Abstract
The mitochondrion is the main site of energy production and a hub of key signaling pathways. It is also central in stress-adaptive response due to its dynamic morphology and ability to interact with other organelles. In response to stress, mitochondria fuse into networks to increase bioenergetic efficiency and protect against oxidative damage. Mitochondrial damage triggers segregation of damaged mitochondria from the mitochondrial network through fission and their proteolytic degradation by mitophagy. Post-translational modifications of the mitochondrial proteome and nuclear cross-talk lead to reprogramming of metabolic gene expression to maintain energy production and redox balance. Chronic obstructive pulmonary disease (COPD) is caused by chronic exposure to oxidative stress arising from inhaled irritants, such as cigarette smoke. Impaired mitochondrial structure and function, due to oxidative stress–induced damage, may play a key role in causing COPD. Deregulated metabolic adaptation may contribute to the development and persistence of mitochondrial dysfunction in COPD. We discuss the evidence for deregulated metabolic adaptation and highlight important areas for investigation that will allow the identification of molecular targets for protecting the COPD lung from the effects of dysfunctional mitochondria.
Keywords: mitochondrial dynamics, metabolic reprogramming, oxidative stress, metabolic adaptation
Chronic exposure to inhaled irritants, such as cigarette smoke (CS) and biomass fuels, and the resulting oxidative stress are major triggers of chronic obstructive pulmonary disease (COPD) inflammation and the associated pathology, which includes emphysema, chronic bronchitis, and small airways remodeling (1). Accelerated cellular aging and senescence are important in the development of emphysema, fibrosis, and inflammation, whereas airway smooth muscle (ASM) thickening contributes to small airways remodeling (1, 2). There is evidence of impaired mitochondrial function in the lungs of patients with COPD, possibly caused by chronic exposure to oxidative stress (3–8). Defective metabolic adaptation in COPD may lead to the development and persistence of mitochondrial dysfunction. We review what is known of the potential mechanisms of adaptation to mitochondrial dysfunction and how these may be deregulated in COPD, while highlighting the areas that require further investigation.
Mitochondria Are Hubs of Energy Production, Biosynthesis, and Redox Regulation
Mitochondria likely originated from α-proteobacteria that were engulfed by primitive eukaryotes, establishing a symbiotic relationship (9). Their bacterial ancestry is reflected by their structure, which is comprised of an outer and an inner membrane, enclosing the matrix and intermembrane space, and a small circular genome. Mitochondrial DNA (mtDNA) encodes for 13 proteins involved primarily in mitochondrial respiration. During evolution, a considerable proportion of the bacterial genome was transferred to the eukaryotic nucleus. Thus, the majority of mitochondrial proteins are encoded by the nuclear genome (10). Because mitochondria cannot be created de novo, mitochondrial biogenesis involves the growth and division of pre-existing mitochondria. Mitochondria divide by the process of fission, and subsequently fuse together to form networks (11). Mitochondrial biogenesis is induced in response to changes in energy demand and environmental cues, by the key transcription factor, peroxisome proliferator–activated receptor γ coactivators (PGCs) 1α and β (12).
Mitochondria provide most of the cell’s energy requirements in the form of ATP through oxidation of glucose, fatty acids, and amino acids. Lung mitochondria primarily use glucose as a substrate; however, they also oxidize fatty acids, glutamine, and lactate (13, 14).
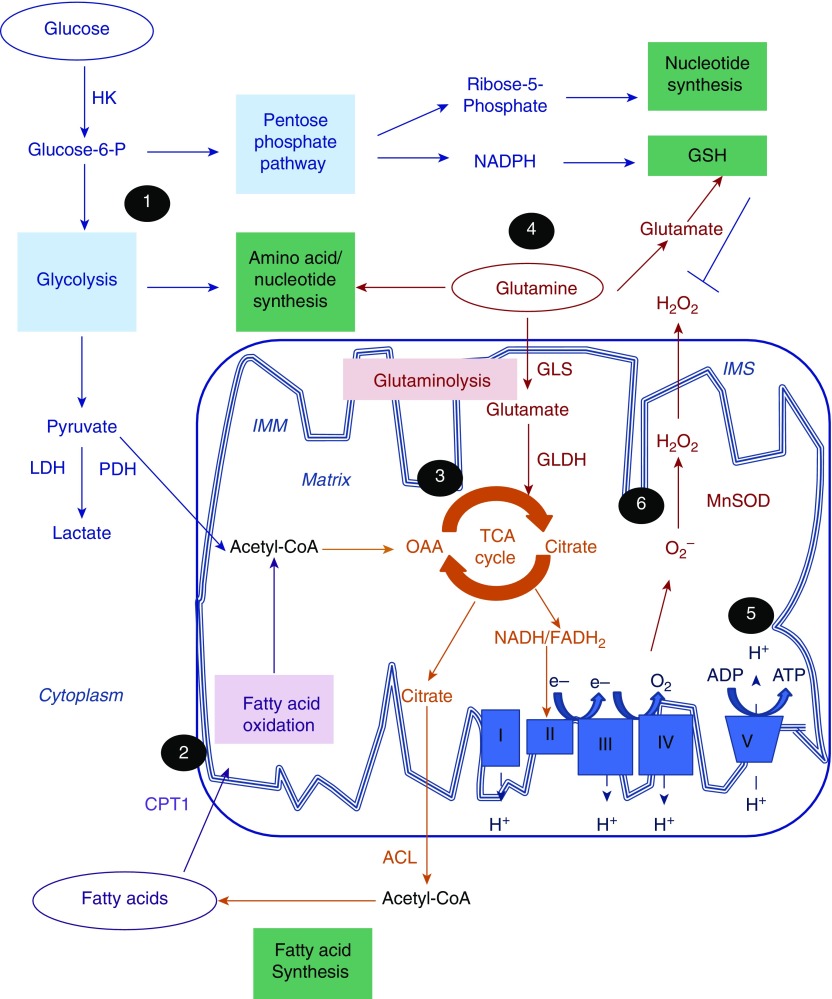
Glucose is taken up by the cells via glucose transporters, and is phosphorylated by hexokinase to glucose-6-phosphate, which enters the glycolytic pathway in the cytoplasm, leading to the production of pyruvate. Most pyruvate is transported through the mitochondrial inner membrane to the mitochondrial matrix, where it is decarboxylated into acetyl-coenzyme A (CoA) by pyruvate dehydrogenase (PDH), whereas a small proportion of it is converted to lactate in the cytoplasm. PDH is negatively regulated by PDH kinase, which acts as a gatekeeper, controlling the flow of pyruvate into the mitochondrion. Fatty acids are taken up by the cells via plasma membrane transporters or they are synthesized de novo. Fatty acids are activated by acetyl-CoA in the cytoplasm and associate with carnitine molecules, forming acylcarnitines that are transported into the mitochondrion, where fatty acid oxidation (FAO) occurs, producing acetyl-CoA (Figure 1) (15).
Figure 1.
Central role of mitochondria in energy production, biosynthesis, and redox regulation. Mitochondria integrate energy metabolism, biosynthesis, and redox balance. (1) Glucose is phosphorylated by hexokinase (HK) to glucose-6-phosphate (Glucose-6-P), which undergoes glycolysis in the cytoplasm to produce pyruvate. Under normal, aerobic conditions, most of the pyruvate that enters is converted to acetyl-coenzyme A (CoA) by the pyruvate dehydrogenase (PDH) complex in the mitochondrial matrix, whereas a small proportion of it is converted to lactate by lactate dehydrogenase (LDH). Glycolytic intermediates also feed into biosynthetic pathways. Glucose-6-phosphate is redirected into the pentose phosphate pathway to produce the nucleotide precursor, ribose-5-phosphate, and reduced nicotinamide adenine dinucleotide phosphate (NADPH) required for the maintenance of reduced glutathione (GSH) levels. (2) Fatty acids are transported into the mitochondrion after their conjugation to carnitine by carnitine palmitoyltransferase 1 (CPT1), to undergo fatty acid oxidation, leading to acetyl-CoA production. (3) Acetyl-CoA combines with oxaloacetate (OAA) to form citrate, which enters the tricarboxylic acid (TCA) cycle in the mitochondrial matrix, generating electron carriers, nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2). Citrate produced by the TCA cycle is converted, by ATP-citrate lyase (ACL), to cytoplasmic acetyl-CoA, which is required for fatty acid synthesis. (4) Cells with increased energy demands, such as rapidly proliferating cells, use glutamine for energy production. Glutamine is converted to glutamate by glutaminolysis through the action of glutaminase (GLS). Glutamate acts as a precursor for glutathione synthesis, but can also be converted, by glutamate dehydrogenase (GLDH), into α-ketoglutarate, which feeds into the TCA cycle. Glutamine also provides nitrogen for amino acid and nucleotide synthesis. (5) Electrons (e−) are transferred from NADH and FADH2 to molecular oxygen (O2) through redox reactions facilitated by a series of electron carrier protein complexes (I–IV) located in the inner mitochondrial membrane (IMM), a process termed “oxidative phosphorylation” (OXPHOS). The energy released by the electron flow drives the movement of protons into the intermembrane space (IMS) creating a membrane potential (ΔΨm). ΔΨm drives the influx of protons back into the mitochondrial matrix via the ATP synthase complex (complex V), which phosphorylates ADP to ATP. Electron leakage during OXPHOS leads to univalent reduction of oxygen to form superoxide anion (O2−). O2− is converted to the less reactive oxidant, hydrogen peroxide (H2O2), which is eliminated through the action of catalase and GSH. (6) In response to oxidative stress and/or mitochondrial dysfunction, adaptive metabolic reprogramming, involving increased glycolysis, fatty acid oxidation, and glutaminolysis, ensures maintenance of ATP production, biosynthesis, and redox balance required for cell survival and normal function. Impaired metabolic reprogramming in chronic obstructive pulmonary disease may lead to prolonged mitochondrial dysfunction and an inability of cells to respond to stress, leading to cell senescence and death. Conversely, extensive metabolic reprogramming, due to overadaptation to hypoxia or inflammation, could lead to increased survival and proliferation of airway smooth muscle cells and myofibroblast activation. MnSOD = manganese superoxide dismutase.
Acetyl-CoA, from glucose or fatty acid oxidation, enters the tricarboxylic acid (TCA) cycle, a series of enzymatic reactions in the mitochondrial matrix giving rise to the electron carriers reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2), which donate electrons to the electron transport chain (ETC) in the inner membrane. The ETC is comprised of four electron-carrier protein complexes (I–IV), which, through a series of redox reactions, transfer electrons to oxygen. During this process, termed oxidative phosphorylation (OXPHOS), complexes I, III, and IV pump protons into the intermembrane space, creating a mitochondrial membrane potential (ΔΨm). ΔΨm drives the influx of protons back into the mitochondrial matrix via a proton channel coupled to the F0F1 ATP synthase (complex V), which facilitates the phosphorylation of ADP to ATP (16). In situations of increased energy demand, such as in proliferating cells, glutamine is also used as a mitochondrial respiration fuel by undergoing glutaminolysis into glutamate, which enters the TCA cycle (Figure 1) (17, 18).
Leakage of electrons during OXPHOS, particularly at complexes I and III, leads to partial reduction of oxygen to produce reactive oxygen species (ROS). ROS have also been shown to be produced as a result of PDH activity, the TCA cycle, and FAO (19, 20). Mitochondrial ROS are maintained at low levels through the action of antioxidants, such as Mn superoxide dismutase and glutathione. Mitochondrial ROS at low levels are involved in the propagation of homeostatic mechanisms, but, at high levels, lead to damage of proteins, lipids, and nucleic acids, and thus cause disease (Figure 1) (16, 19).
Cellular bioenergetic, biosynthetic, and redox processes are highly coordinated to produce energy, while maintaining adequate levels of macromolecules required for biosynthesis and maintenance of redox balance. Citrate from the TCA cycle enters de novo fatty acid synthesis through its conversion to acetyl-CoA by ATP-citrate lyase in the cytoplasm, to replenish fatty acid levels (21). The glycolytic pathway, via glucose-6-phosphate, branches off into the anabolic pentose phosphate pathway (PPP), which produces ribose-5-phosphate, a precursor of nucleotide biosynthesis, and reduced nicotinamide adenine dinucleotide phosphate (NADPH), which is required for fatty acid biosynthesis and antioxidant protection (22). Glutaminolysis also provides nitrogen for nucleotide and protein synthesis, and glutamate, which is a precursor for glutathione synthesis (Figure 1) (17).
Mitochondrial Dysfunction in COPD
There is mounting evidence supporting the presence of mitochondrial dysfunction in the lungs of patients with COPD and its role in the development or progression of the disease (23). Morphologic abnormalities consistent with mitochondrial damage, such as elongated and swollen mitochondria, with poorly defined cristae were reported in bronchial epithelial cells of patients with COPD (4, 5). Impaired mitochondrial function reflected by loss of ΔΨm, increased mitochondrial ROS, and reduced mitochondrial respiration was also reported in ASM cells (ASMCs) and endobronchial biopsies of patients with COPD (8). Mitochondrial damage is possibly caused by oxidative stress as a result of chronic exposure to CS. This is supported by studies showing impaired mitochondrial function in CS- and ozone-induced mouse models of COPD (3, 7, 8). A recent study has provided evidence for increased expression of the iron-responsive element-binding protein 2, a key protein involved in iron homeostasis, that could lead to mitochondrial iron overload and dysfunction in COPD (8, 24). Impaired mitochondrial function has been shown to mediate lung cell apoptosis and senescence and the development of lung inflammation and emphysema, indicating a role in disease pathology (3, 4, 6–8, 25). The effects of mitochondrial dysfunction in disease may be mediated by excessive mitochondrial ROS (3, 4, 6–8) and release of mitochondrial components from damaged cells, such as mtDNA and cardiolipin, which act as danger-associated molecular patterns to induce inflammatory responses (26–28).
The dynamic nature of the mitochondria and their ability to move and communicate with other organelles enables them to adapt to oxidative stress, changes in energy and nutrient levels, and even damage. Loss of these adaptive responses, due to chronic exposure to high levels of oxidative stress or due to inherent defects, may lead to prolonged mitochondrial dysfunction in COPD.
Metabolic Adaptation to Stress and Mitochondrial Dysfunction
Mitochondria not only act as sensors, but are also regulators of metabolic activity. Changes in energy status are signaled by changes in intermediates, such as the ratios of oxidized to reduced NAD (NAD+/NADH) and AMP/ATP, and acetyl-CoA levels (29). These signals are detected by molecular sensors, such as hormones, transcription factors, and kinases, that act to restore metabolic and cellular homeostasis (30). Acute responses to stress, mitochondrial dysfunction, or altered nutrient supply involve changes in mitochondrial morphology, movement and quality control, and post-translational modifications of mitochondrial proteins. Furthermore, retrograde signaling between the mitochondrion and nucleus triggers transcriptional changes that lead to more lasting metabolic changes, termed “metabolic reprogramming” (30, 31).
Mitochondrial Morphology, Movement, and Quality Control
Mitochondria have a very dynamic morphology, and can be found segregated or linked together into linear or branched networks as a result of continuous cycles of fission and fusion, mediated by multidomain dynamin-related GTPases (30). Mitochondrial fission and fusion are pivotal in the ability of cells to survive under conditions of stress (32).
Mitochondrial fusion is induced in the initial stages of cellular stress. The membrane-anchored proteins, mitofusin (Mfn)-1 and -2, facilitate the fusion of the outer membranes, whereas optic atrophy (Opa) 1 mediates the fusion of the inner membranes and controls cristae formation, leading to the formation of networks of elongated mitochondria (33). This leads to complementation between mitochondria through sharing of mtDNA, lipids, and proteins, limiting detrimental mutations and mitochondrial damage. Moreover, fusion maintains OXPHOS efficiency through the formation of ETC super complexes, preventing the proteolytic degradation of mitochondria and facilitating interactions between mitochondria and endoplasmic reticulum (ER) (11). Mitochondrial–ER interactions are important stress response mechanisms in that they allow the movement of Ca2+ that triggers mitochondrial biogenesis as well as transport of essential mitochondrial lipids, such as cardiolipin (34, 35). The proteolytic processing of Opa1 is pivotal in the regulation of mitochondrial dynamics. Under normal conditions, processing by the mitochondrial quality control proteases, ATPases associated with diverse cellular activities proteins, maintains Opa1 in long transmembrane and short, soluble isoforms (36, 37). Under conditions of stress, stomatin-like protein 2, a prohibitin-related scaffolding protein expressed in the mitochondrial inner membrane, maintains Opa1 in the long conformation, promoting mitochondrial hyperfusion (32). Acute exposure to oxidative stress can induce mitochondrial hyperfusion, which possibly acts as a first line of defense against ROS by minimizing cellular damage and increasing metabolic efficiency (38, 39). Thus, mitochondrial hyperfusion has been linked to increased survival and apoptosis resistance (32).
Mitochondrial fission is induced by more prolonged stress and mitochondrial dysfunction. Fission is mediated by the cytosolic dynamin, Drp1, recruited to the fission sites by mitochondrial dynamics factors 49 and 51 or mitochondrial fission factor 1 (33). The fission sites are determined by sites where ER tubules interact with mitochondria in a process termed “ER-associated mitochondrial division” (40). Drp1 forms spiral structures around the mitochondrion, which constricts to split the inner and outer membranes (41). Segregation of mitochondria is required not only for cell division, but also for degradation of damaged mitochondria and induction of apoptosis (11, 30, 33). Loss of ΔΨm leads to Opa1 cleavage into small and inactive isoforms by the metalloprotease, overlapping activity with m–ATPases associated with diverse cellular activities protease 1 (Oma1), halting fusion and allowing the defective mitochondrion to be segregated from the network through fission to allow its efficient removal (36). Defective mitochondria are removed by autophagic degradation, a process termed “mitophagy.” Mitophagy is activated by prolonged reduction in ΔΨm which triggers the recruitment of the serine/threonine kinase, phosphatase and tensin homolog–induced putative kinase 1 (PINK1), from the intermembrane space to the surface of the mitochondria, where it recruits the E3 ubiquitin ligase, Parkin. Parkin ubiquitinates outer membrane proteins, including Mfn-1 and Mfn-2, leading to inhibition of mitochondrial fusion and initiating the formation of autophagosomes, which are degraded by fusing with lysosomes (36, 42, 43).
Another aspect of the dynamic nature of mitochondria is their ability to move across the cell, along microtubules, to interact with other organelles. This process is facilitated by the outer membrane–localized guanosine triphosphatases (GTPases), mitochondrial Rho GTPase 1 and mitochondrial Rho GTPase 2, which interact with the kinesin and dynein motor proteins via adaptor proteins to transport mitochondria toward the cell membrane or the nucleus (44). Perinuclear clustering of mitochondria plays a key role in hypoxia-inducible factor (HIF)-1α–mediated gene activation, and thus to adaptive responses to hypoxia (45).
Acute exposure of alveolar epithelial cells to nontoxic concentrations of CS extract induces elongation and fusion of mitochondria, which appears to be protective, as these cells show increased oxygen consumption and ΔΨm and low mitochondrial ROS levels, suggesting increased mitochondrial respiration efficiency (46). Prolonged exposure (6 mo) of bronchial epithelial cells to CS extract, however, leads to swollen mitochondria with poorly defined cristae, which show both branching and fragmentation. Intriguingly, all these structural changes, apart from fragmentation, persisted after withdrawal of CS extract, indicating that mitochondrial hyperfusion may be a maladaptive mechanism triggered by chronic CS exposure (5). Importantly, a similar morphology, which is characteristic of aging mitochondria (47), was also observed in bronchial epithelial cells of ex-smoking patients with COPD (5). Prohibitin 1, an inner-membrane scaffolding protein that is important in the proteolytic processing of Opa1, was found to be reduced in COPD lungs, suggesting a possible mechanism underlying the impaired morphology and cristae formation (48). A different study in bronchial epithelial cells from patients with COPD reported swollen and cristae-depleted mitochondria, which, however, showed a fragmented morphology (4). The discrepancy in the morphology reported by these studies may be due to differences in cell culturing conditions. Studies of mitochondrial morphology in lung tissue from patients with COPD would therefore be of value.
The hyperfused mitochondrial morphology is a key feature of cells undergoing senescence, and has been attributed to loss of Drp1 activity or fission factor 1 expression (49, 50). Induction of mitochondrial fragmentation by Opa1 knockdown protects HeLa cells from cellular senescence, indicating that fusion/fission imbalance is an important driver of senescence (51). Although studies in epithelial cells have reported changes in fusion/fission protein expression (5, 46) and localization in response to CS exposure (4), no differences have been reported in cells of COPD. Furthermore, the mechanisms linking deregulated mitochondrial morphology and function with cellular dysfunction in COPD are unclear.
The presence of elongated and dysfunctional mitochondria in close proximity to the nuclei of lung fibroblasts exposed to CS extract may provide an important link between mitochondrial dysfunction and cellular senescence (3). Prolonged perinuclear accumulation of damaged mitochondria, which could be a result of impaired mitochondrial mobility mechanisms (52) or prolonged exposure to hypoxic conditions (45), is likely to lead to direct ROS-induced DNA damage (3) or to induce epigenetic modifications in the nucleus (53), ultimately leading to the cellular senescence seen in COPD.
Mitochondrial dysfunction in COPD epithelium may be precipitated by deregulated quality control. Hyperfused mitochondria are spared from mitophagic degradation, possibly due to their large size (54). Indeed, elongated and fused mitochondria were associated with impaired mitophagy, due to suppressed mitochondrial translocation of Parkin-1 by p53, in the lungs of cigarette-exposed mice and of healthy smokers and patients with COPD. Impaired mitophagy was shown to contribute to the development of epithelial cell and fibroblast senescence and emphysema in CS-exposed mice (3). Reduced Parkin expression has also been linked to defective mitophagy in lung tissue from patients with COPD (6). In contrast, another study reported swollen, but increasingly fragmented, mitochondria associated with PINK1-dependent mitophagy in pulmonary epithelial cells exposed to CS extract. The same study reported increased Drp1 and PINK1 in COPD, and demonstrated a role of mitophagy amplifying the mitochondrial damage and contributing to necroptosis of lung epithelial cells and the development of emphysema in a CS-induced mouse model (7). The discrepancy in the findings regarding mitochondrial dynamics and quality in in vitro and in vivo model control may stem from differences in experimental conditions, such as the levels of CS used. A more in-depth study of mechanisms of mitochondrial morphology and mitophagy in cells and lung tissue from patients with COPD is required to understand how these mechanisms are deregulated in disease.
Mitochondrial dysfunction and ER stress are evident in the lungs in response to aging and CS exposure (55, 56). Under conditions of ER stress, ER–mitochondrial coupling is facilitated by Mfn-2 (57). ER stress was shown to contribute to mitochondrial fusion and impaired mitophagy, leading to mitochondrial dysfunction in alveolar epithelial type II cells from aged mice (55). The mechanisms underlying ER-mediated mitochondrial dysfunction are unclear; however, increased Ca2+ flux may play a role. These findings highlight the importance of investigating mitochondrial–ER cross-talk as a possible mechanism of defective metabolic function in COPD.
Post-Translational Modification of Mitochondrial Proteins: Sirtuins
A large proportion of mitochondrial proteins are acetylated, including TCA cycle enzymes and proteins involved in fatty acid, carbohydrate, amino acid, and nucleotide metabolism (58). Protein acetylation acts as a metabolic sensor, detecting acute changes in the cellular bioenergetic status and translating them into adaptive changes in mitochondrial function (31). Acetyl-CoA, produced from glucose or fatty acid oxidation, mediates mitochondrial protein acetylation, whereas, conversely, NAD+, accumulating in conditions of reduced energy, is required as a cofactor for the NAD+-dependent lysine deacetylases, sirtuins. Sirtuins orchestrate stress responses, maintenance of metabolic homeostasis, and antiaging effects (59). Sirtuin (Sirt) 3 is the predominant deacetylase in the mitochondria, where it prevents hyperacetylation of ETC complexes I, II, and V, maintaining OXPHOS and ATP production (60–63). Under conditions of oxidative stress, Sirt3 triggers an adaptive response by driving mitochondrial fusion through deacetylation of Opa1 (64) and mtDNA repair by increasing the stability of the mtDNA repair enzyme, 8-oxoguanine-DNA glycosylase 1 (65). Sirt3 also enhances mitochondrial respiration under conditions of metabolic stress by promoting FAO through activation of long-chain acyl CoA dehydrogenase (66) and glutaminolysis by glutamate dehydrogenase activation (67). Sirt3 also promotes mitochondrial redox balance by contributing to the regeneration of reduced glutathione by promoting NADPH production (68) and activation of Mn superoxide dismutase (69).
Reduced Sirt3 expression has been reported in the lungs of aged mice and in an elastase-induced mouse model of emphysema (70). Sirt3-deficient mice have been shown to demonstrate exaggerated bleomycin-dependent pulmonary fibrosis, inflammation, and mucus production (70–72). Moreover, transforming growth factor-β, a major driver of COPD pathology, inhibits Sirt3 expression in lung fibroblasts, leading to oxidative stress, mtDNA damage, and myofibroblast differentiation (70–72). Thus, defective mitochondrial sirtuin function and deregulated mitochondrial protein acetylation could be key contributors to poor metabolic adaptation to stress and susceptibility to premature aging and COPD, and merit further study.
Metabolic Reprogramming
The communication between the mitochondrion and nucleus allows the sensing and transmission of metabolic signals, in the form of altered metabolic intermediates, to reprogram metabolic gene expression and induce adaptive metabolic and redox changes.
The two key molecular sensors for metabolic adaptation are AMP-activated protein kinase (AMPK) and the sirtuin, Sirt1 (30). AMPK is activated by increased AMP:ATP ratio and increased ADP levels resulting from ATP depletion (73), and also by oxidative stress (74) and mitochondrial ROS due to mitochondrial dysfunction (75, 76). Activation of AMPK leads to inhibition of biosynthetic processes and activation of catabolic metabolism to increase energy production (29). AMPK increases mitochondrial biogenesis through PGC-1α activation (30). At the same time, it promotes production of acetyl-CoA required for mitochondrial respiration by activating FAO via PGC-1α activation, and glycolysis through phosphofructokinase-2 and hexokinase activation (74, 77). Inhibition of the mechanistic target of rapamycin (mTOR) pathway by AMPK also ensures the availability of nutrients and respiration substrates by increasing mitophagy (78). Moreover, AMPK-dependent activation of the cytoprotective transcription factors, nuclear factor E2–related factor 2 and Forkhead box O3 (FoxO3), leads to increased antioxidant gene expression (79, 80), whereas induction of PPP activity provides NADPH required for redox balance (81). AMPK-mediated NAD+ levels activate Sirt1, which induces PGC-1α and FoxO3 activity through deacetylation, to promote catabolic metabolism, ATP production, and antioxidant protection (82).
HIF-1 is another important molecular sensor that mediates adaptive metabolic changes in response to hypoxic conditions (83). Hypoxic cells show an HIF-1–dependent up-regulation of PDH kinase 1 that diverts pyruvate away from mitochondrial respiration and toward glycolysis (84). At the same time, increased expression of glucose transporters and glycolytic enzymes ensure a shift from OXPHOS to glycolysis and the PPP, maintaining ATP production and low ROS levels under hypoxic conditions (83, 85). This switch would lead to reduced flow of acetyl-CoA into the TCA cycle and fatty acid synthesis. Increased glutaminolysis, in response to HIF-1 activation, provides α-ketoglutarate for the TCA cycle and, consequently, citrate for fatty acid synthesis (85, 86)
A reduction in glycolytic flux in type II alveolar epithelial cells of mice exposed to CS for 4–8 weeks is accompanied by increased ETC complex protein activity and FAO (87). At the same time, induction of PPP activity provides NADPH for maintaining redox balance (87). These metabolic changes are reversed upon cessation of CS exposure (87, 88). A more recent study by Cloonan and colleagues (24) also demonstrates that CS-induced OXPHOS impairment in mice is accompanied by a shift toward glycolysis. Thus, CS-mediated stress triggers adaptive changes that allow increased mitochondrial efficiency and activation of alternative pathways to preserve energy and antioxidant levels, and thus cell survival. AMPK activation may facilitate these processes (89). Indeed, AMPK activation has been reported in human bronchial epithelial cells and macrophages, and mouse lungs after CS exposure, in a ROS-dependent manner (90, 91). Nonetheless, the involvement of AMPK in metabolic regulation was not investigated in those studies.
Metabolomic analysis of basal cells from long-term smokers reveals reduced acetyl-CoA levels, reflecting defective glycolysis and/or FAO, and a deficit in succinate, NADH, and FADH2, indicative of reduced TCA cycle activity (92). Moreover, in a model of elastase-induced emphysema, which simulates progressive disease, alveolar epithelial cell senescence and apoptosis were accompanied by a reduction in L-carnitine levels, possibly reflecting impaired FAO. L-carnitine supplementation reduced alveolar epithelial apoptosis and protected from emphysema development (93). This suggests that prolonged exposure to oxidative stress may lead to loss of metabolic flexibility in epithelial cells, rendering them unable to respond to the bioenergetic demands of stress, and thus leading to senescence, apoptosis, and impaired regenerative capacity in the COPD epithelium (94–96). This effect may be a result of deregulated nutrient sensing. Sirt1 (97, 98) and FoxO3 (90, 99, 100) are reduced in the lungs of patients with COPD as a result of chronic exposure to CS, suggesting an impairment of the AMPK–Sirt1–FoxO axis, with potentially detrimental effects on the ability of the cells to reprogram their metabolism and respond to stress. A number of studies have demonstrated a protective role for these pathways against the development of emphysema and inflammation in mouse models of COPD (76, 89, 97–99); however, their role in metabolic function in the lungs is still elusive. Conversely, prolonged activation of adaptive mechanisms could also drive pathogenic processes. CS-induced up-regulation of FAO, via a family with sequence similarity 13 member A (FAM13A)/Sirt1–dependent pathway, was shown to promote lung epithelial cell apoptosis through increased ROS production (101). AMPK has been shown to promote inflammation through NF-κB activation in CS-exposed mice and human macrophages (89–91), and to induce cell senescence (102, 103).
On the other hand, extensive metabolic reprogramming resulting from overadaptation to hypoxia, exposure to inflammatory mediators, or disease-specific differences in metabolic regulation may also lead to aberrant cellular function. In cancer and pulmonary hypertension, a shift from OXPHOS to the use of glycolysis and glutaminolysis as energy sources leads to the production of intermediates for synthesis of lipids, amino acids, nucleotides, and antioxidants required for maintaining proliferation and survival (104, 105). We have demonstrated mitochondrial dysfunction and impaired OXPHOS in ASMCs from patients with COPD (8). Preliminary data in our laboratory suggest that COPD ASMCs do not show senescence, but, rather, increased proliferation in response to growth factors (unpublished data), suggesting that a similar maladaptive metabolic reprogramming may contribute to ASMC hyperplasia. Moreover, increased glycolysis in response to HIF-1 activation drives myofibroblast differentiation and contractility in lung fibrosis (106, 107). Recent studies have demonstrated an integral role of metabolic reprogramming in T cell activation (108) and macrophage phenotype switching (109). Metabolic reprogramming may, therefore, provide another level of regulation of airway inflammation and remodeling in COPD.
Concluding Remarks and Future Directions
Cells respond to stress and mitochondrial dysfunction through immediate changes in their morphology and localization, but also through post-translational modifications of nuclear and mitochondrial proteins and transcriptional changes leading to metabolic reprogramming. These responses are now recognized to be integrally linked and regulated in a coordinated manner (110, 111). Defective metabolic adaptation leads to increased susceptibility to mitochondrial damage in COPD, and may be associated with accelerated aging and disease pathogenesis. Detailed studies on the regulation of mitochondrial dynamics and movement, mitochondrial–ER cross-talk, as well as the mitochondrial proteome and metabolome in cells and lung tissue of COPD will allow us to gain a more complete picture of metabolic adaptation in COPD. This will allow the identification of molecular targets for protecting mitochondria in disease.
Supplementary Material
Footnotes
Supported by the National Institute for Health Research Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield National Health Service Foundation Trust, and Imperial College London; Medical Research Council–Association of the British Pharmaceutical Industry COPD-MAP consortium grant G1001367/1 (C.M., K.F.C., and I.M.A.); by the Dunhill Medical Trust (C.M.); the Wellcome Trust grant 093080/Z/10/Z (I.M.A.); and by British Heart Foundation grant PG/14/27/3067 (S.M. and I.M.A.). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
Author Contributions: All authors participated in the concept, design, drafting, and critical revision of the manuscript. All authors approved the final version of the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 2.Chung KF, Adcock IM. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J. 2008;31:1334–1356. doi: 10.1183/09031936.00018908. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad T, Sundar IK, Lerner CA, Gerloff J, Tormos AM, Yao H, Rahman I. Impaired mitophagy leads to cigarette smoke stress–induced cellular senescence: implications for chronic obstructive pulmonary disease. FASEB J. 2015;29:2912–2929. doi: 10.1096/fj.14-268276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hara H, Araya J, Ito S, Kobayashi K, Takasaka N, Yoshii Y, Wakui H, Kojima J, Shimizu K, Numata T, et al. Mitochondrial fragmentation in cigarette smoke–induced bronchial epithelial cell senescence. Am J Physiol Lung Cell Mol Physiol. 2013;305:L737–L746. doi: 10.1152/ajplung.00146.2013. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann RF, Zarrintan S, Brandenburg SM, Kol A, de Bruin HG, Jafari S, Dijk F, Kalicharan D, Kelders M, Gosker HR, et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res. 2013;14:97. doi: 10.1186/1465-9921-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, Yoshida M, Hara H, Minagawa S, Wakui H, Fujii S, et al. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy. 2015;11:547–559. doi: 10.1080/15548627.2015.1017190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko GR, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124:3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiegman CH, Michaeloudes C, Haji G, Narang P, Clarke CJ, Russell KE, Bao W, Pavlidis S, Barnes PJ, Kanerva J, et al. COPDMAP. Oxidative stress–induced mitochondrial dysfunction drives inflammation and airway smooth muscle remodeling in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015;136:769–780. doi: 10.1016/j.jaci.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Archibald JM. Endosymbiosis and eukaryotic cell evolution. Curr Biol. 2015;25:R911–R921. doi: 10.1016/j.cub.2015.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Gabaldón T, Huynen MA. Shaping the mitochondrial proteome. Biochim Biophys Acta. 2004;1659:212–220. doi: 10.1016/j.bbabio.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Shutt TE, McBride HM. Staying cool in difficult times: mitochondrial dynamics, quality control and the stress response. Biochim Biophys Acta. 2013;1833:417–424. doi: 10.1016/j.bbamcr.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 12.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 13.Fisher AB. Intermediary metabolism of the lung. Environ Health Perspect. 1984;55:149–158. doi: 10.1289/ehp.8455149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lottes RG, Newton DA, Spyropoulos DD, Baatz JE. Lactate as substrate for mitochondrial respiration in alveolar epithelial type II cells. Am J Physiol Lung Cell Mol Physiol. 2015;308:L953–L961. doi: 10.1152/ajplung.00335.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberg SE, Chandel NS. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 20.Rosca MG, Vazquez EJ, Chen Q, Kerner J, Kern TS, Hoppel CL. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes. 2012;61:2074–2083. doi: 10.2337/db11-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353–1363. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riganti C, Gazzano E, Polimeni M, Aldieri E, Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic Biol Med. 2012;53:421–436. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Yue L, Yao H. Mitochondrial dysfunction in inflammatory responses and cellular senescence: pathogenesis and pharmacological targets for chronic lung diseases. Br J Pharmacol. 2016;173:2305–2318. doi: 10.1111/bph.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cloonan SM, Glass K, Laucho-Contreras ME, Bhashyam AR, Cervo M, Pabón MA, Konrad C, Polverino F, Siempos II, Perez E, et al. Mitochondrial iron chelation ameliorates cigarette smoke–induced bronchitis and emphysema in mice. Nat Med. 2016;22:163–174. doi: 10.1038/nm.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aravamudan B, Kiel A, Freeman M, Delmotte P, Thompson M, Vassallo R, Sieck GC, Pabelick CM, Prakash YS. Cigarette smoke–induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2014;306:L840–L854. doi: 10.1152/ajplung.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pouwels SD, Zijlstra GJ, van der Toorn M, Hesse L, Gras R, Ten Hacken NH, Krysko DV, Vandenabeele P, de Vries M, van Oosterhout AJ, et al. Cigarette smoke–induced necroptosis and DAMP release trigger neutrophilic airway inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2016;310:L377–L386. doi: 10.1152/ajplung.00174.2015. [DOI] [PubMed] [Google Scholar]
- 27.Pouwels SD, van Geffen WH, Jonker MR, Kerstjens HA, Nawijn MC, Heijink IH. Increased neutrophil expression of pattern recognition receptors during COPD exacerbations. Respirology. 2017;22:401–404. doi: 10.1111/resp.12912. [DOI] [PubMed] [Google Scholar]
- 28.Pouwels SD, Hesse L, Faiz A, Lubbers J, Bodha PK, Ten Hacken NH, van Oosterhout AJ, Nawijn MC, Heijink IH. Susceptibility for cigarette smoke–induced DAMP release and DAMP-induced inflammation in COPD. Am J Physiol Lung Cell Mol Physiol. 2016;311:L881–L892. doi: 10.1152/ajplung.00135.2016. [DOI] [PubMed] [Google Scholar]
- 29.Wu SB, Wu YT, Wu TP, Wei YH. Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochim Biophys Acta. 2014;1840:1331–1344. doi: 10.1016/j.bbagen.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 30.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148:1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao AW, Cantó C, Houtkooper RH. Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO Mol Med. 2014;6:580–589. doi: 10.1002/emmm.201303782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28:1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voss C, Lahiri S, Young BP, Loewen CJ, Prinz WA. ER-shaping proteins facilitate lipid exchange between the ER and mitochondria in S. cerevisiae. J Cell Sci. 2012;125:4791–4799. doi: 10.1242/jcs.105635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhotra JD, Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol. 2011;3:a004424. doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187:1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Redpath CJ, Bou Khalil M, Drozdzal G, Radisic M, McBride HM. Mitochondrial hyperfusion during oxidative stress is coupled to a dysregulation in calcium handling within a C2C12 cell model. PLoS One. 2013;8:e69165. doi: 10.1371/journal.pone.0069165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan MT, Stojanovski D. Mitofusins ‘bridge’ the gap between oxidative stress and mitochondrial hyperfusion. EMBO Rep. 2012;13:870–871. doi: 10.1038/embor.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mears JA, Lackner LL, Fang S, Ingerman E, Nunnari J, Hinshaw JE. Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission. Nat Struct Mol Biol. 2011;18:20–26. doi: 10.1038/nsmb.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 43.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fransson A, Ruusala A, Aspenström P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- 45.Al-Mehdi AB, Pastukh VM, Swiger BM, Reed DJ, Patel MR, Bardwell GC, Pastukh VV, Alexeyev MF, Gillespie MN. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal. 2012;5:ra47. doi: 10.1126/scisignal.2002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballweg K, Mutze K, Königshoff M, Eickelberg O, Meiners S. Cigarette smoke extract affects mitochondrial function in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2014;307:L895–L907. doi: 10.1152/ajplung.00180.2014. [DOI] [PubMed] [Google Scholar]
- 47.Jendrach M, Pohl S, Vöth M, Kowald A, Hammerstein P, Bereiter-Hahn J. Morpho-dynamic changes of mitochondria during ageing of human endothelial cells. Mech Ageing Dev. 2005;126:813–821. doi: 10.1016/j.mad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Soulitzis N, Neofytou E, Psarrou M, Anagnostis A, Tavernarakis N, Siafakas N, Tzortzaki EG. Downregulation of lung mitochondrial prohibitin in COPD. Respir Med. 2012;106:954–961. doi: 10.1016/j.rmed.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 49.Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ, Cho H. Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J Cell Sci. 2010;123:619–626. doi: 10.1242/jcs.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon YS, Yoon DS, Lim IK, Yoon SH, Chung HY, Rojo M, Malka F, Jou MJ, Martinou JC, Yoon G. Formation of elongated giant mitochondria in DFO-induced cellular senescence: involvement of enhanced fusion process through modulation of Fis1. J Cell Physiol. 2006;209:468–480. doi: 10.1002/jcp.20753. [DOI] [PubMed] [Google Scholar]
- 51.Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen TT, Oh SS, Weaver D, Lewandowska A, Maxfield D, Schuler MH, Smith NK, Macfarlane J, Saunders G, Palmer CA, et al. Loss of Miro1-directed mitochondrial movement results in a novel murine model for neuron disease. Proc Natl Acad Sci U S A. 2014;111:E3631–E3640. doi: 10.1073/pnas.1402449111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smiraglia DJ, Kulawiec M, Bistulfi GL, Gupta SG, Singh KK. A novel role for mitochondria in regulating epigenetic modification in the nucleus. Cancer Biol Ther. 2008;7:1182–1190. doi: 10.4161/cbt.7.8.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jorgensen E, Stinson A, Shan L, Yang J, Gietl D, Albino AP. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer. 2008;8:229. doi: 10.1186/1471-2407-8-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J, et al. Increased ER–mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci. 2011;124:2143–2152. doi: 10.1242/jcs.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finley LW, Haas W, Desquiret-Dumas V, Wallace DC, Procaccio V, Gygi SP, Haigis MC. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS One. 2011;6:e23295. doi: 10.1371/journal.pone.0023295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu YT, Lee HC, Liao CC, Wei YH. Regulation of mitochondrial F(o)F(1)ATPase activity by Sirt3-catalyzed deacetylation and its deficiency in human cells harboring 4977bp deletion of mitochondrial DNA. Biochim Biophys Acta. 2013;1832:216–227. doi: 10.1016/j.bbadis.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 63.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC, Gupta MP. SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol. 2014;34:807–819. doi: 10.1128/MCB.01483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng Y, Ren X, Gowda AS, Shan Y, Zhang L, Yuan YS, Patel R, Wu H, Huber-Keener K, Yang JW, et al. Interaction of Sirt3 with OGG1 contributes to repair of mitochondrial DNA and protects from apoptotic cell death under oxidative stress. Cell Death Dis. 2013;4:e731. doi: 10.1038/cddis.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi J, Koh E, Lee YS, Lee HW, Kang HG, Yoon YE, Han WK, Choi KH, Kim KS. Mitochondrial Sirt3 supports cell proliferation by regulating glutamine-dependent oxidation in renal cell carcinoma. Biochem Biophys Res Commun. 2016;474:547–553. doi: 10.1016/j.bbrc.2016.04.117. [DOI] [PubMed] [Google Scholar]
- 68.Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 70.Sosulski ML, Gongora R, Feghali-Bostwick C, Lasky JA, Sanchez CG. Sirtuin 3 deregulation promotes pulmonary fibrosis. J Gerontol A Biol Sci Med Sci. 2017;72:595–602. doi: 10.1093/gerona/glw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sundaresan NR, Bindu S, Pillai VB, Samant S, Pan Y, Huang JY, Gupta M, Nagalingam RS, Wolfgeher D, Verdin E, et al. SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating glycogen synthase kinase 3β. Mol Cell Biol. 2015;36:678–692. doi: 10.1128/MCB.00586-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bindu S, Pillai VB, Kanwal A, Samant S, Mutlu GM, Verdin E, Dulin N, Gupta MP. SIRT3 blocks myofibroblast differentiation and pulmonary fibrosis by preventing mitochondrial DNA damage. Am J Physiol Lung Cell Mol Physiol. 2017;312:L68–L78. doi: 10.1152/ajplung.00188.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hardie DG, Carling D, Gamblin SJ. AMP-activated protein kinase: also regulated by ADP? Trends Biochem Sci. 2011;36:470–477. doi: 10.1016/j.tibs.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Wu SB, Wei YH. AMPK-mediated increase of glycolysis as an adaptive response to oxidative stress in human cells: implication of the cell survival in mitochondrial diseases. Biochim Biophys Acta. 2012;1822:233–247. doi: 10.1016/j.bbadis.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 75.Mackenzie RM, Salt IP, Miller WH, Logan A, Ibrahim HA, Degasperi A, Dymott JA, Hamilton CA, Murphy MP, Delles C, et al. Mitochondrial reactive oxygen species enhance AMP-activated protein kinase activation in the endothelium of patients with coronary artery disease and diabetes. Clin Sci (Lond) 2013;124:403–411. doi: 10.1042/CS20120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng XY, Li YY, Huang C, Li J, Yao HW. AMP-activated protein kinase reduces inflammatory responses and cellular senescence in pulmonary emphysema. Oncotarget. 2017;8:22513–22523. doi: 10.18632/oncotarget.15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP–activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol (1985) 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- 78.Zhang CS, Lin SC. AMPK promotes autophagy by facilitating mitochondrial fission. Cell Metab. 2016;23:399–401. doi: 10.1016/j.cmet.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 79.Liu XM, Peyton KJ, Shebib AR, Wang H, Korthuis RJ, Durante W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am J Physiol Heart Circ Physiol. 2011;300:H84–H93. doi: 10.1152/ajpheart.00749.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, Coselli JS, Chen L, Wang XL, Zhang Y, et al. Activation of the AMPK–FOXO3 pathway reduces fatty acid–induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2009;58:2246–2257. doi: 10.2337/db08-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–3671. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1–mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 85.Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agarwal AR, Zhao L, Sancheti H, Sundar IK, Rahman I, Cadenas E. Short-term cigarette smoke exposure induces reversible changes in energy metabolism and cellular redox status independent of inflammatory responses in mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2012;303:L889–L898. doi: 10.1152/ajplung.00219.2012. [DOI] [PubMed] [Google Scholar]
- 88.Agarwal AR, Yin F, Cadenas E. Short-term cigarette smoke exposure leads to metabolic alterations in lung alveolar cells. Am J Respir Cell Mol Biol. 2014;51:284–293. doi: 10.1165/rcmb.2013-0523OC. [DOI] [PubMed] [Google Scholar]
- 89.Zhang Z, Cheng X, Yue L, Cui W, Zhou W, Gao J, Yao H. Molecular pathogenesis in chronic obstructive pulmonary disease and therapeutic potential by targeting AMP-activated protein kinase. J Cell Physiol. doi: 10.1002/jcp.25844. [online ahead of print] 4 Feb 2017; DOI: 10.1002/jcp.25844. [DOI] [PubMed] [Google Scholar]
- 90.Ko HK, Lee HF, Lin AH, Liu MH, Liu CI, Lee TS, Kou YR. Regulation of cigarette smoke induction of IL-8 in macrophages by AMP-activated protein kinase signaling. J Cell Physiol. 2015;230:1781–1793. doi: 10.1002/jcp.24881. [DOI] [PubMed] [Google Scholar]
- 91.Tang GJ, Wang HY, Wang JY, Lee CC, Tseng HW, Wu YL, Shyue SK, Lee TS, Kou YR. Novel role of AMP-activated protein kinase signaling in cigarette smoke induction of IL-8 in human lung epithelial cells and lung inflammation in mice. Free Radic Biol Med. 2011;50:1492–1502. doi: 10.1016/j.freeradbiomed.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 92.Deeb RS, Walters MS, Strulovici-Barel Y, Chen Q, Gross SS, Crystal RG. Smoking-associated disordering of the airway basal stem/progenitor cell metabotype. Am J Respir Cell Mol Biol. 2016;54:231–240. doi: 10.1165/rcmb.2015-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Conlon TM, Bartel J, Ballweg K, Günter S, Prehn C, Krumsiek J, Meiners S, Theis FJ, Adamski J, Eickelberg O, et al. Metabolomics screening identifies reduced L-carnitine to be associated with progressive emphysema. Clin Sci (Lond) 2016;130:273–287. doi: 10.1042/CS20150438. [DOI] [PubMed] [Google Scholar]
- 94.van der Toorn M, Slebos DJ, de Bruin HG, Leuvenink HG, Bakker SJ, Gans RO, Koëter GH, van Oosterhout AJ, Kauffman HF. Cigarette smoke–induced blockade of the mitochondrial respiratory chain switches lung epithelial cell apoptosis into necrosis. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1211–L1218. doi: 10.1152/ajplung.00291.2006. [DOI] [PubMed] [Google Scholar]
- 95.Staudt MR, Buro-Auriemma LJ, Walters MS, Salit J, Vincent T, Shaykhiev R, Mezey JG, Tilley AE, Kaner RJ, Ho MW, et al. Airway basal stem/progenitor cells have diminished capacity to regenerate airway epithelium in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:955–958. doi: 10.1164/rccm.201406-1167LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kosmider B, Messier EM, Chu HW, Mason RJ. Human alveolar epithelial cell injury induced by cigarette smoke. PLoS One. 2011;6:e26059. doi: 10.1371/journal.pone.0026059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakamaru Y, Vuppusetty C, Wada H, Milne JC, Ito M, Rossios C, Elliot M, Hogg J, Kharitonov S, Goto H, et al. A protein deacetylase SIRT1 is a negative regulator of metalloproteinase-9. FASEB J. 2009;23:2810–2819. doi: 10.1096/fj.08-125468. [DOI] [PubMed] [Google Scholar]
- 98.Rajendrasozhan S, Yang SR, Kinnula VL, Rahman I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:861–870. doi: 10.1164/rccm.200708-1269OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hwang JW, Rajendrasozhan S, Yao H, Chung S, Sundar IK, Huyck HL, Pryhuber GS, Kinnula VL, Rahman I. FOXO3 deficiency leads to increased susceptibility to cigarette smoke–induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J Immunol. 2011;187:987–998. doi: 10.4049/jimmunol.1001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ganesan S, Unger BL, Comstock AT, Angel KA, Mancuso P, Martinez FJ, Sajjan US. Aberrantly activated EGFR contributes to enhanced IL-8 expression in COPD airways epithelial cells via regulation of nuclear FoxO3A. Thorax. 2013;68:131–141. doi: 10.1136/thoraxjnl-2012-201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang Z, Knudsen NH, Wang G, Qiu W, Naing ZZ, Bai Y, Ai X, Lee CH, Zhou X. Genetic control of fatty acid β-oxidation in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2017;56:738–748. doi: 10.1165/rcmb.2016-0282OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Phadke M, Krynetskaia N, Mishra A, Krynetskiy E. Accelerated cellular senescence phenotype of GAPDH-depleted human lung carcinoma cells. Biochem Biophys Res Commun. 2011;411:409–415. doi: 10.1016/j.bbrc.2011.06.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang W, Yang X, López de Silanes I, Carling D, Gorospe M. Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem. 2003;278:27016–27023. doi: 10.1074/jbc.M300318200. [DOI] [PubMed] [Google Scholar]
- 104.Boehme J, Sun X, Tormos KV, Gong W, Kellner M, Datar SA, Kameny RJ, Yuan JX, Raff GW, Fineman JR, et al. Pulmonary artery smooth muscle cell hyperproliferation and metabolic shift triggered by pulmonary overcirculation. Am J Physiol Heart Circ Physiol. 2016;311:H944–H957. doi: 10.1152/ajpheart.00040.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bernard K, Logsdon NJ, Ravi S, Xie N, Persons BP, Rangarajan S, Zmijewski JW, Mitra K, Liu G, Darley-Usmar VM, et al. Metabolic reprogramming is required for myofibroblast contractility and differentiation. J Biol Chem. 2015;290:25427–25438. doi: 10.1074/jbc.M115.646984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, Bernard K, Thannickal VJ, Liu G. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med. 2015;192:1462–1474. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Byersdorfer CA. The role of fatty acid oxidation in the metabolic reprograming of activated t-cells. Front Immunol. 2014;5:641. doi: 10.3389/fimmu.2014.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kelly B, O’Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015;25:771–784. doi: 10.1038/cr.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O, Braas D, van der Windt GJ, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212:379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.