Abstract
Objectives
Dysfunctions in stress biology are hypothesized to contribute to anxiety disorders, and to be ameliorated during successful treatment, but limited clinical data exist to support this hypothesis. We evaluated whether increases in morning cortisol and the diurnal cortisol slope, markers of stress biology, are associated with clinical response to chamomile therapy among subjects with generalized anxiety disorder (GAD).
Methods
Among 45 subjects with DSM-IV diagnosed GAD in an open-label clinical trial of chamomile, salivary cortisol was assessed for three days each pre- and post-treatment, at 8am, 12pm, 4pm, and 8pm. Mixed model analyses assessed whether GAD symptom change predicted the degree to which cortisol levels changed during treatment.
Results
Symptom improvement during treatment was significantly associated with pre-to-post treatment changes in cortisol. Subjects who experienced more symptomatic improvement experienced significant increases in their morning salivary cortisol (β = 0.48, p < 0.001), and a greater decrease in cortisol from morning to the rest of the day (β = 0.55, p <.001). In addition, at baseline a lower cortisol level (β = −0.24, p = 0.023) and a lesser decrease in cortisol after morning (β = 0.30, p = 0.003) were associated with greater symptomatic improvement.
Conclusion
Increases in morning salivary cortisol and the diurnal cortisol slope are associated with symptom improvement in chamomile treatment of GAD. Response to treatment for GAD could partially stem from normalization of stress biology dysfunction, but further work involving establishing abnormalities within-sample, ruling out of confounds (e.g., sleep), and a placebo control is necessary to conclude an amelioration effect.
Keywords: GAD, cortisol, stress, psychopharmacology, chamomile, clinical trial
While many effective psychopharmacological (Baldwin et al., 2011) and psychosocial treatments (Cuijpers et al., 2014) for Generalized Anxiety Disorder (GAD) exist, the neurobiological mechanisms of these therapies remain poorly defined. Amelioration of dysregulated stress biology has been proposed as a candidate mechanism for the effects of these treatments (Bandelow et al., 2016; Elnazer and Baldwin, 2014). Acute and chronic release of cortisol is a widely-studied component of hypothalamic-pituitary-adrenal axis (HPA) activity. For healthy individuals without psychiatric co-morbidities, circulating cortisol is at its peak at the time surrounding awakening, with an increase in the 30–45 minutes after awakening, and a sharp decline throughout the day. This pattern is known as the cortisol awakening response (CAR), of which awakening cortisol is a component (Steptoe, 2007). Lower awakening cortisol has been characterized as a prognostic marker for the development of a new anxiety disorder (Nederhof et al., 2015) and the persistence of existing anxiety disorders (Vreeburg et al., 2013).
Studies often find adults with GAD have a lower awakening salivary and plasma cortisol levels and a less steep diurnal cortisol slope relative to psychiatrically healthy controls (Bandelow et al., 2016; Elnazer and Baldwin, 2014; Hoehn-Saric et al., In press; Vreeburg et al., 2010). Null non-replications or even opposite results have been reported (Phillips et al., 2011; Steudte et al., 2011; Wang et al., 2017). Further research is clearly required to disambiguate a conflicting body of literature.
More consistently, individuals with GAD have been found to have relatively lower levels of hair cortisol, which may be indicative of hypocortisolism (Miller et al., 2007; Steudte et al., 2011; Wells et al., 2014). While long periods of acute stress in non-psychiatric populations may be associated with hypercortisolism or overactivation of the HPA axis (Chrousos and Gold, 1992), literatures on chronically stressed individuals such as those with PTSD and victims of childhood sexual abuse often find evidence for diminished cortisol release and/or sensitivity (Herane Vives et al., 2015; Morris et al., 2012; Steudte et al., 2013). In particular, chronic stress that consistently activates the HPA axis may eventually promote downregulation of cortisol release, the density of cortisol receptors, and even the activity of cortisol receptors (Fries et al., 2005).
In a recent report identifying cortisol abnormalities in GAD patients, peripheral blood mononuclear cells in patients with GAD (relative to healthy controls) were found to exhibit diminished sensitivity to cortisol (Wang et al., 2017). Furthermore, GAD patients in this study had elevated methylation of the NR3C1 promoter gene, which correlated with diminished transcription of the GRα glucocorticoid receptor (Wang et al., 2017). Both sets of findings (i.e., regarding hair cortisol and diminished glucocorticoid sensitivity) are consistent with the perspective that the cortisol system is desensitized in GAD, possibly through chronic overactivation of the HPA through the stress of GAD symptomatology.
Minimal research has examined how cortisol indicators of any kind track symptomatic improvements in GAD treatment for adolescents and adults (Elnazer and Baldwin, 2014). Among adolescents and adults, the few findings that exist come from cognitive-behavioral therapy (CBT), an evidence-based treatment for GAD (Cuijpers et al., 2014). Child and adolescent anxiety patients who were treatment responders to CBT for several anxiety disorders (including GAD) tended to exhibit a relative increase in morning cortisol during treatment compared to non-responders, in addition to a greater decline in cortisol throughout the day (Dierckx et al., 2012). In a small study comparing a group of 20 adults who received CBT for anxiety compared to 8 untreated but anxiety-symptomatic adults, greater decreases in afternoon cortisol were observed among treated as compared to untreated subjects (Tafet et al., 2005). If subjects with GAD suffer from a chronic overactivation of the HPA axis that leaves them with a dampened release of cortisol, treatment-related increases in awakening cortisol and a greater absolute decrease in cortisol throughout the day may reflect a biological “normalization” of cortisol dynamics.
To date, there are no clear published data on changes in cortisol associated with degree of symptom change during psychopharmacological GAD treatment in an adult sample. Furthermore, current studies have generally not controlled for covariation between baseline symptom levels and changes in cortisol, which means that obtained results could have been due to simultaneous regression to the mean of both symptom and cortisol levels.
To investigate whether changes in cortisol are associated with symptomatic improvement in the psychopharmacological treatment of GAD, we measured salivary cortisol multiple times throughout the day across three consecutive days before and after treatment in a clinical trial of chamomile extract for GAD (Mao et al., 2014). We hypothesized that, concordant with small-sample findings in CBT for adults and adolescents (Dierckx et al., 2012; Tafet et al., 2005), patient increases in morning cortisol and the diurnal cortisol slope following treatment would be associated with superior symptomatic change, controlling for covariation between baseline symptom severity and cortisol levels pre- and post-treatment. We also explored whether baseline cortisol levels predicted degree of treatment response.
METHODS
Subjects
Subjects were adults (>18 years) with a DSM-IV diagnosis of GAD as a primary disorder recruited from a psychiatric clinic at a major research hospital and from primary care practices. All diagnoses were determined using the MINI-SCID/P structured interview to assess for the presence of specific DSM-IV Axis I disorders (First et al., 2001). Discrepancies in diagnostic assessment for inclusion into the study were resolved by conferencing and consensus between the investigators of the trial. Subjects diagnosed with Axis I psychosis, bipolar disorder, or substance abuse or dependence were excluded from participation. In addition, while past history of major depressive disorder and current mood symptom elevation were allowable, patients could not be in a current, SCID-diagnosed major depressive episode.
Overall, 179 subjects began the trial. The last 49 subjects entering the trial were assessed for salivary cortisol at baseline and after 8 weeks of chamomile treatment.
Trial setting
The details of the trial design have been published previously (Mao et al., 2014), and the trial is registered at ClinicalTrials.gov (NCT01072344). The overall study is a randomized-placebo controlled trial (RCT) to evaluate whether long-term use of chamomile will result in decreased relapse of GAD symptoms as compared to placebo. In the open-label phase, chamomile was associated with a clinically significant reduction in core GAD symptomatology (Keefe et al., 2016), and the second, randomized phase found that a blinded switch to placebo predicted return of GAD symptoms relative to chamomile continuation (Mao et al., 2016). In addition, a prior RCT found a significant advantage for chamomile over placebo in acute-phase treatment of GAD (Amsterdam et al., 2009) with a response rate comparable to that of tested anxiolytic and antidepressant therapies for GAD. For this manuscript, we analyzed an exploratory subset of the data from phase I, when all participants were given an open-label administration of pharmaceutical-grade, standardized chamomile extract capsules totaling 1,500 mg/daily for 8 weeks (Mao et al., 2014).
Biological measures
Subjects were requested to collect saliva samples at home using Cortisol-Salivettes® (Sarstedt, Nümbrecht, Germany). All subjects were instructed by study personnel on how to properly sample saliva by a research staff member (approximately 0.5mL minimum), in addition to being given detailed written instructions. For an hour before collection, subjects were instructed to avoid any food or drink aside from tap water, any brushing of teeth, and any smoking. Subjects were also instructed to rinse their mouth 3–5 minutes prior to every collection for 30 seconds with very cold water. Finally, they were instructed to store as soon as possible all samples in the freezer until bringing them in for collection by research staff.
Salivary cortisol was collected at upon awakening at 8am, 12pm, 4pm, and 8pm for three concurrent days prior to the initiation of treatment (Baseline), and subsequently for concurrent three days prior to their final assessment for the open-label phase of treatment (Week 8). Multiple days were sampled to more reliably assess a patient’s cortisol profile across different situational factors (Hellhammer et al., 2007). A limitation of the sampling approach is that because cortisol samplings were performed by the patient during their day-to-day lives, the precise timing of the measurements cannot be guaranteed (e.g., that the subject indeed woke up at 8:00am as instructed and took the measurement quickly thereafter). However, cortisol patterns from ambulatory assessments tend to be similar to those obtained in laboratory studies, and timing problems with ambulatory assessment are likely to be more random (i.e., adding noise and reducing statistical power) rather than systemic (i.e., adding bias) (Kudielka et al., 2012). We did not sample cortisol 30–45 minutes after awakening, which would have permitted measurement of the full CAR response. Every subject had a maximum of 24 measurements, corresponding to four measurements per day across three-days each pre- and post-treatment.
Saliva samples were frozen and stored at −20°C until analysis. After thawing, salivettes were centrifuged at 3,000 rpm for 5 minutes, which resulted in a clear supernatant of low viscosity. Salivary concentrations were measured using commercially available chemiluminescence immunoassay with high sensitivity (functional sensitivity range = 0.008 μg/dL to 0.017 μg/dL) (IBL International, Hamburg, Germany) (Westermann et al., 2004). The reportable range of salivary cortisol was 0.005–4 μg/dL. The intra- and inter-assay coefficients for cortisol were below 8%.
The average subject had 94.3% of pre-treatment measurements completed (mean = 11.3), and 92.8% of post-treatment measurements completed (mean = 11.1). Three different cortisol indices were examined: (1) morning cortisol (i.e., 8am); (2) post-awakening cortisol (i.e., 12/4/8pm); (3) and the change in cortisol between these two measurement sets (i.e., the diurnal cortisol slope). For all analyses, cortisol levels (nmol/L) were log-10 transformed with a plus one constant to ameliorate non-normality, which substantially improved normality (Shapiro-Wilk W untransformed = 0.64; W transformed = 0.96).
Psychological measures
GAD-7
The GAD-7 was the primary outcome measure in the trial. The GAD-7 is a brief subject-report measure of GAD symptomatology and its functional burden, as per DSM-IV criteria for the disorder. It has been shown to have good internal consistency, criterion validity, and sensitivity to treatment (Löwe et al., 2008). Within this sample, the GAD-7 exhibited excellent internal consistency (alpha = 0.90). Subjects reported on their symptoms using the GAD-7 at Baseline, Week 2, Week 4, and Week 8 of treatment. Total GAD-7 symptom change was calculated as the difference between Week 8 and Baseline GAD-7 totals.
Mao Expectancy of Treatment Effects (METE)
The instrument is a 4-question subject-report questionnaire rated on a scale of 1–5 (wherein 1 is total disagreement with a statement and 5 is total agreement), which assesses a subject’s expectation that treatment will relieve his/her primary anxiety symptoms and increase his/her coping abilities and vitality (Keefe et al., 2017). Sample items include a subject’s relative agreement with the statements that with chamomile treatment “I will be able to cope with my anxiety better” and that “The symptoms of my anxiety will disappear.” The METE had good internal consistency in our sample (Cronbach’s alpha = 0.88), and has been found to predict symptom relief during treatment (Keefe et al., 2017). Subjects completed the METE at baseline.
Analyses
General analysis
All analyses were conducted in the R statistical computing language (R Development Core Team, 2016). Primary analyses were conducted in a mixed regression framework using the R packages “lmer” (Bates et al., 2016) and “lmerTest” (Kuznetsova et al., 2016). Fixed effect coefficients are presented as standardized betas. 95% bootstrapped percentile confidence intervals were constructed for mixed model fixed effect estimates using 1,000 replicates.
Modeling change in cortisol over time
A random subject-specific intercept was included to account for within-subject correlations in cortisol measurements. Two time indicators for cortisol were modeled as fixed effects nested within subjects. The first time indicator modeled the change in salivary cortisol from 8am (morning cortisol) to the rest of the day (i.e., Within-day time). As there was relatively little absolute change in cortisol between 12pm, 4pm, and 8pm and more regular measurements throughout a day would be necessary to construct a full cortisol curve, these last three time points were assigned the same within-day time indicator coding (i.e., 1). Measurements on different consecutive days were modeled as correlated samples of an underlying trait-like cortisol pattern. The second time indicator indicated whether a given measurement was taken pre- or post-treatment (i.e., Pre-to-post treatment). An interaction between the two time indicators indicated whether cortisol at each measurement point and the change in cortisol throughout the day was significantly different post-treatment as compared to pre-treatment. All collected awakening and post-awakening measurements were used in the model, under the assumption that any given unobserved measurement was missing at random (Rubin and Little, 2002).
Hypothesis testing framework
A single omnibus model was used to test all hypotheses. The primary subject-level predictor in the model was baseline-to-post treatment change in GAD-7 score (i.e., GAD-7 change). Baseline GAD-7 scores were entered as a covariate, including interactions with time. If baseline GAD-7 were not included in the model, any obtained relationships between changes in cortisol and symptomatic improvements could easily be due to regression to the mean simultaneously occurring for both variables. In addition, subject pretreatment expectancies for treatment improvement were entered as a clarifying covariate of the relationship between symptom change and cortisol change to control for this component of the placebo effect, as expectancies predict symptom change in this trial (Keefe et al., 2017).1 The predicted variable in the mixed model was a given salivary cortisol measurement.
Our primary subject-level predictor of interest (GAD-7 change) and the two subject-level covariates (baseline GAD-7, expectancies) were included as both main effects and as interactions with time. All three subject-level variables were allowed to interact in a two-way interaction with each time indicator (i.e., Within-day time or the Pre-to-post treatment), and together as a three-way interaction between each predictor and each time indicator (e.g., GAD-7 change × Within-day time × Pre-post treatment). As such, each predictor was allowed to predict both pre-treatment cortisol levels (i.e., cortisol in the morning measurements, and the within-day change in cortisol) and how those cortisol levels changed pre- to post-treatment.
Due the exploratory nature of these analyses, we employed the Benjamini-Yekutieli correction method using the R function “p.adjust” to robustly control for the false discovery rate with an alpha = 0.05 (Benjamini and Yekutieli, 2001), which are reported as adjusted p-values. Our primary tests concerned the covariance between GAD-7 symptom change and both baseline CORT (morning and slope) and change in CORT pre-to-post treatment, representing p-values to be adjusted.
RESULTS
Demographics and clinical information on subject subsample with CORT measurements
Forty-five subjects were trial completers with pre- and post-treatment measurements of salivary cortisol, and four subjects did not have usable post-treatment cortisol measurements. Of these subjects, two were lost to follow-up, one was withdrawn from the trial due to an unstable preexisting medical condition, and one was withdrawn due to medication non-compliance. The average subject in this subsample experienced a change of 9.4 (SD 5.5) points on the GAD-7, which was the outcome criterion used in the following analyses. 71.1% of subjects (n = 32) qualified as a clinical responder to treatment using the a priori criteria defined by the trial protocol (≥50% reduction from baseline GAD-7 score and final Clinical Global Impression score of ≤3) (Mao et al., 2014).
The average subject reported that they had experienced their current episode of GAD for at least 10 years, and had tried at least 1 other treatment for their GAD prior to entrance into the trial. 33.3% of patients had a past history of major depressive disorder, but did not have a current diagnosis. Other information on subject demographics and clinical characteristics can be seen in Table 1.
Table 1.
Baseline characteristics of subject subsample with CORT measurements (n = 45)
| Baseline Variables | No. (%) or Mean (SD) |
|---|---|
| Age, y | 45.60 (16.40) |
| Gender (% Female) | 29 (64%) |
| Race (% Caucasian) | 28 (62%) |
| Ethnicity (% Hispanic) | 3 (7%) |
| % Unemployed | 11 (24%) |
| % Married | 10 (22%) |
| % Prior MDD diagnosis | 15 (33.3%) |
| Baseline BDI | 20.9 (10.5) |
| Age at first GAD episode | 18.82 (13.75) |
| Duration of current GAD episode (years) | 10.77 (17.43) |
| Number of previous treatments for GAD | 1.11 (1.11) |
| Baseline GAD-7 | 15.82 (2.77) |
| Pre-Post GAD-7 Improvement | 9.40 (5.48) |
BDI = Beck Depression Inventory; GAD-7 = Generalized Anxiety Disorder 7-Item Scale; MDD = Major Depressive Disorder
Basic CORT dynamics and change
At baseline, the average subject in the trial was estimated as having a salivary cortisol level of 6.1 nmol/L at awakening (8am), and a diurnal cortisol slope of decreasing cortisol to 3.6 nmol/L at 12pm, 2.6 nmol/L at 4pm, and 1.1 nmol/L at 8pm.
Controlling for depression symptom severity using the Beck Depression Inventory, which covaried with GAD symptom severity (r = 0.31), patients with more severe GAD symptoms had significantly lower morning cortisol (β = −0.26 [−0.43 to −0.08], SE = 0.09, df = 130.3, t = −2.78, p = 0.006) and less steep cortisol slopes from morning to the rest of the day (β = 0.22 [0.07 to 0.40], SE = 0.08, df = 462.0, t = 2.71, p = 0.007). The average subject in the trial did not experience any changes in their morning cortisol (β = −0.17 [−0.39 to 0.04], SE = 0.11, df = 104.5, t = −1.60, p = 0.113) or the diurnal cortisol slope (β = 0.18 [−0.06 to 0.42], SE = 0.12, df = 882.9, t = 1.47, p = 0.142) from pre- to post-treatment.
Change in CORT and symptom improvement
Symptomatic improvement in GAD was significantly correlated with changes in cortisol levels during treatment. Subjects who experienced a relative increase in their awakening cortisol level during treatment experienced significantly more GAD symptom improvement (β = 0.48 [0.22 to 0.73], SE = 0.13, df = 955.9, t = 3.68, p < 0.001, adjusted p = 0.001). Furthermore, subjects whose cortisol levels decreased more during the day post-treatment as compared to pretreatment (i.e., had a greater diurnal cortisol slope) had significantly more symptom improvement (β = −0.55 [−0.85 to −0.26], SE = 0.15, df = 954.9, t = −3.74, p < 0.001, adjusted p = 0.001).2 However, the estimated correlation between morning cortisol level and diurnal cortisol slope was high (r = 0.70), indicating that these findings are overlapping in information. Notably, neither relationship differed as a function of having a history of major depressive disorder (interaction ps = 0.483 and 0.738 for morning cortisol and diurnal cortisol slope, respectively).
Baseline CORT correlations with symptom improvement
Baseline cortisol levels correlated with subsequent symptomatic outcomes. Subjects who had less of a decline in cortisol during the day at baseline tended to have greater symptomatic improvements (β = 0.30 [0.09 to 0.51], SE = 0.10, df = 955.9, t = 2.97, p = 0.003, adjusted p = 0.009). Furthermore, subjects with lower awakening cortisol at baseline responded better to treatment (β = −0.24 [−0.45 to −0.02], SE = 0.10, df = 291.0, t = −2.28, p = 0.023, adjusted p = 0.049). Further information on all predictors and covariates with time can be found in Table 2.
Table 2.
Mixed model coefficients for omnibus model
| Term | Standardized Beta (95% CI) |
|---|---|
| Morning CORT at pre-treatment | |
| Baseline GAD-7 | −0.06 [−0.26 to 0.12] |
| GAD-7 Change* | −0.24 [−0.43 to −0.04] |
| METE Patient Expectancies | 0.11 [−0.06 to 0.27] |
| Within-day CORT slope pre-treatment | |
| Baseline GAD-7 × Within-day CORT Slope | 0.00 [−0.18 to 0.21] |
| GAD-7 Change × Within-day CORT Slope** | 0.30 [0.10 to 0.51] |
| METE Patient Expectancies × Within-Day CORT Slope | −0.07 [−0.25 to 0.09] |
| Change in morning CORT pre-to-post treatment | |
| Baseline GAD-7 × Pre-post CORT Change** | −0.32 [−0.53 to −0.07] |
| GAD-7 Change × Pre-post CORT Change*** | 0.48 [0.21 to 0.71] |
| METE Patient Expectancies × Pre-post CORT Change** | −0.32 [−0.55 to −0.09] |
| Change in within-day CORT slope pre-to-post treatment | |
| Baseline GAD-7 × Within-day CORT Slope x Pre-post CORT Change† | 0.24 [−0.05 to 0.50] |
| GAD-7 Change × Within-day CORT Slope × Pre-post CORT Change*** | −0.56 [−0.84 to −0.26] |
| METE Patient Expectancies × Within-Day CORT Slope x Pre-post CORT Change** | 0.38 [0.13 to 0.64] |
= p <.10;
= p <.05;
= p < .01;
= p < .001
DISCUSSION
While many hypothesize that stress biology dysregulation is implicated in the etiology of GAD, few data exist to support a relationship between stress biology and clinical response to psychopharmacological drugs (Bandelow et al., 2016; Elnazer and Baldwin, 2014). In this exploratory investigation conducted in the context of a trial of chamomile for patients with GAD, we found that greater symptom improvement was associated with pre-to-post treatment increases in morning cortisol levels. In addition, patients with more symptom improvement tended to experience a relatively steeper diurnal cortisol slope after awakening post-treatment as compared to pre-treatment, although the diurnal cortisol slope was strongly linked to morning cortisol levels.
Our findings regarding cortisol and symptom change are consistent with small-sample reports of cortisol changes in CBT for GAD, which have found that increases in awakening cortisol or increases in the diurnal cortisol slope correlated with treatment success (Dierckx et al., 2012; Tafet et al., 2005). Contrary to our findings, in studies of geriatric-onset GAD subjects, reduced cortisol over the course of taking escitalopram (a selective serotonin reuptake inhibitor) or diazepam (a benzodiazepine) were associated with greater improvements in anxiety symptomatology (Lenze et al., 2011; Pomara et al., 2005). The discordant findings between adolescent/adult and geriatric GAD treatment studies are potentially because the neurobiology of GAD differs between geriatric-onset GAD versus early or adult onset-GAD (Mantella et al., 2008; Vreeburg et al., 2013). It is also possible that the biomechanisms by which chamomile and CBT work to treat GAD differ from those of SSRIs and benzodiazepines, which may imply divergent influences on stress biology.
Patients with more severe GAD symptoms had lower morning cortisol and a less steep diurnal cortisol slope prior to treatment. If lower awakening cortisol and a less steep diurnal cortisol slope are indeed biomarkers of GAD, increases in these cortisol indicators among particularly successful cases may reflect an amelioration of cortisol abnormalities related to the disorder. However, within-study comparisons with psychiatrically healthy subjects are necessary to firmly establish the existence of a cortisol abnormality that is “normalized,” especially given the inconsistency in the GAD cortisol literature (Bandelow et al., 2016; Elnazer and Baldwin, 2014; Wang et al., 2017).
It was also found that subjects who began the trial having a lower morning cortisol and a less steep diurnal cortisol slope had significantly greater benefit from treatment. This pattern of findings—wherein lower morning cortisol at baseline predicts treatment success, and those whose morning cortisol increases over treatment tend to do best—may suggest that chamomile could especially benefit subjects with these biomarkers. A placebo- or active-treatment controlled trial would be necessary to establish whether these cortisol patterns generally describe successful GAD therapies, or are more specific to particular active treatments.
Future investigations of chamomile might also measure other neural- and bio-markers that could elucidate the mechanism(s) of chamomile’s possible effects on stress biology, such as glucocorticoid sensitivity or receptor transcription (Wang et al., 2017). Chamomile extract and its flavonoid compounds (e.g., apigenin) exhibit anxiolytic effects in animal models (McKay and Blumberg, 2006), downregulating the HPA axis (Reis et al., 2006; Yamada et al., 1996). Apigenin specifically binds to GABA receptors, though not at benzodiazepine receptors (Avallone et al., 2000; Campbell et al., 2004; Losi et al., 2004). Apigenin may also act in part through inhibiting NMDA-glutamatergic neurotransmission and glutamatergic release, decreasing overall network excitation (Chang et al., 2015; Losi et al., 2004).
Limitations
The present exploratory study has several limitations, which restrict the interpretation and generalizability of our findings. Firstly, our sample consisted of only 45 subjects. While this is larger than many reviewed GAD cortisol studies (Elnazer and Baldwin, 2014; Tafet et al., 2005), it is nevertheless a relatively small sample. However, our use of multiple days and time-points of cortisol measurements per subject pre-and-post treatment allows for more reliable cortisol estimates, compared to designs using single measurements (Hellhammer et al., 2007). Secondly, due to the nature of the follow-up phase in the parent trial, entailing a smaller sample of only treatment responders who were randomized to chamomile continuation or placebo, we were unable to examine the long-term predictive clinical value or stability of cortisol changes. Lastly, although chamomile has evidenced controlled efficacy (Amsterdam et al., 2009; Mao et al., 2016), without a placebo control group in this phase of treatment we cannot conclude that changes are attributable to active effects of chamomile, even as we controlled for patient expectancies (a component of placebo effects) in our analysis.
Moreover, a placebo group would protect against the possibility that the observed findings represent a correlated regression to the mean of GAD-7 scores and cortisol levels. On the other hand, we included baseline levels of GAD-7 scores in the model and allowed them to predict change in cortisol, which partially protects against the possibility that our obtained findings are epiphenomenal. Indeed, higher baseline GAD-7 scores predicted changes in cortisol that were opposite those predicted by GAD-7 symptom improvement, which is the opposite direction that would be expected by a regression to the mean of GAD-7 scores (see Table 2).
Furthermore, due to the pre-post design we cannot conclude whether GAD symptom improvement leads to changes in cortisol, whether cortisol changes precede GAD symptom improvement, or whether they occur relatively simultaneously due to a shared causal pathway. For example, treatment-related improvements in sleep quality and regularity (e.g., from reduced stress, somnolent effects of chamomile) could promote shifts in awakening and daily cortisol secretion, which would make cortisol changes biomarkers of treatment success rather than a mechanism (Meerlo et al., 2008). More regular measurement of cortisol levels throughout treatment would allow for a mediational test of the relationship between cortisol and symptom change, as would simultaneous examination of other factors changing alongside cortisol. Additional cortisol measurements during the day would have also allowed greater precision and description of cortisol dynamics associated with GAD (e.g., measuring the full CAR).
Future directions
Our findings provide preliminary evidence consistent with the framework that changes in stress biology correlate with therapeutic response to chamomile for GAD. These results inform a need for a comprehensive study of stress biology changes in the treatment of GAD, including more frequent cortisol measurements during treatment, use of placebo- and active treatment comparators, and an examination of what baseline cortisol abnormalities may exist through use of a healthy control sample within the same study. Future work should investigate whether change in cortisol may be a common or unique biomarker or mechanism for treatment-related GAD improvements. Furthermore, given that psychopharmacological and psychological treatments for GAD are on average equally efficacious, it would be intriguing to examine whether particular cortisol profiles at baseline may help predict whether specific subjects will especially benefit from a particular treatment for GAD.
Figure 1.
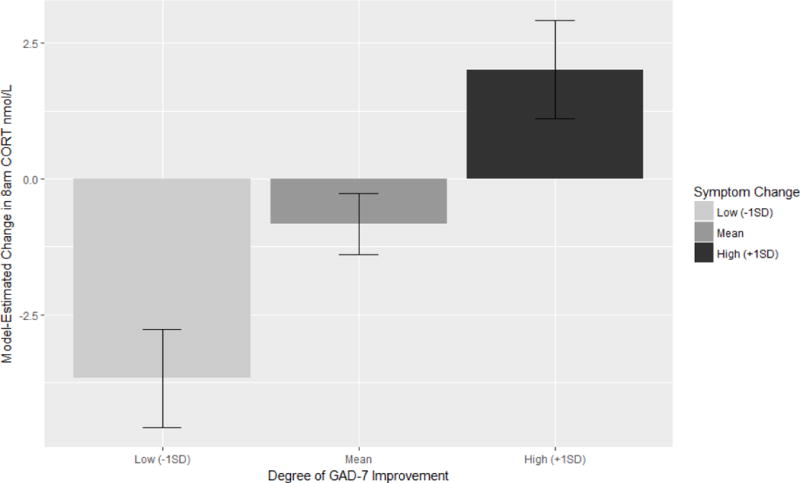
Model-estimated change in 8am (i.e., awakening) salivary cortisol levels from pre- to post-chamomile treatment, as a function of GAD-7 symptom improvement during treatment. Model covariates have been set to their mean values, and bars are standard errors for the estimates. Positive values indicate that CORT was higher at 8am post-treatment as compared to pre-treatment. Increased symptom improvement was associated with relative increases in 8am CORT (p = 0.002).
Acknowledgments
We would like to thank Dr. Bernard Carroll for his helpful commentary and review of an earlier version of this manuscript.
The parent clinical trial for this study was funded by an NIH/NCCAM R01 grant (AT005074) to Jun J. Mao. This research was also funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 and by the Rockefeller Translational and Integrative Medicine Research Fund at the Memorial Sloan-Kettering Cancer Center.
Appendix Table 1.
| Baseline Variables | Relationship with Pre-Post Change in Morning CORT | Relationship with Pre-Post Change in Diurnal CORT Slope |
|---|---|---|
| Age, y | ns | ns |
| Gender (Female) | ns | ns |
| Race (Caucasian) | ns | ns |
| Age at first GAD episode | ns | ns |
| Duration of current GAD episode (years) | β = −0.23, p = 0.024 | ns |
| Number of previous treatments for GAD | ns | ns |
| Prior major depressive disorder diagnosis | ns | ns |
| Beck Depression Inventory score | β = −0.20, p = 0.022 | β = 0.28, p = 0.024 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors declare any conflicts of interest as regards this manuscript.
Results without expectancies as a covariate show the same direction and pattern of statistical significance as when they are included. Analyses controlling for expectancies are presented here as they were the planned analyses for this manuscript.
As a check on our findings, we furthermore examined whether other baseline variables predicted pre-to-post treatment changes in cortisol (see Appendix 1). Both the duration of the current GAD episode and baseline depression severity as measured by the Beck Depression Inventory predicted pre-to-post cortisol changes. However, when including these interactions in the overall model, all originally obtained patterns were retained and remained statistically significant.
Contributor Information
John R. Keefe, University of Pennsylvania
Wensheng Guo, Perelman School of Medicine at the University of Pennsylvania
Qing S. Li, Memorial Sloan-Kettering Cancer Center
Jay D. Amsterdam, Perelman School of Medicine at the University of Pennsylvania
Jun J. Mao, Memorial Sloan-Kettering Cancer Center
References
- Amsterdam JD, Li Y, Soeller I, Rockwell K, Mao JJ, Shults J. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. Journal of clinical psychopharmacology. 2009;29(4):378–382. doi: 10.1097/JCP.0b013e3181ac935c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avallone R, Zanoli P, Puia G, Kleinschnitz M, Schreier P, Baraldi M. Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochemical pharmacology. 2000;59(11):1387–1394. doi: 10.1016/s0006-2952(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Baldwin D, Woods R, Lawson R, Taylor D. Efficacy of drug treatments for generalised anxiety disorder: systematic review and meta-analysis. BMJ (Clinical research ed) 2011;342:d1199. doi: 10.1136/bmj.d1199. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Baldwin D, Abelli M, Bolea-Alamanac B, Bourin M, Chamberlain SR, Cinosi E, Davies S, Domschke K, Fineberg N, Grunblatt E, Jarema M, Kim YK, Maron E, Masdrakis V, Mikova O, Nutt D, Pallanti S, Pini S, Strohle A, Thibaut F, Vaghi MM, Won E, Wedekind D, Wichniak A, Woolley J, Zwanzger P, Riederer P. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2016:1–53. doi: 10.1080/15622975.2016.1190867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Grothendieck G, Green P. lme4: Linear Mixed-Effects Models using ‘Eigen’ and S4. 2016 1.1-12 ed. [Google Scholar]
- Benjamini Y, Yekutieli D. The Control of the False Discovery Rate in Multiple Testing under Dependency. The Annals of Statistics. 2001;29(4):1165–1188. [Google Scholar]
- Campbell EL, Chebib M, Johnston GAR. The dietary flavonoids apigenin and (−)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABAA receptors. Biochemical pharmacology. 2004;68(8):1631–1638. doi: 10.1016/j.bcp.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Chang CY, Lin TY, Lu CW, Wang CC, Wang YC, Chou SSP, Wang SJ. Apigenin, a natural flavonoid, inhibits glutamate release in the rat hippocampus. European Journal of Pharmacology. 2015;762:72–81. doi: 10.1016/j.ejphar.2015.05.035. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- Cuijpers P, Sijbrandij M, Koole S, Huibers M, Berking M, Andersson G. Psychological treatment of generalized anxiety disorder: A meta-analysis. Clinical Psychology Review. 2014;34(2):130–140. doi: 10.1016/j.cpr.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Dierckx B, Dieleman G, Tulen JH, Treffers PD, Utens EM, Verhulst FC, Tiemeier H. Persistence of anxiety disorders and concomitant changes in cortisol. Journal of anxiety disorders. 2012;26(6):635–641. doi: 10.1016/j.janxdis.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Elnazer HY, Baldwin DS. Investigation of cortisol levels in patients with anxiety disorders: a structured review. Current topics in behavioral neurosciences. 2014;18:191–216. doi: 10.1007/7854_2014_299. [DOI] [PubMed] [Google Scholar]
- First MS, Spitzer L, Gibbon M, Williams J. Biometrics Research. New York State Psychiatric Institute; New York, NY: 2001. Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I/P W/PSY Screen) [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2007;32(1):80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Herane Vives A, De Angel V, Papadopoulos A, Strawbridge R, Wise T, Young AH, Arnone D, Cleare AJ. The relationship between cortisol, stress and psychiatric illness: New insights using hair analysis. Journal of psychiatric research. 2015;70:38–49. doi: 10.1016/j.jpsychires.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, McLeod DR, Lee YB, Zimmerli WD. Cortisol levels in generalized anxiety disorder. Psychiatry research. 38(3):313–315. doi: 10.1016/0165-1781(91)90021-g. In press. [DOI] [PubMed] [Google Scholar]
- Keefe JR, Amsterdam J, Li QS, Soeller I, DeRubeis R, Mao JJ. Specific expectancies are associated with symptomatic outcomes and side effect burden in a trial of chamomile extract for generalized anxiety disorder. Journal of psychiatric research. 2017;84:90–97. doi: 10.1016/j.jpsychires.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe JR, Mao JJ, Soeller I, Li QS, Amsterdam JD. Short-term open-label chamomile (Matricaria chamomilla L.) therapy of moderate to severe generalized anxiety disorder. Phytomedicine. 2016;23(14):1699–1705. doi: 10.1016/j.phymed.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Gierens A, Hellhammer DH, Wüst S, Schlotz W. Salivary Cortisol in Ambulatory Assessment—Some Dos, Some Don’ts, and Some Open Questions. Psychosomatic medicine. 2012;74(4):418–431. doi: 10.1097/PSY.0b013e31825434c7. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests in Linear Mixed Effects Models. 2016 2.0-30 ed. [Google Scholar]
- Lenze EJ, Mantella RC, Shi P, Goate AM, Nowotny P, Butters MA, Andreescu C, Thompson PA, Rollman BL. Elevated cortisol in older adults with generalized anxiety disorder is reduced by treatment: a placebo-controlled evaluation of escitalopram. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2011;19(5):482–490. doi: 10.1097/JGP.0b013e3181ec806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi G, Puia G, Garzon G, de Vuono MC, Baraldi M. Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons. Eur J Pharmacol. 2004;502(1–2):41–46. doi: 10.1016/j.ejphar.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Löwe B, Decker O, Müller S, Brähler E, Schelberg D, Herzog W, Herzberg PY. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Medical Care. 2008;46(3):266–274. doi: 10.1097/MLR.0b013e318160d093. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, Reynolds CF, Lenze EJ. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33(6):773–781. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JJ, Li QS, Soeller I, Rockwell K, Xie SX, Amsterdam JD. Long-term chamomile therapy of generalized anxiety disorder: A study protocol for a randomized, double-blind, placebo-controlled trial. Clinical Trials. 2014;4:5. doi: 10.4172/2167-0870.1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JJ, Xie SX, Keefe JR, Soeller I, Li QS, Amsterdam JD. Long-term chamomile (Matricaria chamomilla L.) treatment for generalized anxiety disorder: A randomized clinical trial. Phytomedicine. 2016;23(14):1735–1742. doi: 10.1016/j.phymed.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay DL, Blumberg JB. A review of the bioactivity and potential health benefits of chamomile tea (Matricaria recutita L) Phytotherapy research : PTR. 2006;20(7):519–530. doi: 10.1002/ptr.1900. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Medicine Reviews. 2008;12(3):197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological bulletin. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: A systematic review and meta-analysis. Clinical Psychology Review. 2012;32(4):301–315. doi: 10.1016/j.cpr.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederhof E, van Oort FV, Bouma EM, Laceulle OM, Oldehinkel AJ, Ormel J. Predicting mental disorders from hypothalamic-pituitary-adrenal axis functioning: a 3-year follow-up in the TRAILS study. Psychological medicine. 2015;45(11):2403–2412. doi: 10.1017/S0033291715000392. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Batty GD, Gale CR, Lord JM, Arlt W, Carroll D. Major depressive disorder, generalised anxiety disorder, and their comorbidity: Associations with cortisol in the Vietnam Experience Study. Psychoneuroendocrinology. 2011;36(5):682–690. doi: 10.1016/j.psyneuen.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Pomara N, Willoughby LM, Sidtis JJ, Cooper TB, Greenblatt DJ. Cortisol response to diazepam: its relationship to age, dose, duration of treatment, and presence of generalized anxiety disorder. Psychopharmacology. 2005;178(1):1–8. doi: 10.1007/s00213-004-1974-8. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. [Google Scholar]
- Reis LS, Pardo PE, Oba E, Kronka Sdo N, Frazatti-Gallina NM. Matricaria chamomilla CH12 decreases handling stress in Nelore calves. Journal of veterinary science. 2006;7(2):189–192. doi: 10.4142/jvs.2006.7.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB, Little RJA. Statistical analysis with missing data. 2nd. Wiley; New York, NY: 2002. [Google Scholar]
- Steptoe A. Cortisol awakening response. In: Fink G, editor. Encyclopedia of Stress. Academic Press; Oxford, England: 2007. pp. 649–653. [Google Scholar]
- Steudte S, Kirschbaum C, Gao W, Alexander N, Schönfeld S, Hoyer J, Stalder T. Hair Cortisol as a Biomarker of Traumatization in Healthy Individuals and Posttraumatic Stress Disorder Patients. Biological Psychiatry. 2013;74(9):639–646. doi: 10.1016/j.biopsych.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, Beesdo-Baum K, Kirschbaum C. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry research. 2011;186(2–3):310–314. doi: 10.1016/j.psychres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Tafet GE, Feder DJ, Abulafia DP, Roffman SS. Regulation of hypothalamic-pituitary-adrenal activity in response to cognitive therapy in patients with generalized anxiety disorder. Cognitive, affective & behavioral neuroscience. 2005;5(1):37–40. doi: 10.3758/cabn.5.1.37. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, DeRijk RH, van Dyck R, Smit JH, Zitman FG, Penninx BW. Salivary cortisol levels and the 2-year course of depressive and anxiety disorders. Psychoneuroendocrinology. 2013;38(9):1494–1502. doi: 10.1016/j.psyneuen.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Zitman FG, van Pelt J, DeRijk RH, Verhagen JCM, van Dyck R, Hoogendijk WJG, Smit JH, Penninx BWJH. Salivary Cortisol Levels in Persons With and Without Different Anxiety Disorders. Psychosomatic medicine. 2010;72(4):340–347. doi: 10.1097/PSY.0b013e3181d2f0c8. [DOI] [PubMed] [Google Scholar]
- Wang W, Feng J, Ji C, Mu X, Ma Q, Fan Y, Chen C, Gao C, Ma X-c, Zhu F. Increased methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of patients with generalized anxiety disorder. Journal of psychiatric research. 2017;91:18–25. doi: 10.1016/j.jpsychires.2017.01.019. [DOI] [PubMed] [Google Scholar]
- Wells S, Tremblay PF, Flynn A, Russell E, Kennedy J, Rehm J, Van Uum S, Koren G, Graham K. Associations of hair cortisol concentration with self-reported measures of stress and mental health-related factors in a pooled database of diverse community samples. Stress (Amsterdam, Netherlands) 2014;17(4):334–342. doi: 10.3109/10253890.2014.930432. [DOI] [PubMed] [Google Scholar]
- Westermann J, Demir A, Herbst V. Determination of cortisol in saliva and serum by a luminescence-enhanced enzyme immunoassay. Clinical laboratory. 2004;50(1–2):11–24. [PubMed] [Google Scholar]
- Yamada K, Miura T, Mimaki Y, Sashida Y. Effect of inhalation of chamomile oil vapour on plasma ACTH level in ovariectomized-rat under restriction stress. Biological & pharmaceutical bulletin. 1996;19(9):1244–1246. doi: 10.1248/bpb.19.1244. [DOI] [PubMed] [Google Scholar]


