Abstract
The lung has a unique relationship to cholesterol that is shaped by its singular physiology. On the one hand, the lungs receive the full cardiac output and have a predominant dependence on plasma lipoprotein uptake for their cholesterol supply. On the other hand, surfactant lipids, including cholesterol, are continually susceptible to oxidation owing to direct environmental exposure and must be cleared or recycled because of the very narrow biophysical mandates placed upon surfactant lipid composition. Interestingly, increased lipid-laden macrophage “foam cells” have been noted in a wide range of human lung pathologies. This suggests that lipid dysregulation may be a unifying and perhaps contributory event in chronic lung disease pathogenesis. Recent studies have shown that perturbations in intracellular cholesterol trafficking critically modify the immune response of macrophages and other cells. This minireview discusses literature that has begun to demonstrate the importance of regulated cholesterol traffic through the lung to pulmonary immunity, inflammation, and fibrosis. This emerging recognition of coupling between immunity and lipid homeostasis in the lung presents potentially transformative concepts for understanding lung disease and may also offer novel and exciting avenues for therapeutic development.
Keywords: oxysterols, innate immunity, lipoproteins, liver X receptors
In recent years, there has been a growing appreciation in the fields of vascular biology and endocrinology of the complex interactions that exist between cholesterol homeostasis and immunity. Cholesterol overload of different subcellular compartments in macrophages in varying contexts can induce endoplasmic reticulum stress–dependent cytokine production (1), augmented proinflammatory signaling by Toll-like receptors (TLRs) (2), inflammasome activation (3), or suppression of proinflammatory gene expression (4). Dynamic changes in cholesterol synthesis are also required for antiviral responses in macrophages and for proliferation of T cells (5). Although abnormal pulmonary accumulation of lipid-laden macrophage “foam cells” has been noted in several lung diseases, the mechanisms that regulate cholesterol levels in lung-resident cells, as well as the connection of these mechanisms to lung disease, remain poorly defined. In this minireview, a brief overview of recent advances in this area is presented. For a more detailed discussion of cholesterol trafficking in lung health and disease, the reader is directed to recent comprehensive reviews (6–9).
Reverse Cholesterol Transport: A Brief Primer for the Pulmonologist
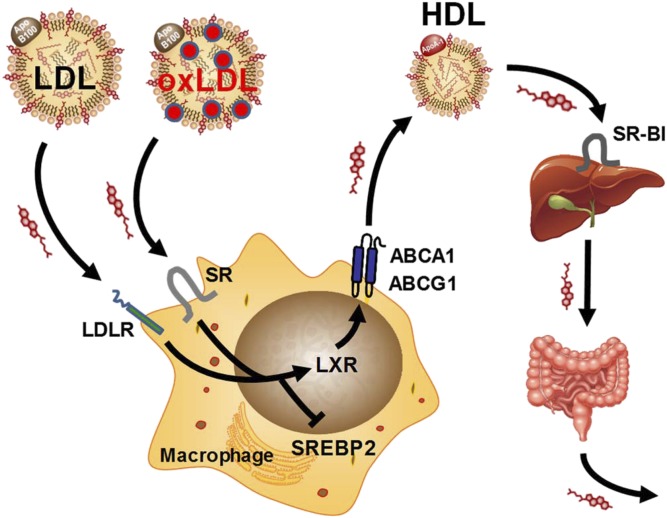
Reverse cholesterol transport (RCT) is the homeostatic pathway by which tissue macrophages and other cells are protected from toxic cholesterol overload (Figure 1) (reviewed in [10]). This occurs through regulated cellular efflux and bodily elimination of cholesterol. At the cellular level, macrophage cholesterol content is controlled through the opposing actions of two families of transcription factors: (1) sterol response element–binding proteins (SREBPs), which induce target genes that promote cholesterol accumulation via uptake (low-density lipoprotein receptor) and synthesis (e.g., hydroxymethylglutaryl coenzyme A reductase); and (2) liver X receptors (i.e., LXR-α and LXR-β), which induce genes that reduce cholesterol uptake (e.g., inducible degrader of low-density lipoprotein receptor) and promote cholesterol efflux (e.g., the ATP-binding cassette transporters A1 and G1 [ABCA1 and ABCG1, respectively]). Oxysterols (i.e., oxidized cholesterol, such as 25-hydroxycholesterol [25-HC] and 27-HC) serve as key integration signals between SREBPs and LXRs. Oxysterols accumulate during sterol overload and effect compensatory cholesterol reduction through activating LXRs (as direct agonists) and inhibiting SREBPs. Additional complex regulatory loops exist, including the intronic microRNA-33, which is cotranscribed with SREBP2 during low-cholesterol states and suppresses ABCA1 and ABCG1 (11). Ultimately, apolipoprotein A-I (apoA-I) and high-density lipoprotein (HDL) serve as plasma acceptors of cellular cholesterol effluxed via ABCA1 and ABCG1, respectively, and transport cholesterol to the liver for scavenger receptor BI (SR-BI)–mediated uptake and subsequent elimination via the bile into the feces.
Figure 1.
Reverse cholesterol transport pathway. Reverse cholesterol transport is the pathway by which tissue macrophages avoid toxic cholesterol overload. Cellular uptake of cholesterol from low-density lipoprotein (LDL) particles or oxidized LDL (oxLDL) particles via the LDL receptor (LDLR) or scavenger receptors (SRs), respectively, inhibits sterol response element–binding protein 2 (SREBP2) and activates liver X receptors (LXRs). The LXRs promote compensatory cholesterol efflux by upregulating ATP-binding cassette transporters A1 and G1 (ABCA1 and ABCG1, respectively). ABCA1 and ABCG1 together increase mobilization of cellular cholesterol to high-density lipoprotein (HDL) particles in the extracellular space. Plasma HDL delivers cholesterol to hepatocytes, where it is internalized via scavenger receptor BI (SR-BI). Cholesterol cleared from the plasma is then exported to the biliary system and from there to the intestinal lumen, where it can be excreted from the host. Apo B100 = apolipoprotein B 100; ApoA-1 = apolipoprotein A-I.
RCT: Ancient Regulator of Immunity and Inflammation
Of interest, RCT regulates not only cellular cholesterol but also innate and adaptive immunity, as recently reviewed (5, 9, 10, 12). LPS, a glycolipid shed from the outer membrane of gram-negative bacteria, partitions into HDL in the plasma, where it is neutralized and then cleared via hepatic SR-BI, along with cholesterol undergoing RCT (10). This finding has suggested that plasma lipoproteins may be an ancient arm of host defense. Intriguingly, sequence homology between HDL-associated plasma proteins that bind microbial lipids (i.e., LPS-binding protein) and host lipids (e.g., phospholipid transfer protein), as well as host defense functions that have been identified for the latter, together support common ancestry between lipid homeostasis and innate immunity (10). Suggesting an intrinsic role for sterols in immunity, recent reports have also shown that several immune receptors are directly ligated and regulated by cholesterol (T-cell receptor-β) and oxysterols (C-X-C chemokine receptor type 2, G protein–coupled receptor 183) (5).
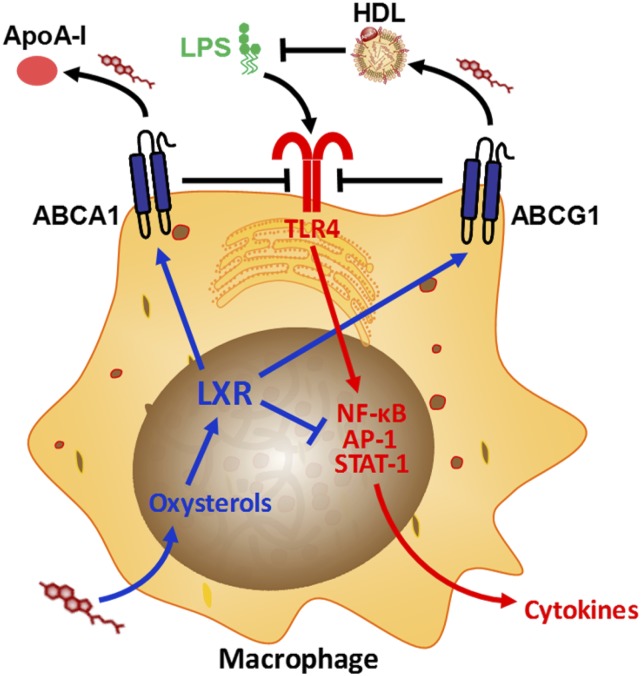
Complex cross-talk exists in macrophages between cholesterol mobilization and inflammatory functions of the cell (Figure 2). ApoA-I and HDL indirectly suppress proinflammatory signaling by TLRs and other receptors through reducing cholesterol in lipid raft microdomains of the plasma membrane (12). LXRs augment this effect through potentiating ABCA1-dependent raft cholesterol efflux (13) and inhibiting proinflammatory gene expression (14), whereas Abca1−/− and Abcg1−/− macrophages have augmented TLR responses as a result of raft cholesterol overload (2, 15). MicroRNA-33 also indirectly augments TLR responses by increasing raft cholesterol through targeting ABCA1 and ABCG1 (16). Conversely, inflammation has been shown to inhibit RCT at multiple steps in the pathway from peripheral macrophage to liver (17). Taken together, RCT and innate immunity appear to be coupled. RCT tonically suppresses inflammation but is itself also a target of inflammation, potentially feeding back during disease to de-repress innate immunity.
Figure 2.
Interactions between cholesterol and proinflammatory Toll-like receptor 4 (TLR4) signaling in the macrophage. Oxysterols accumulate upon cholesterol internalization by the macrophage, activating liver X receptor (LXR). LXR up-regulates the cholesterol efflux proteins ATP-binding cassette A1 and G1 (ABCA1 and ABCG1, respectively) and inhibits proinflammatory cytokine induction by suppressing the activity of transcription factors (i.e., nuclear factor-κB [NF-κB], activator protein [AP]-1, and signal transducer and activator of transcription [STAT]-1) at gene promoters. ABCA1 and ABCG1 inhibit TLR4 activation in the plasma membrane through reducing lipid raft cholesterol (through promoting cholesterol efflux to the extracellular acceptors apolipoprotein A-I [ApoA-I] and high-density lipoprotein [HDL], respectively). HDL also directly suppresses lipopolysaccharide (LPS) signaling by binding and neutralizing LPS.
Good evidence has been amassed for RCT and its effect on immunity outside the lung. Until recently, however, it has remained unclear how cholesterol is regulated in the lung and whether RCT from the lung is linked to pulmonary immune responses and lung disease.
Cholesterol Homeostasis in the Lung?
Cholesterol is the major neutral lipid of pulmonary surfactant (7). Given that modest increases in cholesterol content compromise the surface tension–reducing properties of surfactant (18), it appears clear that cholesterol (and phospholipid) regulation must be a key and perhaps unique requirement of lung biology. Lipid tracer studies in rodents indicate that more than 80% of lung cholesterol is derived from the plasma (i.e., by uptake from lipoproteins) and the remainder, from synthesis in lung-resident cells (19). Plasma lipoprotein-derived fatty acids have also been shown to be incorporated within alveolar epithelial type 2 (AT2) cells into pulmonary surfactant phospholipid (20). Very low–density lipoprotein, LDL, and HDL all induce phosphatidylcholine secretion by AT2 cells (21, 22). Suggesting the possibility that dyslipidemia in humans may dysregulate surfactant lipid composition, researchers have shown that apoE-null mice (which have elevated plasma very low-density lipoprotein and LDL) have abnormal surfactant (20). Interestingly, in addition to potential roles in promoting (retrograde) RCT from the lung, HDL serves as the major vehicle for anterograde delivery of the antioxidant vitamin E to AT2 cells (23) and also potentiates delivery of alpha-1 antitrypsin to the lung (24). Taken together, the lung, perhaps owing to its unique surfactant lipid requirements and highly oxidizing and protease-prone microenvironment, has complex requirements for coordinated delivery by plasma lipoproteins of lipid and protein cargo. Indeed, suggesting a fundamental dependence of lung biology upon lipid trafficking, the lungs of both HDL-deficient Apoai−/− mice and Apoe−/− mice have developmental and physiological abnormalities (25, 26). Specifically, the lungs of naive Apoai−/− mice display increased airway hyperresponsiveness, collagen deposition, and oxidative stress, whereas Apoe−/− mice have reduced developmental alveologenesis associated with accelerated loss of lung recoil with age.
Although authors of one report estimated cholesterol clearance from the air space to be very low (27), more recent genetic approaches have pointed to substantial steady-state flux of cholesterol through the lung. Thus, both Abcg1−/− and Abca1−/− mice have remarkable pulmonary overload with cholesterol and other lipids, along with abnormal accumulation of macrophage foam cells (28–31), suggesting that RCT prevents lipid overload in the lung. Cholesterol handling in the lung appears to be dependent on the maturity of alveolar macrophages insofar as granulocyte macrophage–colony stimulating–factor (GM-CSF)–null mice have ABCG1-deficient alveolar macrophages and pulmonary cholesterol overload (32). Suggesting that the same mechanisms are at play in humans, ABCG1-deficient alveolar macrophages and lung cholesterol overload are also found in patients with pulmonary alveolar proteinosis, a disease driven by neutralizing autoantibodies against GM-CSF (32).
LXR-deficient mice, which have defective RCT, also have increased foam cells in the lung (33). Interestingly, the enzyme that synthesizes the LXR agonistic oxysterol 25-HC (i.e., cholesterol-25-hydroxylase) is most highly expressed in the lungs of mice (34), whereas that which synthesizes the LXR agonists 27-HC and cholestenoic acid (i.e., cytochrome P450 family 27 subfamily A member 1) is highly expressed in the lung in humans (35). This may suggest that locally synthesized oxysterols play an important role in driving LXR-dependent RCT in the lung. The hydrophilic oxysterol cholestenoic acid is thought to play a particularly important role in mediating diffusional sterol export from the human lung; in humans, plasma levels of cholestenoic acid appear to derive largely from synthesis in alveolar macrophages (35). Given this, the finding that plasma cholestenoic acid declines in emphysema, sarcoidosis, and tuberculosis (35) may suggest a unifying mechanism by which lung RCT, as well as local LXR-dependent inflammation suppression, becomes compromised in chronic lung disease.
Increased lipid-laden foam cells have been documented in the lungs of smokers as well as in a wide range of chronic lung diseases (36, 37). Foam cells also increase in the lungs of mice after several experimental exposures, including cigarette smoke, silica, bleomycin, and radiation (38, 39). These findings suggest that pulmonary lipid dysregulation may be a common event in chronic lung disease. Deficient expression of ABC lipid efflux transporters has been noted in lavage cells from patients with sarcoidosis and in alveolar macrophages of mice after multiwall carbon nanotube instillation (40). In both cases, elevated cellular expression of microRNA-33 was noted, suggesting a potential unifying mechanism. Ozone has been shown to suppress LXR in respiratory epithelial cells by causing lipid adduction, suggesting a different mechanism through which environmental oxidants may impair lung RCT (41). Reduced apoA-I is found in bronchoalveolar lavage fluid of patients with idiopathic pulmonary fibrosis (42), pointing to deficient cholesterol acceptor capacity. Taken together, it is intriguing to speculate that defective RCT through a variety of mechanisms may possibly contribute causally in a final common pathway of lung disease pathogenesis and that, if so, its correction might be therapeutic. Outside the lung, plasma HDL has been shown to have a deficient cholesterol-mobilizing function in several systemic diseases, including obesity and diabetes mellitus (43, 44). This raises the broader possibility that systemic metabolic disorders may also potentially impact lung-resident cells and lung disease phenotypes by compromising RCT.
Connections between Lipid Homeostasis and Immunity in the Lung
Recent work done at my laboratory and others has begun to identify intriguing connections in the lung between lipid homeostasis and immunity. Important roles have been shown for several RCT regulatory proteins, including ABCG1, SR-BI, LXRs, apoA-I, and apoE in pulmonary inflammation and host defense. In most cases to date, it has remained unclear to what extent abnormal cholesterol trafficking itself contributes causally to the immune phenotypes of mice in which these genes have been targeted. Nonetheless, reports that a high-cholesterol diet impairs pulmonary host defense against gram-negative bacteria and Mycobacterium tuberculosis (45, 46), reduces neutrophil trafficking to the inflamed lung (45), and augments airway eosinophilia in the ovalbumin model (47) do suggest that primary perturbations of cholesterol balance may be sufficient to alter pulmonary immunity.
Along with striking pulmonary lipid overload, Abcg1−/− mice display increased recruitment of multiple leukocyte subtypes to the lungs in the steady state, along with abnormal induction of air space cytokines and dysregulated maturation of pulmonary dendritic cells (48). Some of this likely arises from increased proinflammatory cytokine induction by Abcg1−/− alveolar macrophages, observed both in the steady state and after LPS stimulation (28, 29). Perhaps owing to accumulation of oxysterols and oxidized phospholipids, the lungs and pleural space of Abcg1−/− mice also exhibit local expansion of innate-like B1-b cells that overproduce natural antibodies targeting oxidation-specific lipid epitopes (49). This finding suggests that lipid handling in the lungs, which is normally managed by ABCG1, may play a direct and specific role in programming of the innate immune system. Indeed, Abcg1−/− mice display an enhanced host defense response during lung infection with Klebsiella pneumoniae (28), a more robust Th17/neutrophilic response to pulmonary allergen challenge (48), and an exacerbated pulmonary fibrotic response to bleomycin challenge (39).
Additional highly complex interactions that exist between lipid trafficking and pulmonary host defense are revealed by mice deficient in SR-BI, an alternate receptor for HDL perhaps best known for its role in RCT through mediating HDL uptake by the liver (Figure 1). Similarly to Abcg1−/− mice, Scarb1−/− mice (null for the gene encoding SR-BI [SCARB1]) exhibit increased neutrophil recruitment to the lungs after LPS exposure and bacterial infection (50). Much of this is likely driven by increased cytokine production by SR-BI–null alveolar macrophages and deficient SR-BI–dependent clearance of LPS from the air space. SR-BI deficiency in the adrenal glands also de-represses neutrophil trafficking to the infected air space by impairing adrenal production of antiinflammatory stress glucocorticoids, a process normally dependent upon SR-BI–dependent supply of HDL cholesterol to the adrenal cortex (51). Unlike Abcg1−/− mice, Scarb1−/− mice demonstrate dramatically increased bacterial overgrowth in the lungs and blood during pneumonia. This likely arises from impaired phagocytic killing, a defect that is independent of the dysregulated HDL cholesterol of the mouse (50). Taken together, SR-BI deletion reveals complex roles for coordinated trafficking of host and microbial lipids, both inside and outside the lung, in the integrated neutrophilic response to bacterial pneumonia. Because functional polymorphisms in human SCARB1 have been identified that phenocopy many features of the Scarb1−/− mouse (52), it seems plausible that similar roles for SR-BI may be at work in the human lung.
The LXRs are expressed by alveolar macrophages and alveolar epithelial cells and also play context-dependent roles in the pulmonary innate immune response, as recently described (53). Specifically, treatment of mice with a synthetic LXR agonist reduces neutrophil accumulation in the air space induced by LPS inhalation and gram-negative bacterial infection. This appears to stem from suppression of air space tumor necrosis factor-α and inhibition of neutrophil chemotaxis and leads to overgrowth of bacteria in the lung and reduced survival. In contrast to this immunosuppressive effect of forced LXR activation, synthetic LXR agonist treatment has been shown to enhance host defense against M. tuberculosis (54) and the nonpulmonary pathogen Salmonella typhimurium (55). This stems from prosurvival and metabolic effects in infected macrophages (55, 56). In yet other settings, LXR-α has recently been shown to promote the fibrotic behavior of idiopathic pulmonary fibrosis fibroblasts and to increase lung fibrosis in the murine bleomycin model (57).
ApoA-I–null mice, which are HDL deficient, have exacerbated alveolar neutrophilia after both adaptive (i.e., ovalbumin sensitization and challenge [58]) and innate (i.e., LPS inhalation [59]) immune challenges. The former effect appears to arise from de-repression of air space GM-CSF and the latter through effects on neutrophil migration to the lung. A wide range of additional interesting pulmonary phenotypes have been noted in dyslipidemic apoE-null mice, including exacerbated neutrophilic lung injury (60), increased airway hyperresponsiveness and goblet cell hyperplasia after house dust mite exposure (61), emphysema while on a high-fat diet (62), and sarcoidosis-like lesions (63) and severe pulmonary hypertension (64) on a cholate-containing high-fat diet. The likelihood that apoE also differentially modifies lung disease in humans is suggested by the finding that mice engineered to express human APOE2, APOE3, or APOE4 (the three major APOE alleles in humans) have differential disease severity in the house dust mite model of asthma (65). Furthermore, APOE4+ human subjects, compared with those who are APOE4 –, have augmented in vivo innate immune responses that may derive from increased lipid raft cholesterol in circulating monocytes (66).
Although in many cases the precise mechanisms by which cholesterol trafficking regulates the pulmonary immune response remain unclear, authors of several clinical reports have found interesting relationships between serum cholesterol and lung disease in human populations. Thus, serum levels of HDL cholesterol and apoA-I are positively associated with FEV1 among patients with atopic asthma, whereas serum LDL cholesterol is negatively correlated (67). Similar relationships between serum lipoproteins and FEV1 were also noted in a U.S. national survey (68). By contrast, authors of a recent report found that higher HDL levels were associated with lower FEV1/FVC ratio and more extensive radiographic emphysema among patients with chronic obstructive pulmonary disease (69). Complex relationships of serum lipoproteins to both asthma and atopy have also recently been noted (70, 71). The potential for lung disease to impact serum cholesterol is also suggested by a recent report that a reduction in serum cholesterol may be a marker of disease progression in patients with nontuberculous mycobacterial lung infection (72).
Closing Remarks
Fundamental roles have been identified for cholesterol trafficking in regulation of the innate and adaptive immune response. Studies of gene-targeted mice have also revealed that there is substantial flux of cholesterol through the lung in the steady state and that this flux is linked in complex ways, likely direct and indirect, to immune homeostasis in the lung. Owing to the unique biophysical properties and environmental susceptibility of surfactant lipid, the alveolar space is almost certainly singular among body compartments in its requirements for and mechanisms of lipid homeostasis. To date, results of studies of cholesterol-reducing statins in several lung diseases, including acute lung injury, chronic obstructive pulmonary disease, and asthma, have unfortunately proven largely negative (73). However, future efforts in rodent models and ideally in patients need to more precisely profile the pulmonary lipidome, perhaps on a cell-type basis, during health and disease, as well as to better define how systemic metabolic perturbations such as obesity, insulin resistance, and dyslipidemia translate to metabolic changes in the lung. Targeted strategies such as this will almost certainly identify more specific opportunities for lipid metabolic therapy in lung disease. The continued emergence of new lipid-targeted strategies for lung disease therapy, such as apolipoprotein mimetic peptides (74), indeed suggests that we are in the very early days of realizing the full therapeutic potential of lipid reprogramming in lung disease.
Supplementary Material
Footnotes
This work was supported by the National Institutes of Health, National Institute of Environmental Health Sciences (Z01 ES102005).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-κB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 2.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via Toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fessler MB. The intracellular cholesterol landscape: dynamic integrator of the immune response. Trends Immunol. 2016;37:819–830. doi: 10.1016/j.it.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaikh SR, Fessler MB, Gowdy KM. Role for phospholipid acyl chains and cholesterol in pulmonary infections and inflammation. J Leukoc Biol. 2016;100:985–997. doi: 10.1189/jlb.4VMR0316-103R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fessler MB, Summer RS. Surfactant lipids at the host-environment interface: metabolic sensors, suppressors, and effectors of inflammatory lung disease. Am J Respir Cell Mol Biol. 2016;54:624–635. doi: 10.1165/rcmb.2016-0011PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabor KA, Fessler MB. Roles of the mevalonate pathway and cholesterol trafficking in pulmonary host defense. Curr Mol Pharmacol. 2017;10:27–45. doi: 10.2174/1874467209666160112123603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fessler MB. Regulation of adaptive immunity in health and disease by cholesterol metabolism. Curr Allergy Asthma Rep. 2015;15:48. doi: 10.1007/s11882-015-0548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azzam KM, Fessler MB. Crosstalk between reverse cholesterol transport and innate immunity. Trends Endocrinol Metab. 2012;23:169–178. doi: 10.1016/j.tem.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Näär AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J Immunol. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito A, Hong C, Rong X, Zhu X, Tarling EJ, Hedde PN, Gratton E, Parks J, Tontonoz P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife. 2015;4:e08009. doi: 10.7554/eLife.08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARγ. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai L, Azzam KM, Lin WC, Rai P, Lowe JM, Gabor KA, Madenspacher JH, Aloor JJ, Parks JS, Näär AM, et al. MicroRNA-33 regulates the innate immune response via ATP binding cassette transporter-mediated remodeling of membrane microdomains. J Biol Chem. 2016;291:19651–19660. doi: 10.1074/jbc.M116.723056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vockeroth D, Gunasekara L, Amrein M, Possmayer F, Lewis JF, Veldhuizen RA. Role of cholesterol in the biophysical dysfunction of surfactant in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;298:L117–L125. doi: 10.1152/ajplung.00218.2009. [DOI] [PubMed] [Google Scholar]
- 19.Turley SD, Andersen JM, Dietschy JM. Rates of sterol synthesis and uptake in the major organs of the rat in vivo. J Lipid Res. 1981;22:551–569. [PubMed] [Google Scholar]
- 20.Ryan AJ, Medh JD, McCoy DM, Salome RG, Mallampalli RK. Maternal loading with very low-density lipoproteins stimulates fetal surfactant synthesis. Am J Physiol Lung Cell Mol Physiol. 2002;283:L310–L318. doi: 10.1152/ajplung.00021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallampalli RK, Salome RG, Bowen SL, Chappell DA. Very low density lipoproteins stimulate surfactant lipid synthesis in vitro. J Clin Invest. 1997;99:2020–2029. doi: 10.1172/JCI119370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voyno-Yasenetskaya TA, Dobbs LG, Erickson SK, Hamilton RL. Low density lipoprotein- and high density lipoprotein-mediated signal transduction and exocytosis in alveolar type II cells. Proc Natl Acad Sci USA. 1993;90:4256–4260. doi: 10.1073/pnas.90.9.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolleck I, Schlame M, Fechner H, Looman AC, Wissel H, Rüstow B. HDL is the major source of vitamin E for type II pneumocytes. Free Radic Biol Med. 1999;27:882–890. doi: 10.1016/s0891-5849(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 24.Moreno JA, Ortega-Gomez A, Rubio-Navarro A, Louedec L, Ho-Tin-Noé B, Caligiuri G, Nicoletti A, Levoye A, Plantier L, Meilhac O. High-density lipoproteins potentiate α1-antitrypsin therapy in elastase-induced pulmonary emphysema. Am J Respir Cell Mol Biol. 2014;51:536–549. doi: 10.1165/rcmb.2013-0103OC. [DOI] [PubMed] [Google Scholar]
- 25.Massaro D, Massaro GD. Apoetm1Unc mice have impaired alveologenesis, low lung function, and rapid loss of lung function. Am J Physiol Lung Cell Mol Physiol. 2008;294:L991–L997. doi: 10.1152/ajplung.00013.2008. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Xu H, Shi Y, Nandedkar S, Zhang H, Gao H, Feroah T, Weihrauch D, Schulte ML, Jones DW, et al. Genetic deletion of apolipoprotein A-I increases airway hyperresponsiveness, inflammation, and collagen deposition in the lung. J Lipid Res. 2010;51:2560–2570. doi: 10.1194/jlr.M004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyder MA, Griffin SM, Worsham DN, Kaneshiro ES. Clearance in vivo of instilled [3H]cholesterol from the rat lung. Biochem Res Int. 2010;2010:965716. doi: 10.1155/2010/965716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Draper DW, Madenspacher JH, Dixon D, King DH, Remaley AT, Fessler MB. ATP-binding cassette transporter G1 deficiency dysregulates host defense in the lung. Am J Respir Crit Care Med. 2010;182:404–412. doi: 10.1164/rccm.200910-1580OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180:4273–4282. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]
- 30.Baldán A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol. 2008;180:3560–3568. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- 31.Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L980–L989. doi: 10.1152/ajplung.00234.2005. [DOI] [PubMed] [Google Scholar]
- 32.Thomassen MJ, Barna BP, Malur AG, Bonfield TL, Farver CF, Malur A, Dalrymple H, Kavuru MS, Febbraio M. ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J Lipid Res. 2007;48:2762–2768. doi: 10.1194/jlr.P700022-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Schuster GU, Parini P, Wang L, Alberti S, Steffensen KR, Hansson GK, Angelin B, Gustafsson JA. Accumulation of foam cells in liver X receptor-deficient mice. Circulation. 2002;106:1147–1153. doi: 10.1161/01.cir.0000026802.79202.96. [DOI] [PubMed] [Google Scholar]
- 34.Lund EG, Kerr TA, Sakai J, Li WP, Russell DW. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J Biol Chem. 1998;273:34316–34327. doi: 10.1074/jbc.273.51.34316. [DOI] [PubMed] [Google Scholar]
- 35.Babiker A, Andersson O, Lindblom D, van der Linden J, Wiklund B, Lütjohann D, Diczfalusy U, Björkhem I. Elimination of cholesterol as cholestenoic acid in human lung by sterol 27-hydroxylase: evidence that most of this steroid in the circulation is of pulmonary origin. J Lipid Res. 1999;40:1417–1425. [PubMed] [Google Scholar]
- 36.Wilson AM, Nair P, Hargreave FE, Efthimiadis AE, Anvari M, Allen CJ ELVIS Research Study Group. Lipid and smoker’s inclusions in sputum macrophages in patients with airway diseases. Respir Med. 2011;105:1691–1695. doi: 10.1016/j.rmed.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Basset-Léobon C, Lacoste-Collin L, Aziza J, Bes JC, Jozan S, Courtade-Saïdi M. Cut-off values and significance of Oil Red O-positive cells in bronchoalveolar lavage fluid. Cytopathology. 2010;21:245–250. doi: 10.1111/j.1365-2303.2009.00677.x. [DOI] [PubMed] [Google Scholar]
- 38.Morissette MC, Shen P, Thayaparan D, Stämpfli MR. Disruption of pulmonary lipid homeostasis drives cigarette smoke-induced lung inflammation in mice. Eur Respir J. 2015;46:1451–1460. doi: 10.1183/09031936.00216914. [DOI] [PubMed] [Google Scholar]
- 39.Romero F, Shah D, Duong M, Penn RB, Fessler MB, Madenspacher J, Stafstrom W, Kavuru M, Lu B, Kallen CB, et al. A pneumocyte-macrophage paracrine lipid axis drives the lung toward fibrosis. Am J Respir Cell Mol Biol. 2015;53:74–86. doi: 10.1165/rcmb.2014-0343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barna BP, McPeek M, Malur A, Fessler MB, Wingard CJ, Dobbs L, Verbanac KM, Bowling M, Judson MA, Thomassen MJ. Elevated microRNA-33 in sarcoidosis and a carbon nanotube model of chronic granulomatous disease. Am J Respir Cell Mol Biol. 2016;54:865–871. doi: 10.1165/rcmb.2015-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speen AM, Kim HH, Bauer RN, Meyer M, Gowdy KM, Fessler MB, Duncan KE, Liu W, Porter NA, Jaspers I. Ozone-derived oxysterols affect liver X receptor (LXR) signaling: a potential role for lipid-protein adducts. J Biol Chem. 2016;291:25192–25206. doi: 10.1074/jbc.M116.732362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim TH, Lee YH, Kim KH, Lee SH, Cha JY, Shin EK, Jung S, Jang AS, Park SW, Uh ST, et al. Role of lung apolipoprotein A-I in idiopathic pulmonary fibrosis: antiinflammatory and antifibrotic effect on experimental lung injury and fibrosis. Am J Respir Crit Care Med. 2010;182:633–642. doi: 10.1164/rccm.200905-0659OC. [DOI] [PubMed] [Google Scholar]
- 43.Aron-Wisnewsky J, Julia Z, Poitou C, Bouillot JL, Basdevant A, Chapman MJ, Clement K, Guerin M. Effect of bariatric surgery-induced weight loss on SR-BI-, ABCG1-, and ABCA1-mediated cellular cholesterol efflux in obese women. J Clin Endocrinol Metab. 2011;96:1151–1159. doi: 10.1210/jc.2010-2378. [DOI] [PubMed] [Google Scholar]
- 44.Farbstein D, Levy AP. HDL dysfunction in diabetes: causes and possible treatments. Expert Rev Cardiovasc Ther. 2012;10:353–361. doi: 10.1586/erc.11.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madenspacher JH, Draper DW, Smoak KA, Li H, Griffiths GL, Suratt BT, Wilson MD, Rudel LL, Fessler MB. Dyslipidemia induces opposing effects on intrapulmonary and extrapulmonary host defense through divergent TLR response phenotypes. J Immunol. 2010;185:1660–1669. doi: 10.4049/jimmunol.0903501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martens GW, Arikan MC, Lee J, Ren F, Vallerskog T, Kornfeld H. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun. 2008;76:3464–3472. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeh YF, Huang SL. Enhancing effect of dietary cholesterol and inhibitory effect of pravastatin on allergic pulmonary inflammation. J Biomed Sci. 2004;11:599–606. doi: 10.1007/BF02256124. [DOI] [PubMed] [Google Scholar]
- 48.Draper DW, Gowdy KM, Madenspacher JH, Wilson RH, Whitehead GS, Nakano H, Pandiri AR, Foley JF, Remaley AT, Cook DN, et al. ATP binding cassette transporter G1 deletion induces IL-17-dependent dysregulation of pulmonary adaptive immunity. J Immunol. 2012;188:5327–5336. doi: 10.4049/jimmunol.1101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baldan A, Gonen A, Choung C, Que X, Marquart TJ, Hernandez I, Bjorkhem I, Ford DA, Witztum JL, Tarling EJ. ABCG1 is required for pulmonary B-1 B cell and natural antibody homeostasis. J Immunol. 2014;193:5637–5648. doi: 10.4049/jimmunol.1400606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gowdy KM, Madenspacher JH, Azzam KM, Gabor KA, Janardhan KS, Aloor JJ, Fessler MB. Key role for scavenger receptor B-I in the integrative physiology of host defense during bacterial pneumonia. Mucosal Immunol. 2015;8:559–571. doi: 10.1038/mi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilibert S, Galle-Treger L, Moreau M, Saint-Charles F, Costa S, Ballaire R, Couvert P, Carrié A, Lesnik P, Huby T. Adrenocortical scavenger receptor class B type I deficiency exacerbates endotoxic shock and precipitates sepsis-induced mortality in mice. J Immunol. 2014;193:817–826. doi: 10.4049/jimmunol.1303164. [DOI] [PubMed] [Google Scholar]
- 52.Vergeer M, Korporaal SJ, Franssen R, Meurs I, Out R, Hovingh GK, Hoekstra M, Sierts JA, Dallinga-Thie GM, Motazacker MM, et al. Genetic variant of the scavenger receptor BI in humans. N Engl J Med. 2011;364:136–145. doi: 10.1056/NEJMoa0907687. [DOI] [PubMed] [Google Scholar]
- 53.Smoak K, Madenspacher J, Jeyaseelan S, Williams B, Dixon D, Poch KR, Nick JA, Worthen GS, Fessler MB. Effects of liver X receptor agonist treatment on pulmonary inflammation and host defense. J Immunol. 2008;180:3305–3312. doi: 10.4049/jimmunol.180.5.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korf H, Vander Beken S, Romano M, Steffensen KR, Stijlemans B, Gustafsson JA, Grooten J, Huygen K. Liver X receptors contribute to the protective immune response against Mycobacterium tuberculosis in mice. J Clin Invest. 2009;119:1626–1637. doi: 10.1172/JCI35288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matalonga J, Glaria E, Bresque M, Escande C, Carbó JM, Kiefer K, Vicente R, León TE, Beceiro S, Pascual-García M, et al. The nuclear receptor LXR limits bacterial infection of host macrophages through a mechanism that impacts cellular NAD metabolism. Cell Rep. 2017;18:1241–1255. doi: 10.1016/j.celrep.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Valledor AF, Hsu LC, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc Natl Acad Sci USA. 2004;101:17813–17818. doi: 10.1073/pnas.0407749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurowska-Stolarska M, Hasoo MK, Welsh DJ, Stewart L, McIntyre D, Morton BE, Johnstone S, Miller AM, Asquith DL, Millar NL, et al. The role of microRNA-155/liver X receptor pathway in experimental and idiopathic pulmonary fibrosis [abstract] J Allergy Clin Immunol. 2017;139:1946–1956. doi: 10.1016/j.jaci.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai C, Yao X, Keeran KJ, Zywicke GJ, Qu X, Yu ZX, Dagur PK, McCoy JP, Remaley AT, Levine SJ. Apolipoprotein A-I attenuates ovalbumin-induced neutrophilic airway inflammation via a granulocyte colony-stimulating factor-dependent mechanism. Am J Respir Cell Mol Biol. 2012;47:186–195. doi: 10.1165/rcmb.2011-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madenspacher JH, Azzam KM, Gong W, Gowdy KM, Vitek MP, Laskowitz DT, Remaley AT, Wang JM, Fessler MB. Apolipoproteins and apolipoprotein mimetic peptides modulate phagocyte trafficking through chemotactic activity. J Biol Chem. 2012;287:43730–43740. doi: 10.1074/jbc.M112.377192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamashita CM, Fessler MB, Vasanthamohan L, Lac J, Madenspacher J, McCaig L, Yao L, Wang L, Puntorieri V, Mehta S, et al. Apolipoprotein E-deficient mice are susceptible to the development of acute lung injury. Respiration. 2014;87:416–427. doi: 10.1159/000358438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao X, Fredriksson K, Yu ZX, Xu X, Raghavachari N, Keeran KJ, Zywicke GJ, Kwak M, Amar MJ, Remaley AT, et al. Apolipoprotein E negatively regulates house dust mite-induced asthma via a low-density lipoprotein receptor-mediated pathway. Am J Respir Crit Care Med. 2010;182:1228–1238. doi: 10.1164/rccm.201002-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldklang M, Golovatch P, Zelonina T, Trischler J, Rabinowitz D, Lemaître V, D’Armiento J. Activation of the TLR4 signaling pathway and abnormal cholesterol efflux lead to emphysema in ApoE-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1200–L1208. doi: 10.1152/ajplung.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samokhin AO, Bühling F, Theissig F, Brömme D. ApoE-deficient mice on cholate-containing high-fat diet reveal a pathology similar to lung sarcoidosis. Am J Pathol. 2010;176:1148–1156. doi: 10.2353/ajpath.2010.090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lawrie A, Hameed AG, Chamberlain J, Arnold N, Kennerley A, Hopkinson K, Pickworth J, Kiely DG, Crossman DC, Francis SE. Paigen diet-fed apolipoprotein E knockout mice develop severe pulmonary hypertension in an interleukin-1-dependent manner. Am J Pathol. 2011;179:1693–1705. doi: 10.1016/j.ajpath.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao X, Dai C, Fredriksson K, Lam J, Gao M, Keeran KJ, Nugent GZ, Qu X, Yu ZX, Jeffries N, et al. Human apolipoprotein E genotypes differentially modify house dust mite-induced airway disease in mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L206–L215. doi: 10.1152/ajplung.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gale SC, Gao L, Mikacenic C, Coyle SM, Rafaels N, Murray Dudenkov T, Madenspacher JH, Draper DW, Ge W, Aloor JJ, et al. APOε4 is associated with enhanced in vivo innate immune responses in human subjects. J Allergy Clin Immunol. 2014;134:127–134. doi: 10.1016/j.jaci.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barochia AV, Kaler M, Cuento RA, Gordon EM, Weir NA, Sampson M, Fontana JR, MacDonald S, Moss J, Manganiello V, et al. Serum apolipoprotein A-I and large high-density lipoprotein particles are positively correlated with FEV1 in atopic asthma. Am J Respir Crit Care Med. 2015;191:990–1000. doi: 10.1164/rccm.201411-1990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cirillo DJ, Agrawal Y, Cassano PA. Lipids and pulmonary function in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;155:842–848. doi: 10.1093/aje/155.9.842. [DOI] [PubMed] [Google Scholar]
- 69.Burkart KM, Manichaikul A, Wilk JB, Ahmed FS, Burke GL, Enright P, Hansel NN, Haynes D, Heckbert SR, Hoffman EA, et al. APOM and high-density lipoprotein cholesterol are associated with lung function and per cent emphysema. Eur Respir J. 2014;43:1003–1017. doi: 10.1183/09031936.00147612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fessler MB, Massing MW, Spruell B, Jaramillo R, Draper DW, Madenspacher JH, Arbes SJ, Calatroni A, Zeldin DC. Novel relationship of serum cholesterol with asthma and wheeze in the United States. J Allergy Clin Immunol. 2009;124:967–974.e15. doi: 10.1016/j.jaci.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fessler MB, Jaramillo R, Crockett PW, Zeldin DC. Relationship of serum cholesterol levels to atopy in the US population. Allergy. 2010;65:859–864. doi: 10.1111/j.1398-9995.2009.02287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hong JY, Yang GE, Ko Y, Park YB, Sim YS, Park SH, Lee CY, Jung KS, Lee MG. Changes in cholesterol level correlate with the course of pulmonary nontuberculous mycobacterial disease. J Thorac Dis. 2016;8:2885–2894. doi: 10.21037/jtd.2016.10.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gowdy KM, Fessler MB. Emerging roles for cholesterol and lipoproteins in lung disease. Pulm Pharmacol Ther. 2013;26:430–437. doi: 10.1016/j.pupt.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao X, Gordon EM, Figueroa DM, Barochia AV, Levine SJ. Emerging roles of apolipoprotein E and apolipoprotein A-I in the pathogenesis and treatment of lung disease. Am J Respir Cell Mol Biol. 2016;55:159–169. doi: 10.1165/rcmb.2016-0060TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.