Abstract
Rationale: Sleep disturbance frequently affects patients with chronic obstructive pulmonary disease (COPD), and is associated with reduced quality of life and poorer outcomes. Data indicate that smokers with preserved pulmonary function have clinical symptoms similar to those meeting spirometric criteria for COPD, but little is known about the driving factors for sleep disturbance in this population of emerging interest.
Objectives: To compare the magnitude and correlates of sleep disturbance between smokers with preserved pulmonary function and those with airflow obstruction.
Methods: Using cross-sectional data from the COPD Outcomes-Based Network for Clinical Effectiveness and Research Translation multicenter registry, we identified participants clinically identified as having COPD with a smoking history of at least 20 pack-years and either preserved pulmonary function or airflow obstruction. We quantified sleep disturbance by T-score measured in the sleep disturbance domain of the Patient-Reported Outcomes Information System questionnaire, and defined a minimum important difference as a T-score difference of two points. We performed univariate and multivariable linear regression to evaluate correlates within each group.
Results: We identified 100 smokers with preserved pulmonary function and 476 with airflow obstruction. The sleep disturbance T-score was 4.1 points greater among individuals with preserved pulmonary function (95% confidence interval [CI], 2.0–6.3). In adjusted analyses, depression symptom T-score was associated with sleep disturbance in both groups (airflow obstruction: β, 0.61 points; 95% CI, 0.27–0.94; preserved pulmonary function: β, 0.25 points; 95% CI, 0.12–0.38). Of note, lower percent predicted FEV1 was associated with greater sleep disturbance among those with preserved pulmonary function (β, –0.19 points; 95% CI, –0.31 to –0.07), whereas higher FEV1 was associated with greater sleep disturbance among individuals with airflow obstruction (β, 0.06 points; 95% CI, 0.01–0.10).
Conclusions: Among smokers with clinically identified COPD, the severity of sleep disturbance is greater among those with preserved pulmonary function compared with those with airflow obstruction. Nonrespiratory symptoms, such as depression, were associated with sleep disturbance in both groups, whereas the relationship of sleep disturbance with FEV1 differed.
Keywords: chronic obstructive pulmonary disease, sleep initiation and maintenance disorders, smoking
Among patients with chronic obstructive pulmonary disease (COPD), sleep disturbance leads to significant reductions in health-related quality of life (1) and has been linked with greater frequency of COPD exacerbations and increased mortality (2, 3). The etiology of significant overlap between sleep disturbance and COPD is unclear, and there are likely to be bidirectional interactions between sleep quality and COPD severity and outcomes (4). An understanding of the contributing factors is necessary to expand understanding and to devise effective therapies. Sleep disturbance in patients with COPD has been linked with daytime hypoxemia, overall symptom burden, and comorbidities including depression and anxiety disorders with severity of airflow obstruction associated in some, but not all, studies (1, 2, 5–7).
Our current knowledge of sleep disturbance in COPD arises from patients with airflow obstruction on spirometry, whereas clinically diagnosed COPD also includes individuals with preserved pulmonary function (8). Although many individuals with clinically diagnosed COPD without airflow obstruction are misclassified, evidence from the SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) cohort indicates that symptomatic smokers with preserved pulmonary function on spirometry have respiratory exacerbations, limited activity, poor sleep quality, and radiographic evidence of airway disease (9, 10). In light of the adverse effects that sleep disturbance has on quality of life, it is important that we understand the burden of sleep disturbance and its impact in this population of emerging interest.
To address sleep disturbance in COPD, we made use of data from the COPD Outcomes-Based Network for Clinical Effectiveness and Research Translation (CONCERT) that include detailed information regarding individuals with clinically diagnosed COPD, including those with airflow obstruction on spirometry and those without obstruction. The primary aim of the current investigation was to compare, among individuals with a significant smoking history, the relative magnitude of sleep disturbance symptoms in participants with preserved pulmonary function compared with those with airflow obstruction. The secondary aim of this investigation was to evaluate correlates of sleep disturbance separately among individuals with preserved pulmonary function and those with airflow obstruction. Our overall hypotheses were that sleep disturbance would be greater among participants with airflow obstruction, and that correlations with sleep disturbance in both groups would be observed with respiratory symptoms and objective respiratory pathology (e.g., hypoxemia and severity of obstruction).
Methods
We performed this cross-sectional study with data from the multicenter CONCERT study (8, 11, 12). Briefly, CONCERT used electronic health data to identify participants with COPD from seven major health care systems throughout the United States. CONCERT included individuals if there was evidence of COPD from clinical data including diagnostic International Classification of Diseases, 9th revision (ICD-9) codes for COPD, prescription of inhaled bronchodilators and corticosteroids, or spirometry consistent with obstruction. Among those with evidence of COPD, a sample was invited to complete in-person evaluations that involved spirometry, ascertainment of medical history, COPD symptoms, and quality of life. We included individuals with a greater than 20–pack-year history of smoking and a self-reported physician diagnosis of COPD or ICD-9 billing diagnosis of COPD. We defined airflow obstruction as a postbronchodilator FEV1/FVC ratio less than 0.7 (13). We defined preserved pulmonary function as the absence of obstruction (FEV1/FVC ratio ≥ 0.7) and an FEV1 greater than 80% predicted.
We used the sleep disturbance domain (consisting of six items) of the Patient-Reported Outcomes Information System (PROMIS) questionnaire, short form version 43, as our primary outcome measure. The PROMIS questionnaire (14, 15) is composed of items assessing seven domains including sleep disturbance, anxiety, depression, and pain interference. Each domain is scored and transformed into a standardized T-score, where a score of 50 identifies the U.S. population mean with a standard deviation of 10. Although clinical cut points for sleep disturbance have not been established, we define a minimum important difference (MID) of 0.2 SD, or a T-score of difference of 2, as has been used previously (16). When exploring these symptoms as binary variables, we used a cutoff T-score of 2, or a minimally important difference above the population mean. Higher scores represent a higher degree of symptoms related to that domain (e.g., a higher score in the sleep disturbance domain relates to a greater degree of sleep disturbance symptoms) (15). The sleep disturbance domain of PROMIS has excellent agreement with the commonly used Pittsburgh Sleep Quality Instrument (6, 17, 18), and consists of six questions that assess participants’ ratings of overall sleep quality, difficulty falling asleep, and the degree to which sleep was restful and refreshing. The PROMIS instrument is available at www.healthmeasures.net.
We used the Functional Assessment of Chronic Illness Therapy (FACIT) 10-item instrument to quantify dyspnea (19). All comorbidities are participant-reported physician diagnoses. For those with self-reported, physician-diagnosed sleep apnea, participants were asked about their usage of positive airway pressure (referred to collectively as continuous positive airway pressure [CPAP]). We defined medications, and oxygen/noninvasive positive pressure ventilation use according to participant report, and used the 6-minute walk test to measure oxygenation (20).
Statistical Analysis
To explore the proportion of participants in each group with a substantial burden of respiratory and nonrespiratory symptoms of interest, we compared self-reported respiratory and nonrespiratory symptoms between participants with preserved pulmonary function and those with airflow obstruction, using χ2 tests. In the event of continuously measured symptoms (e.g., PROMIS T-scores), the population mean T-score of 50 plus the minimum important difference of 2 (T-score of 52) was used as the cutoff. We constructed multivariate linear regression models to explore associations for PROMIS sleep disturbance T-score in participants with airflow obstruction and in participants with preserved pulmonary function. Our multivariate models stratified by group status included candidate variables hypothesized to explain variability in sleep disturbance (21). Specifically, we used the following: age, sex, race, education, sleep apnea, untreated sleep apnea, hypoxia (oxygen saturation as measured by pulse oximetry < 88%), hypoxia without oxygen therapy, cough, paroxysmal nocturnal dyspnea, phlegm production, wheezing, body mass index (BMI), PROMIS domains of depression, anxiety, and pain interference, FACIT dyspnea score, smoking status, FEV1, FEV1/FVC ratio, and use of long- and short-acting β-agonists, inhaled corticosteroids, long- and short-acting anticholinergics, and systemic corticosteroids. Stratified by preserved pulmonary function versus airflow obstruction, we also present correlation coefficients for each variable of interest from univariate linear regression. Finally, we performed these analyses involving the entire sample of 576 participants regardless of pulmonary function, and include those results in the online supplement. There were few missing data in our analysis. Data regarding hypoxemia status was missing among 23 participants, dyspnea data in 5, pain survey data in 3, and data about wheezing in 1. Overall, this led to 29 participants (5%) being excluded from multivariate logistic regression (n = 5, 5% from those with preserved pulmonary function; n = 24, 5% from those with airflow obstruction). Analysis was performed with STATA statistical software (StataCorp, College Station, TX), version 14.2.
Results
Our analysis included 576 individuals with a 20–pack-year smoking history and clinically identified COPD, including 100 individuals with preserved pulmonary function, and 476 with airflow obstruction. Both groups were in late middle age (mean age, 66.5 yr [preserved pulmonary function] vs. 67.8 yr [airflow obstruction]), predominantly male (64.0 vs. 62.0% men), and white (82.0 vs. 81.1%; Table 1). In comparison with participants with airflow obstruction, participants with preserved pulmonary function reported a greater magnitude of sleep disturbance (T-score of 54.2 vs. 50.0). In addition, participants with preserved pulmonary function had more comorbidities, including sleep apnea (38.0 vs. 20.6%), depression (58.0 vs. 37.2%), and diabetes (33.0 vs. 21.4%). As expected, fewer individuals with preserved pulmonary function were hypoxemic (14 vs. 43.2%). Of all 163 participants reporting paroxysmal nocturnal dyspnea, only 37 (22.7%) had a known history of heart failure.
Table 1.
Characteristics of participants with preserved pulmonary function and with airflow obstruction
| |
Preserved Pulmonary Function* |
Airflow Obstruction* |
|---|---|---|
| (n = 100) | (n = 476) | |
| Demographics | ||
| Age, yr | 66.5 (10.6) | 67.8 (9.5) |
| Sex, % women | 36 (36.0) | 181 (38.0) |
| Race, % nonwhite | 18 (18.0) | 90 (18.9) |
| Comorbidities and general health | ||
| Sleep apnea status, % | ||
| No sleep apnea | 62 (62.0) | 378 (79.4) |
| Sleep apnea, nonadherent to CPAP/untreated | 28 (28.0) | 77 (16.2) |
| Sleep apnea, adherent to CPAP | 10 (10.0) | 21 (4.4) |
| Depression diagnosis, % | 58 (58.0) | 177 (37.2) |
| Diabetes mellitus, % | 33 (33.0) | 102 (21.43) |
| Chronic kidney disease, % | 25 (25.0) | 84 (17.7) |
| Coronary disease, % | 47 (47.0) | 175 (36.8) |
| Heart failure, % | 20 (20.0) | 74 (15.6) |
| Sciatica, % | 59 (59.0) | 195 (41.0) |
| Number of comorbidities | 5.7 (2.8) | 4.5 (2.4) |
| BMI, kg/m2 | 30.8 (6.1) | 28.7 (7.0) |
| Sleep disturbance, T-score | 54.2 (9.9) | 50.0 (9.5) |
| Depression, T-score | 50.5 (9.4) | 48.6 (9.3) |
| Anxiety, T-score | 52.2 (9.4) | 50.0 (9.0) |
| Pain interference, T-score | 58.7 (8.6) | 54.0 (9.5) |
| 6-Minute walk distance, ft | 1,089 (390) | 1,112 (349) |
| Respiratory health | ||
| FEV1 % predicted | 94.8 (15.1) | 58.3 (18.9) |
| Hypoxemia status, % | ||
| Nonhypoxic | 81 (85.3) | 260 (56.8) |
| Hypoxemic, using oxygen | 11 (11.6) | 159 (34.7) |
| Hypoxemic, without oxygen | 3 (3.2) | 39 (8.5) |
| FACIT dyspnea, score | 44.4 (8.0) | 44.7 (8.0) |
| Currently smoking, % | 35 (35.0) | 165 (34.7) |
| Respiratory medications | ||
| LABA, % | 13 (13.0) | 164 (34.5) |
| LAMA, % | 5 (5.0) | 149 (31.3) |
| SABA, % | 41 (41.0) | 269 (56.5) |
| SAMA, % | 16 (16.0) | 106 (22.3) |
| ICS, % | 18 (18.0) | 208 (43.7) |
| Systemic corticosteroid, % | 1 (1.0) | 14 (2.9) |
Definition of abbreviations: BMI = body mass index; CPAP = continuous positive airway pressure; FACIT = Functional Assessment of Chronic Illness Therapy; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; SABA = short-acting β-agonist; SAMA = short-acting muscarinic antagonist.
P value from χ2 or unpaired t test for categorical or continuous variables, respectively.
Values shown represent mean (SD) or n (%).
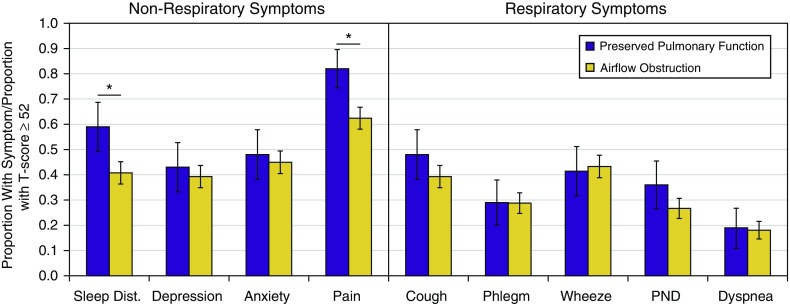
We compared the presence of respiratory and nonrespiratory symptoms by group in Figure 1. For ease of exposition, symptoms measured continuously by T-score are defined as present if the score is greater than or equal to the MID from the population mean (score of 52). Pain and sleep disturbance reported at or above a T-score of 52 (an MID above the population mean) was significantly more frequent among those with preserved pulmonary function than among those with airflow obstruction (pain: 82.0 vs. 62.4%, P < 0.01; sleep disturbance: 59.0 vs. 40.8%, P < 0.01). No significant differences were noted for the presence of respiratory symptoms (Figure 1).
Figure 1.
Symptoms reported among participants with preserved pulmonary function and with airflow obstruction. Shown is the proportion of participants in each group with respiratory and nonrespiratory symptoms of interest. For symptoms measured continuously by T-score, the cutoff is made at a score of 52 (general population mean plus the minimum important difference of 2). Error bars refer to 95% confidence intervals. Proportions compared by χ2 test; *P < 0.05. Dist. = disturbance; PND = paroxysmal nocturnal dyspnea.
We present univariate and multivariate associations with sleep disturbance among participants with preserved pulmonary function and with airflow obstruction in Table 2. Adjusting for all hypothesized factors, increasing severity of depressive symptoms was associated with sleep disturbance both among those with preserved pulmonary function (β, 0.61 increase in sleep disturbance T-score for each increase in depression T-score; P < 0.01) and with airflow obstruction (β, 0.25; P < 0.01). FEV1 was associated with sleep disturbance in both groups, adjusting for other factors. However, lower FEV1 percent predicted was associated with greater sleep disturbance among participants with preserved pulmonary function (β, –0.19 T-score per each percent predicted FEV1; P < 0.01), and greater FEV1 percent predicted was associated with greater sleep disturbance in those with airflow obstruction (β, 0.06; P = 0.02). Only among those with preserved pulmonary function were sleep apnea with adherence to CPAP and greater dyspnea associated with sleep disturbance. Specific to those with airflow obstruction, advancing age, paroxysmal nocturnal dyspnea, and pain interference were associated with sleep disturbance (Table 2).
Table 2.
Adjusted and unadjusted correlation of general and respiratory-specific health status and symptoms with sleep disturbance
| Preserved Pulmonary Function |
Airflow Obstruction |
|||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Age, yr | −0.25 (–0.43, –0.07)* | −0.12 (–0.32, 0.08) | −0.22 (–0.31, –0.14)* | –0.10 (–0.20, –0.01)† |
| Sex, female | 3.82 (–0.21, 7.86) | 2.07 (–1.54, 5.67) | 1.32 (–0.44, 3.08) | 0.70 (–0.89, 2.29) |
| Nonwhite | 0.03 (–5.10, 5.17) | −1.48 (–5.83, 2.87) | 1.96 (–0.21, 4.14) | 1.24 (–0.74, 3.22) |
| BMI, kg/m2 | −0.03 (–0.36, 0.29) | 0.17 (–0.16, 0.49) | 0.05 (–0.07, 0.18) | −0.10 (–0.22, 0.01) |
| Nonhypoxic | Ref | Ref | Ref | Ref |
| Hypoxic, uses oxygen | 0.49 (–5.93, 6.91) | 2.87 (–2.55, 8.29) | 0.57 (–1.32, 2.45) | 0.75 (–1.08, 2.58) |
| Hypoxic, no oxygen | 5.48 (–6.27, 17.23) | 1.53 (–7.78, 10.83) | 2.51 (–0.71, 5.72) | 2.42 (–0.35, 5.19) |
| No sleep apnea | Ref | Ref | Ref | Ref |
| SA, not CPAP adherent | 1.05 (–3.46, 5.55) | 0.11 (–3.81, 4.03) | 4.55 (2.25, 6.85)* | 1.99 (–0.16, 4.14) |
| SA, CPAP adherent | −1.24 (–7.98, 5.50) | –8.71 (–15.09, –2.33)† | 1.75 (–2.37, 5.87) | −0.99 (–4.99, 3.01) |
| PND | 7.17 (3.32, 11.02)* | 1.36 (–2.58, 5.31) | 7.35 (5.53, 9.17)* | 3.79 (1.93, 5.65)† |
| Cough | 3.71 (–0.17, 7.58) | 0.78 (–3.65, 5.20) | 1.5 (–0.25, 3.24) | −0.07 (–2.62, 2.48) |
| Phlegm | 3.13 (–1.17, 7.43) | 2.50 (–2.49, 7.48) | 1.15 (–0.74, 3.04) | −0.40 (–3.09, 2.28) |
| Wheezing | 4.63 (0.71, 8.55)* | 1.19 (–2.36, 4.73) | 4.26 (2.57, 5.94)* | 0.13 (–1.62, 1.87) |
| Depression, T-score | 0.49 (0.3, 0.68)* | 0.61 (0.27, 0.94)† | 0.46 (0.38, 0.54)* | 0.25 (0.12, 0.38)† |
| Pain interference, T-score | 0.65 (0.46, 0.84)* | 0.06 (–0.23, 0.36) | 0.43 (0.35, 0.51)* | 0.22 (0.12, 0.31)† |
| Anxiety, T-score | 0.51 (0.32, 0.69)* | −0.31 (–0.65, 0.03) | 0.42 (0.33, 0.51)* | 0.01 (–0.13, 0.14) |
| Dyspnea, T-score | 0.53 (0.30, 0.75)* | 0.52 (0.23, 0.81)† | 0.40 (0.30, 0.50)* | 0.12 (–0.01, 0.24) |
| Currently smoking | 4.26 (0.22, 8.31)* | 1.30 (–2.92, 5.52) | 2.95 (1.18, 4.73)* | 0.55 (–1.36, 2.46) |
| Number of comorbidities | 1.21 (0.54, 1.88)* | 0.46 (–0.23, 1.15) | 1.01 (0.66, 1.35)* | 0.10 (–0.27, 0.47) |
| Baseline LABA | 0.55 (–5.31, 6.41) | −2.04 (–10.03, 5.95) | −0.50 (–2.30, 1.30) | −1.68 (–3.94, 0.58) |
| Baseline ICS | −0.26 (–5.39, 4.87) | −2.10 (–8.91, 4.7) | 0.71 (–1.02, 2.43) | 0.78 (–1.37, 2.93) |
| Baseline SABA | 3.69 (–0.25, 7.63) | −0.29 (–4.05, 3.47) | 2.03 (0.31, 3.75)* | −0.34 (–2.05, 1.38) |
| Baseline LAMA | −3.76 (–12.78, 5.26) | −1.95 (–9.37, 5.47) | 1.34 (–0.50, 3.18) | 1.18 (–0.67, 3.04) |
| Baseline SAMA | 1.86 (–3.51, 7.23) | −1.17 (–5.64, 3.30) | 1.38 (–0.68, 3.43) | 1.15 (–0.72, 3.02) |
| Oral corticosteroid | −11.77 (–31.45, 7.91) | −0.98 (–17.39, 15.44) | −0.88 (–5.94, 4.18) | −0.32 (–4.78, 4.13) |
| FEV1 % predicted | −0.19 (–0.32, –0.07)* | –0.19 (–0.31, –0.07)† | 0.02 (–0.03, 0.06) | 0.06 (0.01, 0.10)† |
Definition of abbreviations: BMI = body mass index; CPAP = continuous positive airway pressure; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; PND = paroxysmal nocturnal dyspnea; Ref = referent; SA = sleep apnea; SABA = short-acting β-agonist; SAMA = short-acting muscarinic antagonist.
Shown are β coefficients with 95% confidence intervals for each hypothesized correlate of sleep disturbance. β coefficients refer to the magnitude of change in sleep disturbance T-score for a 1-unit change in the exposure outlined in that row. Units for continuous exposures are noted in parentheses. Unadjusted correlates arise from univariate linear regression models (modeling sleep disturbance T-score as outcome). Adjusted models include all other exposures in the table.
Significant unadjusted relationships at P < 0.05 level.
Significant adjusted relationships at the P < 0.05 level, these are also in boldface type.
Discussion
Among participants with a substantial smoking history and a clinical diagnosis of COPD, sleep disturbance was more pronounced in participants with preserved pulmonary function compared with those with evidence of airflow obstruction. In both groups, adjusted analyses demonstrated sleep disturbance to be related to the magnitude of depression. We also found FEV1 to be related to sleep disturbance in both groups, but with contrasting relationships.
The population of participants in our sample with clinically identified COPD and with preserved pulmonary function likely represents a heterogeneous group of participants with a phenotype of COPD without airflow obstruction and those with other, non-COPD causes of dyspnea. Regardless of etiology, the greater sleep disturbance observed in this group may stem from alternative causes of dyspnea and fatigue such as heart failure, diabetes, and obesity that also drive health care utilization (22–25). Sleep disturbance and insomnia have a bidirectional relationship with these three conditions, with greater sleep disturbance contributing to disease severity (26–28), and in turn sleep disturbance may augment symptom perception (29). In addition, heart failure and obesity can both predispose to restrictive ventilatory impairment and may underlie the association of reduced FEV1 and dyspnea with sleep disturbance in this group; residual confounding due to undiagnosed heart failure and truncal obesity may have caused lower FEV1 and dyspnea to remain correlated in adjusted analyses (30, 31). The low-to-normal FEV1 in this group without obstruction may also reflect primarily small-airway disease with sleep-disrupting bronchitic symptoms. Given these putative relationships, further work to explore the role of sleep disturbance in preserved airflow phenotypes of COPD should be pursued.
Although large studies have not found a relationship between insomnia symptoms and severity of obstruction (2, 5, 6), we found that, among those with airflow obstruction, a higher FEV1 was associated with greater sleep disturbance. These findings are consistent with a study of 106 participants showing greater arousal frequency with less severe obstruction (7). This weak but significant association may relate to a reduced predisposition to sleep-related upper airway obstruction and sleep apnea among participants with severe obstruction and hyperinflation (32), and it is worth noting that this relationship was only observed in the adjusted model after accounting for variables that independently predispose to sleep apnea such as BMI, sex, and age.
Contrary to findings where smokers with preserved pulmonary function had appreciable but less severe symptoms than did smokers with airflow obstruction (9), individuals with preserved pulmonary function in our sample tended to have a higher burden of respiratory symptoms such as cough, phlegm production, and dyspnea than those with airflow obstruction, although differences were not statistically significant. The disparities between these two groups in nonrespiratory symptoms were even greater; participants with preserved pulmonary function had substantially greater prevalence and severity of symptoms such as sleep disturbance and pain. These symptoms may independently drive health care utilization, and may also relate to a greater burden of comorbid conditions such as major depressive disorder and sciatica in our group with preserved pulmonary function.
This work highlights the contribution of depression to sleep disturbance in participants with clinically diagnosed COPD. Prior work demonstrated that depressive mood is common among those with COPD (21.4%), and that 90% of patients with COPD with depression complained of symptoms of insomnia (1). As depression is a morbid yet treatable condition that detracts substantially from quality of life (33–35), clinicians should be on alert to inquire about depression among those patients with COPD and sleep disturbance. Concomitant therapy for insomnia alongside depression is also associated with more sustained and effective therapy response in both conditions (36).
Similar to depression, pain was also associated with sleep disturbance in adjusted analyses among those with airflow obstruction. This is not surprising given the high prevalence of pain symptoms in those with COPD (37), and the bidirectional relationship between sleep and pain. Although data support the intuitive causal relationship of pain resulting in sleep disturbance (38), other studies suggest that inadequate or disturbed sleep perpetuates chronic pain (39). It is possible that interventions aimed at improving sleep quality may have salutatory effects on pain and vice versa.
Also consistent with earlier observations, respiratory symptoms such as dyspnea, cough, and phlegm production were associated with overall sleep disturbance symptoms in univariate analyses (5, 6). Prior studies reported composite assessment of respiratory symptoms such as the COPD Assessment Test score (6), whereas we explored individual symptoms. Overall, of the symptoms queried, paroxysmal nocturnal dyspnea was the only respiratory symptom associated with sleep disturbance in the adjusted model—a finding not entirely explained by burden of diagnosed heart failure. Although the test characteristics in patients with COPD are unclear, paroxysmal nocturnal dyspnea has a specificity of 89% for heart failure among the general population of community-dwelling adults (40). Among our participants, the vast majority of those complaining of paroxysmal nocturnal dyspnea did not have a diagnosis of heart failure. Given the high prevalence of undiagnosed heart failure in COPD (41), and the known strong associations of heart failure with sleep disturbance and nocturia (42–44), it is possible that undiagnosed heart failure may at least partially mediate sleep disturbance in COPD.
Among participants with preserved pulmonary function, adherent CPAP use was associated with reduced sleep disturbance even when compared with the referent group of those with no sleep apnea. This observation may be confounded by the fact that those with better sleep continuity are able to tolerate PAP (45). However, as 84–93% of individuals with moderate to severe obstructive sleep apnea remain undiagnosed (46), there is likely a sizeable proportion of individuals in our cohort misclassified as not having sleep apnea whose sleep quality could be improved with identification and management of unrecognized sleep apnea. Because of a higher BMI, the proportion of undiagnosed sleep apnea is likely to be greater among those with preserved pulmonary function, and this could explain why the association with adherent CPAP use was observed only in this group.
Contrary to prior work (21), hypoxemia and supplemental oxygen administration did not emerge as major contributors to poor sleep quality in the adjusted analyses of our study. It is worth noting that sleep disturbance has not been associated with pulse oximetry measures in prior work evaluating sleep quality in COPD. In McSharry and colleagues, sleep disturbance was associated with lower rates of arterial oxygen tension as measured by arterial blood gasses but not oximetry (7). Later, in Budhiraja and colleagues, oxygen use was associated with reduced sleep disturbance, but no relationship with pulse oximetry was reported (5). The absence of an association between hypoxia and sleep disturbance in our analysis would mirror the experience in obstructive sleep apnea where correction of nocturnal hypoxemia does not appear to improve sleep architecture (47) or neuropsychological functioning (48). However, there did appear to be an improvement in insomnia as measured by the Pittsburgh Sleep Quality Instrument score in oxygen users compared with nonusers in the Long-Term Oxygen Treatment Trial (LOTT) (see LOTT description in the online supplement), although the significance of this finding did not persist after Bonferroni adjustment (49).
This investigation has some limitations. First, our assessment of sleep disturbance is based on self-report, and no objective measure of sleep disruption or fragmentation by polysomnography or actigraphy was used. As noted previously, we are also likely underestimating the proportion and potential impact of undiagnosed (and therefore untreated) obstructive sleep apnea. In addition, although we were able to evaluate and adjust for sleep disturbance related to COPD-specific medications such as corticosteroids and inhalers, other medication use was not ascertained in CONCERT and may be a source of residual confounding. Furthermore, given the novel nature of the PROMIS sleep disturbance instrument, a threshold score for abnormal sleep disturbance has not yet been established. Therefore the current analysis allows us to explore associations across the entire range of sleep disturbance as opposed to risk or odds of pathologic sleep disturbance. However, because prior work already established a firm association of COPD with pathologic degrees of sleep disturbance, this is of less concern. Finally, the cross-sectional nature of the work reduces our ability to consider predictors as opposed to consequences of sleep disturbance.
The current work with its large sample size and generalizable population provides insight into the explanatory factors of sleep disturbance in patients with clinically diagnosed COPD. Given that the disease-defining features of COPD did not appear to be associated with sleep disturbance, a focus on the identification and therapy of comorbidities contributing to sleep disturbance, such as sleep apnea and depression, will be of paramount importance. Furthermore, the sizeable contribution of paroxysmal nocturnal dyspnea raises the prospect of undiagnosed heart failure playing a major role in sleep disturbance. Longitudinal studies will be needed to better elucidate causality regarding these potential mediators, and help prioritize therapeutic trials.
Conclusion
Individuals with a significant smoking history and preserved pulmonary function have greater sleep disturbance than those with airflow obstruction. Our study adds to the existing literature that has failed to find a consistent association between greater severity of airflow obstruction and sleep disturbance among those with COPD. Furthermore, the absence of a significant association with hypoxia would support the argument that the central disease processes of COPD—airflow obstruction and impaired gas exchange—are not major drivers of sleep disturbance symptoms. Instead, among those with airflow obstruction, the most robust associations were noted with depression, pain interference, and paroxysmal nocturnal dyspnea, suggesting these symptoms should be prioritized in managing patients with COPD with sleep disturbance. This study also supplements emerging data that individuals with risk factors for COPD who lack airflow obstruction are vulnerable to symptoms that impair quality of life.
Supplementary Material
Acknowledgments
Acknowledgment
The authors acknowledge all of the member sites and investigators in the CONCERT consortium. Without the hard work and dedication of this group, this work would not have been possible.
Footnotes
Supported by Agency for Healthcare Research and Quality (AHRQ) R13 HS017894, National Institutes of Health (NIH) T32 HL007287, and NIH RC2 HL101618 (primary source of funding); and a number of staff are supported by VA Puget Sound Health Services Research and Development. None of the funding sources were involved in the design, conduct, or analysis of this project. The views expressed here are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Author Contributions: Study design and collection of data: S.S.C., J.A.K., P.K.L., R.A.M., E.T.N., D.H.A., and L.M.D. Analysis of data: P.J.R., L.C.F., and L.M.D. Interpretation of data: L.C.F., M.F.G., L.J.S., V.K.K., J.A.K., P.K.L., R.A.M., E.T.N., B.N.P., E.C.P., M.V.V., D.H.A., and L.M.D. Preparation of manuscript: S.S.C., L.C.F., M.F.G., V.K.K., J.A.K., P.K.L., R.A.M., E.T.N., B.N.P., E.C.P., L.J.S., M.V.V., D.H.A., and L.M.D.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the CONCERT Investigators
References
- 1.Ohayon MM. Chronic obstructive pulmonary disease and its association with sleep and mental disorders in the general population. J Psychiatr Res. 2014;54:79–84. doi: 10.1016/j.jpsychires.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Omachi TA, Blanc PD, Claman DM, Chen H, Yelin EH, Julian L, Katz PP. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13:476–483. doi: 10.1016/j.sleep.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geiger-Brown J, Lindberg S, Krachman S, McEvoy CE, Criner GJ, Connett JE, Albert RK, Scharf SM. Self-reported sleep quality and acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:389–397. doi: 10.2147/COPD.S75840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kent BD, McNicholas WT, Verbraecken J. Disturbed sleep and COPD outcomes: cart meets horse. Sleep Med. 2012;13:453–454. doi: 10.1016/j.sleep.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Budhiraja R, Parthasarathy S, Budhiraja P, Habib MP, Wendel C, Quan SF. Insomnia in patients with COPD. Sleep. 2012;35:369–375. doi: 10.5665/sleep.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang CH, Chuang LP, Lin SW, Lee CS, Tsai YH, Wei YF, Cheng SL, Hsu JY, Kuo PH, Yu CJ, et al. Factors responsible for poor sleep quality in patients with chronic obstructive pulmonary disease. BMC Pulm Med. 2016;16:118. doi: 10.1186/s12890-016-0281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McSharry DG, Ryan S, Calverley P, Edwards JC, McNicholas WT. Sleep quality in chronic obstructive pulmonary disease. Respirology. 2012;17:1119–1124. doi: 10.1111/j.1440-1843.2012.02217.x. [DOI] [PubMed] [Google Scholar]
- 8.Prieto-Centurion V, Rolle AJ, Au DH, Carson SS, Henderson AG, Lee TA, Lindenauer PK, McBurnie MA, Mularski RA, Naureckas ET, et al. CONCERT Consortium. Multicenter study comparing case definitions used to identify patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:989–995. doi: 10.1164/rccm.201406-1166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, Gouskova NA, Hansel NN, Hoffman EA, Kanner RE, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez CH, Murray S, Barr RG, Bleecker E, Bowler RP, Christenson SA, Comellas AP, Cooper CB, Couper D, Criner GJ, et al. Subpopulations and Intermediate Outcome Measures in COPD Study Investigators. Respiratory symptoms items from the COPD Assessment Test identify ever-smokers with preserved lung function at higher risk for poor respiratory outcomes: an analysis of the Subpopulations and Intermediate Outcome Measures in COPD Study Cohort. Ann Am Thorac Soc. 2017;14:636–642. doi: 10.1513/AnnalsATS.201610-815OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnan JA, Lindenauer PK, Au DH, Carson SS, Lee TA, McBurnie MA, Naureckas ET, Vollmer WM, Mularski RA COPD Outcomes-Based Network for Clinical Effectiveness and Research Translation. Stakeholder priorities for comparative effectiveness research in chronic obstructive pulmonary disease: a workshop report. Am J Respir Crit Care Med. 2013;187:320–326. doi: 10.1164/rccm.201206-0994WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mularski RA, McBurnie MA, Lindenauer PK, Lee TA, Vollmer WM, Au DH, Carson SS, Krishnan JA CONCERT Investigator Consortium. Comparative effectiveness research in chronic obstructive pulmonary disease. J Comp Eff Res. 2012;1:71–82. doi: 10.2217/cer.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 14.Teresi JA, Ocepek-Welikson K, Kleinman M, Eimicke JP, Crane PK, Jones RN, et al. Analysis of differential item functioning in the depression item bank from the Patient Reported Outcome Measurement Information System (PROMIS): an item response theory approach. Psychol Sci Q. 2009;51:148–180. [PMC free article] [PubMed] [Google Scholar]
- 15.Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, Johnston KL, Shablesky-Cade MA, Pilkonis PA. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33:781–792. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorner JB, Rose M, Gandek B, Stone AA, Junghaenel DU, Ware JE., Jr Method of administration of PROMIS scales did not significantly impact score level, reliability, or validity. J Clin Epidemiol. 2014;67:108–113. doi: 10.1016/j.jclinepi.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartman JE, Prinzen J, van Lummel RC, Ten Hacken NH. Frequent sputum production is associated with disturbed night’s rest and impaired sleep quality in patients with COPD. Sleep Breath. 2015;19:1125–1133. doi: 10.1007/s11325-014-1111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soler X, Diaz-Piedra C, Ries AL. Pulmonary rehabilitation improves sleep quality in chronic lung disease. COPD. 2013;10:156–163. doi: 10.3109/15412555.2012.729622. [DOI] [PubMed] [Google Scholar]
- 19.Yount SE, Choi SW, Victorson D, Ruo B, Cella D, Anton S, Hamilton A. Brief, valid measures of dyspnea and related functional limitations in chronic obstructive pulmonary disease (COPD) Value Health. 2011;14:307–315. doi: 10.1016/j.jval.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 20.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 21.Budhiraja R, Siddiqi TA, Quan SF. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med. 2015;11:259–270. doi: 10.5664/jcsm.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joo MJ, Lee TA, Weiss KB. Geographic variation of spirometry use in newly diagnosed COPD. Chest. 2008;134:38–45. doi: 10.1378/chest.08-0013. [DOI] [PubMed] [Google Scholar]
- 23.Klein OL, Aviles-Santa L, Cai J, Collard HR, Kanaya AM, Kaplan RC, Kinney GL, Mendes E, Smith L, Talavera G, et al. Hispanics/Latinos with type 2 diabetes have functional and symptomatic pulmonary impairment mirroring kidney microangiopathy: findings from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Diabetes Care. 2016;39:2051–2057. doi: 10.2337/dc16-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sin DD, Jones RL, Man SF. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002;162:1477–1481. doi: 10.1001/archinte.162.13.1477. [DOI] [PubMed] [Google Scholar]
- 25.Konstam MA, Kiernan M, Chandler A, Dhingra R, Mody FV, Eisen H, Haught WH, Wagoner L, Gupta D, Patten R, et al. SECRET of CHF Investigators, Coordinators, and Committee Members. Short-term effects of tolvaptan in patients with acute heart failure and volume overload. J Am Coll Cardiol. 2017;69:1409–1419. doi: 10.1016/j.jacc.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest. 2017;152:435–444. doi: 10.1016/j.chest.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowall B, Lehnich AT, Strucksberg KH, Führer D, Erbel R, Jankovic N, Moebus S, Jöckel KH, Stang A. Associations among sleep disturbances, nocturnal sleep duration, daytime napping, and incident prediabetes and type 2 diabetes: the Heinz Nixdorf Recall Study. Sleep Med. 2016;21:35–41. doi: 10.1016/j.sleep.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Nedeltcheva AV, Scheer FA. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2014;21:293–298. doi: 10.1097/MED.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gobin CM, Banks JB, Fins AI, Tartar JL. Poor sleep quality is associated with a negative cognitive bias and decreased sustained attention. J Sleep Res. 2015;24:535–542. doi: 10.1111/jsr.12302. [DOI] [PubMed] [Google Scholar]
- 30.Rocha A, Arbex FF, Sperandio PA, Souza A, Biazzim L, Mancuso F, Berton DC, Hochhegger B, Alencar MCN, Nery LE, et al. Excess ventilation in COPD–heart failure overlap: implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. doi: 10.1164/rccm.201704-0675OC. [online ahead of print] 30 Jun 2017; DOI: 10.1164/rccm.201704-0675OC. [DOI] [PubMed] [Google Scholar]
- 31.Apostolo A, Giusti G, Gargiulo P, Bussotti M, Agostoni P. Lungs in heart failure. Pulm Med. 2012;2012:952741. doi: 10.1155/2012/952741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biselli P, Grossman PR, Kirkness JP, Patil SP, Smith PL, Schwartz AR, Schneider H. The effect of increased lung volume in chronic obstructive pulmonary disease on upper airway obstruction during sleep. J Appl Physiol. 2015;119:266–271. doi: 10.1152/japplphysiol.00455.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manber R, Buysse DJ, Edinger J, Krystal A, Luther JF, Wisniewski SR, Trockel M, Kraemer HC, Thase ME. Efficacy of cognitive–behavioral therapy for insomnia combined with antidepressant pharmacotherapy in patients with comorbid depression and insomnia: a randomized controlled trial. J Clin Psychiatry. 2016;77:e1316–e1323. doi: 10.4088/JCP.15m10244. [DOI] [PubMed] [Google Scholar]
- 34.Laforest L, Roche N, Devouassoux G, Belhassen M, Chouaid C, Ginoux M, Van Ganse E. Frequency of comorbidities in chronic obstructive pulmonary disease, and impact on all-cause mortality: a population-based cohort study. Respir Med. 2016;117:33–39. doi: 10.1016/j.rmed.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 35.Daly EJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Gaynes BN, Warden D, Morris DW, Luther JF, Farabaugh A, Cook I, et al. Health-related quality of life in depression: a STAR*D report. Ann Clin Psychiatry. 2010;22:43–55. [PubMed] [Google Scholar]
- 36.Fava M, McCall WV, Krystal A, Wessel T, Rubens R, Caron J, Amato D, Roth T. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59:1052–1060. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Chen YW, Camp PG, Coxson HO, Road JD, Guenette JA, Hunt MA, Reid WD. Comorbidities that cause pain and the contributors to pain in individuals with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2017;98:1535–1543. doi: 10.1016/j.apmr.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Roehrs T, Roth T. Sleep and pain: interaction of two vital functions. Semin Neurol. 2005;25:106–116. doi: 10.1055/s-2005-867079. [DOI] [PubMed] [Google Scholar]
- 39.Castillo RC, MacKenzie EJ, Wegener ST, Bosse MJ LEAP Study Group. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124:321–329. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Ekundayo OJ, Howard VJ, Safford MM, McClure LA, Arnett D, Allman RM, Howard G, Ahmed A. Value of orthopnea, paroxysmal nocturnal dyspnea, and medications in prospective population studies of incident heart failure. Am J Cardiol. 2009;104:259–264. doi: 10.1016/j.amjcard.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutten FH, Cramer MJ, Grobbee DE, Sachs AP, Kirkels JH, Lammers JW, Hoes AW. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J. 2005;26:1887–1894. doi: 10.1093/eurheartj/ehi291. [DOI] [PubMed] [Google Scholar]
- 42.Lee KS, Lennie TA, Heo S, Song EK, Moser DK. Prognostic importance of sleep quality in patients with heart failure. Am J Crit Care. 2016;25:516–525. doi: 10.4037/ajcc2016219. [DOI] [PubMed] [Google Scholar]
- 43.MacDonald M, Fang J, Pittman SD, White DP, Malhotra A. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med. 2008;4:38–42. [PMC free article] [PubMed] [Google Scholar]
- 44.Redeker NS, Adams L, Berkowitz R, Blank L, Freudenberger R, Gilbert M, Walsleben J, Zucker MJ, Rapoport D. Nocturia, sleep and daytime function in stable heart failure. J Card Fail. 2012;18:569–575. doi: 10.1016/j.cardfail.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eysteinsdottir B, Gislason T, Pack AI, Benediktsdottir B, Arnardottir ES, Kuna ST, Björnsdottir E. Insomnia complaints in lean patients with obstructive sleep apnea negatively affect positive airway pressure treatment adherence. J Sleep Res. 2017;26:159–165. doi: 10.1111/jsr.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, Jackson CL, Williams MA, Redline S. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: a placebo–CPAP-controlled study. Sleep. 2006;29:564–571. doi: 10.1093/sleep/29.4.564. [DOI] [PubMed] [Google Scholar]
- 48.Lim W, Bardwell WA, Loredo JS, Kim EJ, Ancoli-Israel S, Morgan EE, Heaton RK, Dimsdale JE. Neuropsychological effects of 2-week continuous positive airway pressure treatment and supplemental oxygen in patients with obstructive sleep apnea: a randomized placebo-controlled study. J Clin Sleep Med. 2007;3:380–386. [PMC free article] [PubMed] [Google Scholar]
- 49.Albert RK, Au DH, Blackford AL, Casaburi R, Cooper JA, Jr, Criner GJ, Diaz P, Fuhlbrigge AL, Gay SE, Kanner RE, et al. Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617–1627. doi: 10.1056/NEJMoa1604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.