Abstract
Background and Objectives
This study characterizes the multiple morbidities experienced by senior-aged women Veterans so that the Veterans Health Administration (VHA) and other health care systems may be better prepared to meet the health care needs of this growing cohort.
Research Design and Methods
Using the VHA’s Corporate Data Warehouse, we conducted a retrospective observational study of the 38,597 female veteran patients who were at least 65 years old and received care in the VHA during 2013 and 2014. We use a latent class analysis model to cluster diagnoses associated with inpatient and outpatient events over the years.
Results
The senior-aged women Veterans are characterized by six major classes of disease clusters. We defined these classes as: Healthy (16.24% of the cohort); Ophthalmological Disorders (13.84%); Musculoskeletal Disorders (14.22%); At Risk for Cardiovascular Disease (37.53%); Diabetic with Comorbidities (9.05%); and Multimorbid (9.12%). The patterns and prevalence of these condition classes vary by race, age, and marital status.
Discussion and Implications
Each of the six clusters can be used to develop clinical practice guidelines that are appropriate for senior-aged women Veterans. Consistent with past literature, the most common conditions in this cohort are hypertension and hyperlipidemia; together they form the most common class, “At Risk of Cardiovascular Disease (CVD)”. Results also show evidence of race-related disparities, with Blacks being more likely to be in the highest risk classes. Also, members of the cohort who are currently married having improved chances of being in the healthy class. And finally, we see a “healthy survivor” effect with the oldest women in our cohort having low overall rates of disease.
Keywords: Gender issues, Veterans, Women's issues
Translational Significance
The study informs the development of clinical practice guidelines and raises awareness among clinicians to address commonly co-occurring conditions so that treatments and outcomes may be optimized. Recommendations include that care for senior-aged women Veterans be tailored to recognize and address the identified combination of conditions; care teams of senior-aged women should also pay special attention to the risks of potential polypharmacy in the setting of multimorbidity. Patients may benefit from targeted education to prevent or address co-occuring conditions. This study findings also indicate that initiatives specifically aimed at reducing risk of cardiovascular disease would be impactful for the senior-aged women Veterans. For those who are older and unmarried, benefit may be gained by efforts to increase their support network in order to maintain independence. Finally, for Black senior-aged women, may benefit from tailored intervention efforts aimed at avoiding diabetes and its related comorbid conditions.
Background and Objectives
Women Veterans comprise a growing and aging population in the United States. As of 2016, 16% of the two million women Veterans were over age 65 (LaCroix et al., 2016; Reiber & LaCroix, 2016), and there is a projected 83% increase in the number of women Veterans who will be over the age of 65 by the year 2025 (Reiber & LaCroix, 2016). Given these projections, understanding the health needs of women Veterans has been identified as a priority area for the Veterans Health Administration (VHA) (Atkins, 2013; Yano, Bastian, Frayne, Howell, & Lipson, 2006). Although there is a growing body of literature illustrating the different health care use patterns, the costs associated with these patterns, and the disease patterns associated with women Veterans in general, the disease burden and health care needs of older women Veterans warrants further investigation.
One area where additional work needs to be done is in understanding the disease characteristics of the aging women Veteran population. It is especially important to examine the variation that exists across subpopulations of women Veterans in order to plan for the resources and create viable Clinical Practice Guidelines (CPG) that will be needed to care for them (Bean-Mayberry, Batuman, Huang, Goldzweig, & Washington, 2010; Bielawski, Goldstein, Mattocks, Bean-Mayberry, & Yano, 2014; Washington, Bean-Mayberry, Hamilton, Cordasco, & Yano, 2013). Hereafter, the phrase “senior-aged” is referring to ages 65 or older unless otherwise specified. Of particular importance is understanding co-occurring morbidities, or multimorbidity, experienced by senior-aged women Veterans; their specific combinations of conditions are likely to affect which treatments and resources should to be developed or bolstered by the VHA (Akker, Buntix, Metsemakers, Roos, & Knottnerus, 1998; Sambamoorthi, Shen, Findley, Frayne, & Banerjea, 2010; Yoon, Scott, Phibbs, & Frayne, 2012). Thus, in order to inform the development of meaningful clinical guidelines, this study seeks to clarify how health conditions cluster in senior-aged women Veterans.
The Unique Case of Senior-Aged Women Veterans Who Use VHA
It is important to study women Veterans separately because it has been shown that outcomes based on studies of civilian populations cannot always be applied to Veteran populations (Bielawski et al., 2014). There are several theories about how and why Veterans’ health differs from that of non-Veterans. Some argue that military cohorts have higher survival rates (i.e., the “healthy soldier effect”) compared to the general population because of the initial physical screening for military service, the requirements for maintaining a certain fitness standard, and better access to medical care during and after their service (Kang et al., 2014). Others have added a clarification that there is a reduction in the healthy soldier effect as Veterans age (Washington et al., 2016). When it comes specifically to women Veterans, some hypothesize that it is common for women to join the military in order to escape unfavorable or dysfunctional home lives, which is associated with poorer health outcomes (Reiber & LaCroix, 2016).
When considering older generations of women specifically, women Veterans have been shown to differ from civilian women in demographic traits that are relevant to health outcomes. Senior-aged women Veterans are more likely to be college graduates, Caucasian, and less likely to have ever been married or be living with a partner than senior-aged civilian women (LaCroix et al., 2016).
When comparing rates of health conditions, there is some evidence to suggest that senior-aged women Veterans might be in better health than senior-aged women in the United States. Approximately 61.4% of senior-aged women Veterans have a diagnosis of hypertension; in the general U.S. population, 70.8% women ages 65–74 and 80.1% of those over 75 years of age have hypertension (Centers for Disease Control and Prevention, 2015; Frayne, Parker, Christiansen, Loveland, & Seaver, 2014). For osteoporosis, 16.6% of senior-aged women Veterans have the diagnosis; for the United States in general, 25.7% of all U.S. women aged 70–79 and 34.9% of those ages 80 and up have the condition (Centers for Disease Control and Prevention, 2011; Frayne et al., 2014). Rates of diabetes are a bit more similar between the two groups, with 22.2% of senior-aged women Veterans and 19.8% of the general U.S. population of senior-aged women having the condition (Frayne et al., 2014; Go, Mozaffarian, Roger, Benjamin, & Berry, 2013). Finally, 26.4% of senior-aged women Veterans and 31.1% of all U.S. senior-aged women have a diagnosis of cataracts (Frayne et al., 2014; Wright, Looker, Saag, Curtis, & Delzell, 2014).
On the other hand, there are also findings to suggest that women Veterans are in poorer health than their civilian counterparts. A number of studies have gathered evidence showing that non-Veteran women are healthier than Veteran women. Using a self-reported health-related quality of life survey, women Veterans reported worse physical and mental health than non-Veterans (Skinner & Furey, 1998). It has also been noted that women Veterans are more likely to experience sexual trauma than non-Veteran women (Der-Martirosian, Cordasco, & Washington, 2013; Mengeling, Sadler, Torner, & Booth, 2011). And, women Veterans aged 80 or older reported significantly lower perceived health, physical functioning, life satisfaction, social support, and quality of life than non-Veteran women of the same age (LaCroix et al., 2016). While senior-aged women Veterans have a more active lifestyle, they have been shown to experience greater declines in level of physical activity as compared to non-Veteran women (Bastian et al., 2016).
There are previous studies devoted to uncovering the potential causes of health differences (positive or negative) experienced by women Veterans; they highlight several socioeconomic suspects. Der-Martirosian et al. (2013) found that senior-aged women Veterans who are employed, earn higher incomes, are married, and have more education tend to experience better physical health. For women Veterans aged fifty and older, there is evidence showing they have lower rates of ever marrying, are less likely to be currently living with a spouse or partner, and report lower overall levels of social support compared with non-Veterans (LaCroix et al., 2016). Overall, approximately 20% of women Veterans report that they have no one to turn to in times of crisis (Cotton, Skinner, & Sullivan, 2000). Furthermore, women Veterans are also more likely to be members of racial-ethnic minorities than non-Veterans (Goldzweig, Balekian, Rolón, Yano, & Shekelle, 2006). These documented differences (e.g., lower social support and race-based demographic differences) may contribute to health disparities between Veteran and civilian women.
Differences have also been documented between the women Veterans who use VHA and women Veterans who receive their care in the private sector. In terms of demographic characteristics, users of VHA are more likely to have served in combat, be under 65 years old, poorer, African American and urban dwelling (Der-Martirosian et al., 2013; Mengeling et al., 2011; Mooney & Weeks, 2007; Washington, Farmer, Mor, Canning, & Yano, 2015). In terms of health, compared to Veterans who do not use VHA services, VHA users are more likely to rate their health as fair or poor, have a service-connected disability and be uninsured (Der-Martirosian et al., 2013; Mengeling et al., 2011; Mooney & Weeks, 2007; Washington et al., 2015). These findings are confirmed by patterns of attrition from VHA care; women Veterans are more likely to discontinue care in VHA when they are healthier, over 65 years old, have insurance and live in rural areas (Hamilton, Frayne, Cordasco, & Washington, 2013). Given these findings, the population of senior-aged women Veterans using VHA services may represent a particularly condensed population of low resource patients.
The Importance of Understanding Multimorbidity
Multimorbidity refers to the co-occurrence of diseases (acute or chronic) within one person at one point in time (Agur, McLean, Hunt, Cuthrie, & Mercer, 2016; Akker et al., 1998; Marengoni, Rizzuto, Wang, Winbald, & Fratiglioni, 2009). Multimorbidity is associated with adverse health outcomes, greater costs and adverse reactions to treatments (Boyd et al., 2012). When deciding on a course of medical action for any given condition, providers often rely on the U.S. CPG, which are designed by specialty-dominated committees focused on managing a single disease, often without consideration of comorbid conditions (Boyd, Darer, Boult, Fried, & Boult, 2005). When a patient is multimorbid, the various guidelines may be combined in ways that lead to contradictory drug regimens, lack of care coordination, medical errors, insufficient health education, low patient satisfaction, patient confusion, and more (Boyd et al., 2007; Hughes, 2012; Liddy, Blazkho, & Mill, 2014). Therefore, the identification of common multimorbidity clusters for patient cohorts would allow for the creation of guidelines designed to deal with multimorbidity in a way that avoids these pitfalls.
Past literature tells us about the most common physical and mental conditions for senior-aged Veterans. In fiscal years 2007 and 2008, the most common chronic conditions for senior-aged women Veterans were (in descending order): hypertension, hyperlipidemia, arthritis, osteoporosis, and dyspepsia (GERD/PUD) (Steinman et al., 2012). In 2014, the VHA’s Women Health Evaluation Initiative (WHEI) wrote a comprehensive report that compiled a list of the 20 most common conditions specific to senior-aged women Veterans who were receiving care in the VHA; the conditions are listed (in descending order of prevalence) in the method section as well as in Table 3 (Frayne et al., 2014).
Table 3.
Percentage of Cohort or Demographic Subgroup with at Least One Diagnosis for each Condition
| Cohort | Race | Age | Marital status | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (%) | White (%) | Black (%) | Other (%) | Age 65–74 (%) | Age 75–84 (%) | Age 85+ (%) | Married (%) | Not married (%) | |
| Disease Groups | n = 38,597 | n = 31,050 | n = 3,787 | n = 3,760 | n = 20,866 | n = 9,755 | n = 7,956 | n = 11,505 | n = 26,907 |
| Hypertension | 63.40 | 62.63 | 77.45 | 55.53 | 60.38 | 67.87 | 65.84 | 61.19 | 64.58 |
| Lipid Disorders | 56.82 | 57.88 | 55.37 | 49.55 | 59.58 | 59.59 | 46.18 | 58.37 | 56.41 |
| Cataract | 27.10 | 27.28 | 36.12 | 16.57 | 29.64 | 27.59 | 19.86 | 24.04 | 28.52 |
| Joint Disorders—Unspecified or Multiple Joints | 26.43 | 26.70 | 31.11 | 19.57 | 27.06 | 26.66 | 24.52 | 24.22 | 27.50 |
| Eye Disorders—Other | 26.28 | 26.75 | 31.21 | 17.45 | 26.47 | 25.77 | 26.43 | 22.73 | 27.93 |
| Thyroid Disorders | 25.61 | 27.11 | 16.61 | 22.23 | 24.15 | 26.99 | 27.71 | 25.95 | 25.57 |
| Diabetes Mellitus | 24.14 | 23.20 | 36.60 | 19.31 | 27.49 | 24.82 | 14.52 | 22.38 | 24.99 |
| Esophageal Disorders | 23.68 | 24.62 | 23.48 | 16.06 | 25.12 | 23.92 | 19.61 | 23.73 | 23.75 |
| Dermatologic Disorders—Other | 22.75 | 23.62 | 24.03 | 14.28 | 24.43 | 22.40 | 18.78 | 19.66 | 24.16 |
| Refraction Disorders | 22.15 | 22.40 | 27.28 | 14.87 | 25.88 | 20.34 | 14.56 | 20.60 | 22.90 |
| Joint Disorders—Lower Extremity | 21.61 | 21.53 | 31.00 | 14.28 | 24.32 | 20.30 | 16.08 | 19.00 | 22.79 |
| Musculoskeletal Conditions—Other | 21.09 | 21.43 | 24.14 | 15.24 | 23.77 | 20.94 | 14.27 | 20.63 | 21.40 |
| Spine Disorders—Lumbosacral | 18.20 | 18.13 | 24.14 | 12.79 | 21.11 | 16.45 | 12.71 | 16.55 | 18.98 |
| Depression, Possible—Other | 17.61 | 18.40 | 17.35 | 11.30 | 21.98 | 14.29 | 10.19 | 15.50 | 18.59 |
| Hearing Problems | 17.24 | 18.53 | 9.98 | 15.43 | 10.99 | 18.13 | 32.55 | 15.32 | 18.08 |
| Residual Codes | 17.12 | 17.16 | 23.03 | 10.90 | 18.03 | 16.43 | 15.60 | 13.65 | 18.67 |
| Overweight/Obesity | 16.72 | 16.49 | 24.08 | 11.22 | 23.21 | 13.33 | 3.85 | 16.46 | 16.89 |
| Osteoporosis | 15.90 | 16.85 | 9.29 | 14.73 | 11.85 | 18.09 | 23.84 | 14.67 | 16.48 |
| Endocrine, Metabolic, and Nutritional Disorders—Other | 13.40 | 13.65 | 14.15 | 10.64 | 14.19 | 13.58 | 11.12 | 12.49 | 13.84 |
| Coronary Artery Disease—Other | 11.99 | 12.69 | 9.37 | 8.88 | 9.70 | 13.72 | 15.87 | 10.73 | 12.57 |
For women Veterans, previous studies have examined how conditions tend to co-occur in combinations of two or three. Using corrected Chi-Square analyses to study the co-occurrence of chronic conditions among women Veterans over the age of 65, Steinman et al. (2012) found that the two most common sets of triple conditions were (1) 18.4% with diabetes, hypertension and hyperlipidemia and (2) 16.5% with coronary heart disease, hypertension and hyperlipidemia. Narrower in scope, Shen et al. (2010) found that among all-aged women Veterans using VHA services who are diagnosed with diabetes or heart disease, 27% were also diagnosed with depression.
We can make some imperfect comparisons of the findings on multimorbidity among senior-aged women Veterans to general populations. The most common conditions for Swedish women over 77 years old are hypertension, dementia and heart failure (Marengoni, Winblad, Karp, & Fratiglioni, 2008). Using logistic regression and cluster analysis to analyze multimorbid chronic conditions in Swedish men and women over age 77, Marengoni et al. (2009) found the following five clusters: (1) hypertension, heart failure, chronic atrial fibrillation, and Cardiovascular Disease (CVD); (2) thyroid dysfunction, chronic obstructive pulmonary disease, and coronary heart disease; (3) diabetes mellitus, visual impairments, and deafness; (4) dementia, depression, and hip fracture; and (5) malignancy and anemia. And for all ages and sexes of Medicare beneficiaries, 33.7% have a combination of ischemic heart disease, hypertension, and hyperlipidemia; 29.9% have diabetes, hypertension and hyperlipidemia; and 25.7% have arthritis, hypertension, and hyperlipidemia (Centers for Medicare & Medicaid Services, 2012).
This study aims to contribute to our substantive understanding of the health care patterns for senior-aged women Veterans by using a more comprehensive statistical model. We will identify common multimorbid clusters for senior-aged women Veterans who use VHA by applying the statistical technique called Latent Class Analysis (LCA). The objective of LCA is to create a set of latent classes or clusters of individuals determined by a set of comorbid conditions. Once identified, this information can used to design Clinical Practice Guidelines specific to senior-aged women for their most common combinations of conditions in a manner that avoids poor health outcomes, higher costs and lower patient satisfaction.
Design and Methods
With approval from the local VHA Institutional Review Board, data on 41,465 unique female patients were gathered from the VHA Corporate Data Warehouse. Patients were included if they were 65 years old as of January 1, 2014 and if they had at least one outpatient visit or were discharged from at least one inpatient stay between January 1, 2013 and December 31, 2014. Patients (n = 6,703) were excluded if they were marked as deceased or as a non-Veteran.
In addition to age, data on a patient’s marital status and race were included. For marital status, 185 or 0.5% of patients had no mention of their marital status in the patient record. Another 526 or 1.3% of patients had multiple values of marital status; in these cases, the value associated with the most recently entered patient record was selected. When a patient’s demographic information indicated that they were widowed, separated, divorced, single or never married, the patient was characterized as “not currently married”. For race, 2,882 or 7.5% of patients were missing race information. Of the 92.5% with a race entry, about 87% had a consistent indicator for their racial identity, and for the remaining ~13%, we used in hierarchical order, the most commonly occurring value of race, a value of race that matched the records in a secondary table that holds race values, the most recently entered value or the least commonly occurring (population-wise) value. Due to a combination small populations and current VHA coding practices around race, the variable Race was resolved to the categories of White, Black, and Other; the patients who are combined into the “other” race category included 878 American Indians/Alaska Natives/Asians/Native Hawaiians/Pacific Islanders and 2,882 patients with missing information.
Methods defined by the WHEI were used to assemble diagnoses into 202 groupings by organ system (Frayne et al., 2014). Patients were excluded for having diagnoses that are specific to men such as prostate cancer (n = 73 patients) or diagnoses such as menstrual disorders that are associated with younger, premenopausal women when that diagnosis was not marked as historical (n = 68 patients). Diagnoses were excluded if they were modified in a way that indicates they were not final (n = 1 diagnosis) or when the diagnosis was merely a suspected diagnosis marked as the reason for ordering a test as opposed to a confirmed diagnoses based on a test result (n = 1,162 diagnoses). We began with the top 20 condition groupings for senior-aged women Veterans, as defined by WHEI; they are (in descending order): Hypertension; Lipid Disorders; Cataract; Thyroid disorders; Joint Disorders—Unspecified or Multiple Joints; Eye disorders—Other; Diabetes Mellitus; Esophageal disorders; Dermatologic disorders—Other; Refraction disorders; Joint Disorders—Lower Extremity; Osteoporosis; Hearing problems; Depression, possible—Other; Musculoskeletal conditions—Other; Spine Disorders—Lumbosacral; Overweight/Obesity; Coronary Artery Disease—Other; Residual Codes; and Endocrine, Metabolic and Nutritional Disorders—Other (Frayne et al., 2014). WHEI defined the “residual codes” grouping as a domain that “includes miscellaneous diagnoses [that are] not mapped to other domains, such as symptoms, conditions due to external causes, and psychosocial factors” (Frayne et al., 2014).
In LCA, a patient’s health status (the latent variable) defines the specific level of comorbidities (items or health indicators). The patient’s health status can be classified into groups (the latent classes) according to the pattern of comorbidities. LCA estimates the prevalence of each class in the sampled population (class probabilities), the contribution of each comorbidity to the class identification (item probabilities), and the amount of error contributed by each health condition in the estimation of the latent classes (Collins & Lanza, 2010; Funderburk, Maisto, Sugarman, & Wade, 2008; Funderburk, Kenneson, & Maisto, 2014; Green et al., 2010; Muthén, 2001; Pugh et al., 2014).
Model parameters are estimated using the Expectation-Maximization algorithm. A training data set (70% of data) was created to develop the latent class model, which was later validated using the remaining 30% of data. The number of latent classes comprising the latent variable was determined by using information-based criteria, namely Akaike’s Information Criterion and Bayes Information Criterion. An overall statistic was used to assess model fit. Class naming is suggested by the magnitude of the health indicator probabilities (ρ or rho values), in which high values (e.g., 0.45) indicate higher likelihood of a given comorbidity in a chosen class.
Briefly, we use LCA to classify patients based on responses on the 20 diagnosis groups. The probability of a given pattern of indicators/comorbidities, say , where J is the number of indicators, is modeled as a function of the number of classes, C, corresponding class probabilities (γs), and item or indicator probabilities given class . Precisely,
The gamma () parameter estimates the likelihood of a given individual being in class C=c, whereas the corresponds to the probability of the individual response to the indicator being , given membership in class
Finally, we conducted analyses to examine the prevalence of each demographic subgroup (by race, age and marital status) in each condition class. Then, we deconstruct those prevalence patterns by examining percentages of each condition for the same subgroups.
Results
After applying inclusion and exclusion criteria, our study sample included 38,597 patients who were predominately Caucasian (88.5%), unmarried (69.7%), and aged 65–74 (54.1%). See Table 1 for more details.
Table 1.
Cohort Demographics for Senior-aged Women Veterans
| Demo (N = 38,597) | ||
|---|---|---|
| Variable | N | % |
| Race | ||
| Caucasian/White | 31,050 | 88.45 |
| African American/Black | 3,787 | 9.81 |
| Other Racial Groups | 878 | 2.27 |
| Missing | 2,882 | 7.47 |
| Marital status | ||
| Married | 11,505 | 29.81 |
| Not married | 26,907 | 69.71 |
| Missing | 185 | 0.48 |
| Age group | ||
| 65–74 | 20,886 | 54.11 |
| 75–84 | 9755 | 25.27 |
| 85 + | 7,956 | 20.61 |
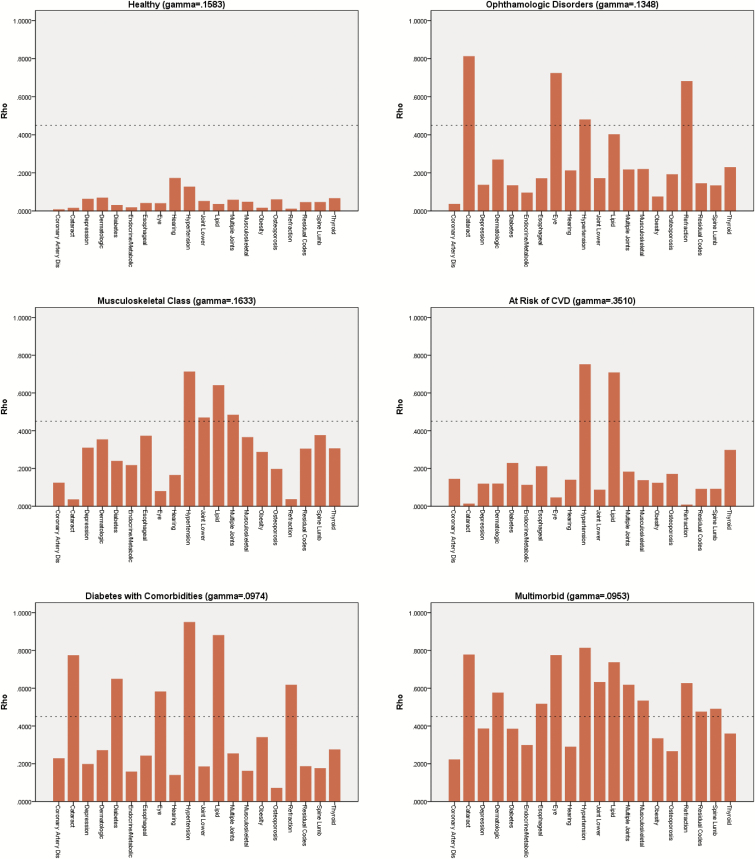
Six latent disease classes were identified for our sample of senior-aged women Veterans. A condition group was assigned to a latent class when it’s Rho estimate indicated that it had over 0.45 or 45% chance of belonging to that class. The first latent class was characterized by a lack of any clinically significant disease; we are labeling this group “Healthy”. All classes included hypertension as a condition except for the “Healthy” class. The second latent class was characterized by hypertension, lipid disorders and joint disorders (unspecified/multiple and lower extremity); we applied a label of “Musculoskeletal Disorders” to this class. The third latent class was characterized by hypertension combined with vision problems such as refraction disorders, cataracts, and other eye disorders; we referred to this group as “Ophthalmologic Disorders”. The fourth latent class was characterized by hypertension and lipid disorders; we labeled this group “At Risk for Cardiovascular Disease (CVD)”. The fifth latent class was characterized by hypertension, lipid disorders, diabetes and ophthalmologic disorders (i.e., refraction abnormalities and cataracts); we labeled this group “Diabetes with Comorbid Conditions”. The sixth latent class was characterized by a large number of multimorbid conditions: hypertension, lipid disorders, all three categories of vision disorders, esophageal disorders, dermatologic disorders, both categories of joint disorders, musculoskeletal conditions, spine disorders, and conditions labeled “residual” by the WHEI (Frayne et al., 2014). We have labeled this sixth latent class “Multimorbid”. Looking at gamma values, there was a 35.1% chance that a senior-aged woman Veteran would be in the “At Risk of CVD” class and a 16.3% chance that they would be in the “Musculoskeletal Disorders” class. See Figure 1 for more details.
Figure 1.
Six diagnosis classes among senior-aged women veterans.
For the cohort as a whole, Table 2 displays the prevalence rates for each latent class. The most common class was “At Risk of Cardiovascular Disease (CVD)”, with 37.5% of the cohort being classified in this group. The next most common class was “Healthy” with approximately 16.2% of the cohort falling into this class. Approximately 9.1% of the cohort fall into the “Diabetes with Comorbid Conditions” and another 9.1% were in the “Multimorbid” class. For demographic subgroups, a diversity of prevalence rates was seen; all differences were statistically significant. When examining the trends by race, our findings indicated that Blacks were disproportionately represented in the classes “Diabetes with Comorbidities” and “Multimorbid”. Blacks were 9.8% of the cohort, but they were 16.4% of the “Diabetes with Comorbidities” class and 13.98% of the “Multimorbid” class. Members of “other” (i.e., American Indians/Alaska Natives, Asians/Native Hawaiians/Pacific Islanders and those with missing race information) racial groups were overrepresented in the healthy class. There are also age-based trends that are notable. Younger seniors aged 65–74 were 54% of the cohort, but they were 63.1% of the “Musculoskeletal Disorders” class and 66.1% of “Diabetes with Comorbid Conditions” class. The oldest group of women (aged 85 or older) were 20.6% of the cohort but only 9.1% of the “Diabetes with Comorbid Conditions” class. Finally, trends by marital status show that those who were married constituted 29.8% of the cohort but only 21.8% of the “Multimorbid” class. Refer to Table 2 for more details.
Table 2.
Prevalence Rates of Latent Condition Classes Within the Cohort and Demographic Subgroups
| Class and class name: | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| (H) Healthy | |||||||||
| (J) Musculoskeletal Disorders | |||||||||
| (O) Ophthalmologic Disorders | |||||||||
| (R) At Risk for Cardiovascular Disease | |||||||||
| (D) Diabetic with Comorbid Conditions | |||||||||
| (M) Multimorbid | |||||||||
| 6,270 | 5,488 | 5,342 | 14,485 | 3,493 | 3,520 | ||||
| Overall prevalence of each class among: | Sample size N | Sample % | (H)% | (J)% | (O)% | (R) % | (D)% | (M)% | Chi-Square |
| Whole Cohort | 38,597 | 100 | 16.24 | 14.22 | 13.84 | 37.53 | 9.05 | 9.12 | |
| Whites | 31,050 | 80.4 | 76.68 | 81.01 | 82.16 | 81.57 | 77.41 | 82.05 | |
| Blacks | 3,787 | 9.8 | 7.18 | 11.92 | 10.28 | 7.39 | 16.38 | 13.98 | |
| Other Races | 3,760 | 9.7 | 16.14 | 7.07 | 7.56 | 11.04 | 6.21 | 3.98 | 912.14* |
| Young Seniors 65–74 | 20,886 | 54.1 | 48.77 | 63.08 | 56.50 | 47.99 | 66.12 | 59.29 | |
| Middle Seniors 75–84 | 9,755 | 25.2 | 23.56 | 21.94 | 24.15 | 27.90 | 24.77 | 24.94 | |
| Older Seniors 85+ | 7,956 | 20.6 | 27.67 | 14.98 | 19.36 | 24.11 | 9.11 | 15.77 | 994.79* |
| Married | 11,505 | 29.8 | 31.53 | 27.68 | 28.10 | 32.90 | 28.06 | 21.68 | |
| Not married | 26,907 | 69.7 | 66.59 | 72.12 | 71.68 | 66.88 | 71.74 | 78.21 | |
| Missing | 185 | .47 | 1.88 | 0.20 | 0.22 | 0.23 | 0.20 | 0.11 | 534.94* |
Note: *p < .0001.
To deconstruct the trends seen in prevalence rates, we explored the differences in percentages of each condition, using a 5% difference as the cutoff, to compare the overall cohort to each demographic subgroup. The overall cohort of senior-aged women Veterans was characterized by high rates of hypertension (68.4%) and lipid disorders (56.8%). Rates of hypertension were even higher for Blacks (77.45%). Blacks also stand apart for having a higher proportion of Diabetes Mellitus (36.6% vs 24.1%), cataracts (36.1% vs 27.1%), and joint disorders of the lower extremity (31.0% vs 21.6%) than the overall cohort; simultaneously, Blacks are less likely than the overall cohort to have diagnoses of osteoporosis (9.3% vs 15.9%), thyroid disorders (16.6% vs 25.6%), and hearing problems (10.0% vs 17.2%). The older members of the cohort, that is, women Veterans aged 85 years and older (n = 7,956), were less likely than the overall cohort to have certain diagnoses: lipid disorders (46.2% of oldest women vs 56.8% of the overall cohort), obesity (3.9% vs 16.72%), diabetes (14.5% vs 24.1%), ophthalmologic disorders (19.9% vs 27.1% cataract and 14.6% vs 22.2% refraction disorders), depression (10.2% vs 17.6%), and musculoskeletal disorders (12.7% vs 18.2% spinal disorder, 14.3% vs 21.1% musculoskeletal conditions, and 16.1% vs 21.6% joint disorders). See Table 3 for more details.
Discussion and Implications
Overall, we found six latent condition classes, with one being “Healthy”. For the five symptomatic classes, we propose that these common condition classes can be used to create CPG that are constructed by multispeciality physician panels. These guidelines should be specific to senior-aged women Veterans, the identified combination of conditions, the mixtures of medications, the educational needs of the patients and more. For example, the latent class “Diabetes with Comorbid Conditions” would develop guidelines that incorporate treatments and interventions for hypertension, lipid disorders, diabetes and ophthalmologic disorders (e.g., refraction abnormalities, cataracts) in such a way as to make them appropriate to senior women. These guidelines would need to be attentive to potential drug interactions between common medications used for the overlapping conditions.
The most common condition for our cohort was hypertension, with 63.4% of all senior-aged women Veterans having a diagnosis in their records; this finding is consistent with the 2013 study of cardiovascular health in women Veterans (Whitehead, 2013). This percentage represents a substantial increase in the rate of hypertension for women Veterans as they age, as Vimalananda et al. (2013) found hypertension in 13% of women Veterans aged 35–44, 28% of women Veterans aged 45–54, and 42% of women Veterans aged 55–64. On the other hand, this rate is lower than in the general population of senior-aged women in the United States, where 70.8% women ages 65–74 and 80.1% of those over 75 years of age have hypertension (Centers for Disease Control and Prevention, 2015). Whether this difference in rates between women Veterans using the VHA and the general population signify better health warrants further comparative studies.
Hyperlipidemia was the second most common condition for our cohort of senior-aged women Veterans (~56%); this result is consistent with Whitehead’s (2013) results, which show that 79.6% of all senior-aged women Veterans have at least one CVD risk factor. Together hypertension and hyperlipidemia form the most common latent class, “At Risk of CVD” (37.5%). This result is consistent with the findings of Vimalananda et al. (2013) who found that rates of CVD disease are shown to increase as women Veterans age (~25% of women aged 45–54 and ~33% of women aged 55–64 in VHA have more than two CVD risk factors).
We find additional support for arguments about intragroup variation among women Veterans, as that variation relates to race. Our results indicated that Blacks were most likely to have diagnoses of hypertension, diabetes, obesity, spine disorders and cataracts. Hence, Blacks have disproportionate representation in the classes that infer the greatest risk of adverse outcomes, “Diabetes with Comorbidities” and “Multimorbid”. This pattern is consistent with past studies on national samples of men and women; they found higher risks of diseases, comorbidities, and mortality for Blacks and Native Americans due to a combination of biological, socioeconomic, environmental, and psychosocial causes (Daw, 2017; Mujahid et al., 2017; Saha, Freeman, Toure, Tippens, & Weeks, 2007; Schoenborn & Heyman, 2009). The VHA may create benefit by specifically targeting Black senior-aged women in their intervention programs that are aimed at reducing obesity and preventing diabetes.
Our findings also reveal a correlation between health status and marital status, which is presumed to be an imperfect proxy for social support. Members of the cohort who are currently married are under-represented in the “Multimorbid” class, while unmarried members are over-represented in the class. Assuming marital status is telling us something about the level of social support the patient has, this pattern is consistent with past literature from the United States, Canada, and India showing the importance of marriage, spouses, family and overall social support in the well-being of senior-aged men and women (Perkins et al., 2016; Ploeg et al., 2017; Schoenborn & Heyman, 2009). However, it is of note that Schoenborn and Heyman (2009) find that the direction of this relationship reverses for both men and women who are 75 years old and older, with marriage actually being associated with poorer health. Of women Veterans aged 65–75, 70.8% are unmarried and 48.1% live alone; these numbers increase to 71.4% and 52.5% respectively for women over the age of 75 (Frayne et al., 2006). Similarly, LaCroix et al. (2016) find that the current cohort of senior-aged women Veterans are less likely to have social support than civilians. In general, services that support older women Veterans may need to plan for an increased demand on institutional services for this population to prolong and preserve their independence, and perhaps marital status can act as something of a prompt for exploring potential patient needs.
Lastly, we found an interesting age-based pattern of prevalence in disease classes. The oldest women in the cohort (ages 85 and older) have relatively low rates of many of the diagnoses examined in this study, such as: lipid disorders, obesity, diabetes, vision disorders, musculoskeletal problems, and depression. They are, however, more likely to have diagnoses such as osteoporosis, hearing problems and thyroid disorders. On the whole, women Veterans aged 85 and older had a disproportionately low probability of being in the “Diabetes with comorbidities” class. A national study performed by Schoenborn and Heyman (2009) found a decreasing probability of diabetes for older cohorts. There may be generational differences underlying these findings; Vimalananda et al. (2013) note a higher prevalence of obesity for younger cohorts as compared to older cohorts, suggesting a possible earlier emergence of diseases such as diabetes in the younger cohort of Veterans. Studies in longevity show that people who survive to the oldest ages (i.e., centenarians) tend to exhibit a compression of disability, an optimal set of health behaviors, stronger social support systems and genetic advantages as compared to people who die at younger ages (Poon & Cheung, 2012; Sebastiani & Perls, 2012); perhaps, we are seeing a healthy survivor effect in the oldest members of the cohort.
This study has several limitations. First, the cohort is restricted to those senior-aged women Veterans who use VHA services. We believe that it is appropriate to examine this cohort separately from non-VHA users, civilian women and younger women Veterans because past studies (as discussed in the introduction) have illuminated each group’s unique health concerns and needs. Furthermore, based simply on growth rates and population size, we believe that an argument can be made to address this expanding segment of VHA patients. In 2016, 32% of women Veterans were enrolled to receive care in VHA (Reiber & LaCroix, 2016). This percentage is the result of a trend of increasing numbers of women Veterans seeking care in VHA, as recorded by Friedman et al. (2011) between 2003 and 2009. A second limitation may be associated with the elimination of the 73 patients that have diagnoses specific to males. While it is possible that this elimination may be obscuring results related to transgendered women, we were unable to consider these 73 patients separately because they did not create a large enough group for use in an LCA model that includes 20 condition groupings. As a final consideration, we believe that it is important to acknowledge the likelihood that future cohorts of senior-aged women Veterans may differ from the current cohort of senior-aged women Veterans because they will be more likely to have engaged in active/combat service and have higher rates of service-connected conditions such as joint disorders (Frayne et al., 2014).
Overall, we found that among older women Veterans, there are six distinct diagnostic clusters with hypertension as a common diagnosis across five of the six. We also identified subgroup trends across the identified clusters relevant to racial minority populations and the oldest women Veterans. These six clusters may assist providers and the health care system in determining what levels and types of clinical services are required to promote optimal well-being and functioning among the aging women Veteran population by providing the prerequisite knowledge necessary for creating clinical guidelines that take multimorbidity into account. Home health and assisted living may greatly benefit the most elderly and unmarried women Veterans, while tailored interventions and education may help Black senior-aged women Veterans avoid obesity, diabetes, and other high-risk conditions. Clinical practice that carefully considers clusters of conditions may better serve the complex needs of senior-aged women Veterans.
Funding
This research was supported by the U.S. Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (project numbers SDR 02-237 and SDR 98-004). K. M. Goldstein is individually supported HSR&D CDA grant #13–263. D. M. Hynes is individually supported by a VA Research Career Scientist Award #98–352.
Conflict of Interest
None reported.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States Government.
References
- Agur K., McLean G., Hunt K., Cuthrie B., & Mercer S. W (2016). How does sex influence multimorbidity? Secondary analysis of a large nationally representative dataset. International Journal of Environmental Research and Public Health, 13, 391–402. doi:10.3390/ijerph13040391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akker M. V. D., Buntix F., Metsemakers J. F. M., Roos S., & Knottnerus J. A (1998). Multimorbidity in general practice: Prevalence, incidence, and determinants of co-occurring chronic and recurrent diseases. Journal of Clinical Epidemiology, 51, 367–375. doi:10.1016/s0895-4356(97)00306-5 [DOI] [PubMed] [Google Scholar]
- Atkins D. (2013). Health services research on women veterans: A critical partner on the road to patient-centered care. Journal of General Internal Medicine, 28 (Suppl 2), S488–S489. doi:10.1007/s11606-013-2472-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian L. A., Hayes P. M., Haskell S. G., Atkins D., Reiber G. E., LaCroix A. Z., & Yano E. M, (2016). Improving our understanding of health issues in older women veterans. The Gerontologist, 56, S10–S13. doi:10.1093/geront/gnv672 [DOI] [PubMed] [Google Scholar]
- Bean-Mayberry B., Batuman F., Huang C., Goldzweig C. L., Washington D. L., Yano E. M., … Shekelle P. G (2010). Systematic review of women Veterans health research 2004–2008. Washington, DC: Department of Veterans Affairs; Retrieved from https://www.hsrd.research.va.gov/publications/esp/reports.cfm. [PubMed] [Google Scholar]
- Bielawski M. P., Goldstein K. M., Mattocks K. M., Bean-Mayberry B., Yano E. M., & Bastian L. A (2014). Improving care of chronic conditions for women veterans: Identifying opportunities for comparative effectiveness research. Journal of Comparative Effectiveness Research, 3, 155–166. doi:0.2217/cer.14.4 [DOI] [PubMed] [Google Scholar]
- Boyd C. M., Boult C., Shadmi R., Leff B., Brager R., Dunbar L., … Wegener S (2007). Guided care for multimorbid older adults. The Gerontologist, 47, 697–704. doi:10.1093/geront/47.5.697 [DOI] [PubMed] [Google Scholar]
- Boyd C. M., Darer J., Boult C., Fried L. P., Boult L., Wu A. W (2005). Clinical practice guidelines and quality of care for older patients with multiple comorbid disease, implications for pay for performance. The Journal of American Medical Association, 294, 716–724. doi:10.1001/jama.294.6.716 [DOI] [PubMed] [Google Scholar]
- Boyd C. M., McNabney M. K., Brandt N., Correa-de-Araujuo R., Daniel K., Epplin J., … Shega J. W, of the American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity (2012). Guiding principles for the care of older adults with multimorbidity: An approach for clinicians. Journal of the American Geriatrics Society, 60, E1–E25. doi:10.1111/j.1532-5415.2012.04188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011). The state of vision, aging, and public health in America. Atlanta, GA: U.S. Department of Health and Human Services; Retrieved from https://www.cdc.gov/visionhealth/resources/publications/index.htm. [Google Scholar]
- Centers for Disease Control and Prevention (2015). Rates of diagnosed diabetes per 100 civilian, non-institutionalized population, by age, United States, 1980–2014. Atlanta, GA: U.S. Department of Health and Human Services; Retrieved from https://www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm. [Google Scholar]
- Centers for Medicare & Medicaid Services (2012). Chronic conditions among Medicare beneficiaries. Baltimore, MD: U.S. Department of Health and Human Services; Retrieved from https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Chartbook_Charts.html. [Google Scholar]
- Collins L. M., & Lanza S. T (2010). Latent class and latent transitional analysis: With applications in the social, behavioral, and health sciences. New Jersey, NJ: John Wiley & Sons. doi:10.1111/j.1751–5823.2010.00122_5.x [Google Scholar]
- Cotton S. R., Skinner K. M., & Sullivan L. M (2000). Social support among women veterans. Journal of Women & Aging, 12, 39–62. doi:10.1300/J074v12n01_04 [DOI] [PubMed] [Google Scholar]
- Der-Martirosian C., Cordasco K. M., & Washington D. L (2013). Health-related quality of life and comorbidity among older women veterans in the United States. Quality of Life Research, 22, 2749–2756. doi:10.1007/s11136-013-0424-7 [DOI] [PubMed] [Google Scholar]
- Daw J. (2017). Contribution of four comorbid conditions to racial/ethnic disparities in mortality risk. American Journal of Preventive Medicine, 52(1S1), S95–S102. doi:10.1016/j.amepre.2016.07.036 [DOI] [PubMed] [Google Scholar]
- Frayne S. M., Parker V. A., Christiansen C. L., Loveland S., Seaver M. R., Kazis L. E., & Skinner K. M (2006). Health status among 28,000 women veterans: The VA Women’s Health Program Evaluation Project. Journal of General Internal Medicine, 21, S40–46. doi:10.1111/j.1525-1497.2006.00373.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayne S. M., Phibbs C. S., Saechao F., Maisel N. C., Friedman S. A., Finlay A., … Iqbal S (2014). Sourcebook: women Veterans in the Veterans Health Administration, volume 3: Sociodemographics, utilization, costs of care, and health profile. Washington, DC: Women’s Health Evaluation Initiative, Women’s Health Services, Veterans Health Administration, U.S. Department of Veterans Affairs; Retrieved from https://www.womenshealth.va.gov/. [Google Scholar]
- Friedman S. A., Phibbs C. S., Schmitt S. K., Hayes P. M., Herrera L., & Frayne S. M (2011). New women veterans in the VHA: A longitudinal profile. Women’s Health Issues, 21, S103–S111. doi:10.1016/j.whi.2011.04.025 [DOI] [PubMed] [Google Scholar]
- Funderburk J. S., Kenneson A., & Maisto S. A (2014). Identifying classes of veterans with multiple risk factors. Military Medicine, 179, 1119–1126. doi:10.7205/MILMED-D-14-00119 [DOI] [PubMed] [Google Scholar]
- Funderburk J. S., Maisto S. A., Sugarman D. E., & Wade M (2008). The covariation of multiple risk factors in primary care: A latent class analysis. Journal of Behavioral Medicine, 31, 525–535. doi:10.1007/s10865-008-9176-1 [DOI] [PubMed] [Google Scholar]
- Go A. S., Mozaffarian D., Roger V. L., Benjamin E. J., Berry J. D., Borden, … Turner M. B, on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. (2013). Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation, 127, e6–e245. doi:10.1161/CIR.0b013e31828124ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldzweig C. L., Balekian T. M., Rolón C., Yano E. M., & Shekelle P. G (2006). The state of women veterans’ health research: Results of a systematic literature review. Journal of General Internal Medicine, 21, S82–S92. doi:10.1111/j.1225-1497.2006.00380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. C., Kershaw T., Lin T., Heimer R., Goulet J. L., Kraemer K. L., … Justice A. C (2010). Patterns of drug use and abuse among aging adults with and without HIV: A latent class analysis of a US veteran cohort. Drug Alcohol Dependence, 110, 208–220. doi:10.1016/j.drugalcdep.2010.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A. B., Frayne S. M., Cordasco K. M., & Washington D. L (2013). Factors related to attrition from VA healthcare use: Findings from the National Survey of Women Veterans. Journal of General Internal Medicine, 28(Suppl 2), S510–S516. doi:10.1007/s11606-013-2347-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes L. D. (2012). Using clinical practice guidelines in multimorbid older adults- A challenging clinical dilemma. Journal of the American Geriatrics Society, 60, 2180–2182. doi:10.1111/j.1532-5415.2012.04223.x [DOI] [PubMed] [Google Scholar]
- Kang H. K., Cypel Y., Kilbourne A. M., Magruder K. M., Serpi T., Collins J. F., … Spiro A III (2014). HealthViEWS: Mortality study of female US Vietnam era veterans (1965–2010). American Journal of Epidemiology, 176, 721–730. doi:10.1093/aje/kwt319 [DOI] [PubMed] [Google Scholar]
- LaCroix A. Z., Rillamas-Sun E., Woods N. F., Weitlauf J., Zaslavsky O., Shih R., … Reiber G (2016). Aging well among women veterans compared with non-veterans in the women’s health initiative. The Gerontologist, 56(Suppl 1), S14–S26. doi:10.1093/geront/gnv124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddy C., Blazkho V., & Mill K (2014). Challenges of self-management when living with multiple chronic conditions. Canadian Family Physician, 60, 1123–1133. Retrieved from http://www.cfp.ca/content/60/12/1123. [PMC free article] [PubMed] [Google Scholar]
- Marengoni A., Rizzuto D., Wang H. Z., Winblad B., & Fratiglioni L (2009). Patterns of chronic multimorbidity in the elderly population. Journal of the American Geriatrics Society, 57, 225–230. doi:10.1111/j.1532-5415.2008.02109.x [DOI] [PubMed] [Google Scholar]
- Marengoni A., Winblad B., Karp A., & Fratiglioni L (2008). Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. American Journal of Public Health, 98, 1198–1200. doi:10.2105/AJPH.2007.121137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengeling M. A., Sadler A. G., Torner J., & Booth M (2011). Evolving comprehensive VA Women’s Health Care: Patient characteristics, needs and preferences. Women’s Health Issues, 21(4S), S120–S129. doi:10.1016/j.whi.2011.04.021 [DOI] [PubMed] [Google Scholar]
- Mooney S. E. & Weeks B. W (2007). Where do women veterans get their inpatient care?. Women’s Health Issues, 17, 367–373. doi:10.1016/j.whi.2007.08.006 [DOI] [PubMed] [Google Scholar]
- Mujahid M. S., Moore L. V., Petito L. C., Kershaw K. N., Watson K., & Diez Roux A. V (2017). Neighborhoods and racial/ethnic differences in ideal cardiovascular health (the multi-ethnic study of atherosclerosis). Health & Place, 44, 61–69. doi:10.1016/j.healthplace.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén B. (2001). Latent variable mixture modeling. In Marcoulides G. A. & Schumacker R. E. (Eds.), New developments and techniques in structural equation modeling (pp. 1–33). UK: Taylor & Francis. doi:10.4324/9781410601858 [Google Scholar]
- Perkins J. M., Lee H., James K. S., Oh K. S., Krishna A., Heo J., … Subramanian S. V (2016). Marital status, widowhood duration, gender and health outcomes: A cross-sectional study among older adults in India. BMC Public Health, 16, 1–12. doi:10.1186/s12889-016-3682-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploeg J., Matthew-Maich N., Fraser K., Dufour S., McAiney C., Kaasalainen S., … Emili A (2017). Managing multiple chronic conditions in the community: A Canadian qualitative study of the experiences of older adults, family caregivers and healthcare providers. BMC Geriatrics, 17, 1–15. doi:10.1186/s12877-017-0431-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon L. W., & Cheung S. L. K (2012). Centenarian research in the past two decades. Asian Journal of Gerontology & Geriatrics, 7, 8–13. Retrieved from ajgg.easyweb.hk/en-ajgg_issue-details-11.html. [Google Scholar]
- Pugh M. J. V., Finley E. P., Copeland L. A., Wang C., Noel P. H., Amuan M. E., … Pugh J. A (2014). Complex comorbidity clusters in OEF/OIF veterans: The polytrauma clinical triad and beyond. Medical Care, 52, 172–181. doi:10.1097/MLR.0000000000000059 [DOI] [PubMed] [Google Scholar]
- Reiber G. E., & LaCroix A. Z (2016). Older women veterans in women veterans health initiative. The Gerontologist, 56(S1), S1–S5. doi:10.1093/geront/gnv673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Freeman M., Toure J., Tippens K. M., & Weeks C (2007). Racial and ethnic disparities in the VA healthcare system: A systematic review. Washington, DC: Evidence Synthesis Pilot Program. U. S. Department of Veterans Affairs; Retrieved from https://www.hsrd.research.va.gov/publications/esp/reports.cfm. [PubMed] [Google Scholar]
- Sambamoorthi U., Shen C., Fidely P., Frayne S., & Banerjea R (2010). Depression treatment patterns among women veterans with cardiovascular conditions or diabetes. World Psychiatry, 9,177–182. doi:10.1002/j.2051–5545.2010.tb00306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn C. A., & Heyman K. M (2009). Health characteristics of adults aged 55 years and over: United States, 2004–2007. National Health Statistics Reports No 16. Hyattsville, MD: National Center for Health Statistics. doi:10.1037/e623972009-001 [PubMed] [Google Scholar]
- Sebastiani P., & Perls P. T (2012). The genetics of extreme longevity: Lessons from the New England Centenarian Study. Frontiers in Genetics, 3, 1–7. doi:10.3389/fgene.2012.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Findley P., Banerjea R., & Sambamoorthi U (2010). Depressive disorders among cohorts of women veterans with diabetes, heart disease and hypertension. Journal of Women’s Health, 19, 1475–1485. doi:10.1089/jwh.2009.1551 [DOI] [PubMed] [Google Scholar]
- Skinner K. M., & Furey J (1998). The focus on women veterans who use veterans administration health care: The Veterans Administration Women’s Health Project. Military Medicine, 163, 761–766. [PubMed] [Google Scholar]
- Steinman M. A., Lee S. J., Boscardin W. J., Miao Y., Fung K. Z., Moore K. L., & Schwatz J. B (2012). Patterns of multimorbidity in elderly veterans. Journal of the American Geriatrics Society, 60, 1872–1880. doi:10.1111/j.1532-5415.2012.04158.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimalananda V. G., Miller D. R., Christiansen C. L., Wang W., Tremblay P., & Fincke B. G (2013). Cardiovascular disease risk factors among women veterans at VA medical facilities. Journal of General Internal Medicine, 28 (Suppl 2), S517–S523. doi:10.1007/s11606-013-2381-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington D. L., Bean-Mayberry B., Hamilton A. B., Cordasco K. M., & Yano E (2013). Women veterans’ healthcare delivery preferences and use by military service era: Findings from the National Survey of Women Veterans. Journal of General Internal Medicine, 28(Suppl 2), 2571–2576. doi:10.1007/s11606-012-2323-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington D. L., Bird C. E., LaMonte M. J., Goldstein K. M., Rillamas-Sun E., Stefanick M. L., … Weitlauf J. C (2016). Military generation and its relationship to mortality in women veterans in women’s health initiative. The Gerontologist, 56(S1), S126–S137. doi:10.1093/geront/gnv669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington D. L., Farmer M. M., Mor S. S., Canning M. & Yano E. M (2015). Assessment of healthcare needs and barriers to VA use experienced by women veterans. Medical Care, 53 (4 Suppl 1), S23–S31. doi:10.1097/MLR.0000000000000312 [DOI] [PubMed] [Google Scholar]
- Whitehead A. M. (2013). State of cardiovascular health in women Veterans, volume 1: VA outpatient diagnosis and procedures in FY 10. Washington, DC: Women’s Health Services, Veterans Health Administration, U. S. Department of Veterans Affairs; Retrieved from https://www.womenshealth.va.gov/WOMENSHEALTH/latestinformation/publications.asp. [Google Scholar]
- Wright N. C., Looker A. C., Saag K. G., Curtis J. R., Delzell E. S., Randall S., & Dawson-Hughes B (2014). The recent prevalence of osteoporosis and low bone mass in the United Stated based on bone mineral density at the femoral neck of lumbar spine. Journal of Bone and Mineral Research, 29, 2520–2526. doi:10.1002/jbmr.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano E. M., Bastian L. A., Frayne S. M., Howell A. L., Lipson L. R., McGlynn G., … Fihn S. D (2006). Toward a VA Women’s Health Research Agenda: Setting evidence-based priorities to improve the health and health care of women veterans. Journal of General Internal Medicine, 21, S93–101. doi:10.1111/j.1525-1497.2006.00381.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Scott J. Y., Phibbs C. S., & Frayne S. M (2012). Trends in rates and attributable costs of conditions among female VA patients, 2000 and 2008. Women’s Health Issues, 22, e337–e344. doi:10.1016/j.whi.2012.03.002 [DOI] [PubMed] [Google Scholar]