Synopsis
Cutaneous T-cell lymphomas comprise a heterogeneous group of diseases characterized by monoclonal proliferations of T-lymphocytes primarily involving skin, modified skin appendages and some mucosal sites. This review addresses the basic clinical, histologic and immunohistochemical characteristics of this group of diseases, with additional attention to evolving literature on dermoscopy, reflectance confocal microscopy, flow cytometry and molecular data that may increasingly be applied to diagnostic and therapeutic algorithms in these diseases. Select unusual phenotypes, or diagnostic examples of classic phenotypes are demonstrated, and flags for consideration while making a pathologic diagnosis of CTCL are suggested.
Keywords: Cutaneous lymphoma, Mycosis fungoides, Sezary syndrome, Anaplastic large cell lymphoma, Lymphomatoid papulosis, Reflective confocal microscopy, Flow cytometry
Introduction
Cutaneous T-cell lymphomas (CTCL) consist of a heterogeneous group of clinicopathologically definable monoclonal T-cell proliferations involving the skin. While the majority of CTCLs are non-aggressive diseases that can be adequately clinically managed with minimal toxicity to the patient, knowledge of the basic classification of these disorders by key clinical and pathologic distinguishing features is important and achievable. The occasionally encountered rapidly progressive and aggressive CTCL subtypes are also identifiable with the use of ancillary studies, allowing appropriate and timely therapeutic intervention.
The 2005 WHO-EORTC classification for cutaneous lymphomas[1], along with the 2008 WHO blue book revised in 2016[2, 3], are the basis for the current standardized classification of CTCL, offering both accepted and provisional categories, (table 1) and the understanding that the appropriate stratification of these diseases is an ongoing process as new technologies help us to diagnose and treat CTCL more effectively.
Table 1.
Cutaneous T-cell lymphomas as per 2016 update of the WHO classification[3]
| Distinct Entity | Variant/subtype |
|---|---|
| Mycosis Fungoides | Pilotropic mycosis fungoides Granulomatous slack skin Localized pagetoid reticulosis |
| Sezary Syndrome | |
| Primary Cutaneous CD30+ T-cell lymphoproliferative disorders | Lymphomatoid papulosis Primary cutaneous anaplastic large cell lymphoma |
| Primary cutaneous gamma-delta T-cell lymphoma | |
|
| |
| Provisional Entity | |
| Primary cutaneous CD8+ aggressive epidermotropic cytotoxic | |
| T-cell lymphoma | |
| Primary cutaneous acral CD8+ T-cell lymphoma | |
| Primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder | |
| Epstein-Barr virus (EBV) positive mucocutaneous ulcer | |
Data from Swerdlow, S.H., et al., The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood, 2016. 127(20): p. 2375–90.
The most common CTCLs (mycosis fungoides (MF)/Sezary syndrome (SS), and the CD30 positive lymphoproliferative disorders, (primary cutaneous anaplastic large cell lymphoma (pcALCL) and lymphomatoid papulosis (LyP)) will be addressed below with consideration to diagnostic modalities including dermoscopy, reflectance confocal microscopy and flow cytometry.
Gamma-Delta T-cell lymphoma (GDTCL), subcutaneous panniculitis like T-cell lymphoma (SPTCL) and provisional entities primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder[4], primary cutaneous acral CD8+ T-cell lymphoma, and primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma will also be addressed briefly to enhance recognition of these less common lymphoproliferative disorders.
Etiology/Pathogenesis
The vast number of T-cells residing in skin (estimated to be 20 billion per normal adult)[5] underlies a significant potential for neoplasia. Genetic factors such as an individual’s human leukocyte antigen (HLA) type may predispose some people to develop CTCL[6] by abetting the inappropriate activation and accumulation of T-cells via antigen presentation. Environmental mechanisms which may deregulate tumor suppressor or pro-oncogenic pathways include viral or other microbial pathogens (HTLV-1, EBV, HSV, Staphylococcus aureus, dermatophytes, Myobacterium leprae, Chlamydia pneumoniae) [7, 8]. Regional variations in incidence of disease, geographic or familial (e.g. married couples) clustering, drug triggers (antihistamines, antiepileptics, anti hypertensives, SSRIs)[9] and occupational/nutritional associations including exposure to aromatic hydrocarbons or vitamin D deficiency support an environmental role in the evolution of CTCL [10–16]. In some patients a shift of TH1 to Th2 cytokines may drive progression of CTCL in a positive feedback loop, e.g. by providing a favorable milieu for Staphylococcus aureus with additional subsequent activation of oncogenic Jak/Stat[17] and T-cell receptor signaling.
Clinical presentation
Mycosis Fungoides/Sezary Syndrome
Mycosis fungoides (MF) is the most common CTCL, can usually be distinguished from Sezary syndrome (SS) with complete clinicopathologic evaluation, and is not a difficult diagnostic challenge when clinical and ancillary information are properly integrated. MF is most often an indolent, progressive monoclonal proliferation of skin-homing memory T-cells that occurs predominantly in patients over the age of 60 years. MF is most typically characterized by a slow evolution from scattered epidermotropic lymphocytic infiltrates manifesting as a few patches or plaques of scaly erythema, to more widespread epidermal involvement resulting in patches, plaques, or erythroderma. In some patients, lymphoid tumors grow to become pandermal and subcutaneous nodules and tumors, rarely spreading to regional or distant lymph nodes, bone marrow, and other organs. Peripheral blood involvement by disease can occur in different degrees, and has a bearing on prognosis and treatment, as reflected in staging algorithms (tables 2 and 3). Late progression of disease is often characterized by an accompanying decrease in the functional immunity of the patient, with susceptibility to widespread infection and tumoral mass and cytokine related clinical distress.
Table 2.
Mycosis Fungoides/Sezary Syndrome staging adapted per ISCL/EORTC revision[55]
| Skin | T1 | Limited patches, papules, plaques <10% body surface area* | |
| T2 | Patches, papules, plaques >10%** | ||
| T3 | One or more tumors >1cm diameter | ||
| T4 | Confluent erythema >80% body surface area | ||
| Node | N0 | No clinically abnormal peripheral lymph nodes; no biopsy required | |
| N1 | N1a | Clinically abnormal peripheral lymph nodes biopsied: Dutch grade 1***/NCI LN0-2; clone negative |
|
| N1b | Clinically abnormal peripheral lymph nodes biopsied: Dutch grade 1/NCI LN0-2; clone positive |
||
| N2 | N2a | Clinically abnormal peripheral lymph nodes biopsied: Dutch grade 2****/NCI LN3; clone negative |
|
| N2b | Clinically abnormal peripheral lymph nodes biopsied: Dutch grade 2/NCI LN3; clone positive |
||
| N3 | Clinically abnormal peripheral lymph nodes biopsied: Dutch grade ¾ *****/NCI LN4; clone positive or negative |
||
| Nx | Clinically abnormal peripheral lymph nodes, not biopsied | ||
| Visceral | M0 | No visceral organ involvement | |
| M1 | Visceral organ involvement (pathologically confirmed)****** | ||
| Blood | B0 | B0a | Absent peripheral blood involvement (<5% Sezary cells); clone negative |
| B0b | Absent peripheral blood involvement (<5% Sezary cells); clone positive | ||
| B1 | B1a | Low blood tumor burden >5% Sezary cells, but not B2; clone negative | |
| B1b | Low blood tumor burden >5% Sezary cells, but not B2; clone positive | ||
| B2 | High blood tumor burden, >1000 Sezary cells/ul^; clone positive |
may be divided into T1a patch or T1b patch/plaque;
may be divided into T2a patch or T2b patch/plaque; ISCL International Society of Cutaneous Lymphoma; EORTC European Organization for the research and treatment of cancer;
Dutch grade 1 includes dermatopathic lymphadenopathy;
Dutch grade 2 includes early presence of cerebriform nuclei in aggregates;
Dutch grade 3, 4 are partial and complete effacement of lymph node architectures;
spleen/liver may be considered involved by imaging;
if Sezary cells cannot be measured, then expanded CD3+/CD4+ cells with CD4:CD8>10:1 or loss of CD7 or CD26 in the presence of T-cell clonality can be used for B2.
From Olsen, E., et al., Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood, 2007. 110(6): p. 1713–22; with permission.
Table 3.
TNM staging as per ISCL/EORTC revision for mycosis fungoides/Sezary syndrome[55]
| Clinical stage | T | N | M | B |
|---|---|---|---|---|
| IA | 1 | 0 | 0 | 0,1 |
| IB | 2 | 0 | 0 | 0,1 |
| IIA | 1–2 | 1,2 | 0 | 0,1 |
| IIB | 3 | 0–2 | 0 | 0,1 |
| IIIA | 4 | 0–2 | 0 | 0 |
| IIIB | 4 | 0–2 | 0 | 1 |
| IVA1 | 1–4 | 0–2 | 0 | 2 |
| IVA2 | 1–4 | 3 | 0 | 0–2 |
| IVB | 1–4 | 0–3 | 1 | 0–2 |
TNM tumor node metastasis, ISCL international society for cutaneous lymphoma; EORTC European organization for the treatment of cancer.
From Olsen, E., et al., Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood, 2007. 110(6): p. 1713–22; with permission.
Characteristic skin lesions in MF patients show a “cigarette paper” wrinkly scale, are flat to slightly indurated, and may be round-to oval or serpiginous in appearance. Later tumor lesions may exhibit less scale and more shiny induration as the infiltrates expand the underlying dermis and cease to extend into the epidermal compartment. Tumors may ulcerate when they are very large. Super-infection is common. Erythroderma encompassing >80% of body surface area (BSA) may mimic severe atopic dermatitis, drug eruption or psoriasis. Clinical variants including follicle-based pilotropic[18], unilesional [19, 20], hyper or hypopigmented[21], erythrodermic or poikilodermatous MF are not uncommon. Less common clinical manifestations include alopecia, leonine facies, nail involvement and acral hyperkeratosis.
SS is a leukemic variant of cutaneous T-cell lymphoma characterized by atypical malignant Sezary cells with a central memory T-cell phenotype, different from MF, in blood, lymph nodes and skin. Patients are typically 55–60 years old and present with erythroderma (>80% BSA), itching, and lymph node enlargement. Median overall survival is 63 months, and 5 year survival may be as low as 28%[22].
CD30 lymphoproliferative disorders
The primary cutaneous CD30+ lymphoproliferative disorders (LPDs) include lymphomatoid papulosis (LyP) and CD30+ pcALCL. While patients can present with lesions of LyP and pcALCL at the same time, the different disorders individually exhibit distinct clinical behaviors and warrant distinct therapeutic interventions. LyP is characterized by a clear pattern of recurrent, regressing crops of macules and papules with superficial ulceration and a variable distribution. LyP may leave scars, but usually goes away without intervention. Conversely, pcALCL most often presents as a solitary nodule, or a few nodules, tumors or plaques in the skin (particularly extremities), which may or may not resolve on their own after a period of time. pcALCL may also ulcerate, and can appear shiny and violaceous as expansion of the skin by the underlying aggregates of lymphocytes occurs. ALCL can occasionally progress to involve the regional lymph nodes, and may recur over time in the skin. It uncommonly progresses to a more aggressive disease process. LyP has been described in the lymph nodes on a rare occasion[23]. Some patients present with lesions with intermediate behavior to these two entities, or with lesions of both entities, and sometimes with another lymphoma such as MF, which may share clonal T-cell gene rearrangements suggesting a common origin of the diseases[24–26]. These patients and their skin lesions need to be approached at a granular level with attention to what is happening with their disease process at the time.
Non MF/SS/CD30 cutaneous lymphoma/lymphoproliferative disorders
Aggressive primary cutaneous lymphomas, aside from the occasional indolent variant that evolves to a more high grade disease, include the gamma delta T-cell lymphomas[27] and the CD8+ aggressive epidermotropic cytotoxic T-cell lymphomas. These lymphomas clinically present with rapidly progressive clinical lesions of variable morphology (patch/plaque/tumor), with wide spread distribution and ulceration of individual lesions. Because of the concomitant vasodestruction that often occurs with these infiltrates, lesions may appear dusky, hemorrhagic, red or violaceous. Either entity may exhibit scale and surface alteration due to extensive epidermal involvement. While a long standing history of patch stage disease is more indicative of progressing mycosis fungoides, there are some cases of cytotoxic CTCL that evolve in the setting of a previously indolent presentation. [28] Primary cutaneous gamma delta lymphomas may be associated wtih autoimmune syndromes and systemic symptoms including hemophagocytic syndrome.
Primary cutaneous CD8+ acral lymphoma and CD4+ small/medium T-cell lymphoproliferative disorders (CD4+ SMPTCL) have been proposed to be immunophenotypic counterparts of the same clinical processes. Both entities may present as a solitary papule, nodule or tumor, often on an acral site (including ears, face, extremities and limbs), which has some bearing on the theory that these processes may represent lymphoid reactions to antigen exposure typical for this type of physical milieu (ear piercing, Borrelia infection, arthropod bite e.g.). CD4+ SMPTCL is particularly more likely to present as several nodules, perhaps in a less distal distribution (face, neck, or upper trunk) in the 6th decade. While both entities are characteristically dermal processes, effacing the overlying epidermis, the CD4+ lesions more frequently involve follicular epithelium and extend into overlying epidermis. Of the 30 or so cases reported of primary cutaneous CD8+ acral lymphoma, presentation on ear, nose, face, feet and hands have been well described. There is a 2:1 male:female predominance and a wide age distribution amongst adults (29–87 years). Clinically a discrete papule or nodule is noted to grow slowly, which may be bilateral and symmetric. Patches or plaques characteristic of MF are not seen in either of these entities, both of which are indolent in course.
Table 4 lists classic clinical features helpful for the clinicopathologic correlation of the CTCLs.
Table 4.
Classic clinical presentation of CTCL
| Mycosis fungoides | Patches, plaques, tumors |
| Anaplastic large cell lymphoma | Tumors |
| Lymphomatoid papulosis | Recurrent papules, crusted, crops |
| CD4+ small/medium T-cell LPD | Solitary or few nodules or tumors |
| CD8+ acral T-cell lymphoma | Solitary nodule or papule |
| Subcutaneous panniculitis-like T-cell lymphoma | Subcutaneous nodules – single or multiple |
| Gamma-delta T-cell lymphoma | Subcutaneous nodules – multiple, ulcerated |
| CD8+ aggressive epidermotropic T-cell lymphoma | Patches, plaques, tumors, ulcerated |
LPD, lymphoproliferative disorder
Dermoscopy
When compared to chronic inflammatory dermatitis, dermoscopic examination of early stage mycosis fungoides has been reported to display a dotted pattern[29], with fine short linear vessels, orange-yellow patchy areas, and vascular structures resembling spermatozoa[30]. A description of poikilodermatous MF included polygonal lobules with white storiform streaks studded with fine dots or hairpin vessels, septal pigmented dots and red and yellow smudges[31]. Dermoscopic examination of alopecia associated with CTCL showed follicular or diffuse scaling, and reduced numbers of follicular openings with broken hairs, short hairs, or keratotic filiform spicules [32].
One study evaluated the varying stages of lymphomatoid papulosis using dermoscopy[33], and found tortuous irregular vessels radiating from the center to the periphery with a surrounding white structureless area in the initial inflammatory lesion. Persistence of the white structureless area but without the vascular central component was seen in more mature hyperkeratotic papules, with a brown gray stuctureless area corresponding to a fibrin-imbued ulcer bed with peripheral vessels in the ulcerated stage, which persisted into the last cicatricial phase.
Reflectance confocal microscopy
It has been suggested that non-invasive imaging such as reflectance confocal microscopy (RCM) could be used to aid in selection of biopsy sites for MF, to decrease false negative pathology test results[34]. Primary diagnosis may be limited at this time using this technique, as most prominent confocal features were in well-developed plaque lesions but not patch lesions of MF and correlation with histology has been moderate in the larger studies. [34, 35]. RCM correlation was found with histologically atypical lymphocytes in the epidermis (weakly refractile oval to round structures in the spinous layer), dermoepidermal junction and dermis, and pautriers microabscesses (vesicle-like dark spaces with monomorphous weakly refractile oval to round cells). Junctional bright roundish and large pleomorphic cells, epidermal disarray and spongiosis were most significantly histologically correlated using RCM, with an 84–90% specificity for MF versus parapsoriasis or normal skin, but not versus other CTCL such as SS or LyP. Other findings include the loss of edged papillae (basal cells around dermal papillae look hyporefractile) in erythematous patch stage MF, hyperreflective dermal papillae in hyperpigmented MF, and disrupted dermal papillae rings in tumor stage MF [34–36]. Limitations at this time include that thick plaques and tumors cannot be analyzed using usual histologic correlates due to the thickened epidermal architecture preventing RCM access to the dermoepidermal junction. Furthermore there is disagreement among researchers as to the sensitivity and specificity of epidermal alteration such as epidermal disarray. In vivo RCM has been described in LyP with a high grade of correspondence to histopathology. Inflammatory cells in the upper epidermis, non-rimmed papillary rings and enlarged bright cells correspond with atypical lymphocytes in the dermis, supporting consideration of the use of RCM in LyP as an aid in biopsy site selection[37] when clinical assessment is insufficient.
Microscopic features
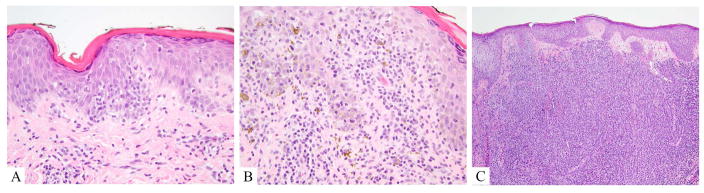
Histologically, MF varies with clinical stage. Patch/plaque lesions are comprised by epidermotropic infiltrates of medium-sized lymphocytes with mildly atypical to hyperconvoluted cerebriform nuclei (fig 1A, 1B). These cells tend to increase in size, and become less epitheliotropic as lesions progress to plaque and tumor stage (fig 1C). Although intraepidermal “Pautrier’s” microabscesses can be seen in any stage, they are most common earlier on and are not sensitive for the diagnosis. Spongiosis, interface dermatitis and intervening histiocytes may be seen. Dermal fibrosis is typical.
Figure 1.
Mycosis fungoides, hematoxylin and eosin A. Patch lesion showing tagging of hyperchromatic lymphocytes out of proportion to spongiosis in a hyperkeratotic epidermis. B. Plaque lesion; hyperchromatic lymphocytes with cerebriform atypia are present in the epidermis as well as in the superficial dermis. C. Tumor lesion; lymphocytes mostly spare the epidermis and are present in sheets extending throughout the dermis, obscuring adnexal (hair) structures).
The most common variant of MF is pilotropic (follicular) MF, (FMF). FMF most often shows a folliculocentric atypical lymphocytic infiltrate with sparing of the interfollicular epidermis and a mixed inflammatory infiltrate. Syringotropism, follicular mucinosis, suppurative folliculitis, cyst formation and granulomatous change may be seen[38].
SS shows similar cytologic features to MF – although sometimes the cerebriform atypia is more striking and epidermotropism less marked. Overlying acanthosis and dermal fibrosis are typical.
Distinct from MF, lesions of ALCL almost always look like tumors, presenting with cohesive sheets of >75% CD30-positive cells in the dermis, often involving intratumoral vessels, or peritumoral intravascular structures. Histologic evidence of ulceration with abundant apoptotic debris is common when the infiltrates are close to the epidermis. Most lesions are comprised of medium to large sized lymphocytes, with intranuclear protrusions, horse-shoe or kidney bean shaped nuclei and multinucleation. A small cell variant of ALCL has been described. Eosinophils and/or neutrophils may be prominent.
LyP can be challenging to commit to diagnostically. At least five histologic subtypes of LyP have been described to date, all of which may occur synchronously or metachronously in the same individual. Type A LyP is a wedge-shaped mixed cell infiltrate with the broad base of the wedge oriented along the epidermis. These lesions may be associated with epidermal hyperplasia, epidermotropism, and ulceration, and epidermotropism. In type A lesions, lymphocytes are small-intermediate and large sized cells in a polymorphous background of neutrophils and eosinophils. The large cells label with CD30 and generally make up less than 50% of the infiltrate. Type B LyP is a lichenoid and epidermotropic infiltrate of cerebriform Lutzner cells, lymphocytes which may or may not be CD30 positive. Type C LYP is made up of sheets of large anaplastic CD30 positive lymphocytes which are histologically indistinguishable from lesions of anaplastic large cell lymphoma. Type D LyP is the lichenoid and epidermotropic form of LyP characterized by CD8 positive, TCR-beta positive, cytotoxic marker positive T-cells, sometimes histologically indistinguishable from a CD8+AETCL or CD8+ MF. LyP type E (angioinvasive LyP) is an oligolesional, characteristically ulcerated, large necrotic eschar-like and self-healing lesion with prominent angioinvasion and angiodestruction by small to medium sized atypical CD8+, CD30+ T-cells.
Of the more aggressive lymphomas, CD8+AETCL can be histologically striking. With these lesions one may initially think of mycosis fungoides, but notice exaggerated features (cell size, epidermotropism). Typically larger (medium to large sized) lymphocytes invade the epidermis in a dense front with high level pagetosis, and may extend into the dermis and/or subcutis with destruction of adnexa, angiocentricity, and angiodestruction. Lymphocytes cluster around dyskeratotic, necrotic basilar epidermis.
GD- TCL can show either a similarly dramatic or a confounding non-specific appearance, particularly when the malignant infiltrates are deeper than the reactive component that is often captured by a superficial biopsy. The lymphocytes are most commonly small to medium in size but may be medium to large. Interface alteration or other features of cytotoxicity including hemorrhage, necrosis and vasculitis may occur. GD-TCL should be considered when there is strong suspicion of CTCL clinically, but the histologic features do not “add up” convincingly for mycosis fungoides.
SPTCL in the most diagnostic cases is comprised of diffuse sheets of atypical lymphocytes which eradicate the fat lobules as in a lobular panniculitis pattern, with some septal involvement. The dermis and epidermis are not involved by lymphoma, although reactive periadnexal lymphocytes are often seen. Classic but not sensitive of specific histologic features include rimming of adipocytes by tumor cells in which lymphoma cells protrude into adipocyte cytoplasm. Cytology of the lymphocytes varies from small to large/pleomorphic and ranges from bland to bizarre with cytotoxic damage including karyorrhexis and cytophagia (causing “bean-bag cells”, emperipolesis), and granulomatous infiltrates. Angiocentricity is often common. Lymphoid follicles with germinal centers and plasma cells are usually absent, and if found, suggest collagen vascular disease.
The acral clustering CD4+SMTCL and CD8+ PCAL suggest reactive infiltrates at first glance. Perivascular dermal diffuse or nodular infiltrates of small to medium-sized lymphocytes predominate in both. The CD4+ lesions are more polymorphous in cell composition and pleomorphic in cell shape, harboring many histiocytes and B cells as well as large cells making up as much as 30% of infiltrates. Adnexal structures may be affected and epidermotropism and Pautrier’s like microabscesses can be seen. CD8+ acral lesions are monomorphous in size and composition, lacking histiocytes and B-cells, and sparing epithelial structures. Large cells are uncommon.
Immunohistochemistry (table 5)
Table 5.
Helpful immunohistochemistry and flags in evaluating CTCL
| CD4/CD8 | CD20 | Pan T-issues | CD68/1 63 | CD30 | TCR | Flags | |
|---|---|---|---|---|---|---|---|
| MF | CD4 | Clusters in advanced MF or TFH IHC |
Loss CD7 | If GSS or GMF, after TX | +/− | AB, rare GD |
NULL-type Loss CD3, retain CD7, strong CD25 strong CD30 TFH markers, EBER |
| SS | CD4 | Scattered | Loss CD7 | +/− | AB, rare GD |
See MF | |
| ALCL | CD8 | No | Loss CD3, Loss TCR beta LossCD45RA |
Yes | + | AB, rare GD |
Strong CD25 Strong FOXp3, EBER Delta TCR |
| LYP | CD4/8 | No | Yes | + | AB, rare GD |
Delta TCR | |
| CD8AETCL | CD8 | No | Retain CD7 | No | Rare, limited | AB | Delta TCR EBER CD30 |
| GDTCL | None | No | Yes | Rare, limited | GD | CD8 CD30 TCRB |
|
| SPTCL | CD8 | No | Yes | Rare, limited | AB | GD EBER CD30 |
|
| CD4SMTCL | CD4 | Clusters | Yes | AB | CD30 EBER GD |
||
| CD8CAL | CD8 | no | Retain CD2/5/7 |
No | No | AB | CD30 EBER GD |
TCR T-cell receptor, MF mycosis fungoides, TFH T-follicle helper, IHC immunohistochemistry, GSS granulomatous slack skin, GMF granulomatous mycosis fungoides, TX therapy, AB alpha-beta, GD gamma-delta, EBER Epstein barr virus encoded Ribonucleic acid, SS sezary syndrome, ALCL anaplastic large cell lymphoma, LYP lymphomatoid papulosis, AETCL aggressive epidermotropic T-cell lymphoma, SPTCL subcutaneous panniculitis like T-cell lymphoma, SMTCL small medium T-cell lymphoproliferative disorder, CAL cutaneous acral lymphoma
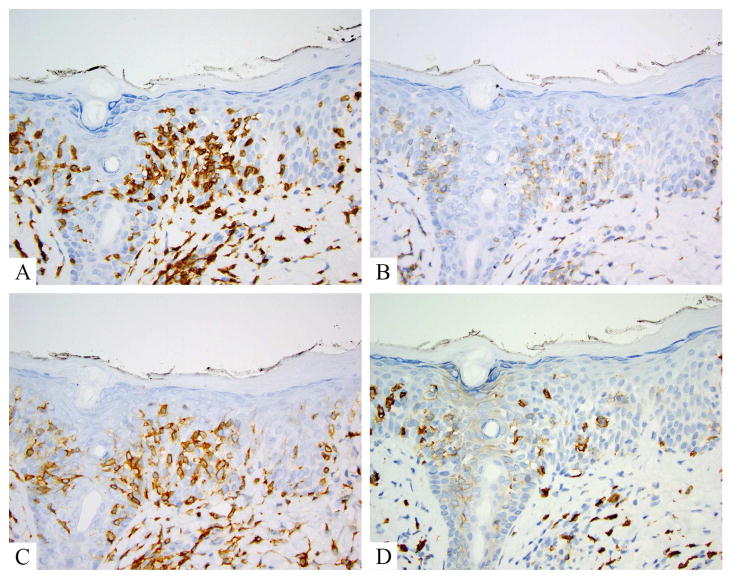
Mycosis fungoides is a CD4+ memory T-helper cell process. Pan-T-cell antigens CD2, CD3 and CD5 are retained in early lesions and lost later, although CD7 is lost early (fig 2A–D). CD8 is the predominant phenotype in some lesions of MF, often in younger patients, and in poikilodermatous, hyper and hypopigmented lesion. TCR beta is typically expressed on the atypical lymphocytes of MF, reflecting a monoclonal rearrangement of the TCR alpha and beta chains. Variants with both TCR beta and TCR gamma or TCR gamma alone have been described (fig3A–C) [39]. TCR delta can be seen on gamma-expressing MF (fig 3D). Ki-67 proliferation indices are not typically high until disease is advanced at which point Ki-67 may correlate with prognosis (fig 4A–C) [40]. CD25 (the interleukin-2 receptor) can be seen in later stage MF, but tends to be mild in intensity, with a vague cytoplasmic staining pattern in contrast to the crisp cytoplasmic and membranous pattern seen in adult T-cell leukemia/lymphoma. The majority of MF is CD30 negative by routine immunohistochemistry (fig 4D). MF with CD30 positivity has been described in a low number of cases of non-transformed MF [40] and in 40% of transformed MF[41] with multiple patterns of labeling and localization noted (scattered, clusters, epidermis, dermis). Higher proportions of dermal CD30 have been found to be associated with higher stage at diagnosis and associated with an adverse prognosis although other studies have shown the converse. [42, 43].
Figure 2.
Mycosis fungoides, classic, immunohistochemistry. A. CD3 labels numerous lymphocytes extending into the mid and upper levels of the epidermis and tagging the dermoepidermal junction. B. CD7 shows complete negativity in the CD3+ cells. C. CD4 highlights the same population labeled by CD3. D. CD8 shows a rare scattered lymphocyte in the epidermis, but more notably highlights scattered reactive dermal cells.
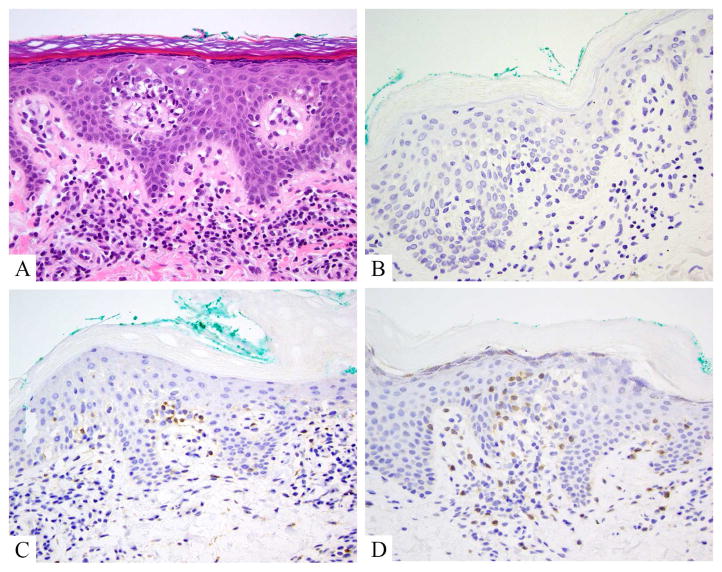
Figure 3.
Mycosis fungoides, aberrant gamma delta phenotype. A. Hematoxylin and eosin shows hyperchromatic atypical lymphocytes tagging the dermoepidermal junction, in mid layers of the epidermis and within papillary dermis. B. TCR beta is negative in the junctional lymphocytes, but positive in some small reactive papillary dermal lymphocytes. C. TCR gamma labels junctional lymphocytes, as does D, TCR delta
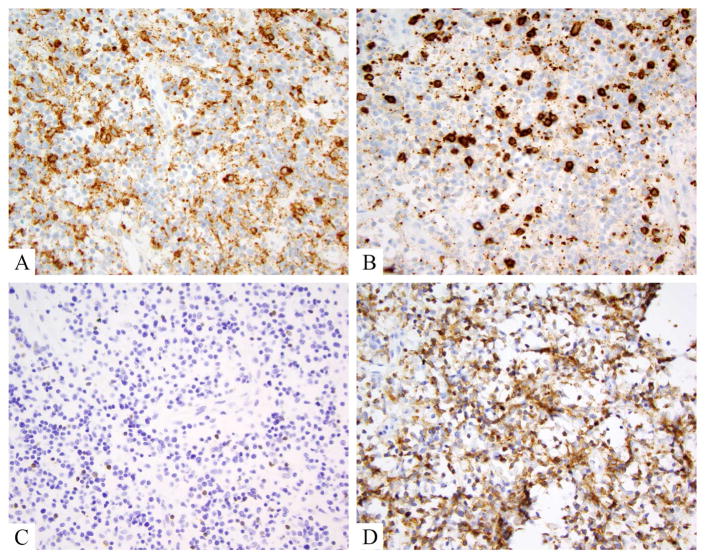
Figure 4.
Tumor stage mycosis fungoides, showing large cell transformation. A, B. Hematoxylin and eosin show large Pautrier’s microabscesses comprised of cells that are 3–4x larger than intervening small reactive lymphocytes. Similar cells efface the dermis in sheets and large clusters. C. Ki-67 proliferation index is high in these lesion, probably 85% in this case. D. Only a rare CD30+ lymphocytes is noted, underscoring the oft absence of CD30 in cases of transformed mycosis fungoides.
PD-1 is the best studied T-follicle helper (TFH) phenotype marker in MF, and appears to be the most sensitive, followed by ICOS, CXCL-13, Bcl-6 and CD10. [44, 45] A TFH phenotype is not uncommon in MF or SS, seems to remain concordant among MF biopsies from the same patient, and is not dependent on disease stage with a cut-off of at least 10% of neoplastic cells expressing at least 3 antigens[46]. Distinct from MF, the loss of pan-T-cell antigens such as CD3, CD5 and CD7 is common in ALCL, which may also be CD45 RA negative[47]. CD2 and CD45RO are often positive. TCR beta is the usual T-cell receptor heterodimer expressed in ALCL, although gamma and/or delta may be occasionally seen in these cases, which can be particularly difficult to interpret when cases also show a CD4/CD8 null immunophenotype [48]. CD30 labels at least 75% of ALCL cells (fig 5A,B) and ALK-1 is typically negative, although positivity in children or with aberrant (cytoplasmic) expression has been noted [49]. Cytotoxic markers TIA-1, perforin or granzyme B are abundant.
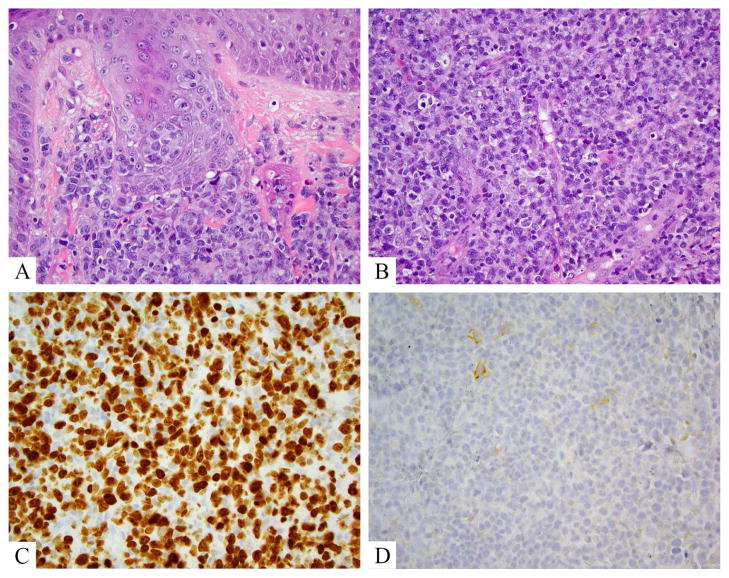
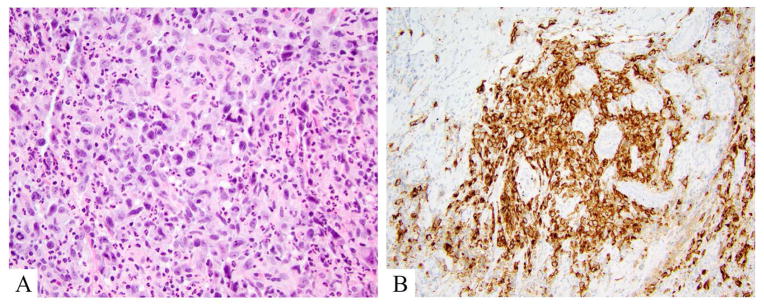
Figure 5.
Anaplastic large cell lymphoma, DUSP22 rearranged. A. Hematoxylin and eosin shows a mixed large and small cell population. Large cells are atypical with kidney bean shaped and donut shaped nuclei. B. CD30 labels >75% of lesional (large) atypical lymphocytes.
Lesions of LYP typically express CD30, regardless of the subtype (A–E). Types A, B, C and E all show a CD4+ predominant but mixed T-cell population while Type D is characteristically CD8 positive. Cytotoxic markers can be positive, including CD56 [50]. TCR beta or gamma [39] can be found.
CD8+ AETCL is strongly CD3 positive and CD8 positive; additional findings include preservation of CD7 (fig 6A–C), and expression of TCR-beta, although it has been suggested that TCR-gamma expression can be seen. As with MF, other pan T-cell markers are lost. [51]. Cytotoxic granules (TIA1+, granzyme B, and perforin) are expressed. CD56 is usually negative.
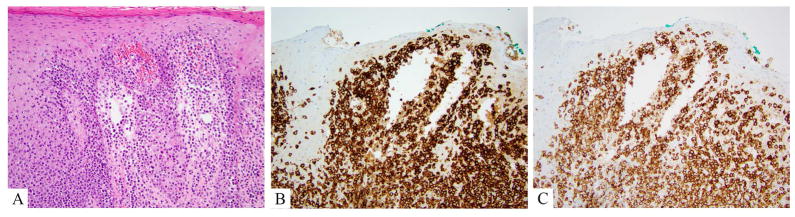
Figure 6.
CD8+ aggressive epidermotropic T-cell lymphoma. A. Hematoxylin and eosin showing a florid front of monomorphic large atypical lymphocytes effacing the dermo-epidermal junction and involving the underlying dermis. B. CD7 is retained, strong and diffuse. C. CD8 is typically strong and diffuse.
GD-TCL may be difficult to diagnose on routine IHC, particularly if sampling is superficial as discussed above. The hallmark finding is the absence of TCR beta expression in the context of positive TCR gamma or delta staining (fig 7C, D). We have found that moderate increases of gamma and delta expression can be seen at all stages of the disease, and should raise suspicion if found in any biopsy specimen. Most GD-TCL shows a null immunophenotype (CD3 positive but CD4 and CD8 negative) (fig 7A,B), although CD8 can be seen in some lesions and may vary with time.
Figure 7.
Gamma delta T-cell lymphoma, immunohistochemistry. A, B, C. CD4, CD8, and TCR beta are negative in the majority of lesional lymphocytes. D. TCR delta is strongly and diffusely positive in lesional lymphocytes.
CD4+ SMPTCL (fig 8A) may express one or more TFH markers PD-1+, ICOS+, CXCL13+, and bcl-6+. Tumors contain numerous CD20+ B-cell aggregates and CD163+ macrophages. There is loss of pan T-cell antigens and a Ki-67 proliferation index less than 30%. Monoclonal T-cell gene rearrangements are common.
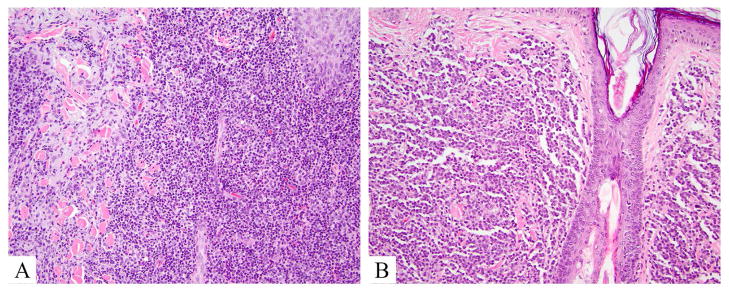
Figure 8.
Indolent lymphoproliferative disorders, hematoxylin and eosin. A. CD4+ small/medium T-cell lymphoproliferative disorder shows a mixed cell morphology with histiocytes and variably sized lymphocytes, effacing epithelial structures. B. Primary cutaneous CD8+ acral lymphoma demonstrates sparing of epithelial structures by a monomorphic small cell population in dermis.
The immunophenotype of primary cutaneous acral CD8+ lymphoma (fig 8B) is that of a non-activated cytotoxic T-cell (Cd8+, CD3+, CD45RO/RA+) which is negative for CD30, CD56, and negative for TFH markers. CD2, CD5, or CD7 may be positive or negative. MIB-1/Ki-67 labeling is often less than 10%. Rare CD20+ B-cells may be seen. Most but not all cases show monoclonal TCR gamma and/or beta gene rearrangements.
Immunophenotypically SPTCL is characterized by a CD3+ CD8+ betaF1+, often TIA-1+ phenotype. EBV, CD30 and TCR-gamma and delta are negative. Clonal TCR gene rearrangement is detectable in most cases.
Flow cytometry
Flow cytometric analysis (FCA) is used to evaluate peripheral blood specimens of patients with mycosis fungoides for staging purposes, and in Sezary Syndrome for diagnosis. Patients with SS have higher percentages of CD4+/CD7− cells compared to patients with benign dermatoses, and significantly higher CD4:CD8 ratios than patients with benign dermatoses or no lymphoproliferative disorders. MF patients do not show differences in either ratio or percentage [52]. FCA could detect aberrant CD2, CD3 or CD5 in 66% of SS and 30% of MF patients As with other mature T-cell lymphomas, loss of expression of a pan T-cell marker >25% is abnormal, and of 2 antigens, or a single antigen >50% is worrisome for a T-cell LPD. These abnormalities have been found to have a sensitivity of 78% and specificity of 89% compared to non-MF or indeterminate histologies[53].
The use of CD26 for diagnosis of MF on FCA is challenging as MF can be CD26 positive or negative, and reactive T-cells can show a loss of CD26 [54]. The use of V-beta FCA may make this issue moot. Monoclonal T-cell receptor gene rearrangements can be assessed for with a set of monoclonal antibodies to 23 V-beta segments covering 70% of the known V-beta repertoire. A clonal T-cell population will show either restriction of one of these V-beta chains, or will be negative for all tested chains. V-beta FCA may be more sensitive than molecular clonality analysis in the assessment of a monoclonal T-cell population, and can be used when CD4:CD8 ratio >10 or with CD4+/CD26− or CD7− cells to upstage blood staging to B2[55, 56].
Skin biopsy specimens (e.g. digested with collagenase) from patients with diagnostic International Society for Cutaneous Lymphoma (ISCL) scores for MF seem to have correlative diagnostic findings by FCA, while sub diagnostic ISCL scores or histology were normal by FCA and polyclonal on TCR PCR[57]. Interestingly, FCA has identified both small and large cell shared monoclonal populations in MF not seen by IHC, with CD3+/CD26− T-cells accounting for 70% of MF cases, and absent CD7 only in 57% of cases[58]. The large cell size and complexity identified by increased forward and side scatter properties, or “high scatter T-cells” are have been said to be specific for the FC diagnosis of MF[59].
Gamma Delta T-cell lymphoma can be identified by FCA, with the findings of CD2+, surface (epsilon) CD3+, TCR gamma/delta, and CD4 and CD8 negativity, as seen in IHC. CD5 and CD7 are variable. NK markers CD16, CD56, CD57 and KIRs are said to be acquired by some GDTCL.
Molecular
Karyotypic, cytogenetic and array-comparative genomic hybridization (CGH) studies of SS have revealed a complex karyotype with structural and numerical abnormalities thought to reflect gross chromosomal instability, involving chromosome 10 and 6 as well as 3,7,9, 17, 1, 12, 8, 11, 13. In transformed or tumor MF the most common (but not specific) finding may be loss of 9p21, with gains in 1p, 1q, 7q, and 8/8q. Loss of 9p21/CDKn2A or 19q26 and gains in 8q have been suggested to correlated with worsened outcome in tumor or transformed MF, but these effects may be attributable in part to selection bias[60–62]. Additional products noted to be gained in CTCL include Nav3 (12q21), JunB (chr19), c-MYC/MAX, p53, PTEN/Fas, p15, p16, NFKB, bcl-2 and Stat2. Overall SS shows numerous non-recurrent complex unbalanced translocations, but no disease specific balanced translocations. Copy number variations are typically numerous, and may be recurrent. [22] Microsatellite instability has been reported to be increased in stage IIB MF and in large cell transformation, and has been reported in 24% of CTCL overall. Mutational data continues to be gathered and analyzed on this diverse group of disease showing alterations in multiple pathways including TCR signaling and chromatin modification (e.g. ARID1A, CTCL, DNMT3a) [63].
The ALK/NPM translocation typically seen in systemic ALCL is only rarely seen in pcALCL. Diagnostically, IRF4 alterations identified in 75% of ALCL [64] are helpful, but not entirely specific, as rare MF (Pham-Ledard) showed IRF4 translocation.[65] ALCL/LyP may show DUSP22-IRF4 locus on 6p25.3 rearrangements, with characteristic dimorphic cytology. These are not diagnostic or prognostic but may be helpful in ruling out high grade ALK negative extracutaneous disease. [66] Other characteristic genetic anomalies include Nav3 in SPTCL and Stat5B/STAT3 mutations in GDTCL [67].
Molecular characterization of CTCL is an evolving field and requires close scrutiny in regards to disease course and response to therapy, in relationship to all clinical, laboratory and other pathologic data as it becomes incorporated into current diagnostic and therapeutic algorithms.
Prognosis
Numerous prognostic markers have been sought for CTCL, including age>60, clinical stage, lactate dehydrogenase, beta-2 microglobulin, neutrophil-lymphocyte ratio, Staphylococcus aureus infection, cell size (small or large, depending on subtype), immunohistochemical or flow cytometric documentation of differentiation marker anomalies, proliferation indices e.g. ki-67, and molecular features (p53, CDKN2A[61]) [68–70]. With most cases, the appropriate diagnostic stratification and clinical staging remain the most reliable discriminators at this point, particularly as many studies are small and/or cannot adequately control for the effect of prior therapies. As molecular (e.g. TCR sequence and mutational data) and flow cytometric data are gathered, multivariate analysis of large groups will be needed to better assess prognostic features for CTCL.
Diagnosis
The differential diagnosis of cutaneous lymphomas is broad and has been well-reviewed previously for individual entities. Disease mimics range from infection, autoimmune or hypersensitivity reactions, solid tumors as well as secondary involvement of the skin by other peripheral T-cell lymphoma, and T-cell rich B-cell lymphoma. A good rule of thumb is to maintain alertness for features that do not follow the usual clinical and histopathologic patterns described for these entities here (tables 4 and 5). When the diagnostic features are not clear, the most prudent course is to ensure clear communication between clinicians and pathologists, and to consider the acquisition of additional biopsy material over time as the disease evolves to a more clearly diagnostic phenotype.
Key Points.
Primary cutaneous T-cell lymphoma comprises a group of diseases characterized by different recognizable clinicopathologic patterns
The vast majority of clinically recognizable cutaneous lymphoma can be diagnostically supported by routine morphologic analysis with a limited number of ancillary studies
Dermoscopic, confocal and flow cytometric analysis may add useful information in the evaluation of CTCL in the correct context.
Acknowledgments
Sources of support: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Willemze R, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768–85. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, EC, Harris NL, Jaffe ES. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Lyon, France: IARC Press; 2008. [Google Scholar]
- 3.Swerdlow SH, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltraminelli H, et al. Primary cutaneous CD4+ small-/medium-sized pleomorphic T-cell lymphoma: a cutaneous nodular proliferation of pleomorphic T lymphocytes of undetermined significance? A study of 136 cases. Am J Dermatopathol. 2009;31(4):317–22. doi: 10.1097/DAD.0b013e31819f19bb. [DOI] [PubMed] [Google Scholar]
- 5.Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol. 2010;130(2):362–70. doi: 10.1038/jid.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackow CM, et al. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive Staphylococcus aureus, and oligoclonal T-cell receptor V beta gene expansion. Blood. 1997;89(1):32–40. [PubMed] [Google Scholar]
- 7.Mirvish JJ, et al. Role of infectious agents in cutaneous T-cell lymphoma: facts and controversies. Clin Dermatol. 2013;31(4):423–31. doi: 10.1016/j.clindermatol.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Abrams JT, Balin BJ, Vonderheid EC. Association between Sezary T cell-activating factor, Chlamydia pneumoniae, and cutaneous T cell lymphoma. Ann N Y Acad Sci. 2001;941:69–85. doi: 10.1111/j.1749-6632.2001.tb03712.x. [DOI] [PubMed] [Google Scholar]
- 9.Litvinov IV, et al. Investigating potential exogenous tumor initiating and promoting factors for Cutaneous T-Cell Lymphomas (CTCL), a rare skin malignancy. Oncoimmunology. 2016;5(7):e1175799. doi: 10.1080/2162402X.2016.1175799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013;149(11):1295–9. doi: 10.1001/jamadermatol.2013.5526. [DOI] [PubMed] [Google Scholar]
- 11.Scarisbrick JJ, et al. Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sezary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J Clin Oncol. 2015;33(32):3766–73. doi: 10.1200/JCO.2015.61.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Litvinov IV, et al. Demographic patterns of cutaneous T-cell lymphoma incidence in Texas based on two different cancer registries. Cancer Med. 2015;4(9):1440–7. doi: 10.1002/cam4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litvinov IV, et al. Identification of geographic clustering and regions spared by cutaneous T-cell lymphoma in Texas using 2 distinct cancer registries. Cancer. 2015;121(12):1993–2003. doi: 10.1002/cncr.29301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghazawi FM, et al. Comprehensive analysis of cutaneous T-cell lymphoma (CTCL) incidence and mortality in Canada reveals changing trends and geographic clustering for this malignancy. Cancer. 2017 doi: 10.1002/cncr.30758. [DOI] [PubMed] [Google Scholar]
- 15.Slodownik D, et al. Occupational mycosis fungoides - a case series. Int J Dermatol. 2017 doi: 10.1111/ijd.13589. [DOI] [PubMed] [Google Scholar]
- 16.Talpur R, et al. Vitamin D deficiency in mycosis fungoides and Sezary syndrome patients is similar to other cancer patients. Clin Lymphoma Myeloma Leuk. 2014;14(6):518–24. doi: 10.1016/j.clml.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Litvinov IV, et al. Analysis of STAT4 expression in cutaneous T-cell lymphoma (CTCL) patients and patient-derived cell lines. Cell Cycle. 2014;13(18):2975–82. doi: 10.4161/15384101.2014.947759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demirkesen C, et al. The clinical features and histopathologic patterns of folliculotropic mycosis fungoides in a series of 38 cases. J Cutan Pathol. 2015;42(1):22–31. doi: 10.1111/cup.12423. [DOI] [PubMed] [Google Scholar]
- 19.Kempf W, et al. Unilesional follicular mycosis fungoides: report of two cases with progression to tumor stage and review of the literature. J Cutan Pathol. 2012;39(9):853–60. doi: 10.1111/j.1600-0560.2012.01965.x. [DOI] [PubMed] [Google Scholar]
- 20.Amitay-Laish I, et al. Unilesional folliculotropic mycosis fungoides: a unique variant of cutaneous lymphoma. J Eur Acad Dermatol Venereol. 2016;30(1):25–9. doi: 10.1111/jdv.12851. [DOI] [PubMed] [Google Scholar]
- 21.Castano E, et al. Hypopigmented mycosis fungoides in childhood and adolescence: a long-term retrospective study. J Cutan Pathol. 2013;40(11):924–34. doi: 10.1111/cup.12217. [DOI] [PubMed] [Google Scholar]
- 22.Izykowska K, et al. Genetic rearrangements result in altered gene expression and novel fusion transcripts in Sezary syndrome. Oncotarget. 2017 doi: 10.18632/oncotarget.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eberle FC, et al. Nodal involvement by cutaneous CD30-positive T-cell lymphoma mimicking classical Hodgkin lymphoma. Am J Surg Pathol. 2012;36(5):716–25. doi: 10.1097/PAS.0b013e3182487158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basarab T, et al. Lymphomatoid papulosis in association with mycosis fungoides: a study of 15 cases. Br J Dermatol. 1998;139(4):630–8. [PubMed] [Google Scholar]
- 25.de la Garza Bravo MM, et al. Shared clonality in distinctive lesions of lymphomatoid papulosis and mycosis fungoides occurring in the same patients suggests a common origin. Hum Pathol. 2015;46(4):558–69. doi: 10.1016/j.humpath.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Zackheim HS, et al. Lymphomatoid papulosis associated with mycosis fungoides: a study of 21 patients including analyses for clonality. J Am Acad Dermatol. 2003;49(4):620–3. doi: 10.1067/s0190-9622(03)01577-9. [DOI] [PubMed] [Google Scholar]
- 27.Ralfkiaer E, et al. T-cell receptor gamma delta-positive peripheral T-cell lymphomas presenting in the skin: a clinical, histological and immunophenotypic study. Exp Dermatol. 1992;1(1):31–6. doi: 10.1111/j.1600-0625.1992.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 28.Berti E, et al. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. A distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol. 1999;155(2):483–92. doi: 10.1016/S0002-9440(10)65144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosseila M, et al. Evaluation of Angiogenesis in Early Mycosis Fungoides Patients: Dermoscopic and Immunohistochemical Study. Dermatology. 2015;231(1):82–6. doi: 10.1159/000382124. [DOI] [PubMed] [Google Scholar]
- 30.Lallas A, et al. Dermoscopy of early stage mycosis fungoides. J Eur Acad Dermatol Venereol. 2013;27(5):617–21. doi: 10.1111/j.1468-3083.2012.04499.x. [DOI] [PubMed] [Google Scholar]
- 31.Xu P, Tan C. Dermoscopy of poikilodermatous mycosis fungoides (MF) J Am Acad Dermatol. 2016;74(3):e45–7. doi: 10.1016/j.jaad.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Miteva M, et al. Alopecia universalis associated with cutaneous T cell lymphoma. Dermatology. 2014;229(2):65–9. doi: 10.1159/000360759. [DOI] [PubMed] [Google Scholar]
- 33.Moura FN, et al. Dermoscopy of lymphomatoid papulosis. Arch Dermatol. 2009;145(8):966–7. doi: 10.1001/archdermatol.2009.167. [DOI] [PubMed] [Google Scholar]
- 34.Agero AL, et al. In vivo reflectance confocal microscopy of mycosis fungoides: A preliminary study. J Am Acad Dermatol. 2007;57(3):435–41. doi: 10.1016/j.jaad.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Mancebo SE, et al. Reflectance confocal microscopy features of mycosis fungoides and Sezary syndrome: correlation with histopathologic and T-cell receptor rearrangement studies. J Cutan Pathol. 2016;43(6):505–15. doi: 10.1111/cup.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange-Asschenfeldt S, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17(1):016001. doi: 10.1117/1.JBO.17.1.016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ardigo M, et al. Concordance between in vivo reflectance confocal microscopy and optical histology of lymphomatoid papulosis. Skin Res Technol. 2013;19(3):308–13. doi: 10.1111/srt.12046. [DOI] [PubMed] [Google Scholar]
- 38.Gerami P, Guitart J. The spectrum of histopathologic and immunohistochemical findings in folliculotropic mycosis fungoides. Am J Surg Pathol. 2007;31(9):1430–8. doi: 10.1097/PAS.0b013e3180439bdc. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Pinilla SM, et al. TCR-gamma expression in primary cutaneous T-cell lymphomas. Am J Surg Pathol. 2013;37(3):375–84. doi: 10.1097/PAS.0b013e318275d1a2. [DOI] [PubMed] [Google Scholar]
- 40.Edinger JT, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33(12):1860–8. doi: 10.1097/PAS.0b013e3181bf677d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arulogun SO, et al. Long-term outcomes of patients with advanced-stage cutaneous T-cell lymphoma and large cell transformation. Blood. 2008;112(8):3082–7. doi: 10.1182/blood-2008-05-154609. [DOI] [PubMed] [Google Scholar]
- 42.Barberio E, et al. Transformed mycosis fungoides: clinicopathological features and outcome. Br J Dermatol. 2007;157(2):284–9. doi: 10.1111/j.1365-2133.2007.08008.x. [DOI] [PubMed] [Google Scholar]
- 43.Pulitzer M, et al. Mycosis fungoides with large cell transformation: clinicopathological features and prognostic factors. Pathology. 2014;46(7):610–6. doi: 10.1097/PAT.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyerson HJ, et al. Follicular center helper T-cell (TFH) marker positive mycosis fungoides/Sezary syndrome. Mod Pathol. 2013;26(1):32–43. doi: 10.1038/modpathol.2012.124. [DOI] [PubMed] [Google Scholar]
- 45.Guitart J, Gammon B. The difficulties in defining follicular T helper phenotype in cutaneous lymphomas. Am J Dermatopathol. 2013;35(6):691. doi: 10.1097/DAD.0b013e31827eaebd. [DOI] [PubMed] [Google Scholar]
- 46.Bosisio FM, Cerroni L. Expression of T-follicular helper markers in sequential biopsies of progressive mycosis fungoides and other primary cutaneous T-cell lymphomas. Am J Dermatopathol. 2015;37(2):115–21. doi: 10.1097/DAD.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 47.Fierro MT, et al. CD45RA+ immunophenotype in mycosis fungoides: clinical, histological and immunophenotypical features in 22 patients. J Cutan Pathol. 2001;28(7):356–62. doi: 10.1034/j.1600-0560.2001.280704.x. [DOI] [PubMed] [Google Scholar]
- 48.Hodak E, et al. CD4/CD8 double-negative epidermotropic cutaneous T-cell lymphoma: an immunohistochemical variant of mycosis fungoides. J Am Acad Dermatol. 2006;55(2):276–84. doi: 10.1016/j.jaad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 49.Pulitzer M, et al. ALK-positive (2p23 rearranged) anaplastic large cell lymphoma with localization to the skin in a pediatric patient. J Cutan Pathol. 2015;42(3):182–7. doi: 10.1111/cup.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poppe H, et al. Childhood mycosis fungoides with a CD8+ CD56+ cytotoxic immunophenotype. J Cutan Pathol. 2015;42(4):258–64. doi: 10.1111/cup.12452. [DOI] [PubMed] [Google Scholar]
- 51.Lee AD, Cohen PR. What is Woringer-Kolopp disease? Skinmed. 2013;11(1):17–20. [PubMed] [Google Scholar]
- 52.Harmon CB, et al. Detection of circulating T cells with CD4+CD7− immunophenotype in patients with benign and malignant lymphoproliferative dermatoses. J Am Acad Dermatol. 1996;35(3 Pt 1):404–10. doi: 10.1016/s0190-9622(96)90605-2. [DOI] [PubMed] [Google Scholar]
- 53.Jokinen CH, et al. Flow cytometric evaluation of skin biopsies for mycosis fungoides. Am J Dermatopathol. 2011;33(5):483–91. doi: 10.1097/DAD.0b013e31820595da. [DOI] [PubMed] [Google Scholar]
- 54.Pierson DM, et al. Utility of CD26 in flow cytometric immunophenotyping of T-cell lymphomas in tissue and body fluid specimens. Cytometry B Clin Cytom. 2008;74(6):341–8. doi: 10.1002/cyto.b.20431. [DOI] [PubMed] [Google Scholar]
- 55.Olsen E, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110(6):1713–22. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 56.Feng B, et al. Flow cytometric detection of peripheral blood involvement by mycosis fungoides and Sezary syndrome using T-cell receptor Vbeta chain antibodies and its application in blood staging. Mod Pathol. 2010;23(2):284–95. doi: 10.1038/modpathol.2009.175. [DOI] [PubMed] [Google Scholar]
- 57.Oshtory S, et al. Usefulness of flow cytometry in the diagnosis of mycosis fungoides. J Am Acad Dermatol. 2007;57(3):454–62. doi: 10.1016/j.jaad.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Novelli M, et al. Flow cytometry immunophenotyping in mycosis fungoides. J Am Acad Dermatol. 2008;59(3):533–4. doi: 10.1016/j.jaad.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 59.Clark RA, et al. High-scatter T cells: a reliable biomarker for malignant T cells in cutaneous T-cell lymphoma. Blood. 2011;117(6):1966–76. doi: 10.1182/blood-2010-05-287664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laharanne E, et al. Genome-wide analysis of cutaneous T-cell lymphomas identifies three clinically relevant classes. J Invest Dermatol. 2010;130(6):1707–18. doi: 10.1038/jid.2010.8. [DOI] [PubMed] [Google Scholar]
- 61.Laharanne E, et al. CDKN2A-CDKN2B deletion defines an aggressive subset of cutaneous T-cell lymphoma. Mod Pathol. 2010;23(4):547–58. doi: 10.1038/modpathol.2009.196. [DOI] [PubMed] [Google Scholar]
- 62.Salgado R, et al. Oligonucleotide array-CGH identifies genomic subgroups and prognostic markers for tumor stage mycosis fungoides. J Invest Dermatol. 2010;130(4):1126–35. doi: 10.1038/jid.2009.306. [DOI] [PubMed] [Google Scholar]
- 63.Choi J, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47(9):1011–9. doi: 10.1038/ng.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kiran T, et al. The significance of MUM1/IRF4 protein expression and IRF4 translocation of CD30(+) cutaneous T-cell lymphoproliferative disorders: a study of 53 cases. Leuk Res. 2013;37(4):396–400. doi: 10.1016/j.leukres.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Pham-Ledard A, et al. IRF4 gene rearrangements define a subgroup of CD30-positive cutaneous T-cell lymphoma: a study of 54 cases. J Invest Dermatol. 2010;130(3):816–25. doi: 10.1038/jid.2009.314. [DOI] [PubMed] [Google Scholar]
- 66.Karai LJ, et al. Chromosomal rearrangements of 6p25.3 define a new subtype of lymphomatoid papulosis. Am J Surg Pathol. 2013;37(8):1173–81. doi: 10.1097/PAS.0b013e318282d01e. [DOI] [PubMed] [Google Scholar]
- 67.Kucuk C, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from gammadelta-T or NK cells. Nat Commun. 2015;6:6025. doi: 10.1038/ncomms7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eren R, et al. Evaluation of neutrophil-lymphocyte ratio in patients with early-stage mycosis fungoides. Ann Hematol. 2016;95(11):1853–7. doi: 10.1007/s00277-016-2779-7. [DOI] [PubMed] [Google Scholar]
- 69.Takahashi Y, et al. Clinicopathological analysis of 17 primary cutaneous T-cell lymphoma of the gammadelta phenotype from Japan. Cancer Sci. 2014;105(7):912–23. doi: 10.1111/cas.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cengiz FP, et al. Prognostic Evaluation of Neutrophil/Lymphocyte Ratio in Patients with Mycosis Fungoides. Ann Clin Lab Sci. 2017;47(1):25–28. [PubMed] [Google Scholar]