1 Introduction
Cancer metabolism has been an active research area for developing biomarkers for cancer diagnosis and treatment response. Early studies have systematically investigated the glucose uptake and spatial distribution of glucose in breast cancer animal models using a enzymatic bioluminescent imaging assay [1]. Heterogeneity in glucose uptake was shown in tumor xenografts [1,2]. Our previous work [3, 4] revealed that the mitochondrial redox state of tumor tissue was a sensitive indicator of tumor aggressiveness and the more aggressive tumors have localized core regions with a more oxidized redox state. To understand the nature of the oxidized tumor core of the aggressive tumors, we performed both H&E and TUNEL assays. The H&E staining revealed that the cells in the tumor core appeared to be pinkish, indicative of necrosis. However, the TUNEL and DAPI staining for both melanoma [5] and breast tumor [4] indicated a low level of cell death. So what is the metabolic status of the cells in the tumor core?
To answer this question, one can measure tumor glucose metabolism simultaneously with the mitochondrial redox state. The mitochondrial redox state of tissue can be measured by using the low-temperature NADH/Fp fluorescence redox scanner [6, 7]. Both NADH and Fp including FAD are intrinsic fluorescent molecules in the electron transport chain. The Fp redox ratio represented by Fp/(NADH + Fp) is a sensitive indicator of mitochondrial redox state [8], and was linked to tumor metastatic potential as described in our previous work [3, 4]. FDG-PET provides information on glucose uptake/metabolism in cells and has been widely used for cancer diagnosis and staging because the abnormally high glucose uptake/consumption is a well-established metabolic hallmark of cancer cells. However, FDG-PET cannot be used for such a purpose due to its relatively low spatial resolution (4–6 mm for humans and 1–2 mm for small animals). The typical size of a tumor core can be ~3 mm or less [3, 4].
Previously, Zheng and co-workers have utilized the high-resolution redox scanner (resolution down to 50 μm) in combination with a near-infrared fluorescence imaging agent Pyro-2DG to simultaneously image both the redox state and glucose uptake of tumors in animal models [9]. It was shown that Pyro-2DG was selectively accumulated in tumor cells via the glucose transporters. Their results showed that the glucose uptake correlated well with the reduced redox state in 9 L glioma [9] and c-MYC-induced mammary tumor on transgenic mouse [10]. It was also shown that Pyro-2DG did not affect the redox state of tumor tissue [9].
Based on these evaluations, we aimed to employ the same method to quantitatively image Pyro-2DG uptake simultaneously with the mitochondrial redox state in the breast cancer mouse xenografts. Knowing the metabolic status of the tumor xenografts may help to gain further insight into breast tumor aggressiveness and progression.
2 Methods
The animal protocol of this study was approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. MDA-MB-231 tumors were grown in athymic nude mice (NCr nu/nu from US National Cancer Institute) as previously described [4]. Pyro-2DG was administered to the mice through a tail vein at ~2.5 mg/kg after the mice were starved for 24 h followed by anesthetization. In approximately 2 h, the mice were snap-frozen using liquid nitrogen to maintain the in vivo mitochondrial redox state for the ex vivo redox scanning. The preparation of samples for the redox scan were performed as previously described [4, 11]. Frozen reference standards (one NADH (1318 μM), one Fp (719 μM), and one pyro-2DG (21 μM)) were placed adjacent to the tissue for redox scanning.
Two MDA-MB-231 tumors (>10 mm in diameter and with an apparent necrotic center, average tumor volume = 989 mm3) were imaged under the following instrument setup. The Fp excitation and emission channel filters were 430 nm ± 25 nm and 525 nm ± 32 nm, respectively; the NADH ones were 360 nm ± 13 nm and 430 nm ± 25 nm, respectively; the pyro-2DG ones were 430 ± 28 nm and 670DF23 nm. Multi-slice fluorescence images of NADH, Fp, and pyro-2DG were obtained section by section with 400 μm spacing. The scanning matrix was 128 × 64 or 128 × 128 with a resolution of 200 μm.
Customized-matlab software was used to analyze the data and construct the images. The nominal concentrations of NADH, Fp, and pyro-2DG in tissue were interpreted using the reference standards. The nominal concentrations of NADH, Fp, and Pyro-2DG were used to calculate the redox ratios, i.e., Fp/(NADH + Fp) and NADH/(NADH + Fp), and normalized Pyro-2DG, i.e., Pyro-2DG/(NADH + Fp). Normalization of Pyro-2DG signals with respect to NADH + Fp is to account for the cell or mitochondria density difference at different regions of tumors.
3 Results
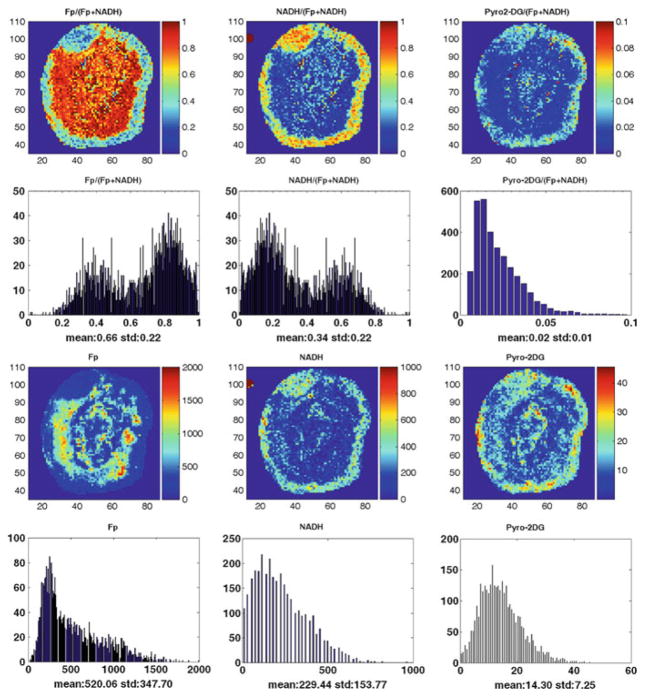
Figure 26.1 shows the typical redox and Pyro-2DG images of the mouse xenografts of MDA-MB-231 tumor lines, where the NADH, Fp, and Pyro-2DG images are shown in their nominal concentrations. The aggressive MDA-MB-231 tumors clearly showed bimodal distribution of NADH and Fp redox ratios indicating a more oxidized tumor core [4].
Fig. 26.1.
Typical mitochondrial redox state and Pyro-2DG uptake images and their corresponding histograms of a representative MDA-MB-231 tumor (the tumor section had no scar). The Fp redox ratio, NADH redox ratio, and normalized Pyro-2DG range between 0 and 1; the Fp, NADH, and Pyro-2DG images are in the unit of μM in reference to the corresponding standards. The mean values and the corresponding standard deviations of these indices are also shown on the x-axes of the histograms. The y-axes represent the number of pixels in the tumor section having a specific value of the indices shown by the x-axes of the histograms
The Pyro-2DG images also revealed heterogeneity in Pyro-2DG uptake. In the tumor rim, the pattern of Pyro-2DG uptake appears to correlate more with the NADH distribution. Interestingly, the oxidized tumor core (higher Fp redox ratio) also showed areas with significant Pyro-2DG uptake.
4 Discussion
Tumor heterogeneity has long been recognized and should be addressed in cancer diagnosis and treatment. From our preliminary imaging results the heterogeneity in glucose uptake and redox state was clearly seen at 200 μm spatial resolution, which demonstrates the advantage of using the redox scanner for tissue imaging. Much of the image details would have been lost had the resolution been on the order of 1 mm or above.
The normalized Pyro-2DG uptake (Pyro-2DG/(NADH + Fp)) image may better represent the cellular metabolic activity than Pyro-2DG uptake, because the cell density in the tumor core is significantly less than that in the rim, particularly for large tumors (diameter >10 mm) as shown by the H&E staining of MDA-MB-231 tumor xenografts.
The typical Pyro-2DG images showed higher glucose uptake in the tumor rim and pronounce uptake in some regions of the tumor core, indicating that the cells in these regions are metabolically active. This is quite interesting and could be rather surprising as cells in the tumor core are often considered necrotic due to limited blood perfusion that usually happens for aggressive tumors in mouse xenografts [3, 12–14]. This result might support the notion that some cells in the core of aggressive tumor might be viable and in the redox state of State 3, where cells had sufficient nutrient resulting in rapid oxidative metabolism, instead of State 2, where cells were nutrient-starved [15–17]. We need to image more tumor samples to confirm the observation.
We recognize that the animal models here hardly resemble/represent the human tumors in the clinic. However, the animal models provide a research basis for us to understand the tumor metabolism and its roles in tumor progression and develop novel imaging methods/markers that may be translatable into the clinic eventually.
5 Conclusions
In this paper we reported our preliminary imaging findings of simultaneous mapping of mitochondrial redox state and glucose uptake in aggressive human breast tumor xenografts using the redox scanner. It was also observed that some areas in the oxidized core had significant Pyro-2DG uptake. Imaging the metabolic states of tumors with high spatial resolution is significant for cancer diagnosis and treatment, such as developing imaging biomarkers for tumor stratification and treatment design.
Acknowledgments
This work was supported by KG081069, NIH (R01 CA155348), NIHRR02305, and 2U24-CA083105. We thank Mr. Aron Roxin for synthesizing Pyro-2DG, Dr. Anatoliy Popov and Dr. Zhihong Zhang for Pyro-2DG solution preparation techniques.
Contributor Information
H.N. Xu, Department of Radiology, School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
G. Zheng, Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada
S. Nioka, Johnson Research Foundation, Department of Biochemistry and Biophysics, School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
B. Chance, Johnson Research Foundation, Department of Biochemistry and Biophysics, School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
L.Z. Li, Department of Radiology, School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
References
- 1.Kallinowski F, Vaupel P, Runkel S, et al. Glucose uptake, lactate release, ketone body turnover, metabolic micromilieu, and pH distributions in human breast cancer xenografts in nude rats. Cancer Res. 1988;48:7264–7272. [PubMed] [Google Scholar]
- 2.Dearling JLJ, Flynn AA, Sutcliffe-Goulden J, et al. Analysis of the regional uptake of radiolabeled deoxyglucose analogs in human tumor xenografts. J Nucl Med. 2004;45:101–107. [PubMed] [Google Scholar]
- 3.Li LZ, Zhou R, Xu HN, et al. Quantitative magnetic resonance and optical imaging biomarkers of melanoma metastatic potential. Proc Natl Acad Sci U S A. 2009;106:6608–6613. doi: 10.1073/pnas.0901807106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu HN, Nioka S, Glickson JD, et al. Quantitative mitochondrial redox imaging of breast cancer metastatic potential. J Biomed Opt. 2010;15:036010. doi: 10.1117/1.3431714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu HN, Zhou R, Nioka S, et al. Histological basis of MR/optical imaging of human melanoma mouse xenografts spanning a range of metastatic potentials. Adv Exp Med Biol. 2009;645:247–253. doi: 10.1007/978-0-387-85998-9_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quistorff B, Haselgrove JC, Chance B. High spatial resolution readout of 3-D metabolic organ structure: an automated, low-temperature redox ratio-scanning instrument. Anal Biochem. 1985;148:389–400. doi: 10.1016/0003-2697(85)90244-1. [DOI] [PubMed] [Google Scholar]
- 7.Li LZ, Xu HN, Ranji M, et al. Mitochondrial redox imaging for cancer diagnostic and therapeutic studies. J Innov Opt Health Sci. 2009;2:325–341. doi: 10.1142/S1793545809000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chance B, Schoener B, Oshino R, et al. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples NADH and flavoprotein fluorescence signals. J Biol Chem. 1979;254:4764–4771. [PubMed] [Google Scholar]
- 9.Zhang M, Zhang Z, Blessington D, et al. Pyropheophorbide 2-deoxyglucosamide: a new photosensitizer targeting glucose transporters. Bioconjug Chem. 2003;14:709–714. doi: 10.1021/bc034038n. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Liu Q, Luo Q, et al. 3D Imaging of the metabolic state of c-MYC-induced mammary tumor with the cryo-imager. Proceedings of in Ed SPIE. 2003;4955:647–655. [Google Scholar]
- 11.Xu HN, Wu B, Nioka S, et al. Quantitative redox scanning of tissue samples using a calibration procedure. J Innov Opt Health Sci. 2009;2:375–385. doi: 10.1142/S1793545809000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley DP, Tessier JJ, Ashton SE, et al. Correlation of MRI biomarkers with tumor necrosis in Hras5 tumor xenograft in athymic rats. Neoplasia. 2007;9:382–391. doi: 10.1593/neo.07145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedley RB, Hill SA, Boxer GM, et al. Eradication of colorectal xenografts by combined radioimmunotherapy and combretastatin a-4 3-O-phosphate. Cancer Res. 2001;61:4716–4722. [PubMed] [Google Scholar]
- 14.Ranney D, Antich P, Dadey E, et al. Dermatan carriers for neovascular transport targeting, deep tumor penetration and improved therapy. J Control Release. 2005;109:222–235. doi: 10.1016/j.jconrel.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Chance B, Williams GR. Simple and rapid assay of oxidative phosphorylation. Nature. 1955;175:1120–1121. doi: 10.1038/1751120a0. [DOI] [PubMed] [Google Scholar]
- 16.Chance B, Baltscheffsky H. Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide. J Biol Chem. 1958;233:736–739. [PubMed] [Google Scholar]
- 17.Chance B, Schoener B. High and low energy states of cytochromes. J Biol Chem. 1966;241:4567–4573. [PubMed] [Google Scholar]