Abstract
Osteoporosis, characterized by low bone mass and micro-architectural deterioration of bone tissue with increased risk of fracture, can be categorized into two forms: primary or secondary, depending on whether it occurs as part of the natural aging process (estrogen-deficiency) or as part of disease pathology. In both forms bone loss is due to an imbalance in the bone remodeling process with resorption/formation skewed more toward bone loss. Recent studies and emerging evidence consistently demonstrate the potential of the intestinal microbiota to modulate bone health. The current chapter discusses the process of bone remodeling and the pathology of osteoporosis and introduces the intestinal microbiota and its potential to influence bone health. In particular, we highlight recent murine studies that examine how probiotic supplementation can both increase bone density in healthy individuals as well as protect against primary (estrogen-deficiency) as well as secondary osteoporosis. Potential mechanisms are described to account for how probiotic treatments could be exerting their beneficial effect on bone health.
Keywords: Microbiota, Probiotic, Bone, Osteoporosis, Menopause, diabetes
THE SKELETON
The adult human skeleton is comprised of 206 bones, excluding the sesamoid bones (1). The bones are subdivided into four general types: long bones, shorts bones, flat bones and irregular bones. Long bones, such as the femur, are comprised of a hollow diaphysis which flairs at the end to form the metaphysis, the region below the growth plate, and the epiphyses, the region above the growth plate. The diaphysis, also known as the shaft, is mainly composed of dense, solid bone known as cortical bone, whereas, the metaphysis and epiphysis contain a honeycomb-like network of interconnected trabecular plates surrounding bone marrow known as cancellous or trabecular bone (1).
Bone is critical for structural support and movement, protection of vital organs as well as for maintenance of mineral homeostasis and hematopoiesis. Bone is a dynamic organ and is continuously undergoing remodeling. Control of bone remodeling is a highly complex process that involves integrative signals from not only the different bone cells but also signals from other systems including immune, neuronal and hormonal (2,3). While it has been known for a long time that the gastrointestinal system plays a critical role in bone homeostasis via regulation of calcium absorption, recent studies underscore the emerging role of the gut microbiota in regulating bone remodeling. Thus modification of the gut microbiota, by ingesting probiotics, could be a viable therapeutic strategy to regulate bone remodeling under a variety of conditions that lead to bone loss and osteoporosis. In this chapter we provide a comprehensive analysis of recent studies that have examined the effectiveness of probiotic for the treatment of bone loss and osteoporosis.
Bone Remodeling
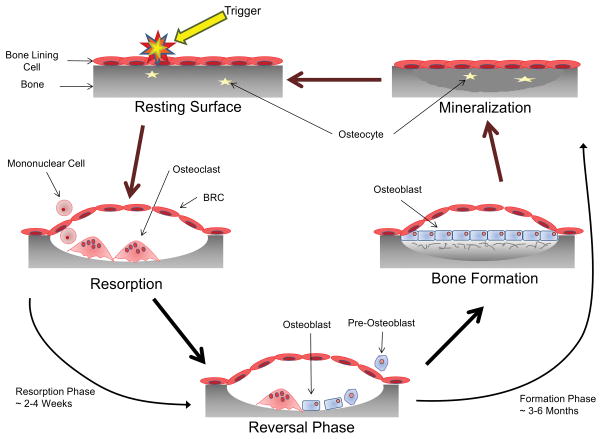
Throughout a lifetime the skeleton is subjected to a variety of stresses and strains leading to the formation of cracks and micro-damage. To maintain the integrity of the skeleton it is continuously remodeled; in the adult human skeleton, 5 – 10% of the existing bone is replaced every year (4). Remodeling is accomplished by the coupled activities of a group of cells collectively termed the bone remodeling unit (BRU) (5). The cells that constitute the BRU are the osteoblasts, cells that produce the organic bone matrix and facilitate bone mineralization (6); the osteoclasts, cells responsible for the degradation of bone and extracellular matrix (7); the osteocytes, osteoblast-derived cells that lies within the bone matrix and act as mechanosensors and endocrine cells (8); and the bone lining cells, cells that form the canopy of the trabecular bone remodeling compartment (BRC) and help to couple bone formation to resorption (9). There are four distinct phases in the bone remodeling cycle: i) initiation, ii) resorption, iii) reversal and iv) formation (see Fig 1 for more details).
Fig 1. The Bone Remodeling Cycle.
A symplified representation of the bone remodeling cycle. The initiation phase of bone remodeling is induced by mechanical strain, damage or by signals from cytokines or systemic factors. This generates local signals that lead to the bone lining cells separating from the bone surface and forming a canopy over the site to be resorbed (94). Osteoclasts and their precursors are then recruited to the site of bone remodeling from the circulatory system via capillaries that are closely associated with the BRC (95). The signals for the initiation of osteoclast differentiation and resorption; macrophage colony stimulating factor (MCSF) and receptor activator of NF-κB ligand (RANKL), are provided by cells of the osteoblast lineage including osteocytes as well as T and B cells (95–98). Once the remodeling process is initiated resorption of the bone occurs. OC attach to the exposed surface of the mineralized matrix where they polarize and form a sealed microenvironment. This sealed microenvironment is then acidified to breakdown the inorganic component of bone followed by release of the enzymes cathepsin K, matrix metalloproteinase-9 (MMP-9) and tartrate resistant acid phosphatase (TRAP) which breakdown the organic component (7,99). Following resorption of the old damaged bone the process undergoes reversal. Toward the end of the resorption phase of the bone remodeling cycle mononuclear cells of osteoblast-lineage move into the resorption pit. These mononuclear cells remove the old demineralized collagen while laying down a new thin layer (100). During this phase the process of ‘coupling’ bone resorption to bone formation occurs to ensure that the volume of bone removed is replaced. Coupling of bone resorption to bone formation is a multifaceted process with numerous regulator molecules derived from the matrix, secreted or membrane-bound contributing (94,101,102). Bone formation is a two-step process and proceeds slowly, taking approximately 3 months (compared to resorption which typically takes 3 weeks). The osteoblast first secretes the unmineralized osteoid which is then mineralized through the incorporation of hydroxyapatite (103). When the osteoblast has completed the matrix formation they undergo a number of possible fates. The majority of osteoblasts become apoptotic; however, some get trapped in the mineralized matrix and undergo further differentiation into the osteocyte while others may become inactive bone lining cells (104). Through the production of sclerostin (SOST), an inhibitor of Wnt signalling, the osteocyte can regulate the amount of new bone formation that takes place (8).
Osteoclasts and Osteoblasts
Osteoclasts are the principal cells responsible for bone resorption while osteoblasts mediate bone formation. The osteoclast is a terminally differentiated, highly motile, multinucleated cell formed by the fusion of monocyte/macrophage precursors derived from hematopoietic origin (10) (Fig 2). In contrast, osteoblasts arise from mesenchymal stem cells (MSC) which are pluripotent cells that have the potential to differentiate into numerous cell types including adipocytes, chondrocytes and osteoblasts (11) (Fig 3). Control of osteoclast and osteoblast differentiation is regulated by numerous cytokines, hormones, growth factors and transcription factors (12,13).
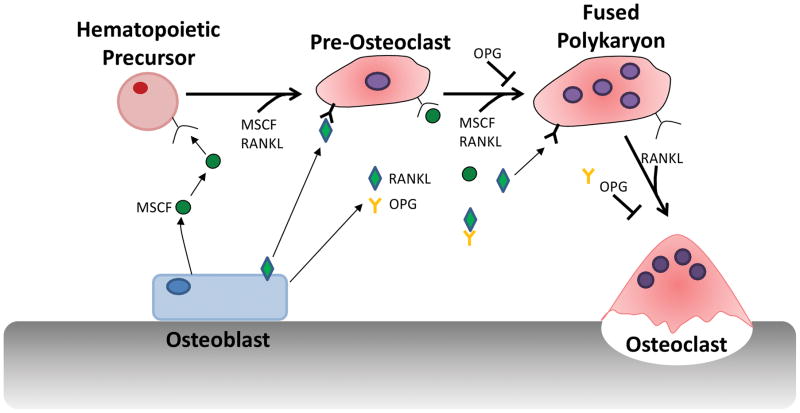
Fig 2. Osteoclast Differentiation.
Osteoclast differentiation is the process by which mononuclear cells undergo fusion into the multinucleated osteoclast. Three cytokines are critical for osteoclast differentiation: MCSF, RANKL and osteoprotegerin (OPG), a soluble decoy receptor for RANKL (105–108). In the initial stages of differentiation, precursor cells proliferate in response to MCSF signaling through its receptor c-FMS (109). RANKL, expressed as a membrane bound or soluble form then binds to its receptor, receptor activator of nuclear factor κB (RANK), present on the precursor cells (110,111). This results in the transcription and activation of numerous osteoclast specific genes; cathepsin K, tartrate resistant acid phosphatase (TRAP; an osteoclast marker), calcitonin receptor and B3 integrin (112). The precursor cells then migrate along chemokine gradients and fuse together to form the multinucleated osteoclast. Control of osteoclast differentiation is via the soluble receptor OPG, which competes with RANK for RANKL binding, thus inhibiting OC differentiation (113,114).
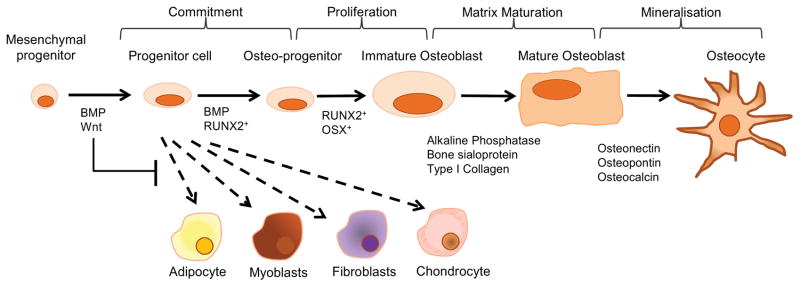
Fig 3. Osteoblast Differentiation.
Signaling by members of the canonical Wnt/β-catenin pathway, such as Wnt10b, and BMP2 and BMP4, direct the MSC cell fate towards the osteoblast lineage. This is achieved by suppressing the adipogenic transcription factors C/EBPα and PPARγ while inducing the osteogenic transcription factors Runx2 and osterix (28,115,116). This immature osteoblast still has the potential to divide and expresses low levels of alkaline phosphatase (ALP) activity, as well as synthesize type I collagen which makes up to 90% of the organic component of bone (117). Differentiation to the non-proliferating mature cuboidal osteoblast that actively mineralizes bone matrix is dependent on the transcription factors osterix (118). Before the newly laid matrix can be mineralized however it must first undergo maturation. Matrix maturation is associated with increased expression of alkaline phosphatase and several non-collagen proteins (NCPs), including osteocalcin, osteopontin, and bone sialoprotein (119). Mineralization of bone is completed by the incorporation of hydroxyapatite (Ca10(PO4)6(OH)2) into the newly deposited osteoid. Membrane bound extracellular bodies (extracellular matrix vesicles) released from the osteoblast facilitate initial mineral deposition by accumulating calcium and phosphate ions in a protected environment. Clusters of these ions come together to form the first stable crystals. Addition of ions to these crystals follows, resulting in their growth (103,120). At the completion of bone formation a subset of osteoblasts can undergo further differentiation, upon being entombed in the bone matrix, and become osteocytes. The remaining osteoblasts are thought to either undergo apoptosis or become inactive bone-lining cells (104).
Osteoimmunology
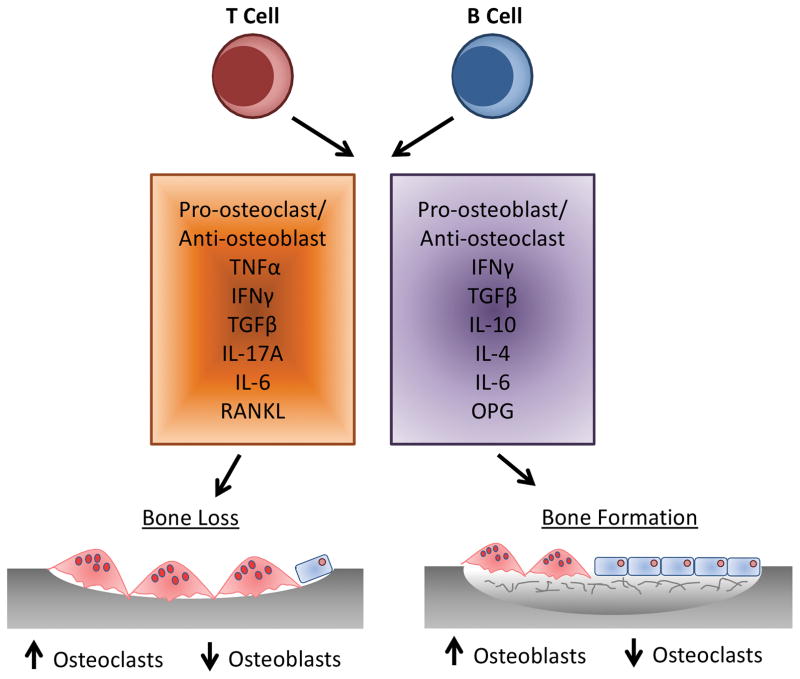
While cells of the osteoblast and osteoclast lineage modulate each other’s differentiation and function through cell-cell contact and diffusible paracrine factors, it is now well recognized that immune cells, including lymphocytes (T and B) and dendritic cells, also play a key role in modulating bone remodeling in both health and disease. This modulation is via direct and indirect measures through expression of a large number of cytokines which can have both a pro-osteoclastogenic effect, resulting in bone loss or pro-osteogenic effect, resulting in bone formation (Fig 4) (12,14).
Fig 4. Cross-talk Between Osteoclasts, Osteoblasts and the Immune System.
Activated T lymphocytes, specifically T helper (Th) 17 cells have been identified as osteoclastogenic through the expression of RANKL and the cytokine interleukin (IL)-17, which induces RANKL expression on osteoblasts (121). Furthermore, expression of IL-17 enhances local inflammation, driving expression of other pro-inflammatory cytokines promoting additional RANKL expression (12). In addition to IL-17, T cell TNFα production has been demonstrated to affect the balance of bone remodeling. Increased T cell TNFα enhances osteoclastogenesis while inhibiting osteoblast differentiation and collagen synthesis (24,122,123). In addition to pro-osteoclastogenic cytokines, T-lymphocytes also secrete IL-10, IL-4 and interferon (IFN)-γ that are potentially anti-osteoclastogenic (12,124). A role for B-lymphocytes in bone homeostasis has been suggested as B cell-deficient mice exhibit an osteoporotic phenotype (114). B-lymphocytes are responsible for 64% of total bone marrow OPG production, with 45% of this derived from mature B cells (114).
BONE DISEASE
Diseases of the bone are typically characterized by direct or indirect effect on the balance of remodeling, with increased or decreased bone resorption/formation. Osteoporosis is a typical example of an imbalance in resorption/formation that is skewed more toward bone loss. By definition, osteoporosis is characterized by low bone mass and micro-architectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture (15). It affects approximately 54 million Americans, with studies suggesting that one in three women and one in five men, age 50 and older, will break a bone due to osteoporosis (16). In 2005 more than 2 million incident fractures were reported in the USA alone at a cost of approximately $17 billion. This number is predicted to rise in excess of $25 billion by 2025 (17). Osteoporosis can be defined as two forms: primary osteoporosis which occurs as part of the normal human aging process or secondary osteoporosis when bone loss is caused by a medical condition/disease or treatment.
Primary Osteoporosis
In females, the onset of menopause is a major factor that contributes to development of post-menopausal osteoporosis. Loss of estrogen gives rise to two stages of bone loss: an early rapid loss of trabecular and cortical bone due to increased osteoclast activity and decreased osteoclast apoptosis, and a second slower prolonged loss due to decreased osteoblast activity (18). The mechanisms behind this uncoupling of bone resorption and bone formation are complex and multifactorial. Loss of estrogen has been observed to increase expression of pro-inflammatory and osteogenic cytokines namely, IL-1, IL-6, IL-7, TNFα, MCSF and RANKL from osteoblasts, T cells and B cells (19–23). Of these cells, T lymphocytes are believed to play a particularly critical role in the bone loss associated with estrogen deficiency. This was demonstrated using T-cell deficient mice wherein these mice were protected from ovariectomy (OVX)-induced bone loss (24). Furthermore, dysregulation of T cell CD40L signaling following estrogen deficiency leads to increased stromal cell expression of osteoclastogenic cytokines while decreasing expression of OPG (25). Asides from its pro-osteoclastogenic effects, the increased expression of TNFα following estrogen deficiency has also been shown to increase expression of sclerostin, a secreted Wnt antagonist (26). The subsequent decrease in Wnt signaling results in a shift of MSC differentiation away from osteoblasts and towards adipocytes resulting in reduced bone formation (27,28). Changes in death receptor signaling on osteoclasts and osteoblasts following estrogen-deficiency is further thought to contribute to the overall bone loss. OVX has been shown to increase osteoblast expression of Fas (CD95), a well characterized “death” receptor, resulting in suppressed osteoblast differentiation and increased apoptosis (29). Interestingly Fas-deficient mice are protected from OVX induced bone loss due to enhanced osteoblast differentiation and activity (30).
Secondary Osteoporosis
As previously mentioned secondary osteoporosis is bone loss caused by a variety of medical and pathological factors including but not limited to: smoking; type 1 diabetes; hyperparathyroidism; inflammatory bowel disease; arthritis and glucocorticoid treatment. The incidence of secondary osteoporosis is difficult to discern however, it has been suggested to occur in almost two-thirds of men and one-fifth of postmenopausal women with osteoporosis (31,32). Various gastrointestinal diseases are known to cause secondary osteoporosis, particularly inflammatory bowel disease (IBD); up to three quarters of IBD patients may have a reduced bone mineral density (BMD) (32). Several mechanisms contribute to the bone loss in IBD patient including malnutrition; malabsorption of vitamin D, calcium and vitamin K; immobilization; and increased expression of inflammatory cytokines such as TNFα, IL-1β and IL-6 (33).
The autoimmune disease type 1 diabetes (T1D) is also associated with secondary osteoporosis (34,35). Through the use of T1D animal models several mechanisms that may contribute to T1D osteoporosis have been identified. In the streptozotocin-induced murine model of T1D, gene expression of TNFα, IL-1β and IL-6 in the bone marrow are up-regulated, leading to increased osteoblast death directly (36) and suppressed wnt10b expression (37,38). Suppression of Wnt10b is known to further decrease osteoblast viability, maturation and lineage selection. Consistent with this finding, expression of the critical osteoblast transcription factor Runx2 is reduced while the adipogenic markers, aP2 (FABP4) and PPARγ, are increased in T1D mouse bone (37). These data suggest that an anabolic defect is a major contributor to the secondary osteoporosis observed in T1D.
Osteoporosis Treatments
Numerous therapies have been developed for the treatment of osteoporosis with the aim of reducing bone loss and correcting the imbalance in bone remodeling. In addition to lifestyle modifications (increased physical activity, reduced alcohol intake and cessation of smoking) current baseline therapies for the prevention and treatment of osteoporosis comprise vitamin D and calcium supplementation (39). In patients that have a higher risk of fracture, pharmacological interventions are employed. The drugs fall into two classes: i) drugs that inhibit bone resorption (anti-resorptive) and ii) drugs that stimulate bone formation (anabolic) See Table 1.
Table 1.
Established Treatments for Osteoporosis
| Anti-Resorptive | Bisphosphonates |
| Raloxifene | |
| HRT | |
| Denosumab | |
| Bone Forming | Teriparatide (Parathyroid Hormone) |
| Other | Calcium |
| Vitamin D | |
| Strontium Ranelate | |
| Calcitonin | |
| Calcitriol | |
| Exercise |
Of the anti-resorptive drugs, bisphosphonates constitute the largest class. Bisphosphonates can be administered orally or intravenously and have a high affinity for bone. In addition, they are inexpensive and have a long safety record. Bisphosphonates inhibit osteoclast activity either by a direct toxic effect or by altering their cytoskeleton (42). Anti-resorptive drugs however, have unintended effects in some patients including upper gastrointestinal irritation due to oral bisphosphonates as well as, osteonecrosis of the jaw and atypical subtrochanteric femoral fractures (43).
While numerous anti-resorptive drugs exist, in the US there is only one approved anabolic drug that builds up new bone, parathyroid hormone (PTH). PTH is used either as a full-length (PTH 1–84) or N-terminal fragment (teriparatide, PTH 1–34). PTH treatment is administered daily via a subcutaneous injection and stimulates increased bone density through an increase in the bone remodeling rate which favors bone formation. Therapeutic courses of PTH are limited to 24 months due to safety concerns related to an increase in risk of osteosarcoma as well as the high cost of the drug (43,44).
These limitations in treatments for osteoporosis underscore the need for novel therapies that have fewer side effects. Interestingly, recent studies have identified the intestinal microbiota as an important link in modulating bone health (45–47). The focus of this chapter will be on probiotics and bone health. In the next subsections, we will examine the how microbiota and its modulation by probiotics is beneficial in osteoporosis, at least in animal models of disease.
MICROBIOTA
It is now clear from both human and animal studies that the intestinal microbiota is needed for the health of its host, and plays a crucial role in many aspects of host physiology including metabolism, nutrition, pathogen resistance, and immune function. While different parts of the intestinal tract exhibit differential densities of microbiota, the colon usually has the highest content, 1011 cfu/mL (48). The human body is thought be a host for ~100 trillion microbes comprising ~1000 species and 28 different phyla (49). In addition to the sheer number of microbes outnumbering host cell number (estimated at ~60 trillion), gut microbiota also express 100 fold more genes compared to the human genome. (49). Thus, as the microbiome coevolves with us, changes in that population can have both beneficial and harmful consequences on human health (50). Therefore, gaining knowledge of the microbiome-host relationship with respect to physiology is highly critical to not only understand disease pathogenesis but also to target the microbiome for therapeutic purposes.
Role of Intestinal Microbiota in Influencing Bone
Previous studies have clearly demonstrated that the intestinal tract can profoundly influence the health of the bone. One way this occurs is through the regulation of mineral absorption which is required for healthy bone and includes calcium, phosphorous and magnesium. In addition, endocrine factors that influence the absorption of these minerals as well as gut-derived factors such as incretins and serotonin can also influence bone turnover (51,52). More recent studies using germ-free mice and probiotics have demonstrated the influence of the intestinal microbiome in modulating bone physiology (53,54).
Early evidence that the intestinal microbiota could affect bone was provided by Sjögren et al (53). In their study, germ free mice, conventional mice and germ free mice colonized with a normal microbiota were used to investigate the role of the microbiota in bone health. Bone mass was observed to be higher in germ free mice compared to that of the conventional mice; germ free mice additionally had reduced number of osteoclasts per bone surface and decreased frequency of CD4+ T cells and osteoclast precursors in their bone marrow. These findings were normalized following colonization of the germ free intestine with a conventional microbiota. The exact role that the microbiota plays in the development of bone however, is not without controversy as subsequent studies have shown either no difference in bone density between conventional mice and germ free mice (54) or that while initial colonization acutely reduces bone density, long-term colonization results in an increase in bone formation (55). This suggests that the effects of the microbiota on bone health are complex and time dependent. The evidence for a role of probiotic supplementation in modulating bone health however, is much stronger. Numerous studies have revealed that modulating the intestinal microbiota with probiotic bacteria can have a beneficial effect. These will be discussed in detail further in this chapter.
PROBIOTICS
Probiotics are defined as dietary supplements that contain live non-pathogenic microorganisms that when administered in adequate amounts can be beneficial in the treatment as well as in the prevention of pathological conditions (56). Many genera of bacteria such as Lactobacillus, Enterococcus, Bacillus, Escherichia, and Bifidobacterium have been used for their beneficial effects as probiotics. Although, most probiotics are bacteria, yeast such as Saccharomyces, have also been found to present probiotic characteristics (57). Probiotic bacteria are naturally found in the mucous membranes such as the mouth, skin, urinary and genital organs, and in the intestines. They are also commonly found in dietary supplements, fermented products (e.g., meat, milk products, beer) and in non-conventional products such as toothpaste and ice cream. The Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) has develop guidelines for the assessment and use of probiotic bacteria for consumption (58). For a microbe to be classified as probiotic it needs to present specific characteristics such as: survival in the gastrointestinal system (acid and bile tolerance), phenotype and genotype stability, adhesion to mucosal surface, antibiotic resistance, production of antimicrobial substances and ability to inhibit known pathogens. In addition, probiotic bacteria or their fermented products cannot be harmful to the host, cannot induce an immune system response unless induced against pathogenic microorganisms, and bacteria that contain transmissible drug resistance genes should not be used.
In recent years, multiple studies have been published indicating the potential benefits of probiotic supplementation on bone health in both healthy and pathological states. These beneficial effects have been observed with multiple strains of bacteria and in numerous experimental animal models of disease (table 2). The mechanism through which probiotics exert their effects however, hasn’t been fully elucidated. It is known that, probiotics can influence the gut through regulation of luminal pH; secretion of antimicrobial peptides; enhancement of barrier function by increasing mucus production and modulation of the host immune system; and by modifying the gut microflora (59–62). Which of these mechanisms are important for the beneficial effects on bone is not yet well known.
Table 2.
Effect of Probiotics on Bone (Animal Studies)
| Probiotic Strain | Animal Model | Duration | Analysis Method | Bone Effects | Ref |
|---|---|---|---|---|---|
| Bacillus licheniformis and Bacillus subtilis | Broiler | 6 weeks | Measuring Calipers | ↑ Tibia lateral and medial wall thickness | (63) |
| Brewer’s yeast | Broiler | 9 weeks | Visual | ↓ Tibial dyschondroplasia | (64) |
| Lactobacillus | Broiler | 28 days | Atomic absorption spectrophotometry Phosphomolybdic Acid method |
↑ Ca and P retention | (65) |
| Lactobacillus reuteri (ATCC 6475) | Male mice | 4 weeks | μCT | ↑ Bone volume fraction ↑ Trabecular number ↑ Trabecular thickness ↑ Bone mineral content ↑ Bone mineral density ↑ Osteocalcin ↑ Bone formation rate |
(66) |
| Female mice (OVX) | 4 weeks | μCT | ↑ Bone volume fraction ↑ Bone mineral content ↑ Bone mineral density ↓ RANKL gene expression ↓ TRAP5 expression |
(67) | |
| Female Mice (Dorsal Surgery) | 8 weeks | μCT | ↑ Bone volume fraction ↑ Mineral apposition rate ↓ RANKL gene expression ↑ OPG gene expression ↑ IL-10 gene expression |
(68) | |
| Male Mice (STZ-induced Type 1 Diabetes) | 4 weeks | μCT | ↑ Bone volume fraction ↑ Bone mineral content ↑ Bone mineral density ↑ Mineral apposition rate ↑ Serum osteocalcin ↑ Wnt10b expression |
(38) | |
| Lactobacillus casei, L. reuteri and L. gasseri | Rats | ↑ Bone mineral content ↑ Ca absorption |
(69) | ||
| Bifidobacterium longum – fermented broccoli | Male Wistar Rats | 12 weeks | Histology | ↓ TRAP+ osteoclasts | (70) |
| Bifidobacterium longum (ATCC 15707) | Male Wistar Rats | 28 days | Texture analyzer Plasma emission spectrophotometry |
↑ Tibial Ca, P and Mg content ↑ Fracture strength |
(71) |
| Lactobacillus rhamnosus (HN001) | Male Sprague-Dawlry Rats | 3 weeks | DEXA | ↑ Ca and Mg retention | (72) |
| Female Sprague-Dawlry Rats (OVX) | 3 months | DEXA | ↓ Bone loss | (72) | |
| Female Mice (OVX) | 4 weeks | μCT | ↑ Bone volume fraction ↑ Serum osteocalcin ↓ RANKL gene expression ↓ TNFα gene expression ↓ IL-17 gene expression |
(54) | |
| L. paracasei (NTU 101) or L. plantarum (NTU 102)-fermented soy milk | Female mice (OVX) | 8 weeks | μCT SEM |
↑ Bone volume fraction ↑ Trabecular number |
(73) |
| L. paracasei or L. paracasei and L. plantarum | Female mice (OVX) | 6 weeks | μCT | ↑ Cortical Bone mineral content ↑ Cortical area ↑ OPG Expression |
(74) |
| L. casei 393-fermented milk | Female Sprague-Dawlry Rats (OVX) | 6 weeks | DEXA Texture Analyzer Plasma emission spectrophotometry |
↑ Bone mineral density ↑ Fracture strength ↑ Ca content ↓ TRAP activity |
(75) |
| L. helveticus-fermented milk | Spontaneously hypertensive male rats | 14 weeks | DEXA Plasma emission spectrophotometry |
↑ Bone mineral density ↑ Bone mineral content |
(76) |
| L. casei and L. acidophilus | Female wistar rat (adjuvant-induced arthritis) | 12 days | X-ray | ↓ Bone loss | (77) |
| Enterococcus faecium (with methotrexate) | Male Lewis rat (adjuvant-induced arthritis) | 50 days | DEXA X-ray |
↑ anti-inflammatory effects ↑ anti-arthritic effects |
(78) |
Probiotics and Bone in Non-Pathological Animal Models
The use of healthy non-pathogenic animal models has been used to evaluate the safety, efficacy and mechanism of probiotic supplementation. Interestingly, the effect of probiotics under healthy non-pathological conditions has been shown to be dependent on many variables including strain and sex of the animal.
In a study using Lactobacillus reuteri ATCC 6475, oral administration of the probiotic for a period of 4 weeks to specific pathogen free healthy male mice, but not female mice, resulted in a significant increase in femoral and vertebral trabecular bone density, trabecular number, trabecular thickness, bone mineral content and bone mineral density when compared to untreated controls (66). This increase in bone density was attributed to an increase in osteoblast bone formation as evidenced by elevated levels of the osteoblast marker osteocalcin and increased bone formation rate; no difference was observed in serum TRAP levels. While in this study the mechanism of action was not fully identified, supplementation with L. reuteri 6475 was observed to decrease expression of the inflammatory cytokine TNFα in the jejunum and ileum. (66). Interestingly, while intact healthy female mice did not respond to oral L. reuteri in terms of bone health, further studies revealed that they subsequently responded to the probiotic if the health status was skewed towards a mild inflammatory state (68). This mild inflammatory state was induced via a dorsal surgical incision (DSI) and following probiotic supplementation resulted in the female mice exhibiting increased bone density. However, this took longer than the males (8 week treatment in females versus 4 weeks in males). In addition to an increase in femoral trabecular bone density, DSI female mice (treated with probiotic) exhibited higher trabecular number as well as mineral apposition rate in comparison with non-treated DSI female mice and treated intact female mice (68). These results suggest that under naïve healthy conditions, females are likely at their maximal anti-inflammatory state and therefore L. reuteri is unable to influence inflammation and bone formation. Whereas, slight inflammation induced by DSI, skews the females (in spite of intact estrogen) towards a pro-inflammatory state, therefore L. reuteri is able to have a beneficial effect on bone density. However, the precise mechanisms of L. reuteri 6475 effects on bone density are still under investigation.
In studies parallel to the mouse model, treatment of rats with yogurt containing L. casei, L. reuteri and L. gasseri increased calcium absorption resulting in elevated BMC compared to the control (69). Likewise, supplementation of growing rats with Lactobacillus rhamnosus (HN001) improved magnesium and calcium retention (72). In addition to different strains of Lactobacillus, beneficial effects on bone have also been observed with Bifidobacterium longum. Supplementation of male rats with Bifidobacterium longum (ATCC 15707)for 28 days showed an increase in calcium, phosphorus, and magnesium content in the tibia and higher percentage fracture strength than untreated rats (71). In a separate study rats fed a high cholesterol diet supplemented with Bifidobacterium longum-fermented broccoli for 12 weeks presented a reduction in the number of TRAP-positive osteoclasts in comparison with untreated rats (70).
Probiotics and Bone Health in Animal Models of Osteoporosis
A number of studies have utilized animal models to investigate whether probiotics can be used to prevent both primary and secondary osteoporotic bone loss (38,54,67,74). These studies have mainly used different species of the Lactobacillus and Bifidobacterium genus.
Primary Osteoporosis
In a recent study from our lab (67) the bacterium L. reuteri ATCC 6475 was used in the primary osteoporosis mouse menopause (OVX) model. 12 Week old Balb/c mice were provided with L. reuteri ATCC 6475 3 times a week by gavage (1×109 cfu/ml) and constantly in the drinking water (1.5×108 cfu/ml) for four weeks following OVX surgery and femoral and vertebral bones analyzed by μCT. OVX mice supplemented with L. reuteri were found to be completely protected from bone loss resulting in a BV/TV that was comparable to the control mice. Furthermore, significant increases in trabecular BMD and BMC were observed in the OVX treated mice compared to the OVX controls. The protective effect of L. reuteri was attributed to a decrease in bone mRNA levels of RANKL and TRAP5. Serum TRAP5 levels were also modestly changed by L. reuteri treatment. However, osteoblast markers such as osteocalcin were not affected. These results suggested that the protective effect of L. reuteri observed in this model is via an anti-osteoclastogenic effect. This was supported by ex vivo bone marrow cultures where the osteoclastogenic potential of the OVX L. reuteri treated bone marrow were significantly reduced, compared to the OVX bone marrow cultures. These data supported an earlier study by Chiang and Pan et al (73) who revealed that OVX mice treated with either L. paracasei (NTU 101) or L. plantarum (NTU 102)-fermented soy milk had significantly increased BV/TV and trabecular number compared to OVX controls.
Further support for the beneficial effects of Lactobacilli treatment preventing estrogen-deficiency-induced trabecular bone loss has been provided by Li et al (54). In their study they utilized both the OVX model in specific pathogen free mice as well as an ovarian sex steroid inhibitor (Leuprolide) in germ free mice. These animals were treated with either Lactobacillus rhamnosus GG (LGG) or the commercially available probiotic supplement VSL#3 (containing four species of Lactobacilli; three species of Bifidobacteria; and Streptococcus salivarius subsp. Thermophilus (79)). In both models LGG and VSL#3 markedly prevented the decrease in femoral bone density, trabecular thickness and number compared with the untreated controls. Importantly, non-probiotic bacteria such as Escherichia coli DH5alpha and the LGG pili mutant (LGG (ΔSpaC)) did not provide any protection from bone loss. While the mechanism of action was not fully elucidated in this study, CTX levels in the serum, a marker of osteoclast bone resorption, were decreased in the OVX + LGG and VSL#3 cohorts but not in the non-probiotic groups (54). This suggests that, as with the other studies in the OVX model of bone loss, probiotics mediate their effects on OVX-induced bone loss by inhibiting osteoclast activity.
In an analogous study by Ohlsson et al (74), mice were treated with either a single Lactobacillus paracasei strain (DSM13434) or a mixture of three strains (Lactobacillus paracasei DSM13434, Lactobacillus plantarum DSM 15312 and DSM 1531) in the drinking water for two weeks prior and for four weeks after OVX surgery. L. paracasei DSM13434 as well as the multiple strains increased cortical bone mineral content compared to the vehicle treated OVX mice. Serum levels of the resorption marker C-terminal telopeptides and the urinary fractional excretion of calcium were decreased as was the cortical bone RANKL/OPG ratio in the probiotic treated groups compared with the vehicle treated group. However, mRNA levels of three osteoblast-associated genes (osterix, Col1α1 and osteocalcin) were not affected by the different probiotics. Together these results further support the notion that probiotics prevent bone loss in estrogen-deficient mice by regulating osteoclast resorption but not osteoblast bone formation.
The effectiveness of probiotics to inhibit OVX-induced bone loss has also been investigated in other animal models. In the OVX rat model the effect of Bifidobacterium longum on bone density, bone mineral content, bone remodeling, bone structure, and osteoclast/osteoblast gene expression markers was investigated. Rats were treated with B. longum for 16 weeks after OVX surgery. The B. longum supplemented group presented an increase in bone density, trabecular number, and thickness. Femoral strength was also enhanced by B. longum supplementation. When compared to the sham group, OVX decreased osteoblast but increased osteoclast surface over bone surface in the femur. These effects were prevented by the B. longum treatment, in addition to decreasing levels of serum C-terminal telopeptide, suggesting that similar to the mouse OVX model, probiotics modulate osteoclast formation and activity in the rat OVX model (80).
Secondary Osteoporosis
While the majority of studies have so far investigated the beneficial effects of probiotic supplementation on primary osteoporosis, few studies have investigated the potential effect in conditions of secondary osteoporosis. Specifically, in the context of type 1 diabetes-induced bone loss our lab has revealed some potentially exciting results with the use of probiotic treatment (38).
Type 1 Diabetes (T1D) is a metabolic disease caused by deficiency in insulin secretion. Hyperglycemia as well as other metabolic impairment has devastating consequences to several end organs including the skeleton. In contrast to primary osteoporosis, T1D–induced osteoporosis is characterized by a dysregulation of osteoblast number and activity as well as increased bone marrow adiposity; however, osteoclast activity seems to be mostly unaffected (81). Similar to the effects of L. reuteri in the mouse OVX model, administration of L. reuteri was effective in preventing streptozotocin (STZ)-induced T1D–mediated bone loss in male C57BL/6 (14 weeks old) mice. After 4 weeks (post-STZ injection) diabetic mice displayed a 35% reduction in bone volume fraction, an effect that was inhibited by L. reuteri treatment. This was further supported by the trabecular bone parameter data which revealed that L. reuteri treatment prevented the reduction in trabecular number and the increase in trabecular spacing induced by T1D. Evidence that T1D-bone loss was due to reduced osteoblast activity was revealed by decreased serum markers of bone formation, such as osteocalcin, and also by a reduced mineral apposition rate. L. reuteri 6475 treatment enhanced mouse serum osteocalcin levels as well as mineral apposition rate suggesting that in this model, unlike the OVX model, L. reuteri has an anabolic bone forming effect (38).
Probiotics and Bone Health in Livestock
Treatment of low bone density in humans is not the only potential use of probiotics. Skeletal abnormalities affecting quality and output in livestock cost the agricultural sector millions of dollars per annum (82). This is especially true in the poultry industry where the burden of having to produce large, fast growing, and affordable broilers in large scale rearing facilities has resulted in the development of bone pathologies (83). While traditionally these impediments were treated with growth factors, antibiotics and veterinary medicines, public opinion and government regulations have changed meaning alternatives, such as probiotics, are required.
Treatment of chickens with probiotic supplementation in the feed has been shown to provide numerous benefits including; improved weight gain, reduced mortality, increased egg size, decreased incidence of salmonella infection and improved bone health (65,84–86) (63–65). In one study supplementation of the diet with Bacillus licheniformis and Bacillus subtilis significantly increased the thicknesses of the tibia lateral and medial walls. The probiotic-supplemented diet also slightly improved tibia yield stress and modulus of elasticity. However, the percentage of calcium on the bone was not affected by probiotic consumption, suggesting that the increase in bone density was independent of bone calcium content (63). This data supported an earlier study that observed an increase in bone strength and lower incidence of tibial dyschondroplasia in chickens receiving brewer’s yeast (64). These studies indicate that in addition to the potential treatment of human bone pathologies, probiotics can additionally be utilized for the improvement of livestock.
Probiotics Mechanism of Action
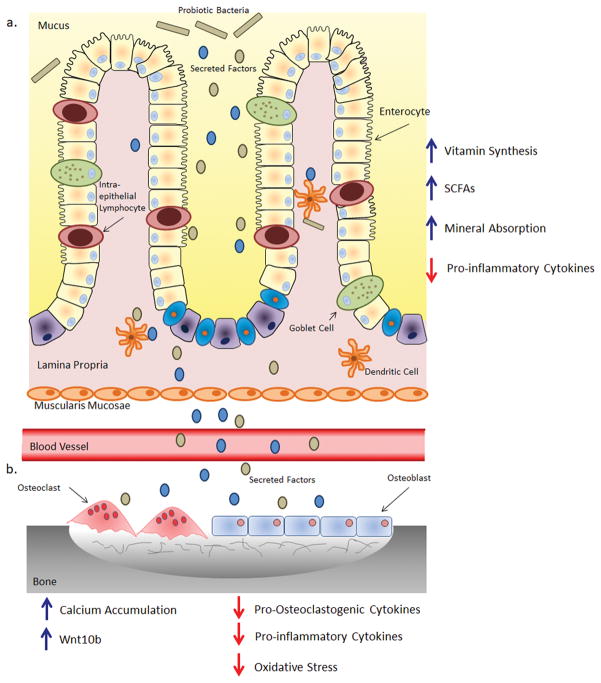
The mechanisms through which probiotic bacteria exert a beneficial effect on bone density are still being investigated. However, both in vitro and in vivo studies have highlighted a complex and multifaceted process by which probiotic bacteria can exert an influence on the host (Fig 5 a and b).
Fig 5.
Potential Mechanism by which Probiotic Bacteria Benefit Bone
a) Probiotic bacteria or their secreted factors interact with the intestinal epithelial barrier and cells in the lamina propria. Within the lamina propria the probiotic bacteria/secreted factors interact with antigen presenting cells, such dendritic cells, modulating their immune response. This results in a reduction of inflammatory cytokines leading to an uptake in minerals from the intestinal lumen. b) The bacterial secreted factors then pass into the blood stream and are transported to the bone. Here they can interact with osteoclasts and osteoblasts as well as immune cells. This could then reduce expression of pro-inflammatory and pro-osteoclastogenic cytokines and oxidative stress while enhancing mineral apposition and Wnt10b expression. This modulation results in reduced osteoclast formation subsequently leading to increased levels of bone.
In Vitro Studies
In vitro studies using probiotics or probiotic-fermented products have been performed to determine whether probiotic secretory products can directly affect the bone cells. These studies have revealed that osteoclast differentiation from monocytic-macrophages was significantly inhibited when cultured with L. reuteri conditioned media. This suggests that the probiotic releases an anti-osteoclastogenic factor that is able to modulate osteoclastogenesis (67). Similar to its effects on osteoclast differentiation, a secreted component of L. reuteri was sufficient to reverse TNFα-induced suppression of Wnt10b expression in the MC3T3 pre-osteoblast cell line (38). The ability of L. reuteri to secrete a modulatory factor is supported by an earlier study which demonstrated that L. reuteri secretes histamine which is capable of suppressing TNFα production from human monocytoid cells (87). Further evidence for probiotics having a direct effect on bone cells has been observed with L. helveticus and L. casei. In MC3T3-E1 cultures L. casei – fermented milk increased proliferation in a dose-dependent manner (75). Also, addition of L. helveticus-fermented milk products to primary bone marrow cultures increased calcium accumulation in osteoblast cultures suggesting that it has the potential to increase osteoblast differentiation. Remarkably, L. helveticus-fermented milk products had no effect on osteoclast differentiation (88); suggesting that different species/strains of bacteria may have cell-specific effects.
In Vivo Studies
The mechanism by which probiotic bacteria exert their effect on bone in vivo is not very well known and most likely complex; with multiple bacterial components affecting different pathways within the host. Bacteria have been shown to synthesize numerous vitamins and enzymes that are required for matrix formation and bone growth including: vitamin D, K, C and folate (89,90). Furthermore, bacteria of the genus Bifidobacteria, produce short chain fatty acids (SCFAs) that can reduce the intestinal tract pH subsequently increasing the absorption of minerals (91).
Studies performed with L. reuteri 6475 have highlighted that this specific probiotic is capable of systemically suppressing gene expression of pro-inflammatory and pro-osteoclastogenic cytokines, in both the intestine and the bone marrow (66–68). This anti-inflammatory effect has also been observed with other species of Lactobacilli. Both LGG and VSL#3 were shown to reduce expression of TNFα, IL-17 and RANKL in cells isolated form the small intestine and bone marrow of OVX mice (54). By reducing intestinal inflammation the probiotic bacteria may directly enhance the transport of calcium across the intestinal barrier.
It is likely that different probiotic bacterial strains acts via distinct and/or overlapping mechanisms. For example, while L. helveticus has been suggested to enhance bone density by increasing calcium uptake, studies have also shown that it is also able to produce the bioactive peptides isoleucyl-prolyl-proline (IPP) and valyl-prolyl-proline (VPP). These peptides are capable of inhibiting angiotensin converting enzyme (ACE), preventing the formation of Angiotensin II (Ang II), a stimulator of OC resorption, from Angiotensin I (Ang I) (76,88).
In contrast, Bifidobacterium longum has been shown to reduce periodontal oxidative stress by decreasing NF-κB gene expression (70). Estrogen-deficiency is associate with an increase in oxidative stress which can potentially inhibit osteoblast differentiation while enhancing osteoclast differentiation (18,92,93). This suggests that Bifidobacterium longum can potentially stimulate osteoblastogenesis while inhibiting osteoclastogenesis.
Conclusions
Osteoporosis is a devastating complication of the skeleton that has profound influence on the quality of life. It is critical that we continue to develop new, safe and effective strategies to prevent or treat osteoporosis associated with different conditions and variables (age, biological sex, disease, genetic background). Effect of probiotics in animal models suggests that oral probiotic supplementation could be a safe and effective alternative for preventing bone loss in various conditions in humans including menopause and T1D as well as enhance bone density under healthy or modestly inflamed conditions.
References
- 1.Rizzo DC. Fundamentals of anatomy and physiology. Cengage Learning. 2015 [Google Scholar]
- 2.Turner RT, Kalra SP, Wong CP, Philbrick KA, Lindenmaier LB, Boghossian S, et al. Peripheral leptin regulates bone formation. J Bone Miner Res. 2013;28(1):22–34. doi: 10.1002/jbmr.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong IPL, Driessler F, Khor EC, Shi YC, Hörmer B, Nguyen AD, et al. Peptide YY regulates bone remodeling in mice: A link between gut and skeletal biology. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009 Dec;5(12):667–76. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 5.Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res. 2001;16(9):1575–82. doi: 10.1359/jbmr.2001.16.9.1575. [DOI] [PubMed] [Google Scholar]
- 6.Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009 Jan;25:629–48. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- 7.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007 Feb;170(2):427–35. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008 May;42(4):606–15. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen TL, Sondergaard TE, Skorzynska KE, Dagnaes-Hansen F, Plesner TL, Hauge EM, et al. A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol. 2009 Jan;174(1):239–47. doi: 10.2353/ajpath.2009.080627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing L, Schwarz EM, Boyce BF. Osteoclast precursors, RANKL/RANK, and immunology. Immunol Rev. 2005 Dec;208:19–29. doi: 10.1111/j.0105-2896.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 11.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 2013 Apr 2;284(1999):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 12.Takayanagi H. New immune connections in osteoclast formation. Ann N Y Acad Sci. 2010 Mar;1192:117–23. doi: 10.1111/j.1749-6632.2009.05303.x. [DOI] [PubMed] [Google Scholar]
- 13.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. 2012 Jan;13(1):27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008 May 15;473(2):201–9. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Various Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993 Jun;94(6):646–50. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 16.Foundation IO. International Osteoporosis Foundation - Facts and Statistics [Internet] International Osteoporosis Foundation; Available from: https://www.iofbonehealth.org/facts-statistics. [Google Scholar]
- 17.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007 Mar;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 18.Manolagas SC. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31(3):266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002 Feb;23(1):90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 20.Bismar H, Diel I, Ziegler R, Pfeilschifter J. Increased cytokine secretion by human bone marrow cells after menopause or discontinuation of estrogen replacement. J Clin Endocrinol Metab. 1995 Nov;80(11):3351–5. doi: 10.1210/jcem.80.11.7593450. [DOI] [PubMed] [Google Scholar]
- 21.D’Amelio P, Grimaldi A, Di Bella S, Brianza SZM, Cristofaro MA, Tamone C, et al. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008 Jul;43(1):92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Weitzmann M, Roggia C. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin …. 2002;110(11) doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003 Apr;111(8):1221–30. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000 Dec;106(10):1229–37. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Tawfeek H, Bedi B, Yang X, Adams J, Gao KY, et al. Ovariectomy disregulates osteoblast and osteoclast formation through the T-cell receptor CD40 ligand. Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):768–73. doi: 10.1073/pnas.1013492108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim BJ, Bae SJ, Lee SY, Lee YS, Baek JE, Park SY, et al. TNF-alpha mediates the stimulation of sclerostin expression in an estrogen-deficient condition. Biochem Biophys Res Commun. 2012;424(1):170–5. doi: 10.1016/j.bbrc.2012.06.100. [DOI] [PubMed] [Google Scholar]
- 27.Foo C, Frey S, Yang HH, Zellweger R, Filgueira L. Downregulation of beta-catenin and transdifferentiation of human osteoblasts to adipocytes under estrogen deficiency. Gynecol Endocrinol. 2007;23(9):535–40. doi: 10.1080/09513590701556483. [DOI] [PubMed] [Google Scholar]
- 28.Bennett CN, Longo Ka, Wright WS, Suva LJ, Lane TF, Hankenson KD, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005 Mar 1;102(9):3324–9. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Moreno C, Catalán MP, Ortiz A, Alvarez L, De La Piedra C. Modulation of survival in osteoblasts from postmenopausal women. Bone. 2004;35(1):170–7. doi: 10.1016/j.bone.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Kovacic N, Grcevic D, Katavic V, Lukic IK, Grubisic V, Mihovilovic K, et al. Fas receptor is required for estrogen deficiency-induced bone loss in mice. Lab Invest. 2010;90(3):402–13. doi: 10.1038/labinvest.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzpatrick LA. Secondary Causes of Osteoporosis. Mayo Clin Proc. 2002;77(5):453–68. doi: 10.4065/77.5.453. [DOI] [PubMed] [Google Scholar]
- 32.Painter SE, Kleerekoper M, Camacho PM. Secondary osteoporosis: a review of the recent evidence. Endocr Pract. 2006;12(4):436–45. doi: 10.4158/EP.12.4.436. [DOI] [PubMed] [Google Scholar]
- 33.Ghishan FK, Kiela PR. Advances in the understanding of mineral and bone metabolism in inflammatory bowel diseases. Am J Physiol Gastrointest Liver Physiol. 2011;300(97):G191–201. doi: 10.1152/ajpgi.00496.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levin ME, Boisseau VC, Avioli LV. Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N Engl J Med. 1976 Jan 29;294(5):241–5. doi: 10.1056/NEJM197601292940502. [DOI] [PubMed] [Google Scholar]
- 35.Coe LM, Zhang J, McCabe LR. Both spontaneous Ins2+/− and streptozotocin-induced type I diabetes cause bone loss in young mice. J Cell Physiol. 2013;228(4):689–95. doi: 10.1002/jcp.24177. [DOI] [PubMed] [Google Scholar]
- 36.Coe LM, Irwin R, Lippner D, McCabe LR. The bone marrow microenvironment contributes to type I diabetes induced osteoblast death. J Cell Physiol. 2011 Feb;226(2):477–83. doi: 10.1002/jcp.22357. [DOI] [PubMed] [Google Scholar]
- 37.Motyl KJ, Botolin S, Irwin R, Appledorn DM, Kadakia T, Amalfitano A, et al. Bone inflammation and altered gene expression with type I diabetes early onset. J Cell Physiol. 2009 Mar;218(3):575–83. doi: 10.1002/jcp.21626. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Motyl KJ, Irwin R, MacDougald Oa, Britton Ra, McCabe LR. Loss of bone and Wnt10b expression in male type 1 diabetic mice is blocked by the probiotic L. reuteri. Endocrinology. 2015;(July):EN20151308. doi: 10.1210/EN.2015-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eastell R. Osteoporosis. Medicine (Baltimore) 2005 Dec;33(12):61–5. [Google Scholar]
- 41.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008 Jun;29(4):441–64. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papapoulos SE. Bisphosphonates: how do they work? Best Pract Res Clin Endocrinol Metab. 2008 Oct;22(5):831–47. doi: 10.1016/j.beem.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Reid IR. Efficacy, effectiveness and side effects of medications used to prevent fractures. J Intern Med. 2015;277(6):690–706. doi: 10.1111/joim.12339. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook P, Cooper C. Osteoporosis. Lancet (London, England) 2006;367(9527):2010–8. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 45.Jones RM, Mulle JG, Pacifici R. Osteomicrobiology: The influence of gut microbiota on bone in health and disease. Bone. 2017 doi: 10.1016/j.bone.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Ohlsson C, Sjögren K. Effects of the gut microbiota on bone mass. Trends Endocrinol Metab. 2015 Feb;26(2):69–74. doi: 10.1016/j.tem.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 47.McCabe L, Britton RA, Parameswaran N. Prebiotic and Probiotic Regulation of Bone Health: Role of the Intestine and its Microbiome. Curr Osteoporos Rep. 2015 Dec;13(6):363–71. doi: 10.1007/s11914-015-0292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loh G, Blaut M. Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes. 2012 Jan;3(6):544–55. doi: 10.4161/gmic.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukuda S, Ohno H. Gut microbiome and metabolic diseases. Semin Immunopathol. 2014 Jan;36(1):103–14. doi: 10.1007/s00281-013-0399-z. [DOI] [PubMed] [Google Scholar]
- 50.Ley R, Turnbaugh P, Klein S, Gordon J. Human Gut microbes associated with obesity. Nature. 2006;(444):1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 51.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007 May;132(6):2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 52.Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schütz G, et al. Lrp5 Controls Bone Formation by Inhibiting Serotonin Synthesis in the Duodenum. Cell. 2008;135(5):825–37. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012 Jun;27(6):1357–67. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J-Y, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016 Jun 1;126(6):2049–63. doi: 10.1172/JCI86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci. 2016;113(47):E7554–63. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ARAYA M, MORELLI L, REID G, SANDERS ME, STANTON C. Guidelines for the Evaluation of Probiotics in Food. London, Ontario, Canada: 2002. [Google Scholar]
- 57.Czerucka D, Piche T, Rampal P. Review article: Yeast as probiotics - Saccharomyces boulardii. Aliment Pharmacol Ther. 2007;26(6):767–78. doi: 10.1111/j.1365-2036.2007.03442.x. [DOI] [PubMed] [Google Scholar]
- 58.FAO and WHO. Probiotics in food. Food and Nutrition Paper. 2006;85 [Google Scholar]
- 59.Broekaert IJ, Nanthakumar NN, Walker WA. Secreted probiotic factors ameliorate enteropathogenic infection in zinc-deficient human Caco-2 and T84 cell lines. Pediatr Res. 2007;62(2):139–44. doi: 10.1203/PDR.0b013e31809fd85e. [DOI] [PubMed] [Google Scholar]
- 60.Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, et al. Lipoteichoic Acids from Lactobacillus Strains Elicit Strong Tumor Necrosis Factor Alpha-Inducing Activities in Macrophages through Toll-Like Receptor 2. Clin Diagn Lab Immunol. 2003;10(2):259–66. doi: 10.1128/CDLI.10.2.259-266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishimura J. Exopolysaccharides Produced from Lactobacillus delbrueckii subsp. bulgaricus. Adv Microbiol. 2014;4(14):1017–23. [Google Scholar]
- 62.Sougioultzis S, Simeonidis S, Bhaskar KR, Chen X, Anton PM, Keates S, et al. Saccharomyces boulardii produces a soluble anti-inflammatory factor that inhibits NF-κB-mediated IL-8 gene expression. Biochem Biophys Res Commun. 2006;343(1):69–76. doi: 10.1016/j.bbrc.2006.02.080. [DOI] [PubMed] [Google Scholar]
- 63.Mutuş R, Kocabagli N, Alp M, Acar N, Eren M, Gezen SS. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult Sci. 2006 Sep;85(9):1621–5. doi: 10.1093/ps/85.9.1621. [DOI] [PubMed] [Google Scholar]
- 64.Plavnik I, Scott ML. Effects of additional vitamins, minerals, or brewer’s yeast upon leg weaknesses in broiler chickens. Poult Sci. 1980 Feb;59(2):459–67. doi: 10.3382/ps.0590459. [DOI] [PubMed] [Google Scholar]
- 65.Nahashon SN, Nakaue HS, Mirosh LW. Production variables and nutrient retention in single comb White Leghorn laying pullets fed diets supplemented with direct-fed microbials. Poult Sci. 1994 Nov;73(11):1699–711. doi: 10.3382/ps.0731699. [DOI] [PubMed] [Google Scholar]
- 66.McCabe LR, Irwin R, Schaefer L, Britton Ra. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 2013 Aug;228(8):1793–8. doi: 10.1002/jcp.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Britton Ra, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, et al. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J Cell Physiol. 2014 Nov;229(11):1822–30. doi: 10.1002/jcp.24636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Collins FL, Irwin R, Bierhalter H, Schepper J, Britton RA, Parameswaran N, et al. Lactobacillus reuteri 6475 Increases Bone Density in Intact Females Only under an Inflammatory Setting. PLoS One. 2016;11(4):e0153180. doi: 10.1371/journal.pone.0153180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghanem KZ, Badawy IH, ABDEL-SALAM AM. Influence of yoghurt and probiotic yoghurt on the absorption of calcium, magnesium, iron and bone mineralization in rats. Milchwissenschaft. 2004;59(9–10):472–475. [Google Scholar]
- 70.Tomofuji T, Ekuni D, Azuma T, Irie K, Endo Y, Yamamoto T, et al. Supplementation of broccoli or Bifidobacterium longum-fermented broccoli suppresses serum lipid peroxidation and osteoclast differentiation on alveolar bone surface in rats fed a high-cholesterol diet. Nutr Res. 2012;32(4):301–7. doi: 10.1016/j.nutres.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 71.Rodrigues FC, Castro ASB, Rodrigues VC, Fernandes SA, Fontes EAF, de Oliveira TT, et al. Yacon flour and Bifidobacterium longum modulate bone health in rats. J Med Food. 2012 Jul;15(7):664–70. doi: 10.1089/jmf.2011.0296. [DOI] [PubMed] [Google Scholar]
- 72.Kruger MC, Fear A, Chua W-H, Plimmer GG, Schollum LM. The effect of Lactobacillus rhamnosus HN001 on mineral absorption and bone health in growing male and ovariectomised female rats. Dairy Sci Technol. 2009;89:219–31. [Google Scholar]
- 73.Chiang SS, Pan TM. Antiosteoporotic effects of lactobacillus-fermented soy skim milk on bone mineral density and the microstructure of femoral bone in ovariectomized mice. J Agric Food Chem. 2011;59:7734–42. doi: 10.1021/jf2013716. [DOI] [PubMed] [Google Scholar]
- 74.Ohlsson C, Engdahl C, Fåk F, Andersson A, Windahl SH, Farman HH, et al. Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One. 2014 Jan;9(3):e92368. doi: 10.1371/journal.pone.0092368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim JG, Lee E, Kim SH, Whang KY, Oh S, Imm JY. Effects of a Lactobacillus casei 393 fermented milk product on bone metabolism in ovariectomised rats. Int Dairy J. 2009;19(11):690–5. [Google Scholar]
- 76.Narva M, Collin M, Lamberg-Allardt C, Kärkkäinen M, Poussa T, Vapaatalo H, et al. Effects of long-term intervention with lactobacillus helveticus-fermented milk on bone mineral density and bone mineral content in growing rats. Ann Nutr Metab. 2004;48:228–34. doi: 10.1159/000080455. [DOI] [PubMed] [Google Scholar]
- 77.Amdekar S, Kumar A, Sharma P, Singh R, Singh V. Lactobacillus protected bone damage and maintained the antioxidant status of liver and kidney homogenates in female wistar rats. Mol Cell Biochem. 2012;368:155–65. doi: 10.1007/s11010-012-1354-3. [DOI] [PubMed] [Google Scholar]
- 78.Rovenský J, Švík K, Mat́ha V, Ištok R, Ebringer L, Ferenčík M, et al. The Effects of Enterococcus faecium and Selenium on Methotrexate Treatment in Rat Adjuvant-induced Arthritis. Clin Dev Immunol. 2004;11(December):267–73. doi: 10.1080/17402520400001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caballero-Franco C, Keller K, Simone C, Chadee K. The VSL # 3 probiotic formula induces mucin gene expression and secretion in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:315–22. doi: 10.1152/ajpgi.00265.2006. [DOI] [PubMed] [Google Scholar]
- 80.Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei ANM, Abdul-Majeed S, et al. Probiotics ( Bifidobacterium longum ) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. Biomed Res Int. 2015;2015:1–10. doi: 10.1155/2015/897639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCabe L, Zhang J, Raehtz S. Understanding the skeletal pathology of type 1 and 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. 2011;21(2):187–206. doi: 10.1615/critreveukargeneexpr.v21.i2.70. [DOI] [PubMed] [Google Scholar]
- 82.Payne JM. Metabolic diseases in farm animals. London: Butterworth-Heinemann; 1977. [Google Scholar]
- 83.Sullivan TW. Skeletal problems in poultry: estimated annual cost and descriptions. Poult Sci. 1994 Jun;73(6):879–82. doi: 10.3382/ps.0730879. [DOI] [PubMed] [Google Scholar]
- 84.Jin LZ, HYW, Abdullah N, Jalaludin S. Probiotics in poultry: modes of action. 1997;53(December) [Google Scholar]
- 85.Nava GM, Bielke LR, Callaway TR, Castañeda MP. Probiotic alternatives to reduce gastrointestinal infections: the poultry experience. Anim Health Res Rev. 2005 Jun 28;6(1):105–18. doi: 10.1079/ahr2005103. [DOI] [PubMed] [Google Scholar]
- 86.Khan RU, Naz S. The applications of probiotics in poultry production. Worlds Poult Sci J. 2013 Sep 5;69(3):621–32. [Google Scholar]
- 87.Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One. 2012 Jan;7(2):e31951. doi: 10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Narva M, Halleen J, Väänänen K, Korpela R. Effects of Lactobacillus helveticus fermented milk on bone cells in vitro. Life Sci. 2004;75:1727–34. doi: 10.1016/j.lfs.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 89.Crittenden RG, Martinez NR, Playne MJ. Synthesis and utilisation of folate by yoghurt starter cultures and probiotic bacteria. Int J Food Microbiol. 2003;80:217–22. doi: 10.1016/s0168-1605(02)00170-8. [DOI] [PubMed] [Google Scholar]
- 90.Arunachalam KD. Role of bifidobacteria in nutrition, medicine and technology. Nutr Res. 1999;19(10):1559–97. [Google Scholar]
- 91.Campbell JM, Fahey GC, Wolf BW. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr. 1997;127(September 1996):130–6. doi: 10.1093/jn/127.1.130. [DOI] [PubMed] [Google Scholar]
- 92.Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, et al. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-??B. Biochem Biophys Res Commun. 2004;314:197–207. doi: 10.1016/j.bbrc.2003.12.073. [DOI] [PubMed] [Google Scholar]
- 93.Suda N, Morita I, Kuroda T, Murota S. Participation of oxidative stress in the process of osteoclast differentiation. Biochim Biophys Acta. 1993;1157:318–23. doi: 10.1016/0304-4165(93)90116-p. [DOI] [PubMed] [Google Scholar]
- 94.Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3(August 2013):481. doi: 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kristensen HB, Andersen TL, Marcussen N, Rolighed L, Delaisse JM. Increased presence of capillaries next to remodeling sites in adult human cancellous bone. J Bone Miner Res. 2013;28(3):574–85. doi: 10.1002/jbmr.1760. [DOI] [PubMed] [Google Scholar]
- 96.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–4. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 97.Fuller K, Wong B, Fox S, Choi Y, Chambers TJ. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med. 1998 Sep 7;188(5):997–1001. doi: 10.1084/jem.188.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–4. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 99.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000 Oct 1;289(5484):1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 100.Everts V, Delaissé JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, et al. The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J Bone Miner Res. 2002 Jan;17(1):77–90. doi: 10.1359/jbmr.2002.17.1.77. [DOI] [PubMed] [Google Scholar]
- 101.Edwards CM, Mundy GR. Eph receptors and ephrin signaling pathways: a role in bone homeostasis. Int J Med Sci. 2008 Jan;5(5):263–72. doi: 10.7150/ijms.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009 Jul;15(7):757–65. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003 Jun;5(3):222–6. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 104.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tanaka S, Takahashi N, Udagawa N, Tamura T, Akatsu T, Stanley ER, et al. Macrophage colony-stimulating factor is indispensable for both proliferation and differentiation of osteoclast progenitors. J Clin Invest. 1993 Jan;91(1):257–63. doi: 10.1172/JCI116179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998 Apr 17;93(2):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 107.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999 Jan 28;397(6717):315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 108.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997 Apr 18;89(2):309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 109.Dai X-M, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002 Jan 1;99(1):111–20. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 110.Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3540–5. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999 Sep 15;13(18):2412–24. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007 Feb;40(2):251–64. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 113.Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y, et al. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology. 2000;141(9):3478–84. doi: 10.1210/endo.141.9.7634. [DOI] [PubMed] [Google Scholar]
- 114.Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109(9):3839–48. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997 May 30;89(5):747–54. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 116.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002 Jan 11;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 117.Boskey AL. Bone composition: relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep. 2013;2(July):447. doi: 10.1038/bonekey.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004 Apr 30;117(3):387–98. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 119.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008 Dec;3(Suppl 3):S131–9. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robey P, Boskey A. Extracellular Matrix and Biomineralisation of Bone. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 2006:12–9. [Google Scholar]
- 121.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673–82. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000 Nov;141(11):3956–64. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 123.Centrella M, McCarthy TL, Canalis E. Tumor necrosis factor-alpha inhibits collagen synthesis and alkaline phosphatase activity independently of its effect on deoxyribonucleic acid synthesis in osteoblast-enriched bone cell cultures. Endocrinology. 1988;123(3):1442–8. doi: 10.1210/endo-123-3-1442. [DOI] [PubMed] [Google Scholar]
- 124.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000 Nov 30;408(6812):600–5. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]