ABSTRACT
Background: The inflammatory milieu in the liver as determined by histopathology is different in individual patients undergoing autologous islet cell transplantation. We hypothesized that inflammation related to fatty-liver adversely impacts islet survival. To test this hypothesis, we used a mouse model of fatty-liver to determine the outcome of syngeneic islet transplantation after chemical pancreatectomy. Methods: Mice (C57BL/6) were fed a high-fat-diet from 6 weeks of age until attaining a weight of ≥28 grams (6–8 weeks) to produce a fatty liver (histologically > 30% fat);steatosis was confirmed with lipidomic profile of liver tissue. Islets were infused via the intra-portal route in fatty-liver and control mice after streptozotocin induction of diabetes. Outcomes were assessed by the rate of euglycemia, liver histopathology, evaluation of liver inflammation by measuring tissue cytokines IL-1β and TNF-α by RT-PCR and CD31 expression by immunohistochemistry. Results: The difference in the euglycemic fraction between the normal liver group (90%, 9/10) and the fatty-liver group (37.5%, 3/8) was statistically significant at the 18th day post- transplant and was maintained to the end of the study (day 28) (p = 0.019, X2 = 5.51). Levels of TNF–α and IL-1β were elevated in fatty-liver mice (p = 0.042, p = 0.037). Compared to controls cytokine levels were elevated after islet cell transplantation and in transplanted fatty-liver mice as compared to either fatty- or islet transplant group alone (p = NS). A difference in the histochemical pattern of CD31 could not be determined. Conclusion: Fatty-liver creates an inflammatory state which adversely affects the outcome of autologous islet cell transplantation.
KEYWORDS: auto-islet transplant, chronic pancreatitis, hepatic steatosis, islet transplant, islets
Introduction
The liver has been the site of choice for islet transplantation in clinical practice. In recent years it has become increasingly recognized that intra-portal infusion of isolated islet cells may not provide the ideal microenvironment for islets due to various factors that contribute to the loss of islet mass early after infusion.1 Many crucial events occurring in the hours and days after islet infusion influence the success of transplantation. During islet infusion an instant blood-mediated inflammatory reaction (IBMIR) is elicited when islets are exposed to blood and involves the coagulation cascade including complement activation.2, 3 The inflammatory process is triggered by tissue factors secreted by endocrine cells, which leads to the generation of thrombin. Thrombin-activated platelets bind to the islet surface and then the amplification loop involving factor XI and activated platelets generate a fibrin capsule surrounding the islets.4-6 Intra-portal islet infusion is also associated with thrombosis and hepatic tissue ischemia caused by islet entrapment in liver sinusoids that leads to sinusoidal endothelial cell activation and functional impairment.7 Finally, the IBMIR culminates in the disruption of islet morphology by infiltrating leukocytes. Polymorphonuclear cells (PMNs) are the predominant cell type infiltrating the islets, attracted by the upregulation and release of ischemia-induced molecules (i.e., tissue factor, IL-1beta, tumor necrosis factor-alpha (TNF-alpha), nitric oxide, high-mobility group box 1 (HMGB1)) and by proinflammatory signals (i.e., monocyte chemoattractant protein (MCP-1), IL-8, IL-6) released from the islet.8-10 After activation, PMNs secrete reactive oxygen species that are short-lived and lead to rapid and direct damage of the islets.11
Revascularization of transplanted islets is crucial for glucose homeostasis, not only because beta cells have a high oxygen consumption rate, but also because of the need for timely response to changes in plasma glucose concentration and nutrient-dependent hormone release directly into the blood.12 Intra-liver islet revascularization is a well-described process in which endothelial cells form intra-islet blood vessels in the first 3 to 5 days post-transplant and full blood circulation is re-established within approximately 10 to 14 days.13-18
In autologous islet cell transplant (AIT) after total pancreatectomy (TP) and infusion of islet cells into the liver via the portal vein, emphasis has been placed on selection of cases, prediction of islet yield, surgery, islet isolation and degree of pancreatic damage and fibrosis.19, 20 The livers of patients with chronic pancreatitis are less scrutinized and may not be normal. For example, many with alcoholic chronic pancreatitis have varying degree of fat or fibrosis in the liver; patients with autoimmune pancreatitis have some peri-portal inflammation. Most of the literature regarding islet engraftment biology does not emphasize the histopathological variation of the liver, nor the reaction to islet implantation. In a prior clinical observation we described three subsets of patients based on their liver histopathology.21 We noted that transaminitis following islet cell infusion, was significantly greater in the fatty-liver group. Characterized by transaminitis inflammation is a known feature of fatty-liver and provides an unstable environment for islet infusion. Steatosis in addition to the IBMIR may produce an exaggerated inflammatory response resulting in an adverse effect on engraftment and vascularization of islets. Histologic evaluation of the liver is not always done pre-AIT and the intrahepatic factors that could influence the successful outcome of islets infusion are not well defined. Factors influencing the success of AIT include the uniformity of the islet yield, isolation factors, and liver pathology. The aim of this study is to evaluate the effect of hepatic steatosis on AIT and the role of inflammation on euglycemia. Since its almost impossible to obtain uniformity in a clinical setting we developed animal experiment to study the effect of having a fatty-liver while keeping other factors uniform.
Results
Establishment of the fatty-liver mouse model
Twenty, 6-week-old, mice fed a high-fat-diet for 6–8 weeks were harvested when their body weights had exceeded 28 grams, and their IPGTT was normal and fat content of the liver was confirmed (Fig. 1). Eight mice satisfied the criteria, weight range 31–37 grams, histologically liver having at least 25–30% (confirmed at 28 days). Based on this criteria, we used other set of 20 mice and performed the experiment. However, only eight of these mice satisfied the liver fat content criteria upon sacrificed and hence those were used for the analysis.
Figure 1.
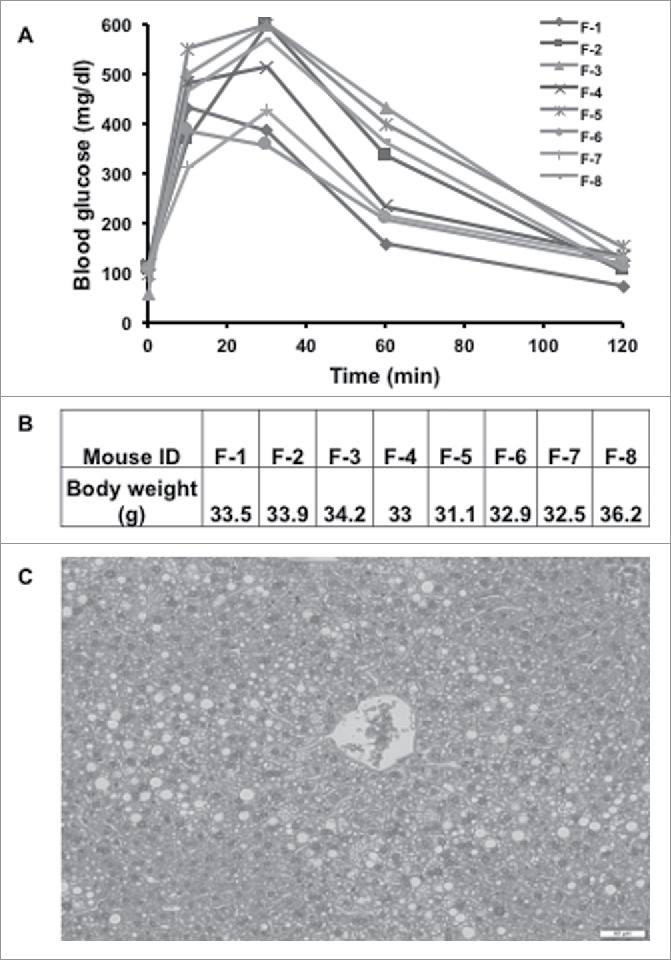
Establishment of mouse fatty liver model. (A) Glucose solution (2g/kg) was injected into the mouse peritoneal cavity after 12 hours of fasting. The blood samples were taken from the tail vein at 0, 10, 30, 60, and 120th minutes after glucose injection. Blood glucose levels were assessed with a Bayor glucometer. (B) Body weight of fatty liver mice. (C) A representative image of H&E stain on the harvested mouse liver.
Glycemic conversion after Islet transplantation
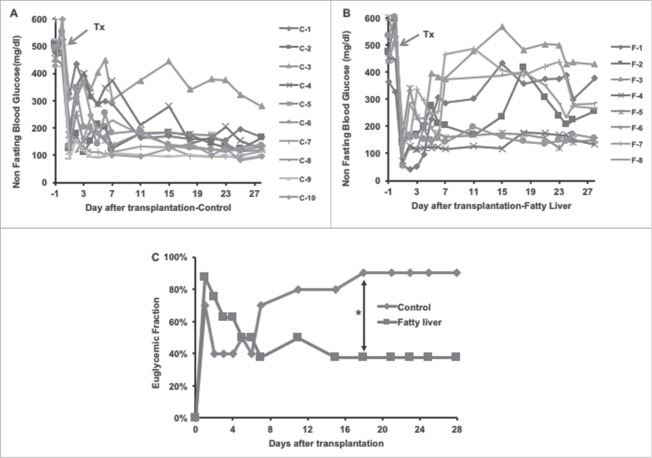
In the normal liver (control) group (Fig. 2 A), 7 out of 10 recipient mice became euglycemic on the first day after the islet transplantation, and 4 out of the 7 mice sustained euglycemia until the termination of this experiment at 28 days after transplantation. Overall, 9 of 10 mice restored normal blood glucose from day 1 to day 18 post-transplantation. In contrast to the control group, 7 out of 8 recipient mice in the fatty-liver group (Fig. 2 B) achieved normal blood glucose levels on the first day. Only 2 of the 7 mice remained euglycemic until 28 days after islet transplantation. Four of 7 mice lost graft function within one week after a short period of restoration of normal blood glucose. One recipient mouse became euglycemic 4 days after transplantation and remained euglycemic til the end of the study. The euglycemic fraction was therefore decreased from 87.5% (7/8) on the first day to 37.5% (3/8) on the 7th day after transplantation in the fatty-liver group, while it was 70% (7/10) on the first day, dropping to 40% (4/10) on the second day, and increasing gradually to 90% (9/10) by the 18th day of transplantation in the normal liver (Fig. 2 C). The difference in the euglycemic fraction between the normal liver group (90%, 9/10) and the fatty-liver group 37.5%, 3/8) was statistically significant at the 18th day post-transplantation and maintained to the end of this study (day 28) (p = 0.019, X2: 5.51).
Figure 2.
Blood glucose profile after syngeneic islet transplantation. The recipient mice were rendered diabetic by streptozotocin (200 mg/kg IP). Diabetic animals from both normal liver control group and the fatty liver group were received 500 islets by intra-portal injection. Serial blood glucose levels were measured. The non-fasting blood glucose results from the normal liver control group and the fatty liver group shown in (A) and (B) respectively. (C) Summary of the euglycemic fraction. The conversion to euglycemia was defined as glucose levels < 200 mg/dL for > 2 consecutive days.
On the H&E examination of livers harvested from mice at 28 days (Fig. 3), graft islets were observed in the recipient livers of both groups. In addition, hepatic steatosis was confirmed in the study group from the mice receiving the high-fat-diet, while the livers from mice in the control group were histologically normal in structure. Immunohistological (CD31) staining demonstrated engrafted islets in recipient livers of both groups (Fig. 4), similarly to the H&E staining result (Fig. 3). However, quantitative analysis was not possible.
Figure 3.
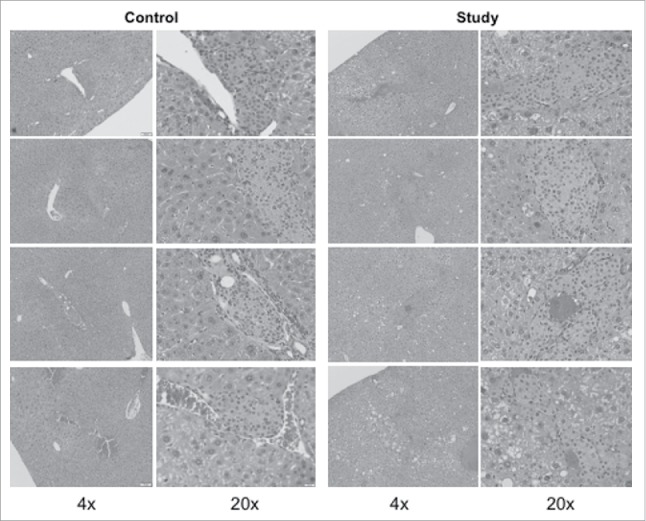
H&E stained mouse islets in the mouse livers after intra-portal transplantation. The livers from the recipient mice in both groups were harvested at 28 days after islet transplantation. The liver samples were fixed in 10% formalin, and embedded in paraffin. Five consecutive liver sections with 5-micron thickness were collected at every 100um intervals. One of 5 sections was subjected to H&E staining.
Figure 4.
Immunohistochemistry stain with CD31 on mouse islets in the mouse liver after intra-portal transplantation. The liver section from the five consecutive sections described previous in the H&E stain on the harvested liver 28 days after islet transplantation.
TNF-α and IL-1β assessment with real time RT-PCR
The expression of proinflammatory cytokines, TNF-α and IL-1β, was increased by over 50% in the fatty-liver group and the fatty-liver group after islet transplantation compared to the normal liver control group. TNF–α mRNA expression in the normal liver group demonstrated only a 1.25 ± 0.363 fold increase compared to baseline, whereas the TNF–α mRNA expression was 2.7 ± 0.499 (p = 0.02) in the fatty-liver group, and 6.68 ± 3.0 (p = 0.04) in the fatty-liver with islet transplant group, respectively. Similarly in the control group IL-1β mRNA expression only exhibited a modest increase of 1.254 ± 0.348 compared to a, 3.781 ± 0.885 (p = 0.037) level in the fatty-liver group, and 5.47 ± 1.39 (p = 0.0428) in the fatty-liver with the islet transplant group (Fig. 5).
Figure 5.
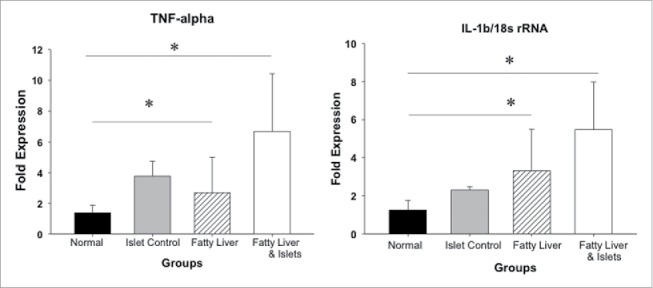
Local inflammatory responses after intra-portal islet transplantation. Five hundred mouse islets were transplanted in both normal liver and the fatty liver groups. Livers were harvested 24 hours after transplantation. mRNA expression of TNF-α and IL-β was quantified by real time RT-PCR. Data are presented as mean ± standard error, *p < 0.05.
Lipodomics study
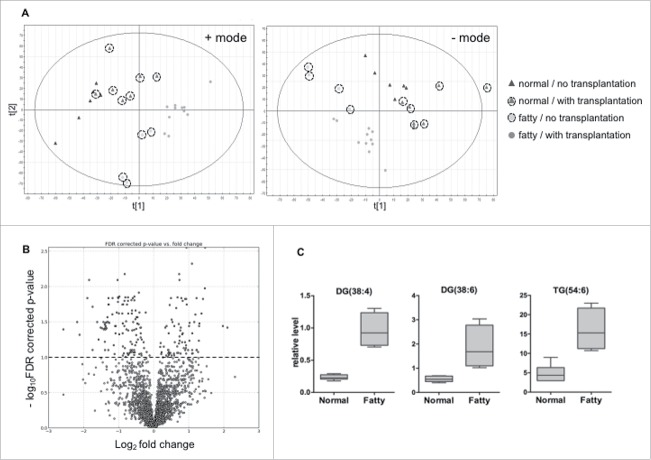
Unsupervised principle component analysis (PCA) plots of the lipid signature showed that the hepatic lipidome of normal liver control mice was segregated from that of fatty-liver mice. PCA also showed clear separation by islet transplantation (Fig. 6A).
Figure 6.
Statistical analysis of liver lipidomics profiling results from mice fed normal diet and high fat diet. (A) Multivariate analysis of lipidomic profiles from normal diet- and high fat-fed mice with or without islet transplantation. Principle components analysis (PCA) unsupervised clustering plots for both positive and negative mode data are shown. (B) Volcano plot displays significant differential ions. Ions labeled above 1.0 on y-axis have significant difference in intensity between high fat-fed mice and normal diet-fed ones. (C) Relative levels of diglyceride and triglyceride ions in high fat-fed and normal diet-fed groups.
Statistical analysis was performed to identify the lipids with statistically significant difference in liver from control mice compared to fatty-liver mice. The volcano plot (Fig. 6B) shows the log10 FDR corrected p-value (y-axis) vs. log2 fold change (fatty-liver/control) for each detected ion. The dots above 1.0 on y-axis are ions with p-values < 0.1 by Welch's T-test. Relative levels of representative ions, two diadylglycerols and one triadylglycerol are shown in Fig. 6C. Levels of these three glycerolipids increased significantly in liver from fatty-liver mice compared to that from control mice (p < 0.05). Putative molecules were designated by screening the accurate mass in metabolite databases, including HMDB, KEGG, LIPIDMAPS, and BioCyc databses. KEGG pathway analysis results indicated the metabolic pathways associated with the differential ions.
Discussion
The purpose of this study was to investigate the role of fatty liver in autologous islet transplantation. We have previously shown that hepatic steatosis is common in patients with chronic pancreatitis.21 In this current investigation we demonstrated that diet-induced hepatic steatosis creates a inflammatory microenvironment that is hostile to islet cells and adversely alters their successful transplantation. We confirmed steatosis histologically and showed that the expression of pro-inflammatory cytokines directly correlated to successful AIT and post-transplantation euglycemic control.
The need for an immune competent animal model for this study and its' uniqueness is easily understood given that in the clinical setting factors such as pancreatic pathology, variations in surgical technique, warm ischemia time and the varying degrees of islet yield in different patients make it difficult to estimate the isolated effect of steatosis on the outcome of TP-AIT.
Inflammation related to IBMIR has been studied in a mouse islet model before. In a study by Wang et al. there was a significant improvement in islet graft survival after intraportal islet transplantation by using alpha-1-antitrypsin.22 The authors maintained that this improvement was by virtue of the mitigation of coagulation in IBMIR and suppression of cytokine-induced JNK and NF-κB activation. As noted by Citro et al. it is not clear which of the many pathways that can result in inflammation are involved in either islet isolation or intraportal islet transplantation.23 In a large part we would argue that these authors and others are underplaying the pre transplant host environment and as we have demonstrated this may be as big a factor as the process of infusion.
Our results show very different euglycemic conversion patterns in the fatty-liver versus control mice after islet transplantation. Fatty-liver mice became euglycemic immediately after islet transplantation but could not sustain good glycemic control at 18 days. The control mice with normal livers had a immediate euglycemic rate that was less impressive, but good glucose control was subsequently maintained. We postulate that this is because in fatty-livers with increased inflammation (which we have demonstrated by increased baseline TNF-α and IL-1β in fatty-livers compared to normal livers), there is greater destruction of islet cells in the early phase and the insulin that is liberated gives the appearance of good glucose control only to fade as the islet cells die off. In normal livers, the islet cell destruction is limited due to less inflammation and since not many cells are destroyed the euglycemic peak is less impressive as compared to the fatty-livers. However with more cells surviving engraftment, glycemic control continues to improve and is sustained. Though not performed at the time, tracking of C-peptide may further support this hypothesis. Elevated levels of TNF-α and IL-1β were noticed in fatty-liver mice more so after islet infusion than in normal liver but the change was not statistically significant. Of note, there are many other inflammatory cytokines/chemokines that could have been tested, but considering the limitation of funding in such a pilot experiment, we selected the most common and representative markers. We tried looking into the engraftment process by CD31 staining but possibly because of sampling limitations we could not appreciate a difference.
Limitations of this study include the small number of mice used for the experiment because it is a pilot study. One of the main challenges was to produce euglycemic fatty-liver mice, i.e., with a normal IPGTT. As mentioned in the methods, we had to sacrifice a considerable number of mice to get the cohort which would satisfy the experimental selection criteria decreasing the number in the actual experiment. It can also be argued that macrosteatosis observed in this study may be little higher than that observed in the clinical setting of islet autotransplantation. Our previous clinical observation does support the notion that many patients have significant fat and fibrosis in their liver .21 It can be also be argued that the diet induced obesity model may not be directly relevant in the clinical setting. This model however provided a histopathologically uniform group that cannot be otherwise reproduced in the clinical setting.We would also concede that portal vein pressure should be measured during the islet infusion, but as shown in our earlier clinical study the liver histopathology has no effect on change in portal pressure from pre- to post- infusion.21
In conclusion, the variation in liver histopathology, in particular steatosis, has a significant adverse effect on achieving sustainable euglycemia after autologous islet transplantation. We aim to direct our efforts towards a more elaborate study with greater differentiation. The clinical application of this study could be appreciated in developing future prospects like patient selection, working on reducing fat content of liver before surgery, pretreatment of fatty liver rather than just generally reducing inflammation with anti-inflammatory drugs.
Materials and methods
Animal model for hepatic steatosis
We compared the outcomes of islet cell transplantation in non-diabetic fatty-liver mice to normal control mice. The mice were rendered chemically diabetic and subjected to syngeneic islet transplantation in this model; male C57BL/6J (B6) mice (5 and 10 weeks old, Charles River Laboratories, MD) were used as islet recipients and donors, respectively. All mouse studies were performed in an ethical fashion and approved by the IACUC committee for animal research by the Georgetown University Comparative Medicine Department.
To create the fatty-liver mice, B6 mice (5-week old) were fed a high-fat-diet with free access to water. The rodent diet high in fat (58Y1, blue) was purchased from TestDiet (St. Louis, MO). This diet was composed of 34.9% fat and with this diet animals obtained 60% of their energy in kilocalories from fat. The fat composition consisted of 13.68% total saturated fatty acids and 14% total monounsaturated fatty acids. The diet was stored in dry refrigerated conditions at 4°C. The control mice received a regular fat diet (#5001) also from TestDiet (St. Louis, MO) with similar but less kilocalories (5 kcal/gm vs 4.1 kcal/gm). This diet contained 6.45% fat with 1.48% total saturated fatty acids and 1.62% total monosaturated fatty acids. Body weight and non-fasting blood glucose (NFBG) of the mice were monitored weekly. Part of the group of mice achieving a body weight ≥28g and normal NFBG, <200mg/dl) were sacrificed and their livers were harvested for histologic examination using Hemotoxylin and Eosin (H&E) stain to assess liver fat content.
Induction of diabetes with streptozotocin
Fatty-liver and control B6 mice were made chemically diabetic by intraperitoneal (IP) injection of 200 mg/kg streptozotocin in citrate buffered saline and screened for the development of diabetes. All mice underwent intraperitoneal glucose tolerance test (IPGTT); after 12 hours of fasting, glucose solution, 2g/kg body weight was injected into the mouse peritoneal cavity with a 1ml syringe. Then blood was sampled (one drop) from the tail vein at 0, 10, 30, 60, and 120th minutes. The blood samples were immediately tested for blood glucose using a Bayer glucometer.
Mice whose NFBG was > 250 mg/dL on two consecutive measurements were considered diabetic.
Harvesting of islets and autologous islet transplantation
Donor B6 mouse pancreata were removed after distension with collagenase RL (1 mg/ml, Roche, Indianapolis, IN) through the common bile duct. Following digestion, islets were purified by a Ficoll discontinuous gradient (1.108, 1.096, and 1.037; Mediatech Inc, Herndon, VA). Isolated islets were cultured for 24 hours in RPMI 1640 supplemented by 10% FCS, L-glutamine (2 mM), and penicillin (100 U/mL), streptomycin (100 µg/mL) and amphotericin B (0.25 µg/mL) (Mediatech Inc, Herndon, VA). Viability was evaluated using a Live/Dead Cell Viability/Cytotoxicity Kit (Molecular Probes, Inc., Eugene, OR) and only isolations with > 90% viability were used for transplantation.
Diabetic fatty-liver and control B6 mice underwent intra-portal islet transplantation under 1% Isoflurane for anesthesia. In brief, 500 hand-picked B6 islets were infused in a total volume of 200 µL into the recipient liver through the portal vein using a 27 Ga insulin syringe, as previously described.24
Mice undergoing islet transplantation were monitored by measuring NFBG daily for two weeks with a Bayer glucometer. Euglycemia was defined as NFBG < 200 mg/dL on two consecutive days.
Evaluation of livers histologically and for inflammation
Livers were harvested 28 days after intraportal islet transplantation from both groups, fixed in 10% formalin, and embedded in paraffin. Five-micron step-sections at 100 µm intervals were obtained. Five consecutive sections were collected from each step section for histological examination; Hematoxylin & Eosin (H&E)and immunohistochemistry.
Immunohistochemical staining of the harvested mouse liver tissue sections was performed with CD31/PECAM (Novus Biological, CO, USA). Five micron sections from formalin fixed paraffin embedded tissues were de-paraffinized with xylene and rehydrated through a graded alcohol series. Heat induced epitope retrieval (HIER) was performed by immersing the tissue sections at room temperature for 20 minutes in Proteinase K. Immunohistochemical staining was performed using a horseradish peroxidase labeled polymer from Vector MP-7444 according to manufacturer's instructions. Briefly, slides were treated with 3% hydrogen peroxide and 2.5% normal goat serum (from Kit) for 20 minutes each, and exposed to primary antibodies for CD31/PECAM, Novus Biological, NBP2-11848(1:75) overnight at 4°C. Slides were exposed to the ImmPRESS Anti-Rat Ig (mouse adsorbed) labeled polymer for 30 minutes and DAB chromagen (Dako) for 5 minutes. Slides were counterstained with Hematoxylin (Fisher, Harris Modified Hematoxylin) at a 1:9 dilution for 2 minutes at RT, blued in 1% ammonium hydroxide for 1 minute at room temperature, dehydrated, and mounted with Acrymount. Sections with omitted primary antibody were used as negative controls.
Hepatic cytokine expression by real-time RT-PCR
Liver samples were harvested 24 hours after islet transplantation and subjected to total RNA extraction to determine levels of TNF-α and IL-1 mRNA. Total RNA of liver samples was isolated and purified using RNeasy Mini Kit (Qiagen, Balencia, CA) according to the manufacturer instructions. Synthesis of complementary DNA (cDNA) was performed by using 1 ug of total RNA and High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA). Quantitative real-time polymerase chain reaction (qPCR) was performed on StepOne Plus (Applied Biosystems, Waltham, MA) with TaqMan Gene Expression Master Mix (Applies Biosystems, Foster City, CA). The amplification was performed in a 20 ul reaction mixture containing 10 ul TaqMan Gene Expresion Master Mix, 4 ul cDNA and 1x of each primer for a specific target. Primers for qPCR were commercially available from Thermo Fisher Scientific (Mm03928990_g1 for 18S ribosomal RNA, Mm00434228_m1 for IL-1b, Mm00443258_m1 for TNF-α). The amplification conditions consisted of one denaturation/activation cycle at 95C for 10min, followed by 40 cycles at 95°C for 15 s and 60° C for 60 s. The individual targets for each sample were quantified by determining the cycle threshold (Ct) and by comparison with the reference samples. The relative amount of the target mRNA was normalized with the reference gene 18s rRNA.
Metabolomic/lipidomic profiling
Snap-frozen liver tissues (10 mg) were homogenized in 300 μL chilled 50% methanol containing trinonadecenoin (TG (19:1/19:1/19:1), with a final concentration of 500 nM. Chloroform (600 μL) was added to the homogenates and vortexed for 10 seconds. HPLC grade water (300 μL)was then added and vortexed for 10 seconds. The tissue homogenates were centrifuged at 13,000 rpm for 15 minutes at 4°C. The upper aqueous phase and bottom organic layer were collected into silica tubes. Supernatants were air dried by speed vacuum. The residues were reconstituted in 100 μL of 50% methanol for analysis. All chemicals were of LC-MS grade and obtained from Sigma-Aldrich (St. Louis, MO).
Acquity CSH C18 50 × 2.1 mm 1.7-μm column (Waters Corp, Milford, MA) was used for lipidomics profiling by UPLC-QTOFMSE. MSE is a technique by which both precursor and fragment mass spectra are acquired by alternating between high and low collision energy during a single chromatographic run using chromatographic and mass spectrometric parameters according to our previous study.25
Raw mass spectrometric data were processed using MarkerLynx software (Waters Corp, Milford, MA) to generate a data matrix that consisted of the retention time, m/z value, and the normalized peak area. Statistical analysis and putative ion identification on the post-processed data were conducted utilizing MetaboLyzer.26 Lipid ions were validated with the fragmentation provided in MSE results. Metabolic pathway information from KEGG as well as BioCyc was further utilized for creating pathway hit histograms and enrichment significance graphs.
Statistical analysis
All data are expressed as mean ± standard deviation. Comparison between groups was performed by a Student's t test. Statistical significance was established at p < 0.05. Analysis of eugylcemic conversion over time was performed by Kaplan-Meier method with a Logrank test to assess statistical significance (Prism Software, GraphPad, Inc.).
Abbreviations page
- °C
Celcisus degree
- cDNA
complementary deoxyribonucleic acid
- dL
deciliter
- DNA
deoxyribonucleic acid
- FCS
Fetal calf serum
- g
gram
- Ga
gauge
- HPLC
high performance liquid chromatography
- IACUC
Institutional Animal Care and Use Committee
- IL-1
Interleukin-1
- KEGG
Kyoto encyclopedia of genes and genomes
- Kg
Kilogram
- LC-MS
Liquid chromatography-mass spectrometry
- mg
milligram
- mL
milliliter
- mM
millimole
- mRNA
Messenger ribonucleic acid
- qPCR
Quantitative polymerase chain reaction
- RNA
Ribonucleic acid
- U
unit
- ug
microgram
- uL
microliter
- um
micrometer
- UPLC-QTOFMS
Ultra performance liquid chromatography-quadrupole time of flight mass spectrometry
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This research was upported by grants from the National Pancreas Foundation (AWD-7771735).
References
- [1].Jirak D, Kriz J, Strzelecki M, Yang J, Hasilo C, White DJ, Foster PJ. Monitoring the survival of islet transplants by MRI using a novel technique for their automated detection and quantification. MAGMA. 2009;22:257-65. doi: 10.1007/s10334-009-0172-4. PMID:19390886 [DOI] [PubMed] [Google Scholar]
- [2].Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587-97. doi: 10.1189/jlb.1104649. PMID:15728243 [DOI] [PubMed] [Google Scholar]
- [3].Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, Bretzel RG, Elgue G, Larsson R, Nilsson B. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907-14. doi: 10.2337/diabetes.48.10.1907. PMID:10512353 [DOI] [PubMed] [Google Scholar]
- [4].Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, Felldin M, Källen R, Salmela K, Tibell A. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54:1755-62. doi: 10.2337/diabetes.54.6.1755. PMID:15919797 [DOI] [PubMed] [Google Scholar]
- [5].Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, Østraat Ø, Salmela K, Tibell A, Tufveson G. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039-45. doi: 10.1016/S0140-6736(02)12020-4. PMID:12504401 [DOI] [PubMed] [Google Scholar]
- [6].Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51:1779-84. doi: 10.2337/diabetes.51.6.1779. PMID:12031965 [DOI] [PubMed] [Google Scholar]
- [7].Bottino R, Fernandez LA, Ricordi C, Lehmann R, Tsan MF, Oliver R, Inverardi L. Transplantation of allogeneic islets of Langerhans in the rat liver: effects of macrophage depletion on graft survival and microenvironment activation. Diabetes. 1998;47:316-23. doi: 10.2337/diabetes.47.3.316. PMID:9519734 [DOI] [PubMed] [Google Scholar]
- [8].Bertuzzi F, Marzorati S, Maffi P, Piemonti L, Melzi R, de Taddeo F, Valtolina V, D'Angelo A, di Carlo V, Bonifacio E. Tissue factor and CCL2/monocyte chemoattractant protein-1 released by human islets affect islet engraftment in type 1 diabetic recipients. J Clin Endocrinol Metab. 2004;89:5724-8. doi: 10.1210/jc.2004-0659. PMID:15531535 [DOI] [PubMed] [Google Scholar]
- [9].Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun. 2003;308:474-9. doi: 10.1016/S0006-291X(03)01392-5. PMID:12914774 [DOI] [PubMed] [Google Scholar]
- [10].Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, Bianchi G, Sica A, Peri G, Melzi R. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51:55-65. doi: 10.2337/diabetes.51.1.55. PMID:11756323 [DOI] [PubMed] [Google Scholar]
- [11].Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195-203. doi: 10.1034/j.1600-065X.2000.17706.x. PMID:11138776 [DOI] [PubMed] [Google Scholar]
- [12].Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. 2007;31:705-14. doi: 10.1007/s00268-006-0719-8. PMID:17347899 [DOI] [PubMed] [Google Scholar]
- [13].Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, Lin PC, Gannon M, Powers AC. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53:1318-25. doi: 10.2337/diabetes.53.5.1318. PMID:15111502 [DOI] [PubMed] [Google Scholar]
- [14].Carlsson PO, Palm F, Mattsson G. Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab. 2002;87:5418-23. doi: 10.1210/jc.2002-020728. PMID:12466329 [DOI] [PubMed] [Google Scholar]
- [15].Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45:749-63. doi: 10.1007/s00125-002-0827-4. PMID:12107718 [DOI] [PubMed] [Google Scholar]
- [16].Lau J, Mattsson G, Carlsson C, Nyqvist D, Kohler M, Berggren PO, Jansson L, Carlsson PO. Implantation site-dependent dysfunction of transplanted pancreatic islets. Diabetes. 2007;56:1544-50. doi: 10.2337/db06-1258. PMID:17400931 [DOI] [PubMed] [Google Scholar]
- [17].Nyqvist D, Kohler M, Wahlstedt H, Berggren PO. Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes. 2005;54:2287-93. doi: 10.2337/diabetes.54.8.2287. PMID:16046293 [DOI] [PubMed] [Google Scholar]
- [18].Vajkoczy P, Olofsson AM, Lehr HA, Leiderer R, Hammersen F, Arfors KE, Menger MD. Histogenesis and ultrastructure of pancreatic islet graft microvasculature. Evidence for graft revascularization by endothelial cells of host origin. Am J Pathol. 1995;146:1397-405. PMID:7539980 [PMC free article] [PubMed] [Google Scholar]
- [19].Desai CS, Stephenson DA, Khan KM, Jie T, Gruessner AC, Rilo HL, Gruessner RW. Novel technique of total pancreatectomy before autologous islet transplants in chronic pancreatitis patients. J Am Coll Surg. 2011;213:e29-34. doi: 10.1016/j.jamcollsurg.2011.09.008. PMID:21996486 [DOI] [PubMed] [Google Scholar]
- [20].Khan KM, Desai CS, Kalb B, Patel C, Grigsby BM, Jie T, Rodriguez-Rilo H. MRI prediction of islet yield for autologous transplantation after total pancreatectomy for chronic pancreatitis. Dig Dis Sci. 2013;58:1116-24. doi: 10.1007/s10620-012-2448-1. PMID:23086123 [DOI] [PubMed] [Google Scholar]
- [21].Desai CS, Khan KM, Megawa FB, Rilo H, Jie T, Gruessner A, Gruessner R. Influence of liver histopathology on transaminitis following total pancreatectomy and autologous islet transplantation. Dig Dis Sci. 2013;58:1349-54. doi: 10.1007/s10620-012-2264-7. PMID:22688185 [DOI] [PubMed] [Google Scholar]
- [22].Wang J, Sun Z, Gou W, Adams DB, Cui W, Morgan KA, Strange C, Wang H. Alpha-1 Antitrypsin Enhances Islet Engraftment by Suppression of Instant Blood-Mediated Inflammatory Reaction. Diabetes. 2017;66:970-80. doi: 10.2337/db16-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Citro A, Cantarelli E, Piemonti L. Anti-inflammatory strategies to enhance islet engraftment and survival. Current diabetes reports. 2013;13:733-44. doi: 10.1007/s11892-013-0401-0. PMID:23912763 [DOI] [PubMed] [Google Scholar]
- [24].Cui W, Wilson JT, Wen J, Angsana J, Qu Z, Haller CA, Chaikof EL. Thrombomodulin improves early outcomes after intraportal islet transplantation. Am J Transplant. 2009;9:1308-16. doi: 10.1111/j.1600-6143.2009.02652.x. PMID:19459803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li HH, Tyburski JB, Wang YW, Strawn S, Moon BH, Kallakury BV, Gonzalez FJ, Fornace AJ Jr. Modulation of fatty acid and bile acid metabolism by peroxisome proliferator-activated receptor alpha protects against alcoholic liver disease. Alcohol Clin Exp Res. 2014;38:1520-31. doi: 10.1111/acer.12424. PMID:24773203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mak TD, Laiakis EC, Goudarzi M, Fornace AJ Jr.. MetaboLyzer: a novel statistical workflow for analyzing Postprocessed LC-MS metabolomics data. Anal Chem. 2014;86:506-13. doi: 10.1021/ac402477z. PMID:24266674 [DOI] [PMC free article] [PubMed] [Google Scholar]