Abstract
Rationale: Obstructive sleep apnea (OSA) has been postulated to contribute to idiopathic pulmonary fibrosis by promoting alveolar epithelial injury via tractional forces and intermittent hypoxia.
Objectives: To determine whether OSA is associated with subclinical interstitial lung disease (ILD) and with biomarkers of alveolar epithelial injury and remodeling.
Methods: We performed cross-sectional analyses of 1,690 community-dwelling adults who underwent 15-channel in-home polysomnography and thoracic computed tomographic imaging in the Multi-Ethnic Study of Atherosclerosis. We measured the obstructive apnea–hypopnea index (oAHI) by polysomnography and high-attenuation areas (HAAs) and interstitial lung abnormalities (ILAs) by computed tomography. Serum matrix metalloproteinase-7 (MMP-7) and surfactant protein-A (SP-A) were measured by ELISA in 99 participants. We used generalized linear models to adjust for potential confounders.
Results: The mean age was 68 years, and the mean forced vital capacity was 97% predicted. The median oAHI was 8.4 events/h, and 32% had an oAHI greater than 15. After adjusting for demographics, smoking, and center, an oAHI greater than 15 was associated with a 4.0% HAA increment (95% confidence interval [CI], 1.4–6.8%; P = 0.003) and 35% increased odds of ILA (95% CI, 13–61%; P = 0.001). However, there was evidence that these associations varied by body mass index (BMI) (P for interaction = 0.08 and 0.04, respectively). Among those with a BMI less than 25 kg/m2, an oAHI greater than 15 was associated with a 6.1% HAA increment (95% CI, 0.5–12%; P = 0.03) and 2.3-fold increased odds of ILA (95% CI, 1.3–4.1; P = 0.005). Among those with a BMI greater than 30 kg/m2, an oAHI greater than 15 was associated with 1.8-fold greater odds of ILA (95% CI, 1.1–2.9; P = 0.01) but was not associated with HAA. There were no meaningful associations detected among those with a BMI of 25–30 kg/m2. Greater oAHI was associated higher serum SP-A and MMP-7 levels, particularly among those with a BMI less than 25 kg/m2.
Conclusions: Moderate to severe OSA is associated with subclinical ILD and with evidence of alveolar epithelial injury and extracellular matrix remodeling in community-dwelling adults, an association that is strongest among normal-weight individuals. These findings support the hypothesis that OSA might contribute to early ILD.
Keywords: lung injury, sleep apnea, interstitial lung disease, epidemiologic studies, biomarkers
Recurrent “microinjuries” of the alveolar epithelium have been postulated to be the antecedent event that leads to aberrant extracellular matrix (ECM) remodeling that precedes idiopathic pulmonary fibrosis (IPF) (1). To date, however, neither pathological evidence of their existence nor the causes of these alveolar microinjuries have been identified.
We and others have attempted to garner evidence of these alveolar microinjuries, using computed tomography (CT)–derived measures of subclinical interstitial lung disease (ILD) in community-dwelling adults. High-attenuation areas (HAAs), the percentage of lung voxels that have CT attenuation values between –600 and –250 Hounsfield units (HU), have been shown to be a quantitative biomarker of subclinical lung injury, inflammation, and ECM remodeling and are associated with the presence of interstitial lung abnormalities (ILAs), a qualitative CT-based subclinical interstitial lung disease phenotype, at 10-year follow-up (2). A growing evidence base suggests that ILA and HAA in the lung fields of community-dwelling adults may, in some cases, capture alveolar microinjuries that precede IPF and other fibrotic ILDs.
“Traction” in the periphery of the lung has been postulated to be a cause of alveolar microinjuries leading to IPF (3). One possible cause of tractional injury is obstructive sleep apnea (OSA) (4, 5), which affects up to 88% of adults with IPF (6, 7). OSA is characterized by repetitive forced inspirations against a completely or partially closed upper airway, occurring up to hundreds of times nightly (8). Wide swings in pleural pressure that occur with obstructive events could exert local strain forces at the periphery of the lung, deforming and stretching alveolar epithelial cells (AECs) in a cyclical fashion, even without changes in lung volume (9). In addition, rapid increases in alveolar volume after an obstructive apnea might stretch the alveolar walls, exerting stress forces on the alveolar epithelium. Such stress and strain forces are established causes of AEC injury and inflammation, which have been hypothesized to lead to lung fibrosis in susceptible individuals (1, 3–5, 10–13). Oxidative stress and inflammation resulting from intermittent hypoxia might also link OSA to AEC injury (14).
In this study, we tested the hypothesis that OSA severity would be associated with two CT-based subclinical ILD phenotypes (HAA and ILA) among community-dwelling adults. We also tested for consistency of this association across categories of body mass index (BMI) and smoking status; and whether serum surfactant protein-A (SP-A) and matrix metalloproteinase-7 (MMP-7) levels, markers of alveolar injury and remodeling, were associated with OSA severity.
Methods
The full text of the methods is available in the online supplement.
Study Design, Data Source, and Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a National Heart, Lung, and Blood Institute–sponsored prospective cohort study designed to investigate subclinical cardiovascular disease. Enrollment criteria have been described previously (15). MESA enrolled 6,814 adults between 45 and 84 years of age, free of clinically evident cardiovascular disease, sampled from six U.S. communities between 2000 and 2002. There were no selection criteria based on respiratory or sleep symptoms, smoking, or lung disease. MESA participants attended follow-up visits, with the most recent visit between 2010 and 2012 (Exam 5).
We performed cross-sectional analyses of participants who underwent polysomnography and full lung or cardiac CT imaging in conjunction with MESA Exam 5 (n = 1,690). Of these, 1,152 full lung CT scans were assessed for ILA, and 99 underwent measurement of MMP-7 and SP-A in serum collected at the Exam 5 visit.
Interstitial Lung Abnormalities
Exam 5 full lung CT scans were performed in the supine position, using the MESA Lung/SPIROMICS protocol (16). Each CT scan was visually examined by one of five expert radiologists for the presence of ILA, defined as involvement of more than 5% of nondependent lung by reticular abnormalities, ground-glass abnormalities, diffuse centrilobular nodularity, honeycombing, traction bronchiectasis, and/or nonemphysematous cysts (2, 17, 18).
High-Attenuation Areas on CT Scans
HAAs and percent emphysema were measured on Exam 5 full lung and cardiac CT scans, using a standardized protocol that has been described previously (19–21). HAA was defined as the percentage of voxels having attenuation values between –600 and –250 HU (2, 20, 22).
Polysomnography
Sleep metrics and methods have been described previously (23). MESA participants, none using continuous positive airway pressure or other nightly treatment for OSA, underwent in-home full polysomnography with a 15-channel monitor (Compumedics Somte system; Compumedics Ltd., Abbotsville, Australia) a median of 300 days after MESA Exam 5, permitting standardized assessments of sleep stages, arousals, and respiratory events.
Our two primary exposures of interest were the obstructive apnea–hypopnea index (oAHI), defined as the average number of all obstructive apneas plus hypopneas with at least 4% oxygen desaturation per hour of sleep, and the nadir oxyhemoglobin saturation during sleep determined by continuous finger pulse oximetry. We also examined the central sleep apnea index (all central apneas per hour of sleep) to determine whether the observed associations were specific for obstructive events.
Statistical Analysis
We used linear regression to examine associations of oAHI categories (<5, 5–15, >15 events/h) and nadir saturation categories (>90, 80–90, <80%) with natural log–transformed HAA with adjustment for age, sex, race/ethnicity, educational attainment, height, smoking status, cigarette pack-years, glomerular filtration rate, and percent emphysema. We tested for effect modification by BMI category: underweight and normal weight (<25 kg/m2), overweight (25–30 kg/m2), and obese (>30 kg/m2), and by smoking status, using likelihood ratio tests. We combined underweight (BMI < 18.5 kg/m2) and normal weight into a single category for our analyses because less than 1% of the cohort were underweight. Because there was evidence of effect modification by BMI, we estimated coefficients from fully adjusted models that included main effects for BMI category and interaction terms for BMI category × oAHI (or nadir saturation). We performed similar analyses for smoking status. We used logistic regression with cluster-robust variance estimation to account for clustering within study sites to examine the associations of both oAHI categories and nadir saturation categories with ILA with and without adjustment for age, sex, race/ethnicity, and smoking. Coefficients were estimated from fully adjusted models, using interaction terms as described above. We used multivariable linear models for analyses of MMP-7 and SP-A, adjusting for age, sex, race/ethnicity, smoking status, and BMI. We tested for effect modification by BMI (dichotomized at 25 kg/m2) as described above. We used multiple imputation with chained equations for missing covariate data in models without interaction terms, as previously described (2). Analyses were performed with Stata version 14.2 (StataCorp, College Station, TX) and the gam package in R version 3.2.2 (R Foundation for Statistical Computing) (24).
Results
Study Participants
A total of 2,261 MESA participants participated in the MESA Sleep Study, and 2,060 had polysomnography data that met study quality criteria (23). Of these, 1,690 also had available CT scans suitable for HAA measurement, and 1,152 had valid ILA assessments on full lung CT scans at Exam 5 (2). Baseline characteristics of the 1,690 participants with available HAA measurement are shown in Table 1. The mean age was 68.3 years, 54% were women, 53% were current or former smokers, 0.7% were underweight, 26% were normal weight, 38% were overweight, 35% were obese, the mean BMI was 28.7 kg/m2, and the mean forced vital capacity was 97% predicted. The median oAHI was 8.4 events/h (interquartile range [IQR], 3.0–18.7; range, 0–103); 32% had moderate or more severe OSA as defined by an oAHI greater than 15. The median nadir saturation was 85% (IQR, 80–89%; range, 40–96%). The median central sleep apnea index was 0 events/h (IQR, 0–0.27; range, 0–8). The median HAA value was 4.4% (IQR, 3.8–5.4%) and the prevalence of ILA was 11.3%. Baseline characteristics of those in the biomarker subset were similar to those of the overall cohort (Table 1).
Table 1.
Baseline characteristics of study participants
| Obstructive AHI Category |
Biomarker Subset* | |||
|---|---|---|---|---|
| <5 Events/h | 5 to <15 Events/h | ≥15 Events/h | ||
| Participants, n | 600 | 547 | 543 | 99 |
| Age, yr | 67 ± 9 | 69 ± 9 | 69 ± 9 | 69 ± 8 |
| Female | 67% | 54% | 38% | 48% |
| Race/ethnicity | ||||
| White | 37% | 38% | 30% | 37% |
| African-American | 29% | 27% | 27% | 31% |
| Hispanic | 21% | 23% | 29% | 18% |
| Asian | 13% | 12% | 14% | 14% |
| Body mass index, kg/m2 | 27 ± 5 | 29 ± 5 | 31 ± 6 | 28 ± 4 |
| <25 kg/m2 | 41% | 23% | 15% | 21% |
| 25–30 kg/m2 | 36% | 43% | 35% | 56% |
| >30 kg/m2 | 23% | 34% | 50% | 22% |
| Height, cm | 164 ± 10 | 165 ± 10 | 167 ± 10 | 166 ± 10 |
| Weight, kg | 73 ± 16 | 78 ± 15 | 86 ± 18 | 76 ± 14 |
| Waist circumference, cm | 94 ± 14 | 100 ± 13 | 104 ± 14 | 97 ± 10 |
| Hip circumference, cm | 103 ± 11 | 106 ± 11 | 109 ± 12 | 103 ± 8 |
| Smoking status | ||||
| Never-smokers | 51% | 45% | 45% | 49% |
| Former smokers | 41% | 47% | 49% | 49% |
| Current smokers | 8% | 8% | 6% | 3% |
| Education | ||||
| Less than high school | 15% | 14% | 17% | 14% |
| High school | 44% | 47% | 47% | 38% |
| College | 41% | 39% | 36% | 48% |
| Resting SpO2, % | 96 ± 1.2 | 96 ± 1.4 | 96 ± 1.3 | 96 ± 1.4 |
| Forced vital capacity, % predicted | 97 ± 17 | 98 ± 19 | 97 ± 17 | 100 ± 17 |
Definition of abbreviations: AHI = apnea–hypopnea index; SpO2 = oxygen saturation as measured by pulse oximetry.
Data are presented as means ± SD and as percentages.
Obstructive Apnea–Hypopnea Index
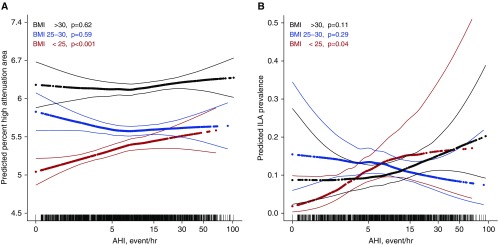
In a precision variable-adjusted model, moderate or more severe OSA (oAHI > 15) was associated with a 7.2% HAA increment (95% CI, 4.5–10%; P < 0.001) compared with those with an oAHI less than 5 (Table 2). This association was somewhat attenuated but remained significant after adjustment for age, sex, race, educational attainment, height, smoking status, cigarette pack-years, glomerular filtration rate, study site, percent emphysema, radiation dose, and total volume of imaged lung (4.0% HAA increment; 95% CI, 1.4–6.8; P = 0.003). In this adjusted model, however, the association between oAHI and HAA appeared to vary by BMI (P for interaction = 0.08), with a stronger association among those with a BMI less than 25 kg/m2. Moderate or more severe OSA was associated with a 6.1% HAA increment (95% CI, 0.5–12.0%; P = 0.03) among normal-weight participants, a 0.8% HAA increment (95% CI, –3.2 to 5.1%; P = 0.40) among overweight participants, and a 2.7% HAA increment (95% CI, –1.6 to 7.3%; P = 0.23) among obese participants; Table 2). Although there was a significant trend across oAHI categories among obese participants (P = 0.03), there was no detectable association among obese participants (P = 0.10; see Table E1 in the online supplement). In modeling the oAHI as a continuous variable, greater oAHI was associated with greater HAA among normal-weight participants (mean, 1.0% HAA increment per 5-unit increment in oAHI; 95% CI, 0.1–1.9%; P = 0.03; Table E1 and Figure 1A), but not among obese participants (P = 0.10).
Table 2.
Associations of the obstructive apnea–hypopnea index with subclinical interstitial lung disease on computed tomography in the Multi-Ethnic Study of Atherosclerosis
| Mean Percent HAA Increment Compared with oAHI < 5 Events/h (95% CI) |
Odds Ratio for ILA Compared with oAHI < 5 Events/h (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|
| <5 Events/h | 5–15 Events/h | >15 Events/h | P Trend | <5 Events/h | 5–15 Events/h | >15 Events/h | P Trend | |
| Precision adjusted | 0 (Ref) | 1.4 (–1.2 to 3.9) | 7.2 (4.5 to 10) | <0.001 | 1 (Ref) | 1.32 (0.87 to 2.00) | 1.64 (1.36 to 1.98) | <0.001 |
| Adjusted | 0 | 0.2 (–0.2 to 2.7) | 4.0 (1.4 to 6.8) | 0.001 | 1 | 0.96 (0.62 to 1.51) | 1.35 (1.13 to 1.61) | <0.001 |
| Adjusted with BMI interaction terms | ||||||||
| <25 kg/m2 | 0 | 4.7 (0.1 to 9.5) | 6.1 (0.5 to 12.0) | 0.03 | 1 | 2.06 (1.16 to 3.6) | 2.31 (1.30 to 4.12) | 0.005 |
| 25–30 kg/m2 | 0 | −1.9 (–5.6 to 2.0) | 0.8 (–3.2 to 5.1) | 0.48 | 0.71 (0.35 to 1.43) | 0.51 (0.23 to 1.12) | 0.10 | |
| >30 kg/m2 | 0 | −3.1 (–7.5 to 1.5) | 2.7 (–1.6 to 7.3) | 0.03 | 0.77 (0.29 to 2.07) | 1.82 (1.13 to 2.93) | <0.001 | |
| Adjusted with smoking interaction term | ||||||||
| Never-smokers | 0 | 3.5 (–0.01 to 7.4) | 9.6 (5.6 to 13.6) | <0.001 | 1 | 0.73 (0.32 to 1.64) | 1.38 (0.84 to 2.28) | 0.01 |
| Ever-smokers | 0 | 0.3 (–3.0 to 3.8) | 5.2 (1.6 to 8.9) | 0.002 | 1 | 1.33 (0.75 to 2.35) | 1.50 (0.99 to 2.27) | 0.050 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; HAA = high-attenuation area; ILA = interstitial lung abnormality; oAHI = obstructive apnea–hypopnea index; Ref = reference category.
The precision-adjusted HAA model includes milliampere (mA) dose, imaged lung volume, and study site for HAA. Note that mA dose is BMI-based, and that this model adjusts for BMI greater than 30 and for BMI less than 20. The precision-adjusted ILA model includes only cluster-robust variance estimation to account for study site. The adjusted HAA model includes adjustment for age, sex, race, educational attainment, height, smoking status, cigarette pack-years, glomerular filtration rate, study site, percent emphysema, milliampere radiation dose, and total volume of imaged lung. The adjusted ILA model includes adjustment for age, sex, race, smoking status, cigarette pack-years, and cluster-robust variance estimation for study site.
For the HAA model: P = 0.08 for the interaction between oAHI and BMI, and P = 0.23 for the interaction between oAHI and smoking. For the ILA model, P = 0.04 for the interaction between nadir oAHI and BMI, and P = 0.46 for the interaction between oAHI and smoking.
Figure 1.
Continuous associations of the obstructive apnea hypopnea index with (A) high-attenuation areas and (B) interstitial lung abnormalities stratified by body mass index (BMI) category. HAA models are adjusted for age, sex, race/ethnicity, smoking status and pack-years, educational attainment, height, glomerular filtration rate, percent emphysema on CT, milliampere dose, total imaged lung volume, and study site. ILA models are adjusted for age, sex, race/ethnicity, smoking status, and pack-years. The thick lines represent the overall adjusted effect estimate; thin lines indicate the 95% confidence bands. Each vertical mark in the rug plot along the x-axis represents one study participant. P values are from generalized additive models and differ somewhat from the P values in Table E2, which are from generalized linear models. AHI = apnea–hypopnea index; CT = computed tomography; HAA = high-attenuation area; ILA = interstitial lung abnormality.
The prevalence of ILA increased across categories of oAHI: 8.9, 11.5, and 13.9% among those with an oAHI less than 5, 5–15, and more than 15, respectively (P for trend < 0.001). After adjustment for age, sex, race, smoking status, and cigarette pack-years, an oAHI greater than 15 was associated with 35% increased odds of ILA (95% CI, 13–61%; P = 0.001) compared with those with an oAHI less than 5 (Table 2). In this model, the association between oAHI and ILA appeared to vary by BMI category (P for interaction = 0.04). Moderate or more severe OSA was associated with 2.3-fold increased odds of ILA (95% CI, 1.3–4.1; P = 0.005) among normal-weight participants and 1.8-fold increased odds of ILA (95% CI, 1.1–2.9; P = 0.01) among obese participants (Table 2). There was no detectable association between OSA and ILA among overweight participants. Among normal-weight participants, each 5-unit increment in oAHI was associated with 29% increased odds of ILA (95% CI, 13–48%; P < 0.001; Table E1 and Figure 1B).
There was no evidence that the association between oAHI and either HAA or ILA varied by smoking status (P values for interaction > 0.20), and these associations were qualitatively similar among never-smokers and among ever-smokers (Table 2 and Table E1).
Sleep-related Hypoxemia
There was a strong inverse correlation between oAHI and nadir saturation (Spearman ρ = –0.75; P < 0.001). More severe sleep-related hypoxemia (measured by lower nadir saturation during sleep) was associated with greater HAA (Table 3). For example, in a precision variable-adjusted model, a nadir saturation less than 80% was associated with a 6.1% HAA increment (95% CI, 2.6–9.8%; P = 0.001; Table 3). In an adjusted model, HAA increased across categories of nadir saturation (P for trend = 0.01; Table 3), and a nadir saturation less than 80% was associated with a 4.6% HAA increment (95% CI, 1.3–8.0%; P = 0.02; Table 3). The association between nadir saturation and HAA, however, varied by BMI category (P for interaction = 0.02), with stronger associations among normal-weight (P for trend = 0.049) and obese participants (P for trend = 0.03) than among overweight participants (P for trend = 0.79; Table 3). Similar associations were found when nadir saturation was treated as a continuous variable (Table E1). Each 5% absolute decrement in nadir saturation was associated with a 1.9% HAA increment (95% CI, 0.3–3.5; P = 0.02) among normal-weight participants and with a 1.2% HAA increment (95% CI, 0.3–2.2; P = 0.01) among obese participants (Table E1).
Table 3.
Associations of sleep-related hypoxemia (nadir saturation) with subclinical interstitial lung disease on computed tomography in Multi-Ethnic Study of Atherosclerosis
| Mean Percent HAA Increment Compared with a Saturation ≥ 90% (95% CI) |
Odds Ratio for ILA Compared with a Saturation ≥ 90% (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|
| <80% | 80–89% | ≥90% | P Trend | <80% | 80–89% | ≥90% | P Trend | |
| Precision adjusted | 6.1 (2.6 to 9.8) | 3.8 (0.9 to 6.8) | 0 (Ref) | 0.003 | 1.64 (1.23 to 2.17) | 1.21 (0.92 to 1.61) | 1 (Ref) | <0.001 |
| Adjusted | 4.6 (1.3 to 8.0) | 2.4 (–0.4 to 5.2) | 0 | 0.01 | 1.35 (0.86 to 2.11) | 1.09 (0.76 to 1.58) | 1 | 0.08 |
| Adjusted with BMI interaction term | ||||||||
| <25 kg/m2 | 6.9 (–0.1 to 14.3) | 3.1 (–1.1 to 7.5) | 0 | 0.049 | 0.71 (0.14 to 3.60) | 2.02 (0.93 to 4.41) | 1 | 0.59 |
| 25–30 kg/m2 | −0.5 (–5.7 to 4.9) | 2.1 (–2.4 to 6.8) | 0 | 0.79 | 0.86 (0.28 to 2.62) | 0.70 (0.28 to 1.76) | 1 | 0.95 |
| >30 kg/m2 | 2.6 (–4.1 to 9.8) | −1.8 (–8.0 to 4.8) | 0 | 0.03 | 1.89 (0.48 to 7.41) | 1.31 (0.27 to 6.30) | 1 | 0.003 |
| Adjusted with smoking interaction term | ||||||||
| Never-smokers | 8.1 (3.3 to 13.2) | 4.4 (0.4 to 8.6) | 0 | 0.002 | 0.97 (0.50 to 1.89) | 0.98 (0.40 to 2.37) | 1 | 0.93 |
| Ever-smokers | 9.0 (4.2 to 14.1) | 5.1 (1.0 to 9.4) | 0 | 0.001 | 2.29 (1.08 to 4.82) | 1.51 (0.72 to 3.16) | 1 | 0.008 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; HAA = high-attenuation area; ILA = interstitial lung abnormality; Ref = reference category; SpO2 = oxygen saturation as measured by pulse oximetry.
The precision-adjusted HAA model includes milliampere (mA) dose, imaged lung volume, and study site for HAA. Note that mA dose is BMI based, and that this model adjusts for BMI greater than 30 and for BMI less than 20. The precision-adjusted ILA model includes only cluster-robust variance estimation to account for study site. The adjusted HAA model includes adjustment for age, sex, race, educational attainment, height, smoking status, cigarette pack-years, glomerular filtration rate, study site, percent emphysema, milliampere radiation dose, and total volume of imaged lung. The adjusted ILA model includes adjustment for age, sex, race, smoking status, cigarette pack-years, and cluster-robust variance estimation for study site.
For the HAA model, P = 0.02 for the interaction between nadir SpO2 and BMI, and P = 0.96 for the interaction between nadir SpO2 and smoking. For the ILA model, P = 0.56 for the interaction between nadir SpO2 and BMI, and P = 0.42 for the interaction between nadir SpO2 and smoking.
The prevalence of ILA increased across descending categories of nadir saturation: 9.3, 10.9, and 14.1% among those with a nadir saturation greater than 90, 80–90, and less than 80%, respectively (P for trend < 0.001). A nadir saturation less than 80% was strongly associated with 1.6-fold higher odds of ILA (95% CI, 1.2–2.2; P = 0.001) in an unadjusted model. This finding was not statistically significant in an adjusted model, and there was no statistically significant evidence that the association varied by BMI category (P for interaction = 0.56) or by smoking (P for interaction = 0.42; Table 3 and Table E1). Nevertheless, in an adjusted model treating nadir saturation as a continuous variable, lower nadir saturation was associated with increased odds of ILA overall (odds ratio [OR], 1.19 per 10% relative decrement in saturation; 95% CI, 1.09–1.30; P < 0.001) and among obese participants (OR, 1.24; 95% CI, 1.15–1.35; P < 0.001; Table E1). A nadir saturation less than 80% was associated with higher odds of ILA among ever-smokers (OR, 2.29; 95% CI, 1.08–4.82; P = 0.03) but not among never-smokers (Table 3).
Central Sleep Apnea Index
The central sleep apnea index was not associated with HAA or ILA in adjusted models (Table E2). There was no evidence of effect modification by BMI category or smoking.
Role of Lung Volume
Among those with a BMI less than 25 kg/m2, we found weak evidence that variation in lung volume explained a small fraction (∼10%) of the association of either HAA or ILA with oAHI (Table 4) (25). There was somewhat stronger evidence that lower lung volumes explained some of the association between HAA and nadir saturation (25% explained by lung volumes), but no evidence that lung volumes explained the association between ILA and nadir saturation (Table 4).
Table 4.
Examination of lung volume as an explanatory factor for associations between subclinical interstitial lung disease and obstructive sleep apnea metrics among those with body mass index less than 25 kg/m2*
| Total Effect Coefficient | Direct Effect Coefficient | Indirect Effect Coefficient | Percentage of Total Effect Explained by Lung Volumes | Interpretation | |
|---|---|---|---|---|---|
| HAA | |||||
| oAHI | 0.75 | 0.67 | 0.08 | 11% | Partial (weak) mediation |
| Nadir saturation | 0.03 | 0.02 | 0.01 | 25% | Partial (weak) mediation |
| ILA | |||||
| oAHI | 0.25 | 0.23 | 0.03 | 10% | Partial (weak) mediation |
| Nadir saturation | −0.0028 | −0.0043 | 0.0015 | N/A | Suppression |
Definition of abbreviations: CT = computed tomography; HAA = high-attenuation area; ILA = interstitial lung abnormality; N/A = not applicable; oAHI = obstructive apnea–hypopnea index.
Lung volumes were measured on full lung CT scans performed at full inspiration. HAA models are adjusted for age, sex, smoking, site, radiation dose, and percent emphysema. ILA models are adjusted for age, sex, and smoking.
Serum MMP-7 and SP-A
The median serum SP-A level was 49 ng/ml (IQR, 34–75 ng/ml), and the median serum MMP-7 level was 3.8 ng/ml (IQR, 3.1–4.8 ng/ml). A greater oAHI was associated with higher levels of MMP-7 (mean, 3.4% MMP-7 increment per 5-unit increase in obstructive AHI; 95% CI, 0.8–60; P = 0.01), and there was a nonsignificant trend toward an association between greater oAHI and higher SP-A levels (mean, 4.1% SP-A increment per 5-unit increase in obstructive AHI; 95% CI, –0.5 to 8.9; P = 0.08) in models adjusted for age, sex, race/ethnicity, BMI, and smoking status (Table 5). Notably, however, both of these associations varied by BMI (P for interaction < 0.001 for MMP-7, and 0.03 for SP-A), with stronger associations among normal-weight participants (Table 5). Lower nadir saturation was associated with higher SP-A levels but was not associated with MMP-7 (Table 5) without evidence of effect modification by BMI. There were no meaningful associations between the central sleep apnea index and either biomarker.
Table 5.
Associations between measures of sleep-disordered breathing and serum biomarkers
| Mean Percent Biomarker Increment* | 95% CI | P Value | P for Interaction with BMI | |
|---|---|---|---|---|
|
Obstructive AHI | ||||
| MMP-7 | <0.001 | |||
| Overall | 3.4 | 0.8 to 6.0 | 0.01 | |
| BMI < 25 | 16.6 | 9.7 to 24.0 | <0.001 | |
| BMI > 25 | 1.6 | −0.9 to 4.1 | 0.21 | |
| SP-A | 0.03 | |||
| Overall | 4.1 | −0.5 to 8.9 | 0.08 | |
| BMI < 25 | 17.5 | 4.5 to 32.2 | 0.008 | |
| BMI > 25 | 1.7 | −3.0 to 6.6 | 0.48 | |
|
Nadir Saturation | ||||
| MMP-7 | 0.19 | |||
| Overall | 4.2 | −0.4 to 9.0 | 0.07 | |
| BMI < 25 | 15.0 | −0.8 to 33.2 | 0.06 | |
| BMI > 25 | 3.6 | −1.2 to 8.7 | 0.15 | |
| SP-A | 0.15 | |||
| Overall | 9.1 | 0.8 to 18.1 | 0.03 | |
| BMI < 25 | 22.8 | −5.3 to 59.1 | 0.12 | |
| BMI > 25 | 6.4 | −2.2 to 15.7 | 0.15 | |
|
Central Sleep Apnea Index | ||||
| MMP-7 | 0.93 | |||
| Overall | 6.8 | −3.8 to 18.5 | 0.21 | |
| BMI < 25 | 5.4 | −8.1 to 21.0 | 0.45 | |
| BMI > 25 | 6.4 | −9.0 to 24.4 | 0.43 | |
| SP-A | 0.95 | |||
| Overall | −2.7 | −19.4 to 17.5 | 0.77 | |
| BMI < 25 | 0.8 | −21.0 to 28.7 | 0.95 | |
| BMI > 25 | −0.4 | −24.5 to 31.5 | 0.98 | |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; CI = confidence interval; MMP-7 = metalloproteinase-7; SP-A = surfactant protein-A.
All models adjusted for age, sex, race, and smoking status. “Overall’ models include adjustment for BMI.
Adjusted mean percent increment in each biomarker per 5-unit absolute increase in obstructive AHI, 5% absolute decrement in nadir saturation, or 1-unit increase in central sleep apnea index.
Discussion
In this study of community-dwelling adults without clinical ILD, we found that the frequency of sleep-related upper airway obstruction and the severity of sleep-related hypoxemia were each associated with two distinct measures of subclinical ILD on CT, particularly among normal-weight adults. OSA measures were also associated with serum biomarker evidence of AEC injury and ECM remodeling among normal-weight adults. Together, our findings provide evidence to support the hypothesis that OSA contributes to subclinical alveolar injury and remodeling, and therefore might be one of the “microinjuries” postulated to contribute to lung fibrosis.
OSA is recognized as a prevalent comorbidity among adults with clinically diagnosed IPF, yet the pathways connecting the two have remained undefined. We and others have previously hypothesized that OSA might cause early AEC injury through one of a variety of mechanisms. For example, cyclic hypoxia–reoxygenation during the intermittent breathing of OSA (14, 26) could contribute to oxidative injury and inflammation of the alveolar epithelium (27). In addition, obstructive apneas and hypopneas might cause stretch-mediated AEC injury. Indeed, stretch is a widely recognized cause of AEC injury in both animal models and in humans subjected to high-tidal volume ventilation during the acute respiratory distress syndrome (28). It is possible that linear stretch of AECs, even in the absence of alveolar inflation, may lead to strain-induced cellular injury. Each obstructive event is a forced inspiration against a closed upper airway, a maneuver that can lead to negative esophageal pressures as large as –60 cm H2O (29, 30). Although simultaneous reductions in pleural and mean alveolar pressure might lead to only small changes in average transpulmonary pressures, large swings in intrathoracic pressure cause regional pressure differences, particularly in peripheral occluded airways (31). The closure of peripheral airways that is thought to occur during obstructive events (32) may therefore facilitate locally large tractional forces at the periphery of the lung even in the absence of overall changes in lung gas volume. This localized alveolar strain may be further exacerbated by the rapid lung inflation with large tidal volumes that occurs at apnea termination. Moreover, cyclic alveolar deformation, as would be expected to occur in OSA, is more injurious than tonic deformation (10). Supporting this hypothesis, inspiratory resistive loading (analogous to an obstructive apnea) causes AEC injury and promotes lung inflammation in mice (33). AECs produce IL-8 and monocyte chemotactic protein-1 in response to strain and deformation in vitro (11–14), providing potential mechanisms linking stretch forces generated by obstructed respiratory events and lung inflammation. Finally, OSA has been postulated to promote gastroesophageal reflux disease (34), which has been implicated as a possible cause of IPF (35). However, data suggest that gastroesophageal reflux disease may not occur during OSA (36–38), making this a less likely mediator of our observed association between OSA and subclinical ILD.
Some have proposed that the lower lung volumes observed in ILD might promote OSA by reducing traction on the trachea and increasing pharyngeal collapsibility or by worsening sleep-related hypoxemia with hypopneic events (increasing the likelihood that those events are identified on the basis of associated desaturation) (39–41). Our data, however, argue against this hypothesis. Importantly, we did not study patients with clinically evident ILD and reduced lung volumes. Rather, we studied a community-based sample of middle-aged and older adults in whom subclinical ILD on CT is not expected to lead to a clinically relevant reduction in lung volumes. In our study, forced vital capacity and resting oxygen saturation were largely in the normal range. Second, we found that variation in lung volumes explained only 10–25% of the association between subclinical ILD and OSA, indicating that the majority of our findings were not due to the small variation in lung volumes observed in our study sample.
We observed a complex relationship between body weight and the association between OSA and subclinical ILD. Body weight appeared to modify many of the associations we observed across three measures of subclinical ILD—HAA, ILA, and serum MMP-7 levels—and for both the obstructive AHI and nadir saturation. The strongest associations between OSA measures and subclinical ILD were consistently found among normal-weight participants, a group in whom the impact of adiposity on lung attenuation, lung volumes, inflammation, and sleep-related hypoxemia is likely small, if not eliminated, perhaps yielding a “cleaner” signal. We have previously detected similar findings, namely that impaired glucose tolerance and OSA are more strongly associated among nonobese than obese individuals (42). Alternatively, because normal-weight individuals are generally more fit and may have stronger respiratory muscles than obese individuals (43), they may be able to generate higher inspiratory pressures terminating periods of airway closure, which we postulate may lead to regional transpleural pressure changes. In contrast, we found that OSA measures were associated with subclinical ILD (particularly ILA) among obese participants in some analyses, and we found no associations among overweight participants. The factors underlying these discrepant findings are not immediately clear. Greater nocturnal desaturation among obese participants may have led to identification of a greater number of hypopneas associated with desaturation (44), easing the ability to detect an association between OSA measures and subclinical ILD.
Two prior studies have identified circulating biomarker evidence of AEC injury in adults with OSA who do not have concomitant lung disease. One study of 11 case subjects with OSA and 10 control subjects, and a second study of 150 case subjects with OSA and 20 control subjects, found higher circulating levels of Krebs von den Lungen-6 (KL-6), a MUC1 glycoprotein found in type 2 AECs, among case subjects with OSA (4, 45). KL-6 is released into the circulation during AEC injury and found at elevated levels in the serum in patients with acute respiratory distress syndrome and in clinically diagnosed ILD (46–48). Similarly, serum levels of SP-A, a glycoprotein produced by type 2 AECs as well as by club cells and airway submucosal gland cells (49), are elevated after lung injury (50, 51) and in IPF (52, 53). Our study provides additional evidence that OSA may contribute to subclinical AEC injury.
Data from animal models and human studies indicate that MMP-7, a mediator of ECM remodeling, plays a key role in IPF, both as a contributor to the disease and as a diagnostic and prognostic serum biomarker (54–56). HAA is associated with elevated serum MMP-7 levels in community-dwelling adults (2). This is the first study, to our knowledge, to show that measures of OSA severity are associated with greater serum MMP-7 levels. Although circulating MMP-7 may arise from extrapulmonary sources, the associations observed between MMP-7 levels and measures of OSA support the hypothesis that OSA may be a contributor to ECM remodeling in the lung even among community-dwelling adults.
Our findings should be interpreted with several caveats. First, the cross-sectional nature of our study limits our ability to determine whether OSA precedes or follows subclinical ILD, or whether both are due to an antecedent event. Second, our study does not provide evidence for treatment of OSA as a means for preventing ILD. Our findings do, however, support the novel hypothesis that OSA may be a modifiable risk factor for ILD, and provide strong support for future investigations testing whether OSA treatment can attenuate lung injury and disease progression in IPF and other ILDs. Finally, we note that although HAA and ILA have physiological, biomarker, and radiological features of ILD, neither has been validated histopathologically.
In summary, our findings show that OSA is associated with subtle structural changes to the alveolus manifest as increased CT lung attenuation, visually identifiable abnormalities on CT, and elevations in serum biomarkers known to represent AEC injury and ECM remodeling in community-dwelling adults. These results suggest that OSA may contribute to “microinjuries” to the alveolar epithelium, which precede clinically evident ILD in susceptible individuals. In the context of the limited number of existing targets, OSA should be considered a therapeutic target for both prevention and treatment of ILD in clinical trials.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Supported by National Institutes of Health contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 and grants UL1-TR-000040, UL1-TR-001079, R01-HL-103676, R01-HL-098433, T32-HL-105323, and K24-HL-131937; by the Pulmonary Fibrosis Foundation; and by the Rocco Guinta Research Fund. This publication was developed under a STAR research assistance agreement, No. RD831697 (MESA Air), awarded by the U.S. Environmental Protection Agency.
This manuscript has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA does not endorse any products or commercial services mentioned in this publication.
Author Contributions: Conception and design of the study: A.J.P., S.M.K., G.R., J.D.K., R.G.B., E.A.H., S.S.R., D.J.L.; data acquisition: J.D.K., R.G.B., K.D.H.S., E.A.H., S.S.R., D.J.L.; analysis of the data: J.S.K., A.J.P., P.B., R.G.B., D.J.G., S.S.R., D.J.L.; J.S.K. drafted the initial manuscript. All authors contributed to data interpretation and edited the manuscript for important scientific content. All of the authors agree to be accountable for all aspects of the work regarding its integrity and accuracy.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.King TE, Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 2.Podolanczuk AJ, Oelsner EC, Barr RG, Hoffman EA, Armstrong HF, Austin JH, Basner RC, Bartels MN, Christie JD, Enright PL, et al. High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J. 2016;48:1442–1452. doi: 10.1183/13993003.00129-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leslie KO. Idiopathic pulmonary fibrosis may be a disease of recurrent, tractional injury to the periphery of the aging lung: a unifying hypothesis regarding etiology and pathogenesis. Arch Pathol Lab Med. 2012;136:591–600. doi: 10.5858/arpa.2011-0511-OA. [DOI] [PubMed] [Google Scholar]
- 4.Lederer DJ, Jelic S, Basner RC, Ishizaka A, Bhattacharya J. Circulating KL-6, a biomarker of lung injury, in obstructive sleep apnoea. Eur Respir J. 2009;33:793–796. doi: 10.1183/09031936.00150708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lederer DJ, Jelic S, Bhattacharya J, Basner RC. Is obstructive sleep apnea a cause of idiopathic pulmonary fibrosis? Arch Pathol Lab Med. 2012;136:470–, author reply 470. doi: 10.5858/arpa.2011-0650-LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancaster LH, Mason WR, Parnell JA, Rice TW, Loyd JE, Milstone AP, Collard HR, Malow BA. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136:772–778. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mermigkis C, Stagaki E, Tryfon S, Schiza S, Amfilochiou A, Polychronopoulos V, Panagou P, Galanis N, Kallianos A, Mermigkis D, et al. How common is sleep-disordered breathing in patients with idiopathic pulmonary fibrosis? Sleep Breath. 2010;14:387–390. doi: 10.1007/s11325-010-0336-5. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glucksberg MR, Bhattacharya J. Effect of alveolar and pleural pressures on interstitial pressures in isolated dog lungs. J Appl Physiol (1985) 1991;70:914–918. doi: 10.1152/jappl.1991.70.2.914. [DOI] [PubMed] [Google Scholar]
- 10.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells: effect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000;162:357–362. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- 11.Hammerschmidt S, Kuhn H, Sack U, Schlenska A, Gessner C, Gillissen A, Wirtz H. Mechanical stretch alters alveolar type II cell mediator release toward a proinflammatory pattern. Am J Respir Cell Mol Biol. 2005;33:203–210. doi: 10.1165/rcmb.2005-0067OC. [DOI] [PubMed] [Google Scholar]
- 12.Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol. 1999;277:L167–L173. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- 13.Kuebler WM, Ying X, Singh B, Issekutz AC, Bhattacharya J. Pressure is proinflammatory in lung venular capillaries. J Clin Invest. 1999;104:495–502. doi: 10.1172/JCI6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 16.Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, Couper D, Goldin J, Guo J, Han MK, et al. SPIROMICS Research Group. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194:794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RS, Lynch DA, Brehm JM, et al. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, Sciurba FC, Hunninghake GM, San José Estépar R, Silverman EK, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol. 2010;17:48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 20.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-Lung Study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, Reinhardt J, Rodriguez J, Stukovsky K, Wong ND, et al. Reproducibility and validity of lung density measures from cardiac CT scans: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein EJ, Barr RG, Austin JHM, Kawut SM, Raghu G, Sell JL, Hoffman EA, Newell JD, Jr, Watts JR, Jr, Nath PH, et al. Rheumatoid arthritis–associated autoantibodies and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis. Thorax. 2016;71:1082–1090. doi: 10.1136/thoraxjnl-2016-208932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, Jackson CL, Williams MA, Redline S. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hastie T. gam: Generalized Additive Models. R package 1.14. Palo Alto, CA: Stanford University; 2016 [accessed 5 July 2017]. Available from: https://cran.r-project.org/package=gam.
- 25.MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1:173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider H, Schaub CD, Chen CA, Andreoni KA, Schwartz AR, Smith PL, Robotham JL, O’Donnell CP. Neural and local effects of hypoxia on cardiovascular responses to obstructive apnea. J Appl Physiol (1985) 2000;88:1093–1102. doi: 10.1152/jappl.2000.88.3.1093. [DOI] [PubMed] [Google Scholar]
- 27.Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–1484. doi: 10.1183/09031936.00086608. [DOI] [PubMed] [Google Scholar]
- 28.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema: respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 29.Buda AJ, Pinsky MR, Ingels NB, Jr, Daughters GT, II, Stinson EB, Alderman EL. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med. 1979;301:453–459. doi: 10.1056/NEJM197908303010901. [DOI] [PubMed] [Google Scholar]
- 30.Tolle FA, Judy WV, Yu PL, Markand ON. Reduced stroke volume related to pleural pressure in obstructive sleep apnea. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1718–1724. doi: 10.1152/jappl.1983.55.6.1718. [DOI] [PubMed] [Google Scholar]
- 31.Loring SH, Topulos GP, Hubmayr RD. Transpulmonary pressure: the importance of precise definitions and limiting assumptions. Am J Respir Crit Care Med. 2016;194:1452–1457. doi: 10.1164/rccm.201512-2448CP. [DOI] [PubMed] [Google Scholar]
- 32.Bijaoui EL, Champagne V, Baconnier PF, Kimoff RJ, Bates JH. Mechanical properties of the lung and upper airways in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:1055–1061. doi: 10.1164/ajrccm.165.8.2107144. [DOI] [PubMed] [Google Scholar]
- 33.Toumpanakis D, Kastis GA, Zacharatos P, Sigala I, Michailidou T, Kouvela M, Glynos C, Divangahi M, Roussos C, Theocharis SE, et al. Inspiratory resistive breathing induces acute lung injury. Am J Respir Crit Care Med. 2010;182:1129–1136. doi: 10.1164/rccm.201001-0116OC. [DOI] [PubMed] [Google Scholar]
- 34.Xiao YL, Liu FQ, Li J, Lv JT, Lin JK, Wen WP, Chen MH. Gastroesophageal and laryngopharyngeal reflux profiles in patients with obstructive sleep apnea/hypopnea syndrome as determined by combined multichannel intraluminal impedance-pH monitoring. Neurogastroenterol Motil. 2012;24:e258–e265. doi: 10.1111/j.1365-2982.2012.01920.x. [DOI] [PubMed] [Google Scholar]
- 35.Tobin RW, Pope CE, II, Pellegrini CA, Emond MJ, Sillery J, Raghu G. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158:1804–1808. doi: 10.1164/ajrccm.158.6.9804105. [DOI] [PubMed] [Google Scholar]
- 36.Kuribayashi S, Massey BT, Hafeezullah M, Perera L, Hussaini SQ, Tatro L, Darling RJ, Franco R, Shaker R. Upper esophageal sphincter and gastroesophageal junction pressure changes act to prevent gastroesophageal and esophagopharyngeal reflux during apneic episodes in patients with obstructive sleep apnea. Chest. 2010;137:769–776. doi: 10.1378/chest.09-0913. [DOI] [PubMed] [Google Scholar]
- 37.Pillai M, Olson AL, Huie TJ, Solomon JJ, Fernandez-Perez ER, Brown KK, Hanna P, Lee-Chiong T, Swigris JJ. Obstructive sleep apnea does not promote esophageal reflux in fibrosing interstitial lung disease. Respir Med. 2012;106:1033–1039. doi: 10.1016/j.rmed.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shepherd KL, James AL, Musk AW, Hunter ML, Hillman DR, Eastwood PR. Gastro-oesophageal reflux symptoms are related to the presence and severity of obstructive sleep apnoea. J Sleep Res. 2011;20:241–249. doi: 10.1111/j.1365-2869.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- 39.Bradley TD, Brown IG, Grossman RF, Zamel N, Martinez D, Phillipson EA, Hoffstein V. Pharyngeal size in snorers, nonsnorers, and patients with obstructive sleep apnea. N Engl J Med. 1986;315:1327–1331. doi: 10.1056/NEJM198611203152105. [DOI] [PubMed] [Google Scholar]
- 40.Heinzer RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, Schory K, White DP. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:114–117. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TC. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep. 2007;30:179–186. doi: 10.1093/sleep/30.2.179. [DOI] [PubMed] [Google Scholar]
- 42.Seicean S, Kirchner HL, Gottlieb DJ, Punjabi NM, Resnick H, Sanders M, Budhiraja R, Singer M, Redline S. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31:1001–1006. doi: 10.2337/dc07-2003. [DOI] [PubMed] [Google Scholar]
- 43.Sahebjami H, Gartside PS. Pulmonary function in obese subjects with a normal FEV1/FVC ratio. Chest. 1996;110:1425–1429. doi: 10.1378/chest.110.6.1425. [DOI] [PubMed] [Google Scholar]
- 44.Peppard PE, Ward NR, Morrell MJ. The impact of obesity on oxygen desaturation during sleep-disordered breathing. Am J Respir Crit Care Med. 2009;180:788–793. doi: 10.1164/rccm.200905-0773OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aihara K, Oga T, Harada Y, Chihara Y, Handa T, Tanizawa K, Watanabe K, Tsuboi T, Hitomi T, Mishima M, et al. Comparison of biomarkers of subclinical lung injury in obstructive sleep apnea. Respir Med. 2011;105:939–945. doi: 10.1016/j.rmed.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 46.Ishizaka A, Matsuda T, Albertine KH, Koh H, Tasaka S, Hasegawa N, Kohno N, Kotani T, Morisaki H, Takeda J, et al. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1088–L1094. doi: 10.1152/ajplung.00420.2002. [DOI] [PubMed] [Google Scholar]
- 47.Sato H, Callister ME, Mumby S, Quinlan GJ, Welsh KI, duBois RM, Evans TW. KL-6 levels are elevated in plasma from patients with acute respiratory distress syndrome. Eur Respir J. 2004;23:142–145. doi: 10.1183/09031936.03.00070303. [DOI] [PubMed] [Google Scholar]
- 48.Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity: sialylated carbohydrate antigen KL-6. Chest. 1989;96:68–73. doi: 10.1378/chest.96.1.68. [DOI] [PubMed] [Google Scholar]
- 49.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Doyle IR, Bersten AD, Nicholas TE. Surfactant proteins-A and -B are elevated in plasma of patients with acute respiratory failure. Am J Respir Crit Care Med. 1997;156:1217–1229. doi: 10.1164/ajrccm.156.4.9603061. [DOI] [PubMed] [Google Scholar]
- 51.Greene KE, Wright JR, Steinberg KP, Ruzinski JT, Caldwell E, Wong WB, Hull W, Whitsett JA, Akino T, Kuroki Y, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160:1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 52.Ohnishi H, Yokoyama A, Kondo K, Hamada H, Abe M, Nishimura K, Hiwada K, Kohno N. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med. 2002;165:378–381. doi: 10.1164/ajrccm.165.3.2107134. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi H, Fujishima T, Koba H, Murakami S, Kurokawa K, Shibuya Y, Shiratori M, Kuroki Y, Abe S. Serum surfactant proteins A and D as prognostic factors in idiopathic pulmonary fibrosis and their relationship to disease extent. Am J Respir Crit Care Med. 2000;162:1109–1114. doi: 10.1164/ajrccm.162.3.9910080. [DOI] [PubMed] [Google Scholar]
- 54.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, Macdonald SD, Pardo A, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA. 2002;99:6292–6297. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.