Abstract
Obesity affects numerous diseases, including asthma, for reasons that remain incompletely understood. Recent research suggests that the asthma of obesity is not a single disease, and that it breaks out into at least two distinct phenotypes. One phenotype is conventional allergic asthma modulated by obesity, whereas another arises solely due to the presence of obesity. The latter is postulated to be a consequence of the chronic lung compression caused by the obese chest wall in individuals with particularly collapsible lungs. Allergic obese asthma, on the other hand, appears to result from the way that obesity affects the immune system, which we hypothesize can be understood in terms of effects on the dynamic regulation of the inflammatory response.
Keywords: lung mechanics, allergic inflammation, acute respiratory distress syndrome, computational model, lung volume
The obesity epidemic that is currently sweeping the world is increasing the incidence of numerous diseases, and asthma is no exception (1–3). Intuitively, this is perhaps not surprising, given the general metabolic dysregulation that accompanies morbid obesity, although exactly why asthma in particular should be impacted, and how to manage it in any given patient, remain poorly understood. Nevertheless, some important recent advances are beginning to shed light on this complex situation.
Dixon and others (4–9) have reported that obesity-associated asthma breaks out into at least two distinct groups. One group is composed of individuals with early-onset atopic (EOA) asthma whose disease does not remit when they lose weight, even though symptoms may improve. These individuals thus appear to have a conventional form of allergic asthma that is modulated by the obese state. There is another group composed of individuals whose asthma is of a late-onset, nonatopic (LONA) form that resolves when they lose weight, and so appears to arise purely as a function of obesity. EOA and LONA obese asthma thus seem to be distinct diseases. Here, we discuss how different pathophysiological processes might underlie EOA and LONA asthma, and how these might account for the similarities and differences in their physiological phenotypes.
LONA Obese Asthma: A Matter of Lung Compression
There seem to be, broadly speaking, two distinct ways in which extreme obesity could affect the severity and nature of asthma. One of these is via alterations in systemic inflammatory status; adipose tissue is metabolically active, and, in particular, is known to produce inflammatory mediators that presumably could play a role in any inflammatory disease, including allergic asthma (10). LONA obese asthma and its associated airway hyperresponsiveness could thus result from such an inflammatory milieu, something that would resolve with weight loss along with the asthma itself (11). Inflammation is not, however, an obvious component of LONA asthma, so it is perhaps more reasonable to assign pathogenesis to the other cardinal effect of obesity on the lung, namely, reduced FRC due both to compression of the thorax by subcutaneous fat over the trunk and to occupation of the thoracic cavity by visceral fat. In support of this hypothesis, we have recently shown that respiratory system impedance is elevated more in obese subjects with LONA asthma compared with obese subjects without asthma, and that major weight loss leads to a greater reduction in lung stiffness in subjects with LONA asthma (10). In fact, it is very well established that airways responsiveness is increased dramatically when lung volume decreases below normal FRC, something that can be explained by the associated reductions in the outward tethering forces that the parenchyma exerts on the airways to which it is attached (12–14). This hyperresponsiveness can result both from increased airway narrowing and from increased closure of peripheral airways, the latter likely due to liquid bridge formation across the lumen (15). It is thus entirely expected that the lung will become hyperresponsive, and thus more “asthma-like,” as a result of being compressed by mass loading of the chest wall.
This hypothesis, however, leaves open the question of why obesity does not transform everyone into an individual with LONA asthma. There are several possible answers to this question, including the issue of how excess adiposity is distributed around the body; preferential allocation to the buttocks would, for example, have less of a lung volume–reducing effect than allocation to the thorax and abdomen. Another possibility is that there may be naturally occurring differences in susceptibility between individuals to become hyperresponsive for a given decrement in lung volume. In this regard, we recently focused on the possibility that there might be a natural degree of interindividual variation in airway wall stiffness, and showed, using computational modeling, that as lung volume becomes progressively depressed with increasing weight, there is a progressive increase in the fraction of the population with more than average compliant airways that becomes hyperresponsive to methacholine challenge (16). It is therefore conceivable that, whereas most individuals in the normal population have airway stiffnesses that are unproblematic at normal weight, when superimposed on a background of progressing obesity this population manifests an increasing prevalence of asthma-like symptoms.
EOA Obese Asthma: A Matter of Aberrant Inflammation
Our computational modeling also revealed, however, that the same kind of progressive fractional increase in asthma among the general population is predicted to occur as the airway walls thicken (16). Such thickening could be due to inflammatory processes that expand the airway epithelium and other components of the airway wall and increase mucus secretion (17), although this seems more likely to explain the increased incidence of EOA rather than LONA asthma among obese individuals. Furthermore, airway inflammation is expected to be somewhat labile, due to variations in the activity of the immune system and the environmental challenges that activate it, whereas airway wall stiffness is likely to be relatively stable over time, because it reflects the nature of the connective tissue and cartilage of which the walls are comprised. This could account for the fact that EOA asthma is the more labile of the two phenotypes, being more associated with disease exacerbations (6, 7). Nevertheless, EOA obese asthma is not as simple as regular (i.e., lean) allergic asthma compounded by a greater severity of systemic inflammation. Indeed, obesity appears to ameliorate some markers of type 2 inflammation (9, 18). Obesity thus might have conflicting effects on individual components of the immune response that combine to produce allergic inflammation. This is likely also true for other diseases, such as acute respiratory distress syndrome (ARDS); although obese individuals are more likely than lean individuals to develop ARDS, they are also less likely to die from it (19).
As a possible route toward understanding this confusing picture, we note that inflammation is a dynamic process that waxes and wanes as the immune system first mounts a defensive response against a perceived threat, and subsequently attempts to return toward a noninflamed state. The resolution of established inflammation is, in fact, as crucially important as its instigation, because failure of resolution places an individual at risk for chronic inflammatory pathology. We recently advanced the hypothesis that the control of the inflammatory response is based on much the same principle as control of skeletal muscle activity, namely, via the repetitive invocation of a self-limited unitary event called a twitch, albeit manifest over very different time scales (20–22). The “inflammatory twitch” that constitutes the unitary event in inflammation is thus posited to follow a characteristic time course that, once initiated, proceeds eventually to the noninflamed state as the organism returns to baseline. A useful aspect of this hypothesis is that it allows one to focus on how extrinsic factors, such as obesity, might affect the various temporal components of the inflammatory twitch. Here, we offer a perspective that might explain how obesity seems to have both exacerbating and protective effects on inflammation.
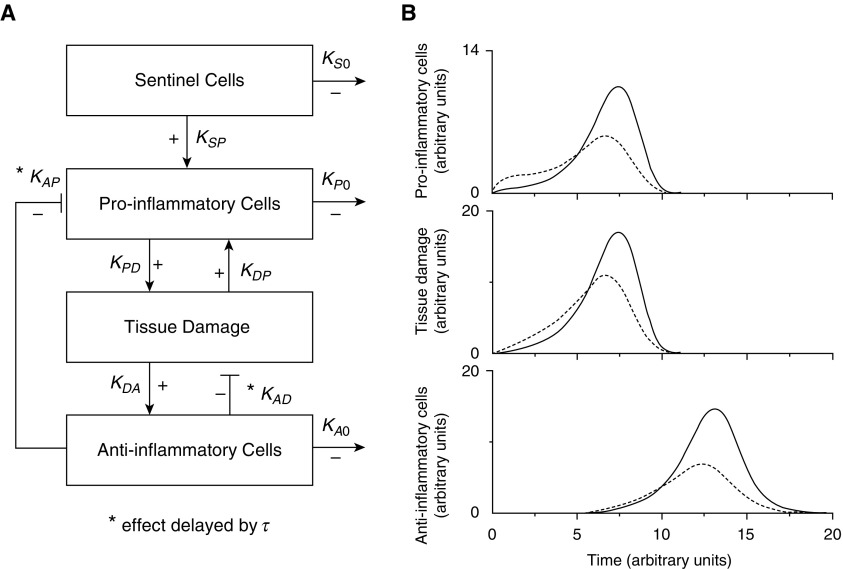
Figure 1A presents a highly simplified model (perhaps the simplest possible) capable of exhibiting an inflammatory twitch response. This model, expressed as a set of coupled differential equations in the online supplement, is a distillation of the essential features of our previously published agent-based model of allergic inflammation in the lung (20, 21). The simplified model consists of a compartment of sentinel cells (e.g., antigen-presenting cells, mast cells, macrophages, epithelium) that initially registers a threat and then sends a signal to a compartment of proinflammatory cells (neutrophils, eosinophils, etc.) that constitute and amplify an inflammatory response. This response is designed to deal with the threat, but at the expense of causing collateral damage to the tissues. Indeed, it is this collateral damage that is probably the main manifestation of pathology in allergic asthma or ARDS. The damage itself then signals a reparative process (the antiinflammatory compartment), involving fibroblasts and other wound-healing machinery, that attempts to repair the damage. Importantly, the action of the antiinflammatory compartment is delayed (by an amount of time designated by τ in the model in Figure 1A), which allows inflammation to ramp up to a biologically effective level before it is eventually reversed and returned to baseline.
Figure 1.
(A) A minimal model of the immune system that can exhibit a twitch-like response to the detection of a threat by sentinel cells (see the online supplement for technical details). (B) Response of the system under “baseline” conditions (solid lines) and during obesity (dashed lines), when the inflammatory milieu is increased, while the functional status of the immune system is decreased (see main text for details). Plus sign indicates pathways that are up-regulating; minus sign indicates down-regulation. Note that the latter applies to the pathways governed by the rate constants, KS0, KP0, and KA0, which indicate compartment sinks. KA0 = sink rate-constant for anti-inflammatory cells (arbitrary units); KAD = inhibitory rate-constant of antiinflammatory cells on tissue damage (arbitrary units); KAP = inhibitory rate-constant of antiinflammatory cells on proinflammatory cells (arbitrary units); KDA = stimulatory rate-constant of tissue damage on antiinflammatory cells (arbitrary units); KDP = stimulatory rate-constant of tissue damage on proinflammatory cells (arbitrary units); KP0 = sink rate-constant for proinflammatory cells (arbitrary units); KPD = stimulatory rate-constant of proinflammatory cells on tissue damage (arbitrary units); KS0 = sink rate-constant for sentinel cells (arbitrary units); KSP = stimulatory rate-constant of sentinel cells on proinflammatory cells (arbitrary units); τ = time delay of antiinflammatory cell action on tissue damage and proinflammatory cells (arbitrary units).
Figure 1B shows the compartmental responses (solid lines) as functions of time from the instant of threat perception at time 0, with all the rate constants arbitrarily assigned a value of 1 while the time delay τ has a value of 5 (all in arbitrary units). The amplitudes of the responses do not mean anything biologic in absolute terms, but they demonstrate that this model exhibits a transient response that eventually returns to baseline in all compartments, as would be expected of a system with appropriate homeostatic control.
Now suppose that obesity results in two opposing effects. On the one hand, because of the general up-regulation in the inflammatory milieu in the obese individual (23), the rate constant connecting the sentinel cells to their target inflammatory cells (KSP) is increased. We represent this effect in the model by increasing KSP arbitrarily from 1 to 4 so that the system is primed to react more vigorously to a threat. On the other hand, because of the adverse effects of obesity on health in general, it seems at least plausible that the immune system just does not work as well in obese compared with lean individuals. We represent this in the model by decreasing the strengths of the interactions between the other compartments (stimulatory rate-constant of proinflammatory cells on tissue damage [KPD], stimulatory rate-constant of tissue damage on proinflammatory cells [KDP], stimulatory rate-constant of tissue damage on antiinflammatory cells [KDA], inhibitory rate-constant of antiinflammatory cells on tissue damage [KAD], and inhibitory rate-constant of antiinflammatory cells on proinflammatory cells [KAP]) arbitrarily from 1.0 to 0.7 (see the online supplement for technical details of the model). The values of these model parameters are not tied to any experimental data, and have thus been chosen merely to make the point that is demonstrated in Figure 1B (dashed lines), which shows an altered inflammatory response that is initially elevated, possibly corresponding to the increased risk of asthma (1–3) or the greater rates of ARDS (24–26) caused by obesity. Subsequently, however, the peak of the response is depressed, which could correspond to the obesity-associated attenuation of type 2 inflammation in asthma (9, 18) or the decreased risk of death in ARDS (24–26). This model is, of course, an enormously simplified representation of an extremely complicated system with innumerable players interacting over many time and length scales. Nevertheless, it serves to illustrate how a factor such as obesity can exert competing risks on a disease process in ways that might initially seem counterintuitive.
Conclusions
Obesity affects the clinical manifestations of asthma in a variety of ways. In some cases, it seems that obesity modulates existing allergic disease, whereas, in other cases, the compressive effects of an obese trunk reduce lung volumes to the point where the airways become unstable, at least in some fraction of the population. The asthma of obesity is thus not a single disease, but in all cases the metabolic activity of excess adipose tissue coupled with dysfunction of immune regulation have the potential to affect how the airways respond to challenge by antigens, bronchial agonists, or the pathogenic insults that cause acute lung injury. The concept of the inflammatory twitch offers a way of thinking about how different facets of the inflammatory response may be impacted by the effects of obesity.
Supplementary Material
Footnotes
Supported by National Institutes of Health grant R01 HL130847 (J.H.T.B and A.E.D.).
The views expressed in this article do not communicate an official position of the University of Vermont or the National Institutes of Health.
Author Contributions: J.H.T.B., M.E.P., and B.T.S. conceived the study; J.H.T.B. performed the computational modeling and drafted the manuscript; and M.E.P., C.M.F., U.P., A.E.D., and B.T.S. revised the manuscript and contributed important intellectual content.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Quinto KB, Zuraw BL, Poon KY, Chen W, Schatz M, Christiansen SC. The association of obesity and asthma severity and control in children. J Allergy Clin Immunol. 2011;128:964–969. doi: 10.1016/j.jaci.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Mosen DM, Schatz M, Magid DJ, Camargo CA., Jr The relationship between obesity and asthma severity and control in adults. J Allergy Clin Immunol. 2008;122:507–511.e6. doi: 10.1016/j.jaci.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Vortmann M, Eisner MD. BMI and health status among adults with asthma. Obesity (Silver Spring) 2008;16:146–152. doi: 10.1038/oby.2007.7. [DOI] [PubMed] [Google Scholar]
- 4.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27:495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 6.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E, Jarjour NN, Moore WC, Peters SP, Yonas M, Teague WG, Wenzel SE. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127:1486–1493.e2. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sutherland ER, Goleva E, King TS, Lehman E, Stevens AD, Jackson LP, Stream AR, Fahy JV, Leung DY Asthma Clinical Research Network. Cluster analysis of obesity and asthma phenotypes. PLoS One. 2012;7:e36631. doi: 10.1371/journal.pone.0036631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pradeepan S, Garrison G, Dixon AE. Obesity in asthma: approaches to treatment. Curr Allergy Asthma Rep. 2013;13:434–442. doi: 10.1007/s11882-013-0354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon AE, Pratley RE, Forgione PM, Kaminsky DA, Whittaker-Leclair LA, Griffes LA, Garudathri J, Raymond D, Poynter ME, Bunn JY, Irvin CG. Effects of obesity and bariatric surgery on airway hyperresponsiveness, asthma control, and inflammation. J Allergy Clin Immunol. 2011;128:508–515.e1–2. doi: 10.1016/j.jaci.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Alwan A, Bates JH, Chapman DG, Kaminsky DA, DeSarno MJ, Irvin CG, Dixon AE. The nonallergic asthma of obesity: a matter of distal lung compliance. Am J Respir Crit Care Med. 2014;189:1494–1502. doi: 10.1164/rccm.201401-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman DG, Irvin CG, Kaminsky DA, Forgione PM, Bates JH, Dixon AE. Influence of distinct asthma phenotypes on lung function following weight loss in the obese. Respirology. 2014;19:1170–1177. doi: 10.1111/resp.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bates JH, Lauzon AM. Parenchymal tethering, airway wall stiffness, and the dynamics of bronchoconstriction. J Appl Physiol (1985) 2007;102:1912–1920. doi: 10.1152/japplphysiol.00980.2006. [DOI] [PubMed] [Google Scholar]
- 13.Cojocaru A, Irvin CG, Haverkamp HC, Bates JH. Computational assessment of airway wall stiffness in vivo in allergically inflamed mouse models of asthma. J Appl Physiol (1985) 2008;104:1601–1610. doi: 10.1152/japplphysiol.01207.2007. [DOI] [PubMed] [Google Scholar]
- 14.Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol (1985) 1987;62:1324–1330. doi: 10.1152/jappl.1987.62.3.1324. [DOI] [PubMed] [Google Scholar]
- 15.Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JH. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med. 2007;175:768–774. doi: 10.1164/rccm.200610-1410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates JH, Dixon AE. Potential role of the airway wall in the asthma of obesity. J Appl Physiol (1985) 2015;118:36–41. doi: 10.1152/japplphysiol.00684.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc. 2009;6:301–305. doi: 10.1513/pats.200808-089RM. [DOI] [PubMed] [Google Scholar]
- 18.van Veen IH, Ten Brinke A, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63:570–574. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhi G, Xin W, Ying W, Guohong X, Shuying L. “Obesity paradox” in acute respiratory distress syndrome: a systematic review and meta-analysis. PLoS One. 2016;11:e0163677. doi: 10.1371/journal.pone.0163677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pothen JJ, Poynter ME, Bates JH. A computational model of unresolved allergic inflammation in chronic asthma. Am J Physiol Lung Cell Mol Physiol. 2015;308:L384–L390. doi: 10.1152/ajplung.00268.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pothen JJ, Poynter ME, Bates JH. The inflammatory twitch as a general strategy for controlling the host response. J Immunol. 2013;190:3510–3516. doi: 10.4049/jimmunol.1202595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pothen JJ, Poynter ME, Lundblad LK, Bates JH. Dissecting the inflammatory twitch in allergically inflamed mice. Am J Physiol Lung Cell Mol Physiol. 2016;310:L1003–L1009. doi: 10.1152/ajplung.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos EJ, Xu Y, Romanova I, Middleton F, Chen C, Quinn R, Inui A, Das U, Meguid MM. Is obesity an inflammatory disease? Surgery. 2003;134:329–335. doi: 10.1067/msy.2003.267. [DOI] [PubMed] [Google Scholar]
- 24.Prescott HC, Chang VW, O’Brien JM, Jr, Langa KM, Iwashyna TJ. Obesity and 1-year outcomes in older Americans with severe sepsis. Crit Care Med. 2014;42:1766–1774. doi: 10.1097/CCM.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wurzinger B, Dünser MW, Wohlmuth C, Deutinger MC, Ulmer H, Torgersen C, Schmittinger CA, Grander W, Hasibeder WR. The association between body-mass index and patient outcome in septic shock: a retrospective cohort study. Wien Klin Wochenschr. 2010;122:31–36. doi: 10.1007/s00508-009-1241-4. [DOI] [PubMed] [Google Scholar]
- 26.Wacharasint P, Boyd JH, Russell JA, Walley KR. One size does not fit all in severe infection: obesity alters outcome, susceptibility, treatment, and inflammatory response. Crit Care. 2013;17:R122. doi: 10.1186/cc12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.