Abstract
A series of phenolic hydrazones were synthesized and evaluated for their inhibition of MIF tautomerase activity. Compound 7 emerged as a potent inhibitor of MIF with an IC50 of 130 nM. Compound 7 dose dependently suppressed TNFα secretion from lipopolysaccharide stimulated macrophages. The therapeutic importance of the MIF inhibition by compound 7 is demonstrated by the significant protection from the lethality of sepsis when administration of the compound was initiated in a clinically relevant time frame.
Graphical Abstract
Introduction
Macrophage migration inhibitory factor (MIF) is a potent pro-inflammatory cytokine, critically involved in the pathogenesis of sepsis and other inflammatory disorders.1,2 We and others have demonstrated that MIF is an important late-acting mediator of systemic inflammation, and that inhibiting its activity in vivo attenuates the lethal consequences of endotoxemia and sepsis in rodents.3,4
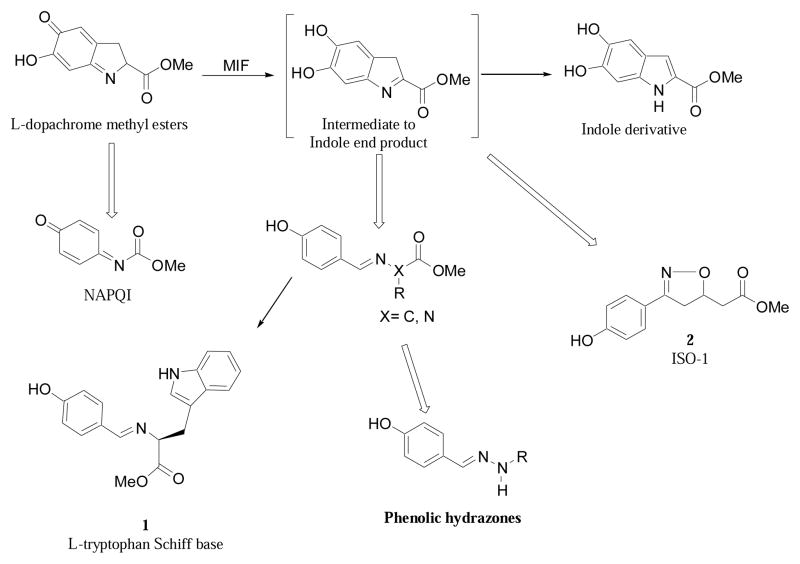
MIF exists as a homotrimer5–8 with the unique ability to catalyze the tautomerization of non-physiological substrates such as D-dopachrome and L-dopachrome methyl ester into their respective indole derivatives (Scheme 1).9 While the physiological role of the tautomerase activity is uncertain, we have previously found that compounds, which are structurally similar to D- and L-dopachrome, could bind to, and thereby block the MIF’s tautomerase active site.4,10–13 We have previously shown that N-acetyl-p-benzoquinone imine (NAPQI) forms a covalent complex with MIF at its active site (Scheme 1) and is capable of irreversibly inhibiting the adverse biological effect of MIF.13 However, the toxicity of NAPQI precluded its use as a viable clinical inhibitor of MIF and the development of non-toxic small molecule inhibitors of MIF tautomerase activity warranted further investigation.
Scheme 1.
Rational Design of MIF Inhibitors.
The indole intermediate of MIF tautomerase catalysis presented itself as a suitable template for the development of potential MIF inhibitors. We reasoned that compounds designed around the phenyl imine scaffold could act as potential MIF antagonists. Indeed, from our rational design we developed the amino acid Schiff bases and the isoxazoline compounds as MIF tautomerase inhibitors.10,12 Among the amino acid Schiff bases tested for their ability to inhibit the tautomerase activity of MIF, it was found that compound 1 was the most potent (Scheme 1, Table 1). Recently, we have reported an isoxazoline inhibitor of MIF “2 (ISO-1)”, which blocks the tautomerase site, inhibits the ability of MIF to overcome anti-inflammatory glucocorticoid activities in vitro and improves survival in animal models of experimental sepsis (Scheme 1).12
Table 1.
Phenolic hydrazones and IC50 values for inhibition of MIF tautomerase activity
| |||
|---|---|---|---|
| Compoundsa | Ar | R | IC50 (μM)b |
|
1 L-tryptophan Schiff base |
1.6 | ||
|
2 ISO-1 |
7 | ||
| 3 |
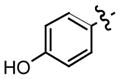
|
H | >500 |
| 4 |

|
CH3 | 43 |
| 5 |

|
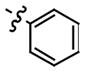
|
2.6 |
| 6c |

|
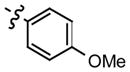
|
0.48 |
| 7c |
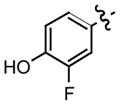
|

|
0.13 |
| 8c |
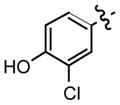
|

|
0.22 |
| 9c |
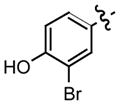
|

|
0.33 |
Compounds were characterized by 1H, 13C NMR and MS.
Spectrophotometric analysis of MIF tautomerase activity on L-dopachrome methyl ester (see experimental).
See experimental for general procedure for the synthesis of 6–9.
In our quest to find more potent inhibitors of MIF, we revisited the phenyl imine scaffold and modified it by adding nitrogen to afford the hydrazone type compound (Scheme 1). Our previous studies on the amino acid Schiff bases and compound 2 revealed that a para-hydroxyl group, as exemplified by a phenolic moiety, is a key structural feature and is required for activity. We have shown that by replacing the phenolic moieties of 1 and 2 with either phenyl or halide substituted phenyl or p-methoxyphenyl groups resulted in the decrease or complete loss of their ability to inhibit MIF tautomerase activity.10,12 In accordance with this result, the chemical structure of the new pharmacophores must contain a phenolic ring (Scheme 1). Utilizing this key structural feature and the observations of 1 and 2 complexed with MIF we propose the synthesis of phenolic hydrazones 3–15 as potential MIF inhibitors.
Results and Discussion
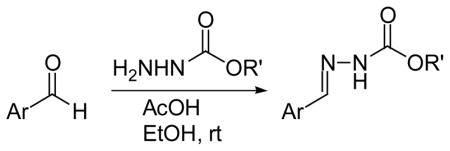
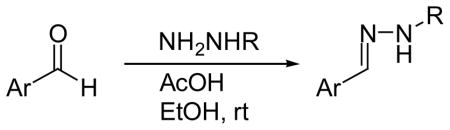
The phenolic hydrazones were obtained in high yields by condensing p-hydroxy-benzaldehyde and the appropriate hydrazide in an alcoholic solvent in the presence of an acid catalyst at room temperature. The hydrazones obtained were assayed for their potency of inhibiting MIF tautomerase activity (Table 1). Briefly, a fresh solution of L-dopachrome methyl ester (2.4 nM) was generated by oxidation of L-3,4-dihydroxyphenylalanine methyl ester with sodium periodate. Activity was determined at room temperature by adding dopachrome methyl ester (0.3 mL) to a cuvette containing 1 μL of MIF solution (850 ng/mL) in 50 mM potassium phosphate buffer (0.7 mL), pH 6, and measuring the decrease in absorbance from 2 to 20 s at 475 nm spectrophotometrically. The inhibitors 3–15 were dissolved in DMSO at various concentrations (0.1–100 μM) and 1 μL was added to the cuvette with the MIF prior to the addition of the dopachrome.
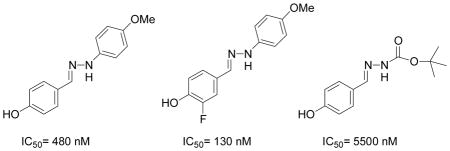
All of the hydrazones prepared in this study 3–15 have one common key structural feature, in that they all possess a “phenolic head” in the form of a 4-hydroxyphenyl ring. We and other workers have shown that the phenolic ring forms key hydrogen bond interactions between the amino acid residue asparagine-97C of the hydrophobic surface within the MIF active site.10,12,14 In addition to this important hydrogen bond interaction, there is a hydrophobic interaction that exist between the aromatic ring of the phenol and the side chains of the amino acid residues, Pro-1, Met-2, Ile-64, Tyr-95, Val-106 and Phe-113, of the hydrophobic pocket, that further contributes to the binding of the inhibitor.14 Supporting evidence for the key interaction between the hydroxyl functionality and the amino acid residue asparagine-97C was obtained by 1) modifying its position and 2) replacing the hydroxyl group with other functional groups. The position of the hydroxyl group is a critical feature of the hydrazones in that changing its position from para (IC50 2.5 μM) to meta (IC50 150 μM) resulted in a dramatic loss of its ability to inhibit MIF tautomerase activity. Furthermore, replacing the hydroxyl group with hydrogen, fluoro, amino, methoxy and nitro functionalities afforded hydrazones that were inactive (data not shown). In developing a structure-activity relationship between the phenolic hydrazones and MIF, we synthesized hydrazones 3–6 from the simple hydrazine, methyl hydrazine, phenyl hydrazine and p-methoxyphenyl hydrazine. One further prerequisite for activity of our phenolic hydrazones is that they require a ydrophobic tail. This is evident from compound 3 (IC50= >500 μM). The hydrogen substituent of the hydrazone in this compound does not offer any hydrophobic interactions and as a result is a very poor inhibitor of MIF tautomerase activity (Table 1). Upon replacing the hydrogen with a methyl group, a pronounced improvement in the ability of the methyl hydrazone 4 to inhibit the tautomerase activity of MIF (IC50= 43 μM) was observed. This suggests that there is a hydrophobic interaction between the methyl group and the surface of MIF. Replacing the methyl group of 4 with the more hydrophobic phenyl ring affords the hydrazone 5 (IC50= 2.6 μM) that is 16 times more potent. Installation of a para-methoxy group on the aromatic ring of 5, gives hydrazone 6 that is 5 fold more potent as an inhibitor than the parent compound. This increased binding of the p-methoxy phenyl hydrazone may be explained by hydrogen bond interactions between the ether oxygen and the known amino acid residues, and the possibility of pi-pi stacking and/or van der Waals interactions between the p-methoxy phenyl ring and the second hydrophobic region of the MIF active site. It has been reported by us and other workers that the amino acids, Pro-33, Tyr-36, Phe-49, Trp-108, and Phe-113, make up the second hydrophobic surface at the rim of the active site of MIF.10,14 These residues further contribute to the hydrophobic and hydrogen bond interactions between the pharmacophore and the active site of MIF.14 In further support of these interactions, we have previously shown that the L-amino acid Schiff bases inhibitory effect was significantly improved by five fold upon changing the amino acid residue from L-phenylalanine (IC50= 50 μM) to L-tyrosine (IC50= 10 μM), respectively. This suggests that the increased potency was attributed to a hydrogen bond interaction within residues at the rim of MIF.10 Hydrazone 6 is twelve times more potent an inhibitor than L-tryptophan Schiff base 1, so far the most potent inhibitor of MIF described in the literature.4,10–12,14,15
Recently, we have discovered that mono-fluorination of 2 improved the inhibition of MIF activity.11 As a consequence, we synthesized the 3-fluoro, 3-chloro, and 3-bromo-4-hydroxyphenyl derivatives of the more potent hydrazone, namely the p-methoxyphenyl hydrazone (6). The synthesis of the mono-halogenated hydrazone derivatives involved treatment of a suspension of the 4-methoxyphenylhydrazine hydrochloride and the 3-halogenated-4-hydroxybenzaldehyde in methanol with aqueous sodium hydroxide (Table 1). A general increase in the inhibition of the MIF tautomerase activity was observed for the 3-halogenated-4-hydroxyphenyl hydrazone derivatives 7–9 (Table 1). Among these hydrazones, compound 7 showed the most potent inhibition with an IC50 value of 130 nM, whilst 8 and 9 gave values of 220 nM and 330 nM, respectively. The significant improvement in the inhibitory effect of these halogenated hydrazones 7–9 may be explained by the inductive effect that may lead to changes in the polarization of the hydroxyl moiety thereby making it a stronger hydrogen bond donor/acceptor.
To determine if the activity would be affected by disubstitution on the nitrogen, compound 10 was synthesized (Figure 1). Methylation of phenyl hydrazine (5) with methyl iodide and sodium amide afforded N-methyl-N-phenyl hydrazine. Hydrazone formation under typical conditions gave 10 in 86% yield. To our disappointment 10 was a very weak inhibitor of MIF activity and displayed an IC50 value of 300 μM. This observation could be accounted for by increased steric hinderance or by the reduced number of hydrogen bonding interactions between the di-substituted hydrazone derivative 10 and the active site of MIF.
Figure 1.
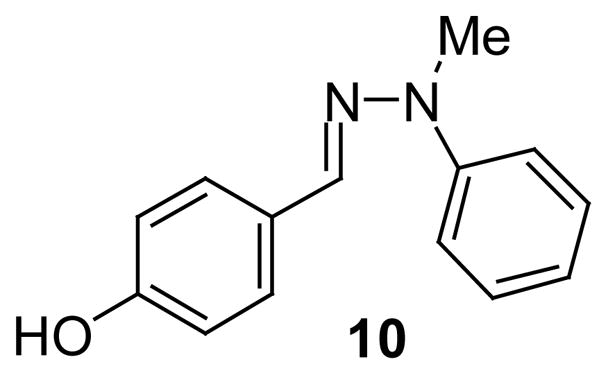
Di-substituted Hydrazone.
To further investigate the structure-activity relationship between the hydrazones and MIF we looked at another class of compounds, namely the phenolic hydrazone carbamates. The phenolic hydrazone carbamates 11–15 were chosen as they displayed key functionalities similar to those of the amino acid Schiff base 1 and 2 (Table 2). These functionalities are the hydroxyl group, phenyl ring and the carboxylate moiety. It has been reported by us that a secondary hydrogen bond interaction exists between the carboxylate moiety of 2 and lysine-32A of the MIF active site.10 Significant improvement, of approximately 10 fold, in the inhibition of MIF tautomerase activity was observed on changing the methyl group of 11 (IC50= 55 μM) to the more lipophilic t-butyl moiety 13 (IC50= 5.5 μM). To determine if phenyl rings improve the inhibition of MIF activity, we replaced the t-butyl carbamate with a benzyl carbamate 14 and p-methoxy benzyl carbamate 15. This change from a bulky alkyl 13 to an aromatic group 14–15 (IC50= 10, 8 μM) resulted in a slight decrease in the inhibitory effect.
Table 2.
Phenolic hydrazone carbamates and IC50 values for inhibition of MIF tautomerase activity
Compounds were characterized by 1H, 13C NMR and MS (see supporting information).
Spectrophotometric analysis of MIF tautomerase activity on L-dopachrome methyl ester.
Biological Activity
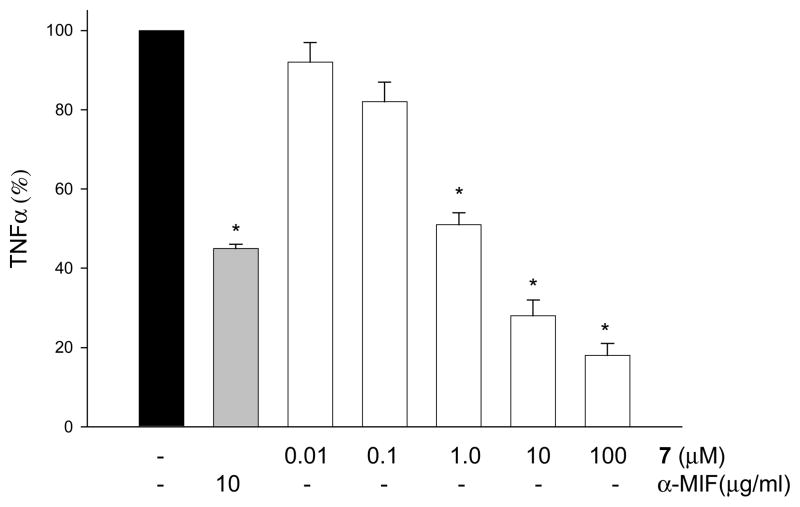
Intracellular MIF occupies a critical role in mediating the cellular responses to pathways activated by lipopolysaccharide (LPS) endotoxin 16 and MIF deficient cells are hyporesponsive to endotoxin.17 We and others have shown that MIF null macrophages can produce 50–60% less TNF compared to wild type.4,18 Previously, we have shown that anti-MIF antibody (10μg/ml) inhibit 50% of TNF release from LPS-treated macrophages.4 We also have found that 2 dose-dependently inhibit LPS-induced TNF release from wild type and not MIF null macrophages suggesting that the chemical inhibitor of MIF is specific. Accordingly, we reasoned that compound 7 binding to MIF would suppress LPS responses in macrophages. Compound 7 dose-dependently inhibited LPS-induced TNF release (Figure 2). Thus compound 7 recapitulates the phenotype of the MIF deficient macrophages and is associated with decreased TNF production in response to LPS.
Figure 2.
Compound 7 inhibits TNF secretion from LPS-treated macrophages. RAW 267.4 macrophages (105) were treated with various concentrations of hydrazone 7 (0.01–100 μM) 30 min prior to LPS addition or 10μg/ml of mouse monoclonal antibody against MIF (XIV.15.5;α-MIF). After 16 h of incubation, cell culture supernatants were collected for determination of TNFα concentration by ELISA. Data are presented as mean ± S.D (n=3, *, p<0.01)
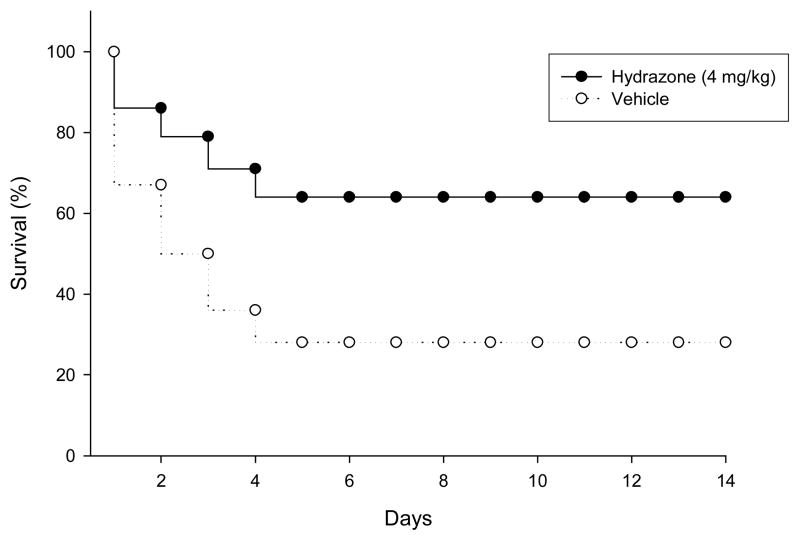
The importance of MIF as a molecular therapeutic target in sepsis has been confirmed by our recent observation that treatment with anti-MIF antibodies (4 mg/kg of monoclonal antibody against MIF (XIV.15.5) daily for three days) or 2 (40 mg/kg bi-daily for three days) significantly improves survival in septic mice.4 We also recently discovered that serum MIF levels increased to 70% of maximum levels within 24 h post CLP, and peaked at 36 h.4 This identified MIF as a late mediator in sepsis and indicated the therapeutic potential of inhibiting MIF in a clinically relevant time frame. Therefore, we reasoned that a delayed treatment with compound 7, consistent with the kinetics of MIF release, could be successfully applied to improve survival in sepsis. We tested the ability of compound 7 to improve the survival rate in cecal ligation and puncture (CLP)-induced peritonitis, a widely used preclinical model of sepsis. Intraperitoneal treatment of 7 (4 mg/kg) initiated 24 h after CLP surgery and continued for 3 days resulted in a survival rate of 65% (p< 0.01) compared to 28% in the control (vehicle-treated) group (Figure 3). Thus, compound 7 treatment provided significant protection against sepsis lethality, comparable to the effect of anti-MIF antibody and compound 2.4 Remarkably, a dose of compound 7, 10-fold less than 2, achieved similar protection. This finding indicates an association between the potency of compound 7 in inhibiting the MIF tautomerase active site and its beneficial effect of improving survival in experimental sepsis.
Figure 3.
Compound 7 is protective after 24 h late treatment in a CLP model: Mice were injected intraperitoneally with compound 7 (4 mg/kg) (n=13, p<0.01) or vehicle 24 h after CLP (n=13). A single injection is composed of 100μg of Compound 7 (equivalent to 4mg/kg) in 200μL of 20% DMSO: 80% saline solution. Additional administrations of compound 7 (bi-daily) were given on days 2 and 3.
In summary, we have designed and synthesized phenolic hydrazones as non toxic, potent MIF antagonists. Our structure-activity relationship study suggests that minor changes in functionalization of the hydrazones affect the binding of these compounds to the MIF active site. Notably, compound 7 exhibits the greatest activity of all the compounds tested and is so far the most potent inhibitor of MIF described in the literature.4,10–12,14,15
Compound 7 exhibited a potent anti-inflammatory activity in vitro as demonstrated by suppression of LPS-induced macrophage activation. Moreover, a relatively low concentration of this small molecule MIF inhibitor improved survival in sepsis when treatment was initiated at 24 hours after the onset of the disease.
Sepsis is a complex inflammatory disorder and its clinical management is a challenging health issue. Therefore, our finding that compound 7 is effective at 24 hours after the onset of the disorder could be of considerable clinical interest.
Experimental Section
General Experimental
All chemicals were obtained from commercial suppliers and used without further purification. Aluminium-backed Silica Gel 60 with a 254 nm fluorescent indicator TLC plates were used. Developed TLC plates were visualized under a short-wave UV lamp, stained with an I2-SiO2 mixture. Flash column chromatography (FCC) was performed using flash silica gel (32–63μm) and usually employed a stepwise solvent polarity gradient, correlated with TLC mobility. Melting points (M. p.) were determined in a Gallenkamp Melting Point Apparatus in open capillaries and are uncorrected. IR spectra were obtained on a Thermo Nicolet IR 100 FT-IR spectrometer. All 1H spectra were recorded either on a Joel spectrometer or a GE QE 300 spectrometer at 270 or 300 MHz. The 13C spectra were recorded on a GE QE 300 spectrometer at 75 MHz. Chemical shifts are relative to the deuterated solvent peak and are in parts per million (ppm). The coupling constants (J) are measured in Hertz (Hz). The 1H signals are described as s (singlet), d (doublet), t (triplet), q (quartet), m (multipet) and br s (broad singlet). Low and high resolution mass spectroscopy was carried out at the Mass Spectrometry Facility at the University of Illinois at Urbana-Champaign.
General Experimental for compounds 3–5 and 10–15
4-Hydroxybenzaldehyde (122 mg, 1 mmol) or 3-fluoro-4-hydroxybenzaldehyde (140 mg, 1 mmol) and the hydrazide (2 mmol) were dissolved in ethanol (10 mL). To this was added acetic acid (1 mmol) and the reaction was stirred overnight at room temperature. Removal of the ethanol in vacuo afforded an oily residue. The residue was taken up with ethyl acetate and washed with water. The organic layer was separated and dried with anhydrous Na2SO4. Concentration in vacuo afforded a residue which was subsequently purified by FCC using hexane and ethyl acetate as eluent (4:1) to give compounds 3–5 and 10–15.
General Procedure for compounds 6–9
4-Hydroxybenzaldehyde (122 mg, 1 mmol) or 3-fluoro-4-hydroxybenzaldehyde (140 mg, 1 mmol) or 3-chloro-4-hydroxydroxybenzaldehyde (156 mg, 1 mmol) or 3-bromo-4-hydroxybenzaldehyde (201 mg, 1 mmol) and 4-methoxyphenylhydrazine hydrochloride (350 mg, 2 mmol) were suspended in methanol (10 mL). To this suspension was added a 2M aqueous solution of sodium hydroxide (60 mg, 1.5 mmol) and the reaction was stirred overnight at room temperature. Upon completion of the reaction, the solution was then acidified to pH 4 by the addition of 1M HCl. Removal of the methanol in vacuo afforded an oily residue. The residue was taken up with ethyl acetate and washed with water. The organic layer was separated and dried with anhydrous Na2SO4. Concentration in vacuo afforded a residue which was subsequently purified by FCC using hexane and ethyl acetate as eluent (4:1) to give compounds 6–9.
Spectrophotometric Assay for Enzymatic Activity
A fresh stock solution of L-dopachrome methyl ester (2.4 nM) was generated by oxidation of L-3,4-dihydroxyphenylalanine methyl ester with sodium periodate, producing an orange-colored solution. Activity was determined at room temperature by adding dopachrome methyl ester (0.3 mL) to a cuvette containing 1 μL of MIF solution (850 ng/mL) in 50 mM potassium phosphate buffer, pH 6, and measuring the decrease in absorbance from 2 to 20 s at 475 nm spectrophotometrically. The inhibitors 3–15 were dissolved in DMSO at various concentrations (0.1–100μM) and 1 μL was added to the cuvette with the MIF prior to the addition of the dopachrome.
Cellular Assay
Compound 7 inhibits TNF secretion from LPS-treated macrophages. RAW 267.4 macrophages (105) were treated with various concentrations of hydrazone3 7 (0.01–100 μM) 30 min prior to LPS addition. After 16 h of incubation, cell culture supernatants were collected for determination of TNF concentration by ELISA. Data are presented as mean ± S.D (n=3, *, p<0.01).
Animal studies
All animal experiments were approved by the Institutional Animal Care and Use Committee of the North Shore-Long Island Jewish Research Institute. Male Balb/C mice, ~8 weeks old, were subjected to cecal ligation and puncture. Details of the CLP procedure has been carried out as follows: In anesthetized male Balb/C mice (ketamine 100 mg/kg and xylazine 8 mg/kg administered intramuscularly) the cecum was ligated and given a single puncture. Abdominal access was gained via a midline incision. The cecum was isolated and ligated with a 6-0 silk ligature below the ileocecal valve, and the cecum punctured once with a 22G needle, stool (approximately 1mm) extruded from the hole, and the cecum placed back into the abdominal cavity. The abdomen was closed with two layers of 6-0 Ethilon sutures. Antibiotics were administered immediately after CLP (Premaxin 0.5 mg/kg, subcutaneously, in a total volume of 0.5 ml/mouse) and single dose of resuscitative fluid (normal saline solution administered subcutaneously (20 ml/kg-body weight) immediately after CLP surgery. 19 Mice were injected intraperitoneally with 4 mg/kg (n= 13, **P< 0.01) or vehicle 24 hours after CLP (n=13). Additional two injections were given on day 2 and 3. Vehicle (aqueous 20% DMSO) or compound 7 (4 mg/kg; intraperitoneally) treatment was started 24 h after the induction of sepsis and repeated twice daily on days 2 and 3. Animal survival was monitored for 14 days.
Acknowledgments
This research was supported by the NIH grant (HL081655) awarded to Dr E. J. Miller and Dr Y. Al-Abed.
Footnotes
Abbreviations: MIF, macrophage migration inhibitory factor; NAPQI, N-acetyl-p-benzoquinone imine; LPS, lipopolysaccharide; CLP, cecal ligation and puncture.
Supporting Information Available: NMR spectral data and elemental analyses of derivatives 3–15. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 3.Calandra T, Echtenacher B, Roy DL, Pugin J, Metz CN, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 4.Al-Abed Y, Dabideen D, Aljabari B, Valster A, Messmer D, et al. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J Biol Chem. 2005;280:36541–36544. doi: 10.1074/jbc.C500243200. [DOI] [PubMed] [Google Scholar]
- 5.Sugimoto H, Suzuki M, Nakagawa A, Tanaka I, Fujinaga M, et al. Crystallization of rat liver macrophage migration inhibitory factor for MAD analysis. J Struct Biol. 1995;115:331–334. doi: 10.1006/jsbi.1995.1057. [DOI] [PubMed] [Google Scholar]
- 6.Sun HW, Bernhagen J, Bucala R, Lolis E. Crystal structure at 2.6-A resolution of human macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. 1996;93:5191–5196. doi: 10.1073/pnas.93.11.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki M, Sugimoto H, Nakagawa A, Tanaka I, Nishihira J, et al. Crystal structure of the macrophage migration inhibitory factor from rat liver. Nat Struct Biol. 1996;3:259–266. doi: 10.1038/nsb0396-259. [DOI] [PubMed] [Google Scholar]
- 8.Taylor AB, Johnson WH, Czerwinski RM, Li HS, Hackert ML, et al. Crystal structure of macrophage migration inhibitory factor-complexed with (E)-2-fluoro-p-hydroxycinnamate at 1.8 angstrom resolution: Implications for enzymatic catalysis and inhibition. Biochemistry. 1999;38:7444–7452. doi: 10.1021/bi9904048. [DOI] [PubMed] [Google Scholar]
- 9.Rosengren E, Bucala R, Aman P, Jacobsson L, Odh G, et al. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol Med. 1996;2:143–149. [PMC free article] [PubMed] [Google Scholar]
- 10.Dios A, Mitchell RA, Aljabari B, Lubetsky J, O’Connor K, et al. Inhibition of MIF bioactivity by rational design of pharmacological inhibitors of MIF tautomerase activity. J Med Chem. 2002;45:2410–2416. doi: 10.1021/jm010534q. [DOI] [PubMed] [Google Scholar]
- 11.Cheng KF, Al-Abed Y. Critical modifications of the ISO-1 scaffold improve its potent inhibition of macrophage migration inhibitory factor (MIF) tautomerase activity. Bioorg Med Chem Lett. 2006;16:3376–3379. doi: 10.1016/j.bmcl.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Lubetsky JB, Dios A, Han J, Aljabari B, Ruzsicska B, et al. The tautomerase active site of macrophage migration inhibitory factor is a potential target for discovery of novel anti-inflammatory agents. J Biol Chem. 2002;277:24976–24982. doi: 10.1074/jbc.M203220200. [DOI] [PubMed] [Google Scholar]
- 13.Senter PD, Al-Abed Y, Metz CN, Benigni F, Mitchell RA, et al. Inhibition of macrophage migration inhibitory factor (MIF) tautomerase and biological activities by acetaminophen metabolites. Proc Natl Acad Sci U S A. 2002;99:144–149. doi: 10.1073/pnas.011569399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orita M, Yamamoto S, Katayama N, Aoki M, Takayama K, et al. Coumarin and chromen-4-one analogues as tautomerase inhibitors of macrophage migration inhibitory factor: Discovery and X-ray crystallography. Journal of Medicinal Chemistry. 2001;44:540–547. doi: 10.1021/jm000386o. [DOI] [PubMed] [Google Scholar]
- 15.Orita M, Yamamoto S, Katayama N, Fujita S. Macrophage migration inhibitory factor and the discovery of tautomerase inhibitors. Curr Pharm Des. 2002;8:1297–1317. doi: 10.2174/1381612023394674. [DOI] [PubMed] [Google Scholar]
- 16.Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001;414:920–924. doi: 10.1038/414920a. [DOI] [PubMed] [Google Scholar]
- 17.Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, et al. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, et al. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci U S A. 2002;99:345–350. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]