Abstract
Background and objectives
Cardiac involvement has been well recognized in patients with dermatomyositis (DM) and polymyositis (PM) with a variable frequency between 9 and 72%. However, clinically significant heart involvement in DM/PM is relatively infrequent and there have been rare reports of cardiac transplantation in DM. Our aims were to describe a case of severe cardiac involvement in DM requiring heart transplantation and review the literature of cardiac disease in DM and PM.
Methods
A patient with dermatomyositis who was referred to our institution with severe heart failure is described. Pathology of the patient’s skeletal and cardiac muscle is reviewed. A MEDLINE database search of reports of cardiac involvement in DM and PM was also conducted.
Results
A 36 year-old man with DM presented with severe heart failure to our institution for evaluation of heart transplantation. After a three month hospitalization he underwent successful cardiac transplantation. Pathological examination of his explant heart revealed a pattern of inflammation and damage similar to DM in skeletal muscle. The patient is currently doing well, 20 months post-transplant, and is maintained on tacrolimus, cellcept, rituximab, and low dose prednisone. To our knowledge, this is the first case report of heart transplantation in dermatomyositis in which the muscle pathology is similar in both heart and skeletal muscle.
Conclusions
Severe cardiac involvement requiring transplantation is rare in dermatomyositis but does occur and appears to be related to a similar inflammatory process as noted in the skeletal muscle.
Keywords: Dermatomyositis, inflammatory myopathy, cardiomyopathy, cardiac transplantation, orthotopic heart transplant
Introduction
Dermatomyositis (DM) and polymyositis (PM) are both idiopathic inflammatory myopathies (IIM) characterized by proximal muscle weakness and inflammatory cell infiltrates within the skeletal muscle.1,2 Cardiac involvement such as conduction abnormalities, arrhythmias, congestive heart failure, valvular/pericardial/coronary artery disease and left ventricular dysfunction has been reported as a common cause of death.3–5 Severe cardiac involvement in IIM is rare and only two cases of cardiac transplant in IIM have been reported, one in a patient with PM and the other in which the cardiac muscle pathology showed giant cell myocarditis. In this report, we describe a patient with severe cardiac involvement in DM requiring heart transplant and review the literature of cardiac disease in DM and PM.
Case Report
A 36 year old African American male previously in good health presented to an outside facility with diffuse muscle pain and proximal muscle weakness. He described difficulty raising his arms above his head and climbing stairs. He had a pruritic, papular rash on his upper back and anterior chest and complained of itching and swelling around his eyes, hoarse voice, and swelling and stiffness of his hands. Labs were significant for a creatine phosphokinase (CPK) of 12,006 and MRI of bilateral femurs showed diffuse muscle edema. He was started on prednisone at 80mg daily for possible myositis. He subsequently developed dysphagia, and a muscle biopsy of his left thigh showed severe inflammatory myopathy with perivascular inflammation and zones of pan- and perifasicular atrophy consistent with dermatomyositis or variant. Two months after starting prednisone, the patient began methotrexate at 15mg weekly and the prednisone was tapered. Due to persistent muscle weakness and CPK elevation after 6 weeks on methotrexate, rituximab was added. Within 6 months of presentation, the patient developed severe fatigue and shortness of breath. He was found to have cardiomyopathy with an ejection fraction of 10–15% and normal coronary arteries. Over the subsequent 4 months he had multiple hospital admissions at an outside facility with heart failure complicated by atrial fibrillation, ventricular tachycardia, gastrointestinal bleeding with hemoptysis, and a lower extremity deep venous thrombosis. The patient was transferred to our facility for evaluation of orthotopic heart transplantation (OHT).
Past medical history included heart palpitations as a teenager and an isolated episode of endocarditis 12 years prior to presentation. The patient had played college basketball and noted that he could not pursue professional basketball because he was unable to pass the “heart evaluations” required. He noted that his muscle weakness was worse in sites of old basketball injuries including his left quadriceps muscle and right shoulder. Six months prior to presentation with muscle weakness he had had onset of Raynaud’s phenomenon and numbness in the hands. Electromyogram and nerve conduction study of the upper extremities at that time revealed bilateral median neuropathy at the wrists and “no electrical instability” of the muscles. Social history was remarkable for no tobacco, IV drugs, or alcohol abuse. The patient worked as a personal trainer.
Upon admission to our facility, the patient had residual lower extremity proximal muscle weakness and a mild hyperpigmented rash on his upper chest and back. He was receiving prednisone 10mg daily, MTX 25mg SQ weekly and rituxan was dosed 7 months prior to admission. CPK was 126 IU/L. Serologic testing showed the presence of an anti-Ku antibody. The patient had a complicated hospital course including cardiogenic shock requiring placement of an intra-aortic balloon pump followed by bi-ventricular assist devices (VADs). Immunosuppressive medications were not increased due to concern regarding biVAD infections by the Cardiology Transplant service which would preclude OHT. A month following his initial admission, the patient had bleeding and purulent discharge from his VAD sites and the methotrexate was held and prednisone was decreased to 7mg daily. The patient’s lower extremity weakness worsened and hoarseness of his voice returned. His methotrexate was re-initiated at 10mg weekly, however after several weeks, his CK level rose to 1400IU/L and moderate dose prednisone at 40mg daily and IVIG were initiated. Within 2 weeks of this DM flare, the patient underwent a successful orthotopic heart transplant.
On examination of the explanted heart valvular circumferences were within the normal high limits6. The tricuspid (13.2 cm in circumference), mitral (11.7 cm in circumference) and aortic cusps (6.8 cm in circumference) appeared mildly thickened. Mitral chorda were attached to both papillary muscles. All three pulmonary cusps were unremarkable and 6.8 cm in circumference. Pathology of the patient’s skeletal and cardiac muscle is shown in Figures 1 and 2 in detail. Tissue samples obtained from explanted heart included left and right ventricular wall and papillary muscle. Histologic examination showed a multifocal chronic and severe fibrosing myocarditis in all areas examined. Active myocardiocyte injury was evaluated using non-specific esterase (NSE), an enzyme reaction with propensity to detect lysosomal activation and active myodegeneration. This consisted of foci of activity and degeneration also multifocal and of variable severity ranging from single fiber necrosis to large areas of perifascicular injury. Perifascicular active myofiber injury and microvascular immunoreactivity with antibodies to membrane-attack-complex (c5b9) were more prominently noted in samples from right ventricular wall. “Active” mononuclear inflammation was present and moderate in density. This consisted mainly of a T-lymphocytes identified in the area immediately adjacent to the fascicles (perimysium) and adjoining connective tissue. Both CD4-helpers and CD8-cytotoxic T cells were present with no apparent predominance. Histiocytes were distributed diffusely and were present in both endomysium and in peripheral connective tissue. Degenerative changes consisted of lipofuscin deposits, myofiber size variation and splitting consistent with ventricular wall hypertrophy, and reactive mitochondrial features seen on light and electron microscopy.
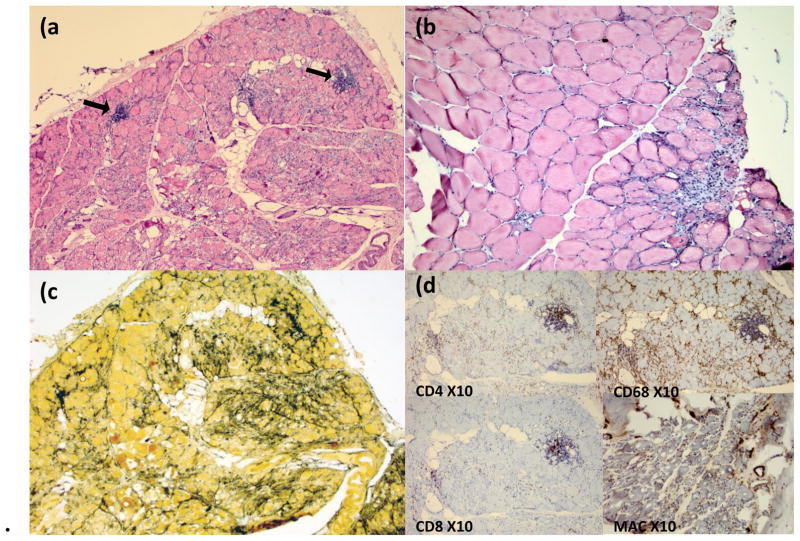
Figure 1. Skeletal muscle.
Skeletal muscle from left thigh showing changes consistent with dermatomyositis (DM) including inflammatory myopathy with perivascular inflammation (arrows), marked fiber damage, and zones of perifascicular atrophy (panels a and b; H&E, original magnification 10X). Additional changes consistent with DM include prominent connective epi-/perimysial tissue reaction with alkaline phosphatase (AP) staining (panel c, AP 10x), and increased membrane attack complex (MAC) expression in the muscle microvasculature (panel d). Presence of prominent CD4 lymphocytes and numerous CD68 positive histiocytes was also noted (panel d).
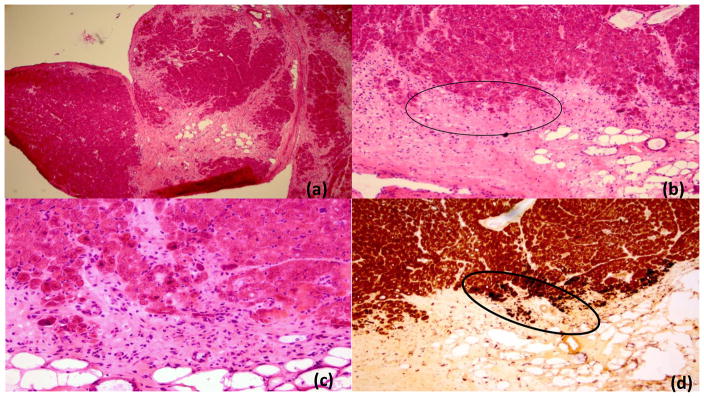
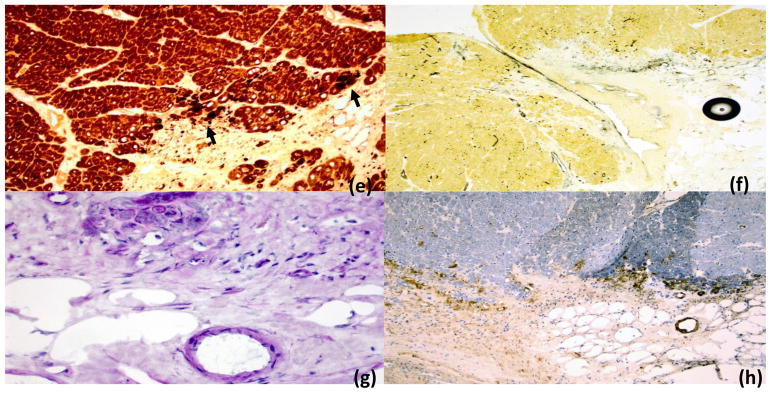
Figure 2. Cardiac muscle.
Cardiac muscle from the right ventricular wall showing prominent scarring and fibrosis and variable myofiber damage among the fascicles (panel a, H&E, low power 4X). Higher power views show inflammatory activity and muscle fiber injury/atrophy in the peripheral areas of several fascicles (panels b-c; H&E 10X and 20X; panel c is magnified view of highlighted area in panel b). Increased nonspecific esterase activity (dark spots) in panels d and e (right and left ventricular walls respectively) reflects ongoing degenerative fiber damage of variable severity seen in these areas in the periphery of the fascicles. Alkaline phosphatase (AP) fibrovascular activity (reaction in black) is increased in epi- and perimysial fibrovascular tissue of cardiac muscle (panel g, AP 10x). Microvascular rarefaction is noted in area of damage (panel g; PAS diastase 40X), and similar to the findings in the skeletal muscle, membrane attack complex (MAC) reactivity was observed in many microvessels (panel h, MAC immunohistochemistry 4X).
The patient is currently 25 months post-transplant and doing well. His immunosuppressive regimen includes cellcept (500mg BID), tacrolimus (1mg qAM/2mg qPM), rituxan (1000mg x 2) every 6 months, and prednisone at 5mg daily. Rituximab was added to the transplantation medications in order to control his DM. The patient currently plays 2 hours of basketball three times a week and beats most of his local competition in Los Angeles.
Discussion
Cardiac involvement in DM and PM has been well described in the literature with a reported incidence between 9 and 72% depending on patient selection and methods of detection.7,8 However, clinically significant cardiac involvement is much less common.
A systematic review reported EKG and Holter monitor abnormalities such as conduction defects, ST-T changes, frequent atrial or ventricular premature beats in up to 85% of IIM cases3,5,7,9,10. Other modalities for cardiac evaluation included echocardiogram showing wall motion, valvular and pericardial abnormalities in up to 62% of patients11,12, Technetium99m-pyrophosphate (99mTc-PYP) scintigraphy, and cardiac MRI showing abnormal enhancement in a study of 4 patients which improved after corticosteroid treatment13. The most frequently reported clinical symptom of cardiac involvement is congestive heart failure7,9,14, although coronary artery disease including acute myocardial infarction is also increased in patients with inflammatory myopathies15. Patients with DM/PM have increased mortality compared to the general population with cardiopulmonary disease as the leading cause of death7,8,16,17
Two case reports have previously been published regarding cardiac transplantation in myositis. The most recent report described a case of fulminant giant-cell myocarditis in a patient with possible DM diagnosed two weeks prior to presentation. The patient was treated with high doses of methylprednisolone and 2 doses of IVIG but progressed to cardiogenic shock rapidly and ultimately underwent a successful orthotopic heart transplant18. Histological examination of the explanted heart revealed foci of mixed inflammatory cells admixed with multinucleated giant cells/degenerating myocardial fibers, consistent with fulminant giant-cell myocarditis. The second case report described a patient with polymyositis who developed severe heart failure over 7 years following diagnosis, and ultimately underwent successful heart transplantation. The pathology of the explant heart was not extensively described in this report. Additional autopsy studies evaluating the histopathology of myocarditis in PM patients have described a diffuse interstitial and perivascular mononuclear cell infiltrate similar to the inflammatory changes seen in the affected skeletal muscle, 9,19 however further detailed descriptions, particularly in DM, are lacking.
In the current work, we detail the cardiac pathology and also report similarities observed between the myocardium and the skeletal muscle particularly those pertaining to characteristic pattern of DM injury. The most notable resemblance was the perifascicular distribution of atrophy and damage in the skeletal muscle which was paralleled by zones of peripheral fiber damage in the cardiac muscle. Intense perifascicular alkaline phosphatase reactivity, characteristic of DM was also noted in both cardiac and skeletal muscle. The multifocal and variable nature of the disease from region to region was similar to the pattern of injury commonly seen in dermatomyositis affecting skeletal muscle. Finally, membrane attack complex (MAC) overexpression in the vasculature, a hallmark sign of DM pathology, was noted in abundance in both skeletal and cardiac muscle microvasculature in the current case. Such histologic similarities suggest that dermatomyositis is indeed a systemic disease that may cause muscular inflammation and damage beyond the skeletal muscle. Myocardial findings were indeed limited in comparison to the features available on skeletal muscle biopsy. However, the latter dated a year prior and therefore not representative of the peripheral skeletal muscle disease at the time of the OHT.
To date, the severity and activity of the underlying myositis has not been clearly shown to correlate with the presence of pathologic cardiac involvement which can develop even when the peripheral symptoms are in remission.20 In the current case, the patient described a history of worse weakness from his DM in sites of old injuries such as the left hip flexor musculature which was injured playing basketball. Interestingly, he also had a history of prior cardiac disease as a teenager with one episode of endocarditis, however, was not limited by this cardiac disease in playing college basketball prior to onset of DM. His skeletal muscle biopsy showed an extensive peri- but also pan-fascicular damage.
Past work in a mouse model of myositis has suggested that injury followed by regeneration could set up an inflammatory milieu in the muscle which facilitates the development of autoimmune myositis21. Mammen and colleagues reported that the expression of Mi-2, an antigen against which autoantibodies are commonly identified in DM patients, is markedly up-regulated during muscle regeneration in a mouse model of muscle injury/repair22. Studies have yet to show whether an autoantibody response against antigens such as Mi-2 is indeed driven by regenerating myofibers, and if so, whether such upregulation plays a role in muscle damage in DM. We hypothesize that our patient’s past cardiac injury may have predisposed him to the development of severe DM heart involvement requiring transplantation. While this remains speculative, staining for the Ku antigen in the patient’s cardiac and skeletal muscle will be pursued.
Conclusion
Although most cases of cardiac involvement in DM are subclinical, it is important to realize that severe cases of cardiac involvement requiring transplantation do occur, in which case transplant may be lifesaving. The histopathologic study suggests a pattern of similar inflammatory damage in the heart as noted in the skeletal muscle.
Footnotes
Conflicts of Interest and source of funding
None of the authors have any financial, consultant, institutional and other relationships that might lead to bias or a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts) The New England journal of medicine. 1975;292:403–407. doi: 10.1056/NEJM197502202920807. [DOI] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) The New England journal of medicine. 1975;292:344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Wang GC, Ma L, Zu N. Cardiac involvement in adult polymyositis or dermatomyositis: a systematic review. Clinical cardiology. 2012;35:686–691. doi: 10.1002/clc.22026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine. 1977;56:255–286. doi: 10.1097/00005792-197707000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AJ, Wortham DC, Burge JR, Rogan KM. The heart in polymyositis: a prospective evaluation of 26 patients. Clinical cardiology. 1993;16:802–808. doi: 10.1002/clc.4960161110. [DOI] [PubMed] [Google Scholar]
- 6.Westaby S, Karp RB, Blackstone EH, Bishop SP. Adult human valve dimensions and their surgical significance. Am J Cardiol. 1984;53:552–556. doi: 10.1016/0002-9149(84)90029-8. [DOI] [PubMed] [Google Scholar]
- 7.Hochberg MC, Feldman D, Stevens MB. Adult onset polymyositis/dermatomyositis: an analysis of clinical and laboratory features and survival in 76 patients with a review of the literature. Seminars in arthritis and rheumatism. 1986;15:168–178. doi: 10.1016/0049-0172(86)90014-4. [DOI] [PubMed] [Google Scholar]
- 8.Danko K, Ponyi A, Constantin T, Borgulya G, Szegedi G. Long-term survival of patients with idiopathic inflammatory myopathies according to clinical features: a longitudinal study of 162 cases. Medicine. 2004;83:35–42. doi: 10.1097/01.md.0000109755.65914.5e. [DOI] [PubMed] [Google Scholar]
- 9.Denbow CE, Lie JT, Tancredi RG, Bunch TW. Cardiac involvement in polymyositis: a clinicopathologic study of 20 autopsied patients. Arthritis and rheumatism. 1979;22:1088–1092. doi: 10.1002/art.1780221007. [DOI] [PubMed] [Google Scholar]
- 10.Lundberg IE. The heart in dermatomyositis and polymyositis. Rheumatology (Oxford) 2006;45(Suppl 4):iv18–21. doi: 10.1093/rheumatology/kel311. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal CS, et al. The heart in polymyositis-dermatomyositis. Journal of neurology. 1989;236:249–250. doi: 10.1007/BF00314509. [DOI] [PubMed] [Google Scholar]
- 12.Strongwater SL, Annesley T, Schnitzer TJ. Myocardial involvement in polymyositis. The Journal of rheumatology. 1983;10:459–463. [PubMed] [Google Scholar]
- 13.Allanore Y, et al. Effects of corticosteroids and immunosuppressors on idiopathic inflammatory myopathy related myocarditis evaluated by magnetic resonance imaging. Annals of the rheumatic diseases. 2006;65:249–252. doi: 10.1136/ard.2005.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrnes TJ, Baethge BA, Wolf RE. Noninvasive cardiovascular studies in patients with inflammatory myopathy. Angiology. 1991;42:843–848. doi: 10.1177/000331979104201010. [DOI] [PubMed] [Google Scholar]
- 15.Tisseverasinghe A, Bernatsky S, Pineau CA. Arterial events in persons with dermatomyositis and polymyositis. The Journal of rheumatology. 2009;36:1943–1946. doi: 10.3899/jrheum.090061. [DOI] [PubMed] [Google Scholar]
- 16.Sultan SM, Ioannou Y, Moss K, Isenberg DA. Outcome in patients with idiopathic inflammatory myositis: morbidity and mortality. Rheumatology (Oxford) 2002;41:22–26. doi: 10.1093/rheumatology/41.1.22. [DOI] [PubMed] [Google Scholar]
- 17.Benbassat J, et al. Prognostic factors in polymyositis/dermatomyositis. A computer-assisted analysis of ninety-two cases. Arthritis and rheumatism. 1985;28:249–255. doi: 10.1002/art.1780280303. [DOI] [PubMed] [Google Scholar]
- 18.Morrissey RP, et al. Case of fulminant giant-cell myocarditis associated with polymyositis, treated with a biventricular assist device and subsequent heart transplantation. Heart Lung. 2011;40:340–345. doi: 10.1016/j.hrtlng.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Haupt HM, Hutchins GM. The heart and cardiac conduction system in polymyositis-dermatomyositis: a clinicopathologic study of 16 autopsied patients. Am J Cardiol. 1982;50:998–1006. doi: 10.1016/0002-9149(82)90408-8. [DOI] [PubMed] [Google Scholar]
- 20.Stern R, Godbold JH, Chess Q, Kagen LJ. ECG abnormalities in polymyositis. Archives of internal medicine. 1984;144:2185–2189. [PubMed] [Google Scholar]
- 21.Kimura N, Hirata S, Miyasaka N, Kawahata K, Kohsaka H. Injury and subsequent regeneration of muscles for activation of local innate immunity to facilitate the development and relapse of autoimmune myositis in C57BL/6 mice. Arthritis Rheumatol. 2015;67:1107–1116. doi: 10.1002/art.39017. [DOI] [PubMed] [Google Scholar]
- 22.Mammen AL, et al. Expression of the dermatomyositis autoantigen Mi-2 in regenerating muscle. Arthritis and rheumatism. 2009;60:3784–3793. doi: 10.1002/art.24977. [DOI] [PMC free article] [PubMed] [Google Scholar]