypes all but one including only one isolate. The comparative analysis showed five single pulsotype clusters (strain identification numbers 3, 5, 6, 8, and 9) and four multiple-pulsotype clusters showing over 93% of similarity (strain identification numbers 1, 2, 4, and 7). Two of the latter contained three strains each (Figure 3). Not all the strains belonging to multiple-strain clusters could be assigned to similar geographic areas. Nevertheless, the two strains showing an indistinguishable profile (pulsotype 2) were recovered from cases from the same district. Also clusters 4 and 7 contained strains isolated from cases residing in the same district and in a neighbouring one (i.e. Iasi and Bacau; Arges and Bucharest) (Figure 1).
Genomic characterisation and typing
In silico WGS-based O and H serotyping assigned the six sequenced strains to the serotype O26:H11, a piece of information that complemented the conventional serotyping which was restricted to O antigen identification. Moreover, WGS confirmed that all these strains were indeed members of ST21 clone.
The results of the virulotyper confirmed the genotypes identified through PCR and additionally identified accessory virulence genes in six strains that were analysed (Table 2). The comparison of the eae gene sequence identified in the tested strains with those in the NCBI Reference Sequence Database (RefSeq) allowed identification of the type β intimin allele in all the genomes. A limited variation in the gene content was observed among the sequenced isolates mainly for genes usually located on plasmids. In detail, only two isolates harboured the plasmid-borne cba gene, encoding colicin B. One strain lacked the ehxA, espP, katP and toxB genes, described to be conveyed by the virulence plasmid of STEC O26 (Table 2) and that were present in all the other genomes analysed.
Table 2. Virulence genes identified in the Shiga toxin-producing Escherichia coli O26 strains subjected to whole genome sequencing, Romania, 2016.
| Strain identification number | Virulence genes | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cba | cif | eae | efa1 | ehxA | epeA | espA | espB | espF | espJ | espP | gad | iha | iss | katP | lpfA | nleA | nleB | nleC | stx1a | stx2a | tir | toxB | |
| 1 | – | + | + | + | + | – | + | + | + | + | + | + | + | + | + | + | + | + | + | – | + | + | + |
| 2 | – | + | + | + | + | – | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 3 | – | + | + | + | + | – | + | + | + | + | + | + | + | + | + | + | + | + | + | + | – | + | + |
| 4 | – | + | + | + | – | – | + | + | + | + | – | + | + | + | – | + | + | + | + | + | + | + | – |
| 5 | + | + | + | + | + | – | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 6 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
+: present; –: absent.
The whole genome SNPs comparison allowed for producing a dendrogram with topology compatible with that obtained through PFGE typing. The high resolution of the SNPs analysis allowed identification of differences between the strains that appeared indistinguishable using PFGE (Figure 2). In detail, two clear clusters were identified in the dendrogram produced from the results of the SNPs analysis (Figure 2), one including the strains 5 and 6 (cluster 2 in Figure 3) and a second one grouping the strains 2 and 4 (part of cluster 4 in Figure 3).
Discussion
This study focuses on the microbiological characterisation of STEC O26 strains isolated from cases identified in Romania during a community-wide outbreak of STEC-associated HUS [13]. The combined use of microbiological and serological techniques, the latter performed with ECDC support, brought evidence that STEC belonging to serogroup O26 were the cause of infection in most of the patients involved in the outbreak [13].
An outbreak was suspected because of the high number of HUS cases observed in a short period of time (January and February 2016) among children from Arges district in Romania; this was a twofold increase compared with cases reported in the six previous years by four regional Romanian hospitals, Bucharest, Cluj, Iasi and Timisoara [13]. This triggered a more thorough investigation on STEC infection in the country. Active case finding was promoted at the national level by the public health authorities on 15 February 2016 and resulted in the identification of cases that could have remained undetected. The enhanced surveillance based on the reporting of cases presenting with HUS or severe diarrhoea to the national health authorities revealed that STEC cases had a wide geographical distribution, with cases in seven districts in addition to those in Arges.
Epidemic cases were defined according to the ECDC case definition [13]. Before outbreak onset, difficulties in monitoring HUS across the country were due to the lack of mandatory reporting of HUS as well as the limited familiarity of many physicians with the clinical course of STEC infection and HUS owing to the rare occurrence of this condition.
STEC O26 strains were obtained from patients involved in the outbreak across the country. One of the strains, strain 2, was isolated from a child from Arges district with a history of exposure to the dairy products initially suspected to be implicated in the transmission of STEC O26 infection to the epidemic cases. The rest of the strains originated from children with no obvious exposure to such products.
All the STEC O26 strains were characterised using molecular typing methods allowing identification of virulence determinants and the clonal relationship. Virulence genes typing showed that the strains from all the children with HUS carried the stx2a gene, which is common in STEC O26 associated with HUS, especially in small children under 5 years of age [3,28-30]. By contrast, STEC O26 strains harbouring only stx1, usually configuring a profile of less severe clinical outcome [3,28,31], were found in children who did not progress to HUS. Irrespective of the clinical features, all the STEC O26 strains possessed the intimin-coding gene eae which indicated that these strains were capable of producing attaching and effacing lesions in the intestinal mucosa [32]. Additionally, most strains were positive for enterohaemolysin-coding gene ehxA, a marker of the large virulence plasmids associated with the most pathogenic STEC strains involved in human illness [33,34]. The first six strains recovered during the outbreak were further characterised through WGS, which allowed identification of additional virulence genes, type all the sequenced strains as O26:H11, and the intimin coding gene as eaeβ. Unfortunately, due to limited resources and time constraints, the rest of the STEC O26 strains could not be sequenced prior to manuscript submission.
The Romanian strains studied were exclusively typed as ST21, the clone that includes the majority of human STEC O26 strains isolated from clinical cases of STEC infection in Europe [11]. With one exception (i.e. strain 4), the sequenced strains were also shown to harbour the classic plasmid gene profile described in the vast majority of STEC O26 belonging to ST21 [11], including positivity for the three virulence genes exhA, katP and espP. The sequence type and the plasmid profile indicate that the strains analysed did not belong to the emerging STEC O26 ST29 clone, which has been increasingly described in Europe [11,35,36].
When analysing strains through PFGE and WGS, they were found to belong to a heterogeneous population. Interestingly, strains with the highest similarity in PFGE (> 97.4%) were isolated from patients residing in the same district (strains 5, 6 from Ialomita patients and strains 2, 11 from Arges patients) suggesting a link among some cases. The heterogeneity of the isolates suggested that the outbreak had a possible multi-clonal aetiology, similarly to the STEC O26 outbreak observed in Italy in 2013 [6] However, the heterogeneity of isolates could also reflect the low specificity of the confirmed case definition adopted at the European level [13], possibly leading to a misclassification of sporadic cases of STEC O26 infection as part of the outbreak.
Multiple aetiology outbreaks (i.e. outbreaks with multiple causative agents or multiple clones belonging to the same agent) are often reported in community-wide outbreaks of non-O157 STEC infection [8]. This highlights the importance of applying molecular typing techniques that are able to discriminate the clonal relationship of strains belonging to the same STEC serotypes, especially for those that are most frequently reported in humans.
A possible selection bias of the STEC O26 strains subjected to characterisation should be mentioned. The strains in this study were not necessarily representative of all the incident cases of STEC O26 infections occurring in Romania during the epidemic period as cultures from the earliest HUS cases were not available and milder clinical cases could have been overlooked. On the other hand, the opportunity to examine a set of clinical isolates from several regions of Romania and the results from the extensive molecular characterisation, including whole genome sequencing, served as reference data for Romanian STEC O26 strains and are suitable for comparison with similar human, food or animal isolates at a national or international level.
The cause(s) of the outbreak observed in Romania remained unknown in spite of evidence of an epidemiological link with the consumption of contaminated dairy products from local producers [13]. None of the dairy products sampled in Romania tested positive for STEC [13]. Interestingly, during the epidemic period in Romania, one case of HUS caused by STEC O26 infection and epidemiologically linked to the cases in Romania was described in Italy [13].
In conclusion, this study provides data on the characterisation of STEC O26 strains isolated from human cases of disease in Romania for first time and it describes a rather genetically heterogeneous population of STEC O26 belonging to the ST21 clone, and possessing virulence gene combinations able to cause severe condition such as HUS.
The 2016 outbreak clearly showed the need to rapidly detect and characterise the STEC strains causing human diseases with molecular typing techniques in order to understand their epidemiology and circulation, and thereby support targeted measures that limit human exposure to the source of infection. As part of an effective strategy for the control of STEC infection in the population, the adoption of a sensitive national surveillance system for STEC infection and HUS is desirable. The system should be capable of providing data on the incidence of HUS and STEC infection at both the national and regional level, and describing the characteristics of both clinical cases and STEC strains. The timely subtyping and assessment of virulence profiles of STEC strains isolated from patients would facilitate the early detection of outbreaks in the communities. For future research the whole genome sequence data will be used to understand the genetic diversity and clonal relatedness of the STEC O26 strains isolated from patients in Romania and in Italy during the 2016 outbreak.
Acknowledgements
We thank Mihaela Andrei and Andrei Popa for their skilful technical assistance in isolation of the E. coli strains. We gratefully acknowledge the contribution of the local health departments, all the laboratories and hospitals, and the National Center for Surveillance and Control of Communicable Diseases (National Institute of Public Health, Bucharest) linked to this study. We thank Ettore Severi, Otilia Mårdh, Emilie Peron and Johanna Takkinen at the European Center for Disease Prevention and Control (ECDC) for believing in our work.
The whole genome sequencing work was technically supported by SC Antisel RO SRL and we especially thank Dr Nikos Davanos (ANTISEL SA, Greece) for his valuable guidance.
Conflict of interest: None declared.
Authors’ contributions: Codruta-Romanita Usein wrote the manuscript, coordinated the microbial characterisation and was involved in the molecular analysis. Cornelia Madalina Militaru participated in the molecular characterisation of the strains. Maria Condei performed PFGE. Sorin Dinu and Mihaela Oprea took part in the molecular investigation and did the whole genome sequencing. Adriana Simona Ciontea and Daniela Cristea performed the phenotypic analysis. Lavinia Cipriana Zota and Alina Zaharia performed surveillance activities. Valeria Michelacci, Gaia Scavia and Stefano Morabito performed the bioinformatics analysis of the whole genome sequencing data, contributed to data interpretation and revised the manuscript critically. All authors read and approved the final manuscript.
References
- 1. Caprioli A, Morabito S, Brugère H, Oswald E. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet Res. 2005;36(3):289-311. 10.1051/vetres:2005002 [DOI] [PubMed] [Google Scholar]
- 2. Adams NL, Byrne L, Smith GA, Elson R, Harris JP, Salmon R, et al. Shiga Toxin-Producing Escherichia coli O157, England and Wales, 1983-2012. Emerg Infect Dis. 2016;22(4):590-7. 10.3201/eid2204.151485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brandal LT, Wester AL, Lange H, Løbersli I, Lindstedt BA, Vold L, et al. Shiga toxin-producing escherichia coli infections in Norway, 1992-2012: characterization of isolates and identification of risk factors for haemolytic uremic syndrome. BMC Infect Dis. 2015;15(1):324. 10.1186/s12879-015-1017-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heiman KE, Mody RK, Johnson SD, Griffin PM, Gould LH. Escherichia coli O157 Outbreaks in the United States, 2003-2012. Emerg Infect Dis. 2015;21(8):1293-301. 10.3201/eid2108.141364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365(9464):1073-86. 10.1016/S0140-6736(05)71144-2 [DOI] [PubMed] [Google Scholar]
- 6. Germinario C, Caprioli A, Giordano M, Chironna M, Gallone MS, Tafuri S, et al. Community-wide outbreak of haemolytic uraemic syndrome associated with Shiga toxin 2-producing Escherichia coli O26:H11 in southern Italy, summer 2013. Euro Surveill. 2016;21(38):30343. 10.2807/1560-7917.ES.2016.21.38.30343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuehne A, Bouwknegt M, Havelaar A, Gilsdorf A, Hoyer P, Stark K, et al. Estimating true incidence of O157 and non-O157 Shiga toxin-producing Escherichia coli illness in Germany based on notification data of haemolytic uraemic syndrome. Epidemiol Infect. 2016;144(15):3305-15. 10.1017/S0950268816001436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luna-Gierke RE, Griffin PM, Gould LH, Herman K, Bopp CA, Strockbine N, et al. Outbreaks of non-O157 Shiga toxin-producing Escherichia coli infection: USA. Epidemiol Infect. 2014;142(11):2270-80. 10.1017/S0950268813003233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Messens W, Bolton D, Frankel G, Liebana E, McLauchlin J, Morabito S, et al. Defining pathogenic verocytotoxin-producing Escherichia coli (VTEC) from cases of human infection in the European Union, 2007-2010. Epidemiol Infect. 2015;143(8):1652-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dallman TJ, Byrne L, Launders N, Glen K, Grant KA, Jenkins C. The utility and public health implications of PCR and whole genome sequencing for the detection and investigation of an outbreak of Shiga toxin-producing Escherichia coli serogroup O26:H11. Epidemiol Infect. 2015;143(8):1672-80. 10.1017/S0950268814002696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bielaszewska M, Mellmann A, Bletz S, Zhang W, Köck R, Kossow A, et al. Enterohemorrhagic Escherichia coli O26:H11/H-: a new virulent clone emerges in Europe. Clin Infect Dis. 2013;56(10):1373-81. 10.1093/cid/cit055 [DOI] [PubMed] [Google Scholar]
- 12. Peron E, Zaharia A, Zota LC, Severi E, Mårdh O, Usein C, et al. Early findings in outbreak of haemolytic uraemic syndrome among young children caused by Shiga toxin-producing Escherichia coli, Romania, January to February 2016. Euro Surveill. 2016;21(11):30170. 10.2807/1560-7917.ES.2016.21.11.30170 [DOI] [PubMed] [Google Scholar]
- 13.European Centre for Disease Prevention and Control (ECDC) and European Food Safety Authority. (EFSA). Multi-country outbreak of Shiga toxin-producing Escherichia coli infection associated with haemolytic uraemic syndrome. Stockholm: ECDC; 5 Apr 2016. Available from: http://ecdc.europa.eu/en/publications/Publications/RRA-Escherichia-coli-O26-Romania-Italy-April2016.pdf
- 14. Schmidt H, Beutin L, Karch H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect Immun. 1995;63(3):1055-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scheutz F, Teel LD, Beutin L, Piérard D, Buvens G, Karch H, et al. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J Clin Microbiol. 2012;50(9):2951-63. 10.1128/JCM.00860-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep. 2013;5(1):58-65. 10.1111/1758-2229.12019 [DOI] [PubMed] [Google Scholar]
- 17. Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, et al. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog Dis. 2006;3(1):59-67. 10.1089/fpd.2006.3.59 [DOI] [PubMed] [Google Scholar]
- 18. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33(9):2233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, et al. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006;60(5):1136-51. 10.1111/j.1365-2958.2006.05172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Union Reference Laboratory VTEC. Advanced Research Infrastructure for Experimentation in Genomics (ARIES) public Galaxy server. Rome: Istituto Superiore di Sanità (ISS). [Accessed 20 Jun 2016]. Available from: https://w3.iss.it/site/aries/
- 21. Joensen KG, Scheutz F, Lund O, Hasman H, Kaas RS, Nielsen EM, et al. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J Clin Microbiol. 2014;52(5):1501-10. 10.1128/JCM.03617-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357-9. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455-77. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joensen KG, Tetzschner AM, Iguchi A, Aarestrup FM, Scheutz F. Rapid and Easy In Silico Serotyping of Escherichia coli Isolates by Use of Whole-Genome Sequencing Data. J Clin Microbiol. 2015;53(8):2410-26. 10.1128/JCM.00008-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inouye M, Dashnow H, Raven LA, Schultz MB, Pope BJ, Tomita T, et al. SRST2: Rapid genomic surveillance for public health and hospital microbiology labs. Genome Med. 2014;6(11):90. 10.1186/s13073-014-0090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gardner SN, Slezak T, Hall BG. kSNP3.0: SNP detection and phylogenetic analysis of genomes without genome alignment or reference genome. Bioinformatics. 2015;31(17):2877-8. 10.1093/bioinformatics/btv271 [DOI] [PubMed] [Google Scholar]
- 27. Wilks SS. The Large-Sample Distribution of the Likelihood Ratio for Testing Composite Hypotheses. Ann Math Stat. 1938;9(1):60-2. 10.1214/aoms/1177732360 [DOI] [Google Scholar]
- 28. Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J Clin Microbiol. 1999;37(3):497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Persson S, Olsen KE, Ethelberg S, Scheutz F. Subtyping method for Escherichia coli shiga toxin (verocytotoxin) 2 variants and correlations to clinical manifestations. J Clin Microbiol. 2007;45(6):2020-4. 10.1128/JCM.02591-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Werber D, Fruth A, Buchholz U, Prager R, Kramer MH, Ammon A, et al. Strong association between shiga toxin-producing Escherichia coli O157 and virulence genes stx2 and eae as possible explanation for predominance of serogroup O157 in patients with haemolytic uraemic syndrome. Eur J Clin Microbiol Infect Dis. 2003;22(12):726-30. 10.1007/s10096-003-1025-0 [DOI] [PubMed] [Google Scholar]
- 31. Buvens G, De Gheldre Y, Dediste A, de Moreau AI, Mascart G, Simon A, et al. Incidence and virulence determinants of verocytotoxin-producing Escherichia coli infections in the Brussels-Capital Region, Belgium, in 2008-2010. J Clin Microbiol. 2012;50(4):1336-45. 10.1128/JCM.05317-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123-40. 10.1038/nrmicro818 [DOI] [PubMed] [Google Scholar]
- 33. Brunder W, Schmidt H, Frosch M, Karch H. The large plasmids of Shiga-toxin-producing Escherichia coli (STEC) are highly variable genetic elements. Microbiology. 1999;145(5):1005-14. 10.1099/13500872-145-5-1005 [DOI] [PubMed] [Google Scholar]
- 34. Fratamico PM, Yan X, Caprioli A, Esposito G, Needleman DS, Pepe T, et al. The complete DNA sequence and analysis of the virulence plasmid and of five additional plasmids carried by Shiga toxin-producing Escherichia coli O26:H11 strain H30. Int J Med Microbiol. 2011;301(3):192-203. 10.1016/j.ijmm.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 35. Marejková M, Bláhová K, Janda J, Fruth A, Petráš P. Enterohemorrhagic Escherichia coli as causes of hemolytic uremic syndrome in the Czech Republic. PLoS One. 2013;8(9):e73927. 10.1371/journal.pone.0073927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Januszkiewicz A, Wołkowicz T, Chróst A, Szych J. Characterization of the Shiga toxin-producing Escherichia coli O26 isolated from human in Poland between 1996 and 2014. Lett Appl Microbiol. 2015;60(6):605-8. 10.1111/lam.12413 [DOI] [PubMed] [Google Scholar]


