Abstract
Rationale: Existing real-time surveillance of influenza morbidity, based primarily on time-trended U.S. hospitalization and death data, is inadequate. These surveillance methods do not accurately predict hospital resource requirements or sufficiently capture the public health impact of the current influenza season.
Objectives: To determine the feasibility and potential usefulness of tracking surrogate markers of influenza morbidity among patients hospitalized with influenza.
Methods: We performed a pilot study at three tertiary care referral hospitals and retrospectively collected and analyzed data on patients admitted with influenza during the 2013–2014 influenza season. We analyzed traditional influenza surveillance metrics, including weekly statistics on admissions and deaths, as well as weekly rates and trends of intensive care unit (ICU), mechanical ventilation, and extracorporeal membrane oxygenation (ECMO) utilization.
Results: In our three-hospital cohort, 431 patients were hospitalized with influenza and spent a total of 1,520 days in ICUs. Eighty-six (20%) of these patients required 1,080 days of mechanical ventilation, and 17 patients (4%) received 229 days of ECMO. Trends of ICU and mechanical ventilation use were similar but differed notably from trends of ECMO use, hospitalization, and death. In particular, at two hospitals, increases in utilization of ICU and mechanical ventilation among patients with influenza occurred several weeks after increases in hospitalization rates. Furthermore, ICU, mechanical ventilation, and ECMO utilization rates at the three-hospital network remained elevated for several weeks after the influenza-associated hospitalization rate declined.
Conclusions: Surrogate markers of influenza severity were feasible to collect and revealed trends of ICU resource utilization that differed notably from trends of hospitalization and death given by traditional influenza surveillance metrics. A national network of sentinel hospitals that prospectively collects, time-trends, and reports additional influenza morbidity data would be useful to hospital administrators, hospital epidemiologists, infection preventionists, and public health officials.
Keywords: extracorporeal membrane oxygenation, mechanical ventilation, infection control, epidemiology
Many experts perceived that the 2013–2014 influenza season was associated with unusually high morbidity and mortality, especially for adults younger than 65 years (1). By the conclusion of the influenza season, several facts supported this assumption: the influenza-associated hospitalization rate in the United States for adults aged 50–64 years was higher in 2013–2014 than in any other season since the 2009 H1N1 pandemic (2); California reported increased intensive care unit (ICU) admissions and deaths among adults aged 41–64 years with influenza (3); and a hospital survey described increased use of extracorporeal membrane oxygenation (ECMO) during the influenza season (4).
Whereas retrospective analyses of influenza severity are common (5, 6), real-time surveillance and reporting of regional and national influenza morbidity is inadequate. The Centers for Disease Control and Prevention (CDC, Atlanta, GA) releases weekly rates of laboratory-confirmed influenza hospitalization and the percentage of deaths caused by either pneumonia or influenza (7). In addition, the CDC’s online Influenza Hospitalization Surveillance Network (FluSurv-NET) database provides the percentage of patients with influenza each season who are admitted to ICUs, require mechanical ventilation, or die (8); however, this database provides these data on influenza morbidity and mortality as annual statistics rather than providing weekly time-trended analysis. Furthermore, these variables are binary and do not capture duration of ICU care and mechanical ventilation.
Important indicators, such as ICU, mechanical ventilation, and ECMO utilization among patients hospitalized with influenza, have not been used as detailed, real-time surrogates for influenza-related morbidity. Such surrogate outcomes are needed, as time-trended rates of hospitalization alone do not accurately predict the need for common and expensive hospital resources, such as ICU beds, ventilators, and ECMO circuits. Only some of the patients admitted with influenza require any of these three aggressive measures (5, 6, 9). Similarly, death metrics may be poor predictors of hospital resource utilization for severe influenza; even patients with respiratory failure due to influenza who are treated with ECMO have in-hospital mortality rates less than 40% (9–11).
We hypothesized that a surveillance system using sentinel hospitals that serially recorded days of ICU admission, mechanical ventilation, and ECMO utilized each week by patients with influenza would provide a more accurate and timely description of influenza-related morbidity and associated hospital resource requirements. Such a system would be especially useful if these surveillance data were coupled with traditional weekly surveillance metrics currently reported by the CDC (7). We conducted a pilot study at three tertiary care U.S. hospitals to determine the feasibility and potential usefulness of tracking these surrogate markers of influenza severity.
Methods
Data were retrospectively collected after the 2013–2014 influenza season at three tertiary care academic hospitals: a 924-bed hospital in Durham, North Carolina (hospital A); a 779-bed hospital in Richmond, Virginia (hospital B); and a 705-bed hospital in Iowa City, Iowa (hospital C). We collected data from the 36-week time period from August 4, 2013, through April 12, 2014, which included weeks with increased influenza activity in North Carolina (12), Virginia (13), and Iowa (14). These data were analyzed by weekly increments corresponding to Morbidity and Mortality Weekly Report (MMWR) week numbers (15), which ranged from Week 32 of 2013 (week beginning August 4, 2013) through Week 15 of 2014 (week beginning April 6, 2014). Week start and end dates were adjusted by 1 day at hospital A to match existing weekly surveillance of hospital admissions and patient-days.
The cohort of patients studied at each hospital included all patients requiring inpatient admission who were diagnosed as having influenza on the basis of respiratory sample (nasopharyngeal swab/wash or bronchoalveolar lavage) testing by polymerase chain reaction (PCR). Influenza testing was performed at the discretion of individual providers and was not influenced by this retrospective study. Some patients with critical illness may have been transferred to study hospitals after receiving a microbiologic diagnosis of influenza. To capture patients with influenza who did not undergo microbiologic testing at study hospitals, we also included patients assigned International Classification of Diseases, Ninth Revision (ICD-9) codes for “influenza with pneumonia” (487.0), “influenza with other respiratory manifestations” (487.1), and “influenza with other manifestations” (487.8). Adults were defined as patients at least 18 years of age on the date of admission.
We recorded the weekly number of patients with influenza who were admitted to the hospital, initiated on mechanical ventilation, or started on ECMO therapy. We also tracked deaths that occurred during index hospitalizations for influenza. Each center additionally counted the number of ICU, mechanical ventilation, and ECMO days accrued each week by patients with influenza. Data were assigned to the weeks that a critical care resource was utilized or a death occurred, independent of the week of hospital admission.
For each hospital, we divided the number of patients hospitalized with influenza each week by the total number of admissions to standardize influenza hospitalization rates across different study sites and throughout the influenza season. We divided the number of ICU days, mechanical ventilation days, and ECMO days accrued each week among patients with influenza by the total number of patient-days for all hospitalized patients to calculate weekly utilization rates. Weekly death rates were given as in-hospital deaths among patients with influenza per total patient-days.
Hospital A staff obtained all data for this study through queries of the electronic medical record (EMR), using Oracle SQL Developer version 3.2 (Oracle, Redwood Shores, CA). These queries did not require review of medical records of individual patients. Hospital B and hospital C staff reviewed the medical records of all patients with positive influenza PCRs or influenza-associated ICD-9 codes to determine ICU, mechanical ventilation, and ECMO utilization, as well as deaths.
The institutional review boards at hospital A and hospital B approved this research and waived the requirement for informed consent. The institutional review board at hospital C declared the study to be exempt research.
Results
We identified 431 patients hospitalized with influenza from August 4, 2013 (start of Week 32 of 2013) through April 12, 2014 (end of Week 15 of 2014), in our three-hospital cohort. These admissions represented 0.6% of 76,968 total admissions. There were 485,358 total patient-days during the 36-week study period. A total of 349 patients (81%) with influenza were adults; the remaining 82 patients (19%) with influenza were children. The diagnosis of influenza was made by PCR for 356 patients (83%); 75 additional patients (17%) had ICD-9 codes indicative of influenza and either were not tested for influenza at our hospitals with a PCR test, or the PCR test was negative.
Patients with influenza spent 1,520 days in intensive care units. Eighty-six patients (20%) received a total of 1,080 days of mechanical ventilation; 17 patients (4%) underwent ECMO, accounting for 229 ECMO days; and 25 (6%) of the 431 patients with influenza died during the hospitalization. Eleven children (13%) required mechanical ventilation, but no children received ECMO therapy, and 2 children (2%) died. We also examined these data stratified by hospital (Table 1).
Table 1.
Method of diagnosis, resource utilization, and death data for all patients with influenza admitted to a network of three tertiary care hospitals during the 2013–2014 influenza season
| Characteristic | Hospital A |
Hospital B |
Hospital C |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 186) |
Adults (n = 150) |
Children (n = 36) |
Total (n = 141) |
Adults (n = 113) |
Children (n = 28) |
Total (n = 104) |
Adults (n = 86) |
Children (n = 18) |
|
| [n (%)] | [n (%)] | [n (%)] | [n (%)] | [n (%)] | [n (%)] | [n (%)] | [n (%)] | [n (%)] | |
| Method of diagnosis | |||||||||
| PCR | 150 (80.6) | 125 (83.3) | 25 (69.4) | 125 (88.7) | 102 (90.3) | 23 (82.1) | 81 (77.9) | 68 (79.1) | 13 (72.2) |
| ICD-9 code | 36 (19.4) | 25 (16.7) | 11 (30.6) | 16 (11.3) | 11 (9.7) | 5 (17.9) | 23 (22.1) | 18 (20.9) | 5 (27.8) |
| Resource utilization | |||||||||
| Mechanical ventilation | 38 (20.4) | 30 (20.0) | 8 (22.2) | 22 (15.6) | 22 (19.5) | 0 | 26 (25.0) | 23 (26.7) | 3 (16.7) |
| ECMO | 10 (5.4) | 10 (6.7) | 0 | 5 (3.5) | 5 (4.4) | 0 | 2 (1.9) | 2 (2.3) | 0 |
| ICU days* | 859 | 738 | 121 | 383 | 380 | 3 | 278 | 266 | 12 |
| Mechanical ventilation days* | 657 | 588 | 69 | 254 | 254 | 0 | 169 | 162 | 7 |
| ECMO days* | 144 | 144 | 0 | 74 | 74 | 0 | 11 | 11 | 0 |
| Death | 8 (4.3) | 8 (5.3) | 0 | 4 (2.8) | 4 (3.5) | 0 | 13 (12.5) | 11 (12.8) | 2 (11.1) |
| Total admissions (all patients)† | 30,023 | 24,443 | 5,590 | 24,530 | 19,889 | 4,641 | 22,415 | 18,250 | 4,165 |
| Total patient-days (all patients)† | 214,472 | 169,857 | 44,615 | 151,665 | 126,943 | 24,712 | 119,221 | 89,445 | 29,776 |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; ICD-9 = International Classification of Diseases, 9th Revision; ICU = intensive care unit; PCR = polymerase chain reaction.
Cumulative ICU, mechanical ventilation, and ECMO days among patients with influenza.
Total admissions and total patient-days among all patients admitted to hospital.
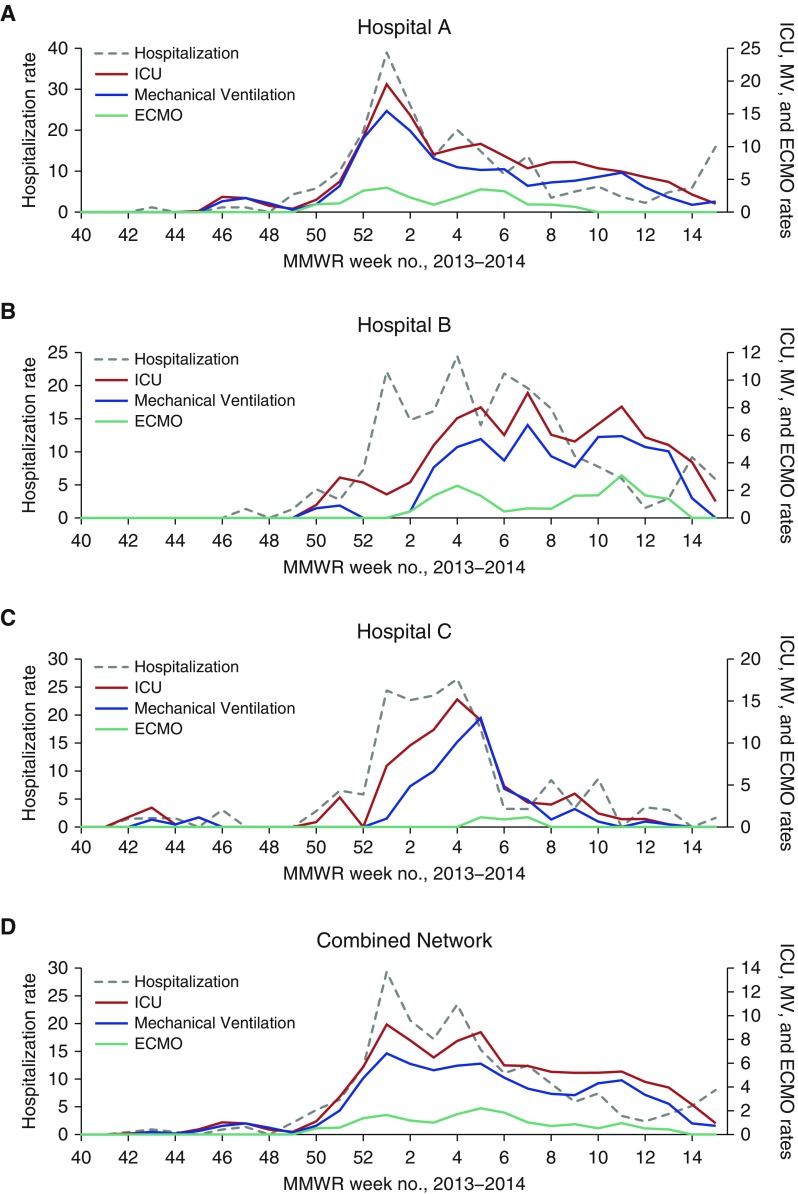
Hospital A had the highest peak rate of hospitalization for patients with influenza (39.0 hospitalizations per 1,000 total admissions), which occurred during Week 1 (Table 2). The peak rates of ICU use (19.5 d per 1,000 total patient-days), mechanical ventilation use (15.4 d per 1,000 total patient-days), and ECMO use (3.7 d per 1,000 total patient-days) among patients with influenza also occurred at hospital A and reached maximum rates during Week 1. The highest rate of hospitalization at both hospital B and hospital C occurred during Week 4. Peak rates of ICU, mechanical ventilation, and ECMO utilization at these two hospitals occurred at different times between Week 4 and Week 11. The highest death rate (0.7 deaths per 1,000 total patient-days) occurred during Week 1 at hospital C, which had more deaths (n = 13) than hospitals A and B combined (n = 12).
Table 2.
Week numbers with corresponding dates of first events and highest event rates for patients with influenza hospitalized at a network of three tertiary care hospitals during the 2013–2014 influenza season*
| Event | Hospital A |
Hospital B |
Hospital C |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week of first event (date) | Highest weekly rate | Week of highest rate (date) | Week of first event (date) | Highest weekly rate | Week of highest rate (date) | Week of first event (date) | Highest weekly rate | Week of highest rate (date) | ||
| Hospitalization† | 39.0 | 1 (12/29) | 24.5 | 4 (1/19) | 26.4 | 4 (1/19) | ||||
| Positive PCR | 46 (11/10) | 49 (12/1) | 42 (10/13) |
|||||||
| ICD-9 code | 43 (10/20) | 39 (9/22) | 50 (12/8) | |||||||
| ICU‡ | 45 (11/3) | 19.5 | 1 (12/29) | 50 (12/8) | 9.1 | 7 (2/9) | 42 (10/13) |
15.2 | 4 (1/19) | |
| Mechanical ventilation‡ | 46 (11/10) | 15.4 | 1 (12/29) | 50 (12/8) | 6.7 | 7 (2/9) | 43 (10/20) | 13.0 | 5 (1/26) | |
| ECMO‡ | 50 (12/8) | 3.7 | 1 (12/29) | 2 (1/5) | 3.1 | 11 (3/9) | 5 (1/26) | 1.2 | 7 (2/9) | |
| Death§ | 45 (11/3) | 0.2 | 1 (12/29) | 3 (1/12) | 0.2 | 13 (3/23) | 45 (11/3) | 0.7 | 1 (12/29) | |
Definition of abbreviations: ECMO = extracorporeal membrane oxygenation; ICD-9 = International Classification of Diseases, 9th Revision; ICU = intensive care unit; PCR = polymerase chain reaction.
Dates are given as Morbidity and Mortality Weekly Report (MMWR) week numbers and corresponding week start dates (15). Weeks 32–52 correspond to the last 21 weeks of 2013, and Weeks 1–15 correspond to the first 15 weeks of 2014.
Rates of hospitalization are given as hospitalizations of patients with influenza per week per 1,000 total admissions.
Rates of ICU, mechanical ventilation, and ECMO use are given as days of utilization per week per 1,000 total patient-days (influenza and noninfluenza patient-days).
Death rates are given as deaths per week per 1,000 total patient-days.
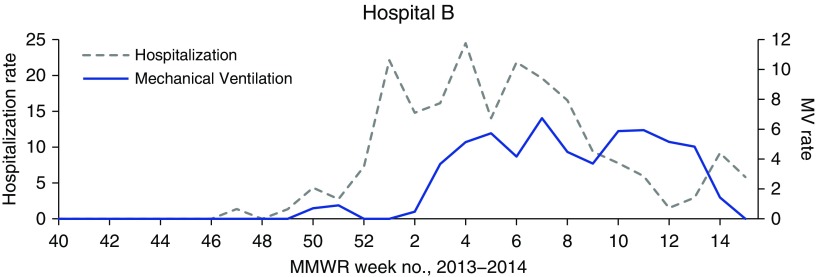
Weekly trends of ICU utilization rates among patients with influenza closely mirrored rates of mechanical ventilation use at all three centers, but these trends differed from trends of hospitalization and ECMO use (Figure 1). These four markers revealed different trends at different hospitals. For example, at hospitals B and C, initial increases in rates of ICU use and mechanical ventilation for care of patients with influenza occurred later in the influenza season than increases in the hospitalization rate. Furthermore, the combined network hospitalization rate for patients with influenza declined rapidly between its peak at Week 1 and nadir at Week 12; however, ICU, mechanical ventilation, and ECMO use for care of patients with influenza remained elevated throughout this 11-week time period. Discrepancies in trends of hospitalization and mechanical ventilation rates were particularly pronounced at hospital B (Figure 2).
Figure 1.
Weekly rates of hospitalization and rates of intensive care unit (ICU), mechanical ventilation (MV), and extracorporeal membrane oxygenation (ECMO) use among patients with influenza admitted to (A) hospital A, (B) hospital B, (C) hospital C, and (D) the combined three-hospital network of tertiary care hospitals during the 2013–2014 influenza season. Week numbers reflect Morbidity and Mortality Weekly Report (MMWR) numbering. Hospitalization rate is given as hospitalizations per week per 1,000 total admissions. ICU, mechanical ventilation, and ECMO rates are given as days of utilization per week per 1,000 total patient-days (influenza and noninfluenza patient-days).
Figure 2.
Weekly rates of hospitalization and mechanical ventilation (MV) utilization among patients with influenza admitted to sentinel hospital B during the 2013–2014 influenza season. Week numbers reflect Morbidity and Mortality Weekly Report (MMWR) numbering. Hospitalization rate is given as hospitalizations per week per 1,000 total admissions. Mechanical ventilation rate is given as days of utilization per week per 1,000 total patient-days (influenza and noninfluenza patient-days).
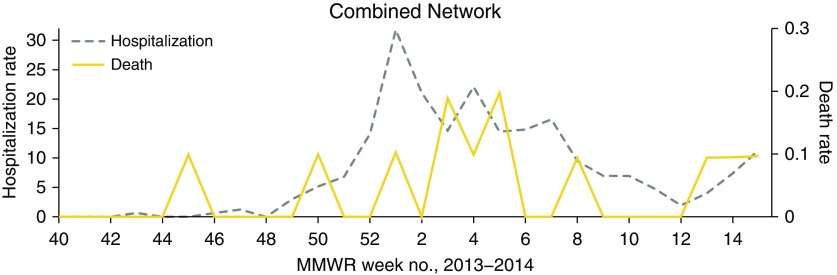
Rates of ECMO use among patients with influenza were low and remained relatively constant throughout the influenza season (Figure 1). The network-wide death rate spiked early at Week 45, several weeks before any of the other studied rates increased; subsequently, the death rate roughly mirrored the hospitalization rate (Figure 3).
Figure 3.
Weekly rates of hospitalization and death among patients with influenza admitted to a three-hospital network of tertiary care hospitals during the 2013–2014 influenza season. Week numbers reflect Morbidity and Mortality Weekly Report (MMWR) numbering. Hospitalization rate is given as hospitalizations per week per 1,000 total admissions. Death rate is given as deaths per week per 1,000 total patient-days (influenza and noninfluenza patient-days).
Discussion
Hospitals treating critically ill patients with influenza utilize numerous resources during the influenza season, including ICU beds and time from physicians, nurses, and other ICU staff. Most patients admitted to ICUs with influenza receive mechanical ventilation (16, 17); thus, adequate numbers of ventilators and specialized support staff, such as respiratory therapists, are also necessary resources. ECMO can be an effective salvage therapy for patients with acute respiratory distress syndrome or cardiopulmonary failure from H1N1 influenza (9, 18–21). The resources required for ECMO include ECMO cannulas and circuits, as well as highly trained nursing and medical staff, including a perfusionist team (22). Furthermore, some hospitals without ECMO capability transfer patients with influenza-related respiratory failure to ECMO referral centers. The transfer of a patient to an ECMO referral center requires additional personnel and associated costs at both the transferring and receiving hospital. The resources needed to care for critically ill patients with influenza vary depending on the timing and severity of each influenza season, the virulence of the circulating strains of influenza virus, and the population affected (5, 6, 23).
We successfully collected data related to surrogate markers of influenza morbidity at three tertiary care referral hospitals during the 2013–2014 influenza season. We retrospectively tracked ICU, mechanical ventilation, and ECMO utilization among patients hospitalized with influenza. The surveillance methods used at hospital A demonstrated the feasibility and simplicity of collecting detailed influenza morbidity data via EMR queries without accessing patient charts. We used advanced coding techniques to build the initial EMR queries at hospital A, but similar queries made during subsequent influenza seasons would require only minimal time and expertise.
A major weakness of current influenza surveillance is that weekly, time-trended data on hospitalization and death from influenza provide a limited “real-time” assessment or estimation of influenza morbidity. These markers do not accurately predict or correlate with hospital resource requirements, nor do they offer a detailed barometer of the current influenza season’s impact on public health. Moreover, the CDC includes deaths from either influenza or pneumonia in the current metric (24), which limits the utility of these death data for assessing the number and burden of influenza-specific complications. Finally, the CDC’s prospectively collected ICU and mechanical ventilation data are stratified by influenza season rather than individual week (8). The binary construction of these variables allows calculation of the percentage of patients who at any time during an influenza season required ICU level care or mechanical ventilation; however, data on the duration of these events would greatly improve hospital resource utilization estimates.
Our study illustrated that hospitalization rate is an insufficient marker of influenza morbidity. ICU and mechanical ventilation utilization by patients with influenza at our three sentinel tertiary care centers had similar trends at all sites, but these trends differed in important ways from data on influenza hospitalizations. For example, aggregate rates of ICU and mechanical ventilation utilization remained elevated for several weeks after rates of hospitalization for influenza care declined. This discrepancy may be explained by patients who were admitted with severe influenza early in the season and had long ICU stays and associated complications.
Rates of death in patients hospitalized with influenza were of limited utility in large part because influenza-associated deaths were relatively infrequent at two of our three centers during this single influenza season. H1N1 influenza was the predominant strain that circulated during the 2013–2014 season, both nationally (25) and in the three states where study hospitals were located (12–14). H1N1 influenza is associated with increased risk of ICU admission, mechanical ventilation, and ECMO (5, 6, 23); however, this strain causes severe disease in younger adults more frequently than other influenza strains (26–28). Even though H1N1 influenza is associated with increased morbidity among younger people, overall mortality rates may not increase when the population affected most commonly is relatively healthy and has few underlying chronic diseases (6, 23). These characteristics of H1N1 influenza may explain why the death rate at hospitals A and B was relatively low in comparison with substantial rates of critical care utilization.
Hospital administrators and public health officials could more accurately predict hospital resource needs and report the burden of influenza using the data we collected, rather than relying primarily on real-time data on hospitalizations and death. Data from a nationwide sentinel network of hospitals, if updated frequently, could help hospital administrators, intensivists, hospital epidemiologists, and infection preventionists assess in real time the local need for costly critical care resources, such as ICU beds, ventilators, ECMO circuits, and perfusionist staff, to care for patients with influenza. Moreover, enhanced surveillance utilizing the metrics we collected could help public health officials perform a more complete, accurate, and timely assessment of the true burden of influenza during the influenza season. The public health response in the midst of an influenza season might be more aggressive if ICU, ventilator, and ECMO use is elevated, even if corresponding influenza hospitalization rates are near baseline.
Our study had three primary limitations. First, we were inspired near the conclusion of the 2013–2014 influenza season to conduct this study because many clinicians perceived that this influenza season was particularly severe, but detailed, real-time data supporting this hypothesis were lacking. Therefore, although we have reported that prospective surveillance of several markers of inpatient influenza morbidity is feasible, we collected our data retrospectively. Second, our case definition for influenza was imperfect. Some patients in our influenza cohort, particularly patients captured via ICD-9 codes, may not have truly had influenza. Likewise, some patients not included in our cohort, particularly those hospitalized with mild cases of influenza, may have had influenza but not undergone laboratory testing. If influenza testing occurred less frequently for patients with less severe influenza, the metrics used in this study may have underestimated total cases of influenza while overestimating relative severity. Third, we included only three centers and reviewed data from a single influenza season, during which utilization of ECMO was relatively low. Data from additional sentinel hospitals collected serially during multiple influenza seasons would allow more detailed future studies on regional and seasonal trends of influenza morbidity. In particular, such data might improve our understanding of how best to collect and report ECMO utilization data to assess severity of the influenza season and associated resource requirements. Analysis of multiple seasons of morbidity data would also help determine how overall influenza incidence rates and activity of different circulating virus subtypes affect influenza morbidity.
We recommend that the CDC augment its current inpatient weekly influenza surveillance of hospitalizations and deaths with time-trended data on two additional markers of influenza morbidity: ECMO use and either ICU utilization or mechanical ventilation utilization, which have parallel trends. Using time-trended data and calculating utilization rates from aggregate days of resource use, instead of reporting only the percentage of patients with influenza who for any length of time required certain therapies, would allow health care facilities and public health agencies to better estimate real-time resource utilization and future needs. Finally, the CDC should report weekly deaths from influenza separately from deaths from pneumonia to improve the utility of surveillance data on deaths.
In summary, a national network of sentinel hospitals from geographically diverse areas could enhance prospective “real-time” surveillance of influenza morbidity by collecting and reporting expanded data on critical care resource utilization among patients with influenza. If sentinel hospitals used standardized electronic data collection methods and regularly reported data to a central agency, such as the CDC, public health officials could assess the severity of the influenza season in real time. In addition, hospitals and public health agencies could improve their allocation of key resources during, rather than after, an individual influenza season. The CDC could request that specific hospitals in its existing FluSurv-NET (8, 24, 29) collect such data. The CDC, in turn, could add data on these surrogate markers of influenza-related morbidity to the data on influenza hospitalization and death currently included in the CDC’s Weekly U.S. Influenza Surveillance Report (7).
Supplementary Material
Footnotes
Supported in part by the Duke University Transplant Infectious Diseases Interdisciplinary Research Training Grant: National Institutes of Health grant 5T32AI100851-02 (A.W.B.).
Author Contributions: A.W.B. initiated and designed the study, collected data, interpreted findings, and drafted the manuscript. M.B.E. proposed the research question, collected data, interpreted findings, and reviewed the manuscript. L.A.H. collected data, interpreted findings, and revised the manuscript. L.F.C. designed the study, interpreted findings, and reviewed the manuscript. S.S. analyzed data and reviewed the manuscript. D.J.S. initiated and designed the study, interpreted findings, and revised the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Edmond M Controversies in Hospital Infection Prevention [blog] Flu: a ground-level view. [9 Feb 2014; accessed 22 Dec 2014]. Available from: http://haicontroversies.blogspot.com/2014/02/flu-ground-level-view.html.
- 2.Epperson S, Blanton L, Kniss K, Mustaquim D, Steffens C, Wallis T, Dhara R, Leon M, Perez A, Chaves SS, et al. Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC) Influenza activity—United States, 2013–14 season and composition of the 2014–15 influenza vaccines. MMWR Morb Mortal Wkly Rep. 2014;63:483–490. [PMC free article] [PubMed] [Google Scholar]
- 3.Ayscue P, Murray E, Uyeki T, Zipprich J, Harriman K, Salibay C, Kang M, Luu A, Glenn-Finer R, Watt J, et al. Centers for Disease Control and Prevention (CDC) Influenza-associated intensive-care unit admissions and deaths—California, September 29, 2013–January 18, 2014. MMWR Morb Mortal Wkly Rep. 2014;63:143–147. [PMC free article] [PubMed] [Google Scholar]
- 4.Palanzo DA, Wise RK, Baer LD. Impact of the 2013/2014 influenza season on extracorporeal membrane oxygenation programs in the United States. Artif Organs. 2014;38:909–913. doi: 10.1111/aor.12411. [DOI] [PubMed] [Google Scholar]
- 5.Chaves SS, Aragon D, Bennett N, Cooper T, D’Mello T, Farley M, Fowler B, Hancock E, Kirley PD, Lynfield R, et al. Patients hospitalized with laboratory-confirmed influenza during the 2010–2011 influenza season: exploring disease severity by virus type and subtype. J Infect Dis. 2013;208:1305–1314. doi: 10.1093/infdis/jit316. [DOI] [PubMed] [Google Scholar]
- 6.Reed C, Chaves SS, Perez A, D’Mello T, Daily Kirley P, Aragon D, Meek JI, Farley MM, Ryan P, Lynfield R, et al. Complications among adults hospitalized with influenza: a comparison of seasonal influenza and the 2009 H1N1 pandemic. Clin Infect Dis. 2014;59:166–174. doi: 10.1093/cid/ciu285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Weekly U.S. Influenza Surveillance Report. [Accessed 3 Dec 2014]. Available from: http://www.cdc.gov/flu/weekly/
- 8.Centers for Disease Control and Prevention. Laboratory-confirmed influenza hospitalizations. [Accessed 3 Jan 2017]. Available from: https://gis.cdc.gov/grasp/fluview/FluHospChars.html.
- 9.Zangrillo A, Biondi-Zoccai G, Landoni G, Frati G, Patroniti N, Pesenti A, Pappalardo F. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. 2013;17:R30. doi: 10.1186/cc12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, Sadique MZ, Sekhon JS, McAuley DF, Firmin RK, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1) JAMA. 2011;306:1659–1668. doi: 10.1001/jama.2011.1471. [DOI] [PubMed] [Google Scholar]
- 11.Pappalardo F, Pieri M, Greco T, Patroniti N, Pesenti A, Arcadipane A, Ranieri VM, Gattinoni L, Landoni G, Holzgraefe B, et al. Italian ECMOnet. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Med. 2013;39:275–281. doi: 10.1007/s00134-012-2747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.North Carolina Department of Health and Human Services. North Carolina Weekly Influenza Surveillance Summary #33; 2013–14 influenza season; Week 20: Ending May 17, 2014. [Accessed 14 June 2014]. Available from: http://epi.publichealth.nc.gov/cd/flu/figures/flu1314.pdf.
- 13.Virginia Department of Health. Weekly Influenza Activity Report; data from week ending date: 5/17/2014. [Accessed 14 June 2014]. Available from: http://www.vdh.virginia.gov/epidemiology/influenza-flu-in-virginia/influenza-surveillance/
- 14.Iowa Department of Public Health. Iowa flu reports. [Accessed 14 June 2014]. Available from: https://idph.iowa.gov/influenza/reports.
- 15.Centers for Disease Control and Prevention National Notifiable Diseases Surveillance System MMWR Week. [Accessed 2 Jan 2015]. Available from: http://wwwn.cdc.gov/nndss/document/MMWR_week_overview.pdf
- 16.Wiesen J, Komara JJ, Walker E, Wiedemann HP, Guzman JA. Relative cost and outcomes in the intensive care unit of acute lung injury (ALI) due to pandemic influenza compared with other etiologies: a single-center study. Ann Intensive Care. 2012;2:41. doi: 10.1186/2110-5820-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuhrman C, Bonmarin I, Bitar D, Cardoso T, Duport N, Herida M, Isnard H, Guidet B, Mimoz O, Richard JC, et al. Adult intensive-care patients with 2009 pandemic influenza A(H1N1) infection. Epidemiol Infect. 2011;139:1202–1209. doi: 10.1017/S0950268810002414. [DOI] [PubMed] [Google Scholar]
- 18.Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, et al. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 19.Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, Iotti GA, Arcadipane A, Panarello G, Ranieri VM, et al. The Italian ECMO Network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37:1447–1457. doi: 10.1007/s00134-011-2301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holzgraefe B, Broomé M, Kalzén H, Konrad D, Palmér K, Frenckner B. Extracorporeal membrane oxygenation for pandemic H1N1 2009 respiratory failure. Minerva Anestesiol. 2010;76:1043–1051. [PubMed] [Google Scholar]
- 21.Bonacchi M, Ciapetti M, Di Lascio G, Harmelin G, Sani G, Peris A. Atypical clinic presentation of pandemic influenza A successfully rescued by extracorporeal membrane oxygenation: our experience and review of the literature. Interv Med Appl Sci. 2013;5:186–192. doi: 10.1556/IMAS.5.2013.4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins AM, Pettilä V, Harris AH, Bailey M, Lipman J, Seppelt IM, Webb SA. The critical care costs of the influenza A/H1N1 2009 pandemic in Australia and New Zealand. Anaesth Intensive Care. 2011;39:384–391. doi: 10.1177/0310057X1103900308. [DOI] [PubMed] [Google Scholar]
- 23.Bauernfeind S, Bruennler T, Ehrenstein B, Langgartner J, Wenzel JJ, Werner S, Lubnow M, Mueller T, Floerchinger B, Salzberger B. Pandemic and post-pandemic influenza A (H1N1) seasons in a tertiary care university hospital: high rate of complications compared to previous influenza seasons. Infection. 2013;41:145–150. doi: 10.1007/s15010-012-0310-1. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Overview of influenza surveillance in the United States. [Accessed 3 Dec 2014]. Available from: http://www.cdc.gov/flu/weekly/overview.htm.
- 25.Centers for Disease Control and Prevention. National and regional level outpatient illness and viral surveillance. [Accessed 3 Jan 2017]. Available from: https://gis.cdc.gov/grasp/fluview/fluportaldashboard.html.
- 26.Shrestha SS, Swerdlow DL, Borse RH, Prabhu VS, Finelli L, Atkins CY, Owusu-Edusei K, Bell B, Mead PS, Biggerstaff M, et al. Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009–April 2010) Clin Infect Dis. 2011;52:S75–S82. doi: 10.1093/cid/ciq012. [DOI] [PubMed] [Google Scholar]
- 27.Jhung MA, Swerdlow D, Olsen SJ, Jernigan D, Biggerstaff M, Kamimoto L, Kniss K, Reed C, Fry A, Brammer L, et al. Epidemiology of 2009 pandemic influenza A (H1N1) in the United States. Clin Infect Dis. 2011;52:S13–S26. doi: 10.1093/cid/ciq008. [DOI] [PubMed] [Google Scholar]
- 28.Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, Bandaranayake D, Breiman RF, Brooks WA, Buchy P, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012;12:687–695. doi: 10.1016/S1473-3099(12)70121-4. [DOI] [PubMed] [Google Scholar]
- 29.Chaves SS, Lynfield R, Lindegren ML, Bresee J, Finelli L. The US Influenza Hospitalization Surveillance Network. Emerg Infect Dis. 2015;21:1543–1550. doi: 10.3201/eid2109.141912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.