Abstract
Rationale: Handgrip strength (HGS) predicts mortality in the elderly, but its determinants and clinical significance in chronic obstructive pulmonary disease (COPD) has not been defined.
Objectives: We tested associations of HGS with pectoralis muscle area (PMA), subcutaneous adipose tissue (SAT), imaging characteristics, and lung function in smokers with COPD, and evaluated the cross-sectional and longitudinal associations of HGS with acute respiratory events.
Methods: We analyzed demographic, clinical, spirometry, HGS, and imaging data of 272 subjects with COPD, obtaining measures of airway thickness, emphysema, PMA, and SAT from chest computed tomography scans. We tested associations of lung function and imaging characteristics with HGS, using linear models. HGS association to acute respiratory events at enrollment and during follow-up (mean, 2.6 years) was analyzed using adjusted logistic models.
Results: HGS correlated with PMA, SAT, forced expiratory volume, and airway thickness, but not with body mass index or emphysema severity. In adjusted regression models, HGS was directly (β, 1.5; 95% confidence interval [CI], 0.1–3.0) and inversely (β, −3.3; 95% CI, −5.1 to −0.9) associated with one standard deviation of PMA and SAT, respectively, independent of body mass index and emphysema. In regression models adjusted for age, sex, body mass index, race, pack-years smoked, current smoking, chronic bronchitis, FEV1% predicted, emphysema, and airway metrics, HGS was associated with exacerbation risk; in cross-sectional analyses, there was an increment of 5% in the risk of exacerbations for each 1-kg decrement in HGS (risk ratio, 1.05; 95% CI, 1.01–1.08), and there was a similar risk during follow-up (risk ratio, 1.04; 95% CI, 1.01–1,07).
Conclusions: In ever-smokers with COPD, HGS is associated with computed tomography markers of body composition and airway thickness, independent of body mass index and emphysema. Higher HGS is associated with lower exacerbation frequency.
Keywords: COPD symptom flare up, adult, cohort studies, Body Mass Index, computed tomography
Persons with chronic obstructive pulmonary disease (COPD), at this time the third cause of mortality among American adults (1), frequently develop different systemic manifestations (2), in addition to the well-known progressive decline in lung function and persistent respiratory symptoms. An important, although frequently overlooked effect, of COPD is the development of markers of global functional deterioration, usually known as geriatric conditions, functional limitations (3, 4), changes in body composition (5), and cognitive impairment (6), which are commonly grouped under the terms “frailty” (7) or markers of accelerated aging. Two important components of frailty models, such as the Fried’s model (8, 9), are weakness (measured by handgrip strength [HGS]) and unintentional weight loss of more than 10 pounds. Low HGS itself is a strong predictor of all-cause and cardiovascular mortality at the population level (10–12), and frailty is prevalent among patients with COPD referred to pulmonary rehabilitation (13). Despite the high frequency and prognostic value of frailty and its components, these functional markers of aging are not part of current guidelines for evaluation, management, and prognostication of patients with COPD (3).
These important gaps in the recognition of extrapulmonary functional measures as significant predictors of disease progression have been addressed by the inclusion of measures of walking ability and body composition in different multidimensional models of disease impact, such as BODE (body mass index [BMI], obstruction, dyspnea, and exercise). Subjects with COPD, particularly those with emphysema-predominant disease, not only lose weight, which may lead to low BMI (14), but also may lose muscle mass much faster than fat (14). Surprisingly, little is known about the associations of HGS in COPD or how it correlates with specific disease phenotypes. Evidence about its association with COPD outcomes is conflicting, in particular regarding exacerbations (15).
Exploring the association of functional measures and BMI as a measure of body composition is limited because BMI does not distinguish between loss of muscle mass versus gain in fat mass (16). A novel approach to this problem, validated in the field of abdominal oncology and surgery, is to use measures of muscle and fat structures captured as part of clinically indicated imaging studies (e.g., abdominal computed tomography [CT]) as surrogates of body composition (17, 18). Although this approach is appealing, the seminal studies in COPD have relied on imaging analysis of lower extremity muscles (19), routinely not part of the imaging tests available in the evaluation of COPD. Recent developments in imaging analysis have taken advantage of the inclusion of extrathoracic structures in chest CT, such as pectoralis muscles and subcutaneous fat. Methods to obtain these measures have been standardized and proven to be associated with measures of airflow obstruction, dyspnea, and 6-minute-walk distance (20). The availability of these methods opens new opportunities to explore associations of body composition with COPD manifestations and outcomes.
In the current study, we aimed to evaluate the associations of imaging-based markers of body composition (subcutaneous fat, pectoralis muscle mass) with HGS in smokers with COPD, and to test whether the associations are independent of BMI, markers of lung function, and severity of emphysema and airway disease. Furthermore, we aimed to identify the clinical relevance of measuring HGS, testing its associations with acute respiratory events or exacerbations.
This study was presented as an abstract at the CHEST 2016: American College of Chest Physicians International Conference, Los Angeles, CA, October 23, 2016 (21).
Methods
Design and Participants
The current analysis is a cross-sectional analysis of baseline data from selected participants with COPD in the NIH-funded Genetic Epidemiology of COPD Study (COPDGene; ClinicalTrials.gov identifier: NCT00608764). COPDGene is an ongoing multicenter cohort study aiming to identify genetic factors in smoking-related lung disease and to assess associations of susceptibility genes with disease phenotypes. The design and protocols for COPDGene have been previously described (22); briefly, COPDGene recruited non-Hispanic white or African American adults, both sexes, current or past smokers, with at least 10 pack-year history and willingness to participate in detailed clinical, physiologic, and imaging evaluation. The protocols for COPDGene have been approved at the University of Michigan, where this analysis was conducted (University of Michigan Health System Research Committee institutional review board approval HUM000014973, July 16, 2010) and all participating centers. All participants provided informed consent to participate in the study.
Subjects included in the current analysis were part of a pilot project implemented in one COPDGene participating center to evaluate HGS in smokers. We included smokers with COPD, diagnosed using the fixed-ratio GOLD definition of postbronchodilator FEV1/FVC ratio below 0.7. COPDGene spirometry is performed using an EasyOne spirometer, with values expressed as percentage based on predictive equations (23).
Handgrip Strength Measurement
Trained personnel measured HGS at enrollment according to a standardized protocol, using a Jamar dynamometer (Fabrication Enterprises, White Plains, NY). Three measurements were made from the participant’s dominant unsupported hand, and the highest value was used in the current analyses. We report and compare age- and sex-specific values, using as normative age and sex values pooled data from 12 studies, as reported by Dodds and colleagues (24)
CT Analysis
COPDGene participants underwent a volumetric CT scan of the chest at full inflation and relaxed expiration. COPDGene imaging protocols are described elsewhere (22). For this study, inspiratory CT data were used. Emphysema was defined as percentage of lung attenuation areas below −950 Hounsfield Units (HU). Wall thickening was measured using Pi10, which is the square root of the wall area of a theoretical airway of 10 mm luminal perimeter (25).
CT Measures of Body Composition
Pectoralis muscle (PMA) and subcutaneous adipose tissue (SAT) areas were measured by trained analysts unaware of participants’ clinical data, using Slicer software (slicer.com) (20). To measure PMA, the first axial image above the aortic arch was used. At that level, the left and right pectoralis major and minor muscles were identified on the anterior chest, and their edges were manually segmented, using an attenuation range of −50 and 90 HU. The interior of the muscle was then filled automatically. SAT was manually segmented between the major pectoralis muscle and the skin, using a range of −200 and 0 HU at same axial slice, with the muscle edges used as lateral boundaries of the SAT measurement. In previous work by our group and others, the intra- and inter-reproducibility of PMA and SAT (two readers) ranged from 0.98 to 1.00 (19, 26), and the current analyses are based on one reader’s measures. To account for differences in height and sex, PMA and SAT areas (in square centimeters) were divided by the square of the individual’s height (in square meters), with all measures being used as cm2/m2; furthermore, the measures were standardized (rescaled to have a mean of zero and a standard deviation of one) separately for each sex, and reported and used in this analysis as standard deviation units.
Acute Respiratory Disease
Baseline history of respiratory disease events or exacerbations (27) were defined as increased cough, phlegm, or shortness of breath that lasted more than 48 hours, requiring management with antibiotics and/or systemic steroids, in the year before enrollment. Acute events during longitudinal follow-up (mean, 2.6 years) were ascertained using a similar question during regular phone contact or a web-based survey applied at 3–6-month intervals (28).
Other covariates
Demographic (age, sex, race) and biometric (weight, height) data were collected at enrollment. Self-administered questionnaires were used to collect data on smoking, respiratory symptoms and medications, and medical history. Comorbid chronic conditions were self-reported, based on the answer to the question, “Have you ever been told by a physician that you have [condition name]?” The modified Medical Research Council Dyspnea Scale scale was used to evaluate the degree of dyspnea.
Statistical Analysis
Demographics and anthropometric and lung function data were described as means with their standard deviation or frequencies, when appropriate. Correlative analysis of HGS (absolute value) with markers of body composition, lung function, and imaging were analyzed with Pearson’s coefficients. In an initial analysis, we used multivariate linear regression to test associations between HGS (outcome) and markers of body composition (PMA and SAT, age and height adjusted and normalized to be analyzed as 1 standard deviation increment) in models additionally controlled for lung function and CT-measured emphysema and Pi10. We also analyzed the associations of HGS with CT measures of body composition in obese and nonobese subjects, defining obesity as BMI ≥30 kg/m2. In these analyses, PMA and SAT were dichotomized as low PMA or SAT for those in the lower tertile of their distribution. Finally, we tested the association of HGS with respiratory events at enrollment (cross-sectional) and to any incident event (longitudinal), using negative binomial logistic regression models also adjusted for age, sex, race, pack-years smoked, current smoking status, FEV1% predicted, and models of events during follow-up additionally adjusted for exacerbation history at enrollment. All analyses were performed using Stata v.12 (Stata Corp., College Station, TX.).
Results
Description of Participants
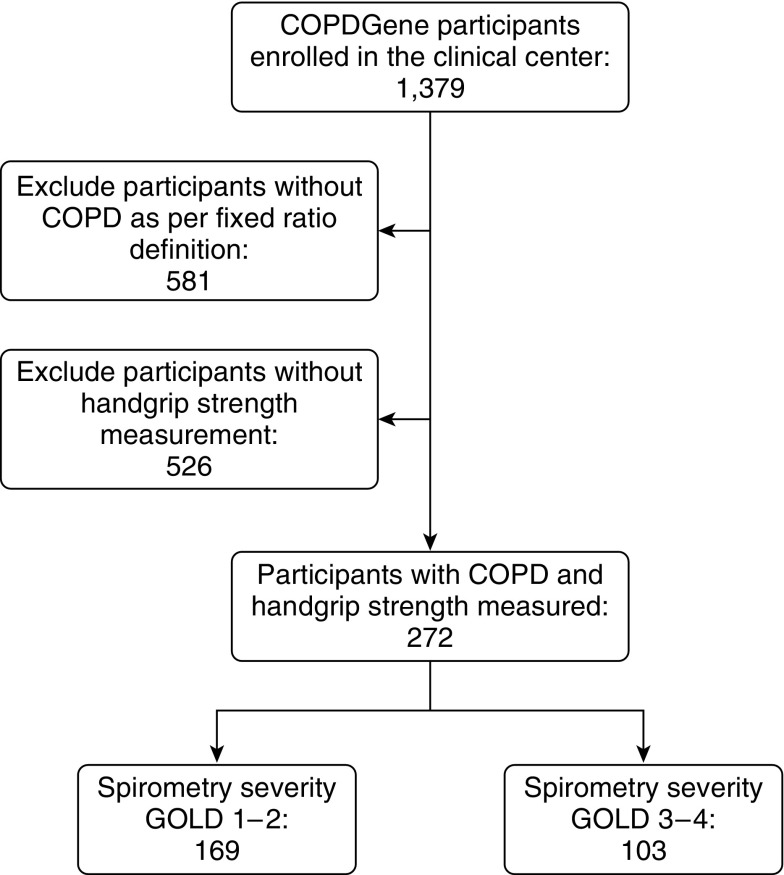
The current analysis included 272 COPD participants with complete measures of HGS, body composition, and longitudinal follow-up, recruited at National Jewish Health in Denver (Figure 1). Overall, subjects were predominantly non-Hispanic whites (92.3%), middle-aged (mean, 64.7 ± 8.0 years; mean ± SD), with a sizeable proportion of women (44.5%), with a substantial smoking history (51.1 ± 25.4 pack-years), and with 25.4% being current smokers (Table 1). A HGS below the 25th percentile of the normative value for age and sex (24) was found in 65.4% of subjects. When comparing participants included in the current analyses with the rest of those with COPD enrolled at the clinical center for whom no HGS data were collected, we did not find significant demographic or tobacco use differences, although those who had HGS measured had slightly better measures of lung function and reported more exacerbations (see Table E1 in the online supplement). A detailed description of participants by HGS tertiles can be found in Table E2.
Figure 1.
Flow of participants included in the study. COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Obstructive Lung Disease.
Table 1.
Description of participants (n = 272)
| Demographics | |
|---|---|
| Age, mean (s.d.) | 64.7 (8.0) |
| Female sex, % | 44.5 |
| African American, % | 7.7 |
| BMI, mean (s.d.) | 28.1 (5.6) |
| Smoking history | |
| Pack-years smoked, mean (s.d.) | 51.1 (25.4) |
| Currently smoking, % | 25.4 |
| Lung function | |
| FEV1 in liters, mean (s.d.) | 1.70 (0.77) |
| FEV1% predicted, mean (s.d.) | 59.0 (22.5) |
| FEV1/FVC, mean (s.d.) | 0.51 (0.13) |
| Spirometry severity GOLD stages 3-4, % | 37.9 |
| Markers of respiratory impact | |
| Annual respiratory events, mean (s.d.) | 0.6 (1.1) |
| Chronic bronchitis symptoms, % | 22.8 |
| mMRC dyspnea score, mean (s.d.) | 1.8 (1.3) |
| Comorbid conditions, % | |
| Obesity (BMI ≥30 kg/m2) | 32.4 |
| Other cardiovascular risk factors | 66.5 |
| Diabetes | 11.0 |
| Cardiovascular disease | 22.1 |
| Musculoskeletal disease | 38.6 |
Definition of abbreviations: BMI = body mass index; GOLD = Global Initiative for Obstructive Lung Disease; mMRC = modified Medical Research Council Dyspnea Scale; s.d. = standard deviation.
Handgrip Associations with Body Composition and Airway Metrics
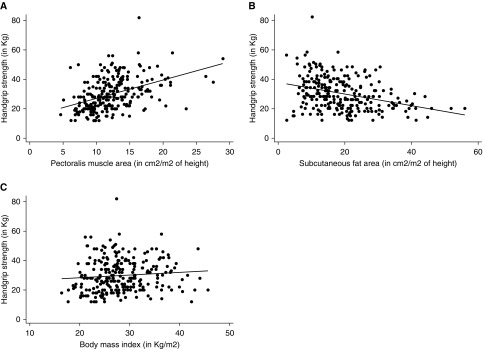
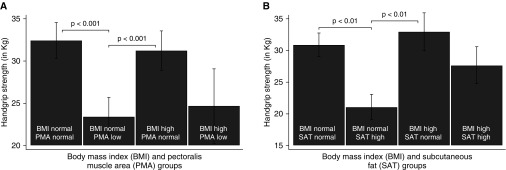
HGS had moderate correlation with PMA (r = 0.42; P < 0.001) and inversely with SAT (r = −0.35; P < 0.001), but not with BMI (r = 0.07; P = 0.25; Table 2 and Figure 2). HGS also moderately correlated with Pi10 (r = −0.32; P < 0.001), but not with emphysema extent (r = −0.07; P = 0.27). Although HGS did only moderately correlate with FEV1% predicted (r = 0.12; P = 0.05), it had a stronger correlation with FEV1 absolute value (r = 0.47; P < 0.001) (Table 2). Furthermore, the associations of higher PMA and low SAT with greater HGS were maintained, regardless of BMI: higher PMA and low SAT showed a positive association with HGS in both participants with and without obesity (defined as BMI ≥30 kg/m2; Figures 3A–3B).
Table 2.
Correlation of handgrip strength with measures of body composition, lung function, and imaging phenotypes
| Markers of Body Composition | Correlation Coefficient | P Value |
|---|---|---|
| Pectoralis muscle area | 0.42 | <0.001 |
| Subcutaneous fat area | −0.35 | <0.001 |
| Body mass index | 0.07 | 0.25 |
| Lung function | ||
| FEV1 in liters | 0.47 | <0.001 |
| FEV1% predicted | 0.12 | 0.05 |
| Total lung capacity | 0.54 | <0.001 |
| Markers of respiratory impact | ||
| Chronic bronchitis symptoms | −0.04 | 0.51 |
| mMRC dyspnea score | −0.17 | <0.001 |
| Distance walked in 6 minutes | 0.30 | <0.001 |
| Imaging characteristics | ||
| Emphysema percentage | −0.07 | 0.27 |
| Pi10 | −0.32 | <0.001 |
Definition of abbreviation: mMRC = modified Medical Research Council Dyspnea Scale.
Figure 2.
Correlation between handgrip strength and imaging markers of body composition. The scatterplots show the correlations between handgrip strength and (A) pectoralis muscle area (r = 0.39), (B) subcutaneous fat area (r = −0.34), and (C) body mass index (r = 0.05).
Figure 3.
Differences in handgrip strength by body composition and imaging groups. The association between low pectoralis muscle area and low handgrip strength (A) is present, regardless of body mass index (BMI). In a similar manner, higher subcutaneous tissue area is associated with low handgrip strength (B), even if the subject has normal body mass index. All comparisons are based on two-way analyses of variances, corrected for multiple comparisons.
Next, we tested whether the associations of body composition and imaging characteristics to HGS were robust to adjustments for BMI, lung function, and comorbidities (Table 3). Because HGS is significantly different by sex (for men, HGS 36.3 ± 9.6 [mean ± standard deviation] and for women, 21.4 ± 5.1 kg), we included in the models all body composition measures adjusted for sex and height, and further models also included adjustment for sex. We found that an increment of 1 standard deviation of PMA (sex-specific and adjusted for height) was associated with higher HGS (β, 1.5; 95% CI, 0.1–3.0), whereas for 1 standard deviation increment in SAT (sex-specific and adjusted for height), there was lower HGS (β, −3.3; 95% CI, −5.7 to −0.9). Of notice, the univariate association of HGS with airway metrics was maintained in multivariate models showing that as Pi10 increases, there is lower HGS (β, −3.7 per 1 standard deviation increment of Pi10; 95% CI, −5.0 to −2.3), whereas the association with emphysema was not significant.
Table 3.
Association of handgrip strength with body composition and airway metrics (change in handgrip strength in kilograms [95% CI] per unit increment in predictor)
| Predictor | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Pectoralis muscle area (per 1 s.d.) | 1.7 (0.3 to 3.1) | 1.7 (0.3 to 3.2) | 1.5 (0.1 to 3.0) |
| Subcutaneous fat area (per 1 s.d.) | −0.6 (−1.9 to 0.8) | −0.5 (−1.9 to 0.8) | −3.3 (−5.7 to −0.9) |
| Imaging characteristics | |||
| Pi10 (per 1 s.d.) | −3.5 (−4.8 to −2.2) | −3.7 (−5.0 to −2.3) | |
| Emphysema % (per 1 s.d.) | −0.4 (−1.8 to 1.0) | 0.2 (−1.8 to 1.4) | |
| Covariates | |||
| FEV1% predicted | −0.03 (−0.11 to 0.04) | ||
| Body mass index | 0.7 (0.2 to 1.2) | ||
| Diabetes | −0.6 (−4.7 to 3.5) | ||
| Musculoskeletal disease | −5.7 (−8.2 to −3.1) |
Definition of abbreviations: CI = confidence interval; s.d. = standard deviation.
All entries represent β coefficient and 95% confidence interval (β [95% CI]). Pectoralis muscle area and subcutaneous fat area values were derived by dividing the area (in square centimeters) by the square of the individual’s height (in square meters), with all measures being used as cm2/m2; the measures were also standardized (rescaled to have a mean of zero and a standard deviation of one) separately for each sex, as described in the Materials and Methods. In all analyses, pectoralis muscle area and subcutaneous fat area were included in the same model. Model 1: Bivariate associations. Model 2: Model 1 also adjusted for imaging characteristics. Model 3: Model 2 also adjusted for predicted percentage of FEV1, body mass index, diabetes, and musculoskeletal disease.
Handgrip Is Associated with Acute Respiratory Events
Of the total participants, almost 30.9% reported at least one respiratory event in the year before enrollment. During a follow-up of 2.6 ± 0.73 years (mean ± SD) 34.0% had at least one exacerbation, for an annual rate of 0.6 ± 1.1 (mean ± SD). Frequent events (≥2/year) were present in 12.5% at enrollment and 12.2% during follow-up. We performed analyses testing associations of HGS with acute respiratory events, adjusting for age, sex, BMI, race, pack-years smoked, current smoking, FEV1% predicted, chronic bronchitis, emphysema, and airway metrics. Results showed that for a 1-kg decrement in HGS, participants had a 5% higher relative risk of reporting exacerbations during the year before enrollment (95% CI, 1.01–1.08). In models equally adjusted and with additional adjustment for baseline exacerbation history, each additional kilogram decrement in HGS was associated with higher relative risks of having any incident event during follow-up (rate ratio, 1.04; 95% CI, 1.01–1.07; Table 4). Finally, in similarly adjusted analyses, we also found that the risk for severe events (those requiring emergency visits or hospital admission) during follow-up was also higher per 1-kg decrement in HGS (relative risk, 1.06; 95% CI, 1.01–1.12).
Table 4.
Association of handgrip strength with acute respiratory events
| Any Event at Baseline (Rate Ratio) | Any Event during Follow-Up (Rate Ratio) | Any Severe Event during Follow-Up (Rate Ratio) | |
|---|---|---|---|
| Handgrip strength (per 1 kg decrement) | 1.05 (1.01–1.08) | 1.04 (1.01–1.07) | 1.06 (1.01–1.12) |
| Lung function | |||
| FEV1% predicted | 0.99 (0.98–1.00) | 0.99 (0.98–1.01) | 0.99 (0.98–1.01) |
| Chronic bronchitis | 1.33 (0.80–2.20) | 0.82 (0.48–1.40) | 0.58 (0.22–1.50) |
| Exacerbations at baseline | N/A | 1.18 (1.00–1.38) | 1.29 (1.01–1.65) |
Definition of abbreviation: N/A = not applicable.
All entries represent rate ratios and 95% confidence intervals. All models included all variables presented in the table and were also adjusted for age, sex, body mass index, race, pack-years smoked, currently smoking, emphysema, and airway metrics. Models during follow-up were also adjusted for history of acute events at enrollment.
Discussion
The current analysis of 272 subjects with COPD reveals interesting findings about the associations and effect of HGS: We found that HGS was associated with CT-based markers of body composition, independent of BMI and CT measures of emphysema and airway disease. Furthermore, we found that HGS was modestly associated with cross-sectional and longitudinal respiratory events, independent of common markers of exacerbation risk, including lung function, smoking, and history of bronchitis. Our findings support the concept that systemic effects of disease, identifiable using physical measures such as HGS, are another important dimension of tobacco-related diseases and need to be considered part of its clinical evaluation.
Our results demonstrate the clinical utility of testing HGS, which we selected as a feasible, noncomplex, and validated marker of physical performance in the elderly, already one of the measures included in frailty models (8, 29). HGS is infrequently used or reported as a functional measure in patients with respiratory disease, perhaps because it is commonly considered part of a complex battery of functional tests. Using this simple functional measure, we found that 65.4% of our participants had low HGS, a striking figure compared with 15.4% reported for community-dwelling adults (30), and 33% on subjects with esophageal cancer (31). Because our participants are relatively younger, our findings suggest not only a high frequency but also an early development of a systemic functional decline in COPD. These findings also support the need for early identification and interventions to preserve functional status in subjects with COPD, because maintaining physical function and muscle bulk, for which HGS may be an important surrogate measurement, could be associated with reduced risk for cardiovascular disease many decades later (32).
These findings agree with and expand previous studies showing associations between HGS and markers of COPD severity, such as hyperinflation and FEV1, previously found to be highly correlated with HGS (for hyperinflation, r = −0.51 and for FEV1, r = 0.59) (33). We extend these findings using a larger sample of subjects and by showing similar correlations (FEV1, r = 0.47; TLC, r = 0.54). These findings could be interpreted as additional evidence that functional measures probably depend on the same factors explaining cardiovascular fitness in COPD, such as hyperinflation and airflow obstruction. In addition, we found that HGS was associated with body composition, evaluated by chest CT scans, a very common diagnostic method, which could affect medical practice. Evaluation of body composition is considered by many clinicians to require methods reserved for investigative purposes (34, 35), and hence to be beyond the scope of their practice. By deriving measures of body composition (PMA and SAT) from conventional chest CT scans, and demonstrating that they are associated with HGS, which we in turn showed to be related to conventional COPD outcomes including respiratory event frequency, we highlight a new opportunity to use the information provided by CT scans performed for other clinical reasons. For example, although not the main goal of the current investigation, CT measures of PMA were found to be associated with a lower incidence risk ratio of reporting any exacerbation at baseline (incidence risk ratio, 0.40 per 1 standard deviation PMA) when controlling for demographics, lung function, and smoking history. This could be particularly important with the growing amount of imaging available as part of lung cancer screening programs with low-dose CT scans. Interestingly, we were able to show that both low PMA and high SAT are independently associated with HGS, and that they are also independent of BMI. The fact that PMA and BMI have a separate effect on functional status aligns with growing awareness of a subgroup of ever-smokers with low muscle mass and function, yet normal BMI, as a result of excess fat tissue (36), and the potential for conflicting effects of BMI and muscle mass on clinical outcomes in COPD (37, 38). Concordant with those findings, we demonstrated that the effect of low PMA on HGS is relevant, regardless of the subject’s BMI or SAT.
Interest in how HGS affects patient-centered outcomes in different diseases (31, 39–41) has been strengthened by population-based studies confirming that low HGS is a risk factor for all-cause death and cardiovascular mortality (11). Evidence on the associations of HGS and outcomes in subjects with tobacco-related diseases is growing, linking it to hospital admissions (42) and to walking distance (43). A large study of COPD patients found an association between low HGS and mortality, but not exacerbations (15). We extended the findings by confirming the presence of a modest but significant association between low HGS and acute respiratory events/exacerbations, including more severe exacerbations requiring emergency department visits or hospital admission. Follow-up of this and other cohorts is needed to ascertain the effect of HGS on survival.
Another interesting finding of our study is the association between HGS and CT measures of emphysema and airway disease. Although it could be tempting to hypothesize that low HGS could be more frequent among patients with COPD with characteristics of the emphysematous type, we found that HGS is strongly associated with airway wall thickening, but not with emphysema. The association with airway-predominant imaging phenotype is aligned with recent evidence that nonemphysematous COPD has stronger associations with cardiometabolic diseases than emphysema (44). Because inflammatory processes are pathways to the development of cardiometabolic conditions, and an inflammatory profile is reported among subjects with low HGS at the population level, the associations have biological plausibility that deserve further study (45).
Our analyses are subject to some limitations, including the selection of the measures of body composition. Although we used analysis of chest CTs, instead of the gold standard of dual absorptiometry or other methods such as bioelectrical impedance (16, 34), these novel imaging markers of body composition have proved to be reliable, to be associated with other clinical markers of respiratory disease severity (20), and to have great potential for translation to the clinical practice in subjects with respiratory disease. Furthermore, we made every possible effort to separate the independent associations of muscle and fat, from the most common measure of BMI, with encouraging findings. Another potential limitation is the evaluation of cross-sectional associations between HGS and acute respiratory events, a design that impedes inferences about the direction of the association. However, participants were also followed for more than 2 years, and the association between baseline HGS and subsequent respiratory events was maintained. Using a self-reported outcome of exacerbation could be seen as another limitation, but COPDGene has designed and validated a system that follows participants to ascertain this outcome (28). A final limitation is that we tested a selected group of participants in a cohort study, not a representative sample of the population, including a low burden of emphysema and fewer African American participants. Strengths of the current analyses include its use of a cohort with detailed data on lung function and imaging; the experience of the researchers with the imaging analysis methods; and the inclusion in our models of most of the relevant variables associated with acute respiratory events or exacerbations in subjects with or at risk for COPD.
Conclusions
In conclusion, we show that smokers with COPD frequently have low HGS, that independent of BMI, HGS strongly associates with markers of body composition (PMA and SAT) and with measures of lung function and lung structure, in particular airway thickness, and that HGS independently is associated with increased risk for exacerbations.
Footnotes
COPDGene is supported by National Heart, Lung, and Blood Institute grants R01HL089897 and R01HL089856. Additional grant support from: National Heart, Lung, and Blood Institute R01HL122438-02S1 and K23 HL128936 (C.H.M.), K01HL118714 (A.A.D.), K99HL121087 (M.-L.N.M.), R01HL122438 (M.K.H.), and R01HL107246 and R01HL122464 (G.R.W.). The funding agencies did not have any role in the analysis, interpretation of the findings, or manuscript preparation. J.L.C. has received grant funding from MedImmune, Ltd.
Author Contributions: C.H.M. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, and is the guarantor of this study; C.H.M. and A.A.D. contributed to conceiving and writing the study, data analysis, and clinical interpretation of the data and to approving the final draft of the manuscript; C.A.M., M.-L.N.M., S.M., G.L.K., J.E.H., and J.L.C. contributed to conceiving and writing the manuscript, clinical interpretation of the data, and approving the final draft of the manuscript; C.H.M., R.P.B., and M.K.H. contributed to conceiving the study, collecting the data, writing the manuscript, clinical interpretation of the data, and approving the final draft of the manuscript; G.R.W. contributed to conceiving the study and collecting the data, performing the imaging measures, writing the manuscript, clinical interpretation of the data, and approving the final draft of the manuscript; and E.A.R. contributed to conceiving the study and collecting the data, performing the handgrip measures, writing the manuscript, clinical interpretation of the data, and approving the final draft of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the COPDGene Investigators
References
- 1.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1–51. [PubMed] [Google Scholar]
- 2.Brown JP, Martinez CH. Chronic obstructive pulmonary disease comorbidities. Curr Opin Pulm Med. 2016;22:113–118. doi: 10.1097/MCP.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 3.Janaudis-Ferreira T, Beauchamp MK, Robles PG, Goldstein RS, Brooks D. Measurement of activities of daily living in patients with COPD: a systematic review. Chest. 2014;145:253–271. doi: 10.1378/chest.13-0016. [DOI] [PubMed] [Google Scholar]
- 4.Eisner MD, Iribarren C, Blanc PD, Yelin EH, Ackerson L, Byl N, Omachi TA, Sidney S, Katz PP. Development of disability in chronic obstructive pulmonary disease: beyond lung function. Thorax. 2011;66:108–114. doi: 10.1136/thx.2010.137661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbatecola AM, Fumagalli A, Spazzafumo L, Betti V, Misuraca C, Corsonello A, Cherubini A, Guffanti EE, Lattanzio F. Body composition markers in older persons with COPD. Age Ageing. 2014;43:548–553. doi: 10.1093/ageing/aft196. [DOI] [PubMed] [Google Scholar]
- 6.Martinez CH, Richardson CR, Han MK, Cigolle CT. Chronic obstructive pulmonary disease, cognitive impairment, and development of disability: the health and retirement study. Ann Am Thorac Soc. 2014;11:1362–1370. doi: 10.1513/AnnalsATS.201405-187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57:830–839. doi: 10.1111/j.1532-5415.2009.02225.x. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 9.Op het Veld LP, van Rossum E, Kempen GI, de Vet HC, Hajema K, Beurskens AJ. Fried phenotype of frailty: cross-sectional comparison of three frailty stages on various health domains. BMC Geriatr. 2015;15:77. doi: 10.1186/s12877-015-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36:228–235. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 11.Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A, Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, et al. Prospective Urban Rural Epidemiology (PURE) Study investigators. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386:266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 12.Gosselink R, Troosters T, Decramer M. Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2000;20:353–360. doi: 10.1097/00008483-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Maddocks M, Kon SS, Canavan JL, Jones SE, Nolan CM, Labey A, Polkey MI, Man WD. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71:988–995. doi: 10.1136/thoraxjnl-2016-208460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battaglia S, Spatafora M, Paglino G, Pedone C, Corsonello A, Scichilone N, Antonelli-Incalzi R, Bellia V. Ageing and COPD affect different domains of nutritional status: the ECCE study. Eur Respir J. 2011;37:1340–1345. doi: 10.1183/09031936.00032310. [DOI] [PubMed] [Google Scholar]
- 15.Puhan MA, Siebeling L, Zoller M, Muggensturm P, ter Riet G. Simple functional performance tests and mortality in COPD. Eur Respir J. 2013;42:956–963. doi: 10.1183/09031936.00131612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King S, Wilson J, Kotsimbos T, Bailey M, Nyulasi I. Body composition assessment in adults with cystic fibrosis: comparison of dual-energy X-ray absorptiometry with skinfolds and bioelectrical impedance analysis. Nutrition. 2005;21:1087–1094. doi: 10.1016/j.nut.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Miller BS, Ignatoski KM, Daignault S, Lindland C, Gauger PG, Doherty GM, Wang SC University of Michigan Analytical Morphomics Group. A quantitative tool to assess degree of sarcopenia objectively in patients with hypercortisolism. Surgery. 2011;150:1178–1185. doi: 10.1016/j.surg.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Sheetz KH, Zhao L, Holcombe SA, Wang SC, Reddy RM, Lin J, Orringer MB, Chang AC. Decreased core muscle size is associated with worse patient survival following esophagectomy for cancer. Dis Esophagus. 2013;26:716–722. doi: 10.1111/dote.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 20.McDonald ML, Diaz AA, Ross JC, San Jose Estepar R, Zhou L, Regan EA, Eckbo E, Muralidhar N, Come CE, Cho MH, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease: a cross-sectional study. Ann Am Thorac Soc. 2014;11:326–334. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez CH, Diaz AA, Meldrum C, McDonald ML, Kinney G, Hokanson J, Bowler R, Han MK, Washko G, Regan EA. Hand grip in smokers predicts future acute respiratory events, and is associated with body composition, independently of body mass index and measures of airflow. Presented at the American College of Chest Physicians International Conference. October 23, 2016, Los Angeles, CA. Chest 2016;150(4 Suppl):p917A. [Google Scholar]
- 22.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 24.Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, Der G, Gale CR, Inskip HM, Jagger C, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9:e113637. doi: 10.1371/journal.pone.0113637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Paré PD. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 26.Diaz AA, Zhou L, Young TP, McDonald ML, Harmouche R, Ross JC, San Jose Estepar R, Wouters EF, Coxson HO, MacNee W, et al. ECLIPSE investigators. Chest CT measures of muscle and adipose tissue in COPD: gender-based differences in content and in relationships with blood biomarkers. Acad Radiol. 2014;21:1255–1261. doi: 10.1016/j.acra.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowler RP, Kim V, Regan E, Williams AAA, Santorico SA, Make BJ, Lynch DA, Hokanson JE, Washko GR, Bercz P, et al. Prediction of acute respiratory disease in current and former smokers with and without COPD. Chest. 2014;146:941–950. doi: 10.1378/chest.13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart JI, Moyle S, Criner GJ, Wilson C, Tanner R, Bowler RP, Crapo JD, Zeldin RK, Make BJ, Regan EA For The Copdgene Investigators. Automated telecommunication to obtain longitudinal follow-up in a multicenter cross-sectional COPD study. COPD. 2012;9:466–472. doi: 10.3109/15412555.2012.690010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens PJ, Syddall HE, Patel HP, Martin HJ, Cooper C, Aihie Sayer A. Is grip strength a good marker of physical performance among community-dwelling older people? J Nutr Health Aging. 2012;16:769–774. doi: 10.1007/s12603-012-0388-2. [DOI] [PubMed] [Google Scholar]
- 30.Samper-Ternent R, Al Snih S, Raji MA, Markides KS, Ottenbacher KJ. Relationship between frailty and cognitive decline in older Mexican Americans. J Am Geriatr Soc. 2008;56:1845–1852. doi: 10.1111/j.1532-5415.2008.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CH, Ho-Chang, Huang YZ, Hung TT. Hand-grip strength is a simple and effective outcome predictor in esophageal cancer following esophagectomy with reconstruction: a prospective study. J Cardiothorac Surg. 2011;6:98. doi: 10.1186/1749-8090-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen K, Rasmussen F, Held C, Neovius M, Tynelius P, Sundström J. Exercise capacity and muscle strength and risk of vascular disease and arrhythmia in 1.1 million young Swedish men: cohort study. BMJ. 2015;351:h4543. doi: 10.1136/bmj.h4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortopassi F, Celli B, Divo M, Pinto-Plata V. Longitudinal changes in handgrip strength, hyperinflation, and 6-minute walk distance in patients with COPD and a control group. Chest. 2015;148:986–994. doi: 10.1378/chest.14-2878. [DOI] [PubMed] [Google Scholar]
- 34.Elia M. Body composition by whole-body bioelectrical impedance and prediction of clinically relevant outcomes: overvalued or underused? Eur J Clin Nutr. 2013;67:S60–S70. doi: 10.1038/ejcn.2012.166. [DOI] [PubMed] [Google Scholar]
- 35.Ravaglia G, Forti P, Maioli F, Boschi F, Cicognani A, Gasbarrini G. Measurement of body fat in healthy elderly men: a comparison of methods. J Gerontol A Biol Sci Med Sci. 1999;54:M70–M76. doi: 10.1093/gerona/54.2.m70. [DOI] [PubMed] [Google Scholar]
- 36.Batsis JA, Mackenzie TA, Jones JD, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and inflammation: results from the 1999-2004 National Health and Nutrition Examination Survey. Clin Nutr. 2016;35:1472–1483. doi: 10.1016/j.clnu.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van de Bool C, Rutten EP, Franssen FM, Wouters EF, Schols AM. Antagonistic implications of sarcopenia and abdominal obesity on physical performance in COPD. Eur Respir J. 2015;46:336–345. doi: 10.1183/09031936.00197314. [DOI] [PubMed] [Google Scholar]
- 38.Koo HK, Park JH, Park HK, Jung H, Lee SS. Conflicting role of sarcopenia and obesity in male patients with chronic obstructive pulmonary disease: Korean National Health and Nutrition Examination Survey. PLoS One. 2014;9:e110448. doi: 10.1371/journal.pone.0110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–117. doi: 10.1016/j.nut.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Sato T, Aoyama T, Hayashi T, Segami K, Kawabe T, Fujikawa H, Yamada T, Yamamoto N, Oshima T, Rino Y, et al. Impact of preoperative hand grip strength on morbidity following gastric cancer surgery. Gastric Cancer. 2016;19:1008–1015. doi: 10.1007/s10120-015-0554-4. [DOI] [PubMed] [Google Scholar]
- 41.Mendes J, Azevedo A, Amaral TF. Handgrip strength at admission and time to discharge in medical and surgical inpatients. JPEN J Parenter Enteral Nutr. 2014;38:481–488. doi: 10.1177/0148607113486007. [DOI] [PubMed] [Google Scholar]
- 42.Vilaró J, Ramirez-Sarmiento A, Martínez-Llorens JM, Mendoza T, Alvarez M, Sánchez-Cayado N, Vega A, Gimeno E, Coronell C, Gea J, et al. Global muscle dysfunction as a risk factor of readmission to hospital due to COPD exacerbations. Respir Med. 2010;104:1896–1902. doi: 10.1016/j.rmed.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Dourado VZ, Antunes LC, Tanni SE, de Paiva SA, Padovani CR, Godoy I. Relationship of upper-limb and thoracic muscle strength to 6-min walk distance in COPD patients. Chest. 2006;129:551–557. doi: 10.1378/chest.129.3.551. [DOI] [PubMed] [Google Scholar]
- 44.Hersh CP, Make BJ, Lynch DA, Barr RG, Bowler RP, Calverley PM, Castaldi PJ, Cho MH, Coxson HO, DeMeo DL, et al. COPDGene and ECLIPSE Investigators. Non-emphysematous chronic obstructive pulmonary disease is associated with diabetes mellitus. BMC Pulm Med. 2014;14:164. doi: 10.1186/1471-2466-14-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, Marsh AP, Kritchevsky SB, Nicklas BJ. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]