Abstract
Alveolar epithelial type II (AEII) cells are “professional” secretory cells that synthesize and secrete massive quantities of proteins to produce pulmonary surfactant and maintain airway immune defenses. To facilitate this high level of protein synthesis, AEII cells are equipped with an elaborate endoplasmic reticulum (ER) structure and possess an abundance of the machinery needed to fold, assemble, and secrete proteins. However, conditions that suddenly increase the quantity of new proteins entering the ER or that impede the capacity of the ER to fold proteins can cause misfolded or unfolded proteins to accumulate in the ER lumen, also called ER stress. To minimize this stress, AEII cells adapt by (1) reducing the quantity of proteins entering the ER, (2) increasing the amount of protein-folding machinery, and (3) removing misfolded proteins when they accumulate. Although these adaptive responses, aptly named the unfolded protein response, are usually effective in reducing ER stress, chronic aggregation of misfolded proteins is recognized as a hallmark feature of AEII cells in patients with idiopathic pulmonary fibrosis (IPF). Although mutations in surfactant proteins are linked to the development of ER stress in some rare IPF cases, the mechanisms causing protein misfolding in most cases are unknown. In this article, we review the mechanisms regulating ER proteostasis and highlight specific aspects of protein folding and the unfolded protein response that are most vulnerable to failure. Then, we postulate mechanisms other than genetic mutations that might contribute to protein aggregation in the alveolar epithelium of IPF lung.
Keywords: endoplasmic reticulum stress, unfolded proteins response, impaired nutrient sensing, ATP, lipid synthesis
Alveolar epithelial type II (AEII) cells are arguably the most complex and metabolically active cells in the lung, in large part because of their need to continuously produce pulmonary surfactant. Consistent with having a high basal metabolic rate, it is estimated that a significant minority (nearly 50%) of all mitochondria contained in the lung reside within AEII cells (1–3). Like other professional secretory cells that synthesize large amounts of proteins (e.g., plasma cells, hepatocytes, or pancreatic exocrine cells), AEII cells are estimated to secrete anywhere from several hundred to tens of thousands of proteins per second (4, 5). This astonishing feat is achieved not only by having an elaborate endoplasmic reticulum (ER) structure that contains an abundance of protein-folding machinery but also by maintaining a sophisticated system to recognize and discard misfolded proteins when they accumulate. Although ER proteostasis is usually maintained in AEII cells despite wide fluctuations in the amount of incoming proteins, toxic levels of misfolded proteins have been shown to accumulate in the lungs of patients with idiopathic pulmonary fibrosis (IPF) (6–9). Because abnormal protein accumulation is thought to contribute to functional decline in AEII cells in IPF, it is largely assumed that elucidating the mechanisms by which ER stress develops will ultimately lead to new therapies for this disease. In this article, our objectives are to review the mechanisms regulating ER proteostasis and then discuss why functional decline in AEII cells in IPF, specifically the activities of their mitochondria, might play an important role in compromising protein folding and activation of the unfolded protein response (UPR).
Protein Folding Is Error-Prone and Energy Consuming
Protein folding in the ER is a highly complex process that is dependent on a multitude of factors, including the maintenance of a uniquely oxidizing and calcium-rich environment and the activities of numerous enzymes, such as those required for the formation of disulfide bonds and arranging proteins in their proper cis-trans configuration (Table 1) (10–14). Although the native structure of any protein is ultimately dictated by its amino acid composition, it is also appreciated that many properly translated proteins never reach their native configuration. In fact, it is estimated that in the average cell 30% of all proteins produced in the ER are misfolded under homeostatic conditions (14), implying that even greater rates of protein misfolding might occur when cells are either injured or under stressed conditions. This elevated level of basal protein misfolding is attributable largely to nonspecific hydrophobic interactions that occur between different regions of the same protein or as a result of interactions between neighboring proteins. To minimize these interactions, cells possess high levels of chaperone proteins in their ER lumen, which function to physically block “sticky” regions on unfolded proteins (14). Chaperone proteins are mostly classified according to their molecular weight (e.g., heat shock protein 40 [HSP40], HSP60, HSP70) and, as a group, are critically involved in virtually all aspects of ER protein homeostasis, including de novo folding, refolding, assembly of multimers, and the trafficking of proteins to the secretory or degradation pathways. Importantly, the activities of chaperone proteins are dependent on the hydrolysis of ATP; thus, they require a constant supply of energy to perform their vital functions. This energy dependence probably explains why ER stress is often observed in cells deprived of essential nutrients or have significant metabolic impairments (12, 15). Although recent studies suggest that augmenting the expression of chaperone proteins can by itself reduce ER stress in some experimental model systems, it remains to be determined whether this approach would be equally effective in cells that are depleted of energy stores.
Table 1.
Conditions required for effective protein folding in the endoplasmic reticulum
| • Adequate supply of energy (ATP) |
| • High calcium levels in the ER lumen |
| • Substrate and enzymes for glycosylation of proteins |
| • A rich oxidizing environment to form disulfide bonds |
| • High concentrations of chaperone proteins |
| • Isomerase activity |
| • Ability to synthesize lipids |
Definition of abbreviation: ER = endoplasmic reticulum.
ER Quality Control Is Dependent on the UPR
Because abnormal protein accumulation can be toxic to cells, it should be of no surprise that mammalian cells have evolved an elaborate system to monitor the status of protein folding in the ER and eliminate misfolded proteins when they accumulate. Currently, there are three known branches of this monitoring system, also known as the UPR. These three branches are defined by a single transmembrane ER-resident protein and are called (1) inositol-requiring enzyme 1 (IRE1), (2) activating transcription factor 6 (ATF6), and (3) double-stranded RNA-activated protein kinase–like endoplasmic reticulum kinase (PERK) (4, 13, 16, 17).
The most evolutionarily conserved branch of the UPR is the IRE1 pathway (13, 17). IRE1 is a transmembrane ER-specific resident protein with an N-terminal domain residing in the ER lumen and a C-terminal domain that extends out into the cytoplasm. The N-terminal domain senses ER stress and then transmits this signal across the ER membrane to activate downstream events that lead to the transcription of many UPR target genes. Although the specifics of IRE1 activation are beyond the scope of this article, the C-terminal effector domain has a protein kinase that acts to phosphorylate itself and other IRE1 molecules. These phosphorylation events convert IRE1 to a high-affinity nucleotide-binding protein, which reveals a sequence-specific endoribonuclease that degrades numerous mRNA transcripts. Although endoribonuclease activity serves to globally reduce the amount of proteins entering the ER lumen, one important target of this enzyme is the X-box binding protein 1 (XBP1) mRNA transcript. IRE1 splices XBP1 in exactly two locations, thereby liberating a central component and allowing a neighboring ligase to rejoin the free 3′ and 5′ strands. This recombination event results in a shorter XBP1 transcript which codes for a transcription factor that drives the expression of various UPR genes, including many involved in the biosynthesis of lipids. Lipid synthesis is believed to be important because it enables cells to expand their ER membranes, thereby providing a greater surface area for incoming proteins and also enabling misfolded proteins to be exported out of the ER in lipid vesicles. Because lipid biosynthesis consumes large amounts of energy, it is likely that activating this arm of the UPR is compromised in cells lacking sufficient energy stores.
The second ER-localized transmembrane protein involved in sensing misfolded proteins is ATF6 (13, 16). Like IRE1, ATF6 remains inactive in its membrane-bound form, but in contrast to IRE1 biology, ATF6 activation results in its release from the ER and mobilization to the Golgi. Once at the Golgi complex, ATF6 undergoes proteolytic cleavage by sites 1 and 2 proteases to liberate its N-terminal fragment and allow this new, smaller protein to mobilize to the nucleus to perform its transcriptional activities. Among ATF6’s known target genes are several key ER-resident proteins, including various chaperone proteins and isomerases as well as many of the same lipid biosynthesis enzymes activated by XBP1. It is likely that this redundancy in the ability to induce lipid synthesis was an important evolutionary advancement which ensured that cells could elongate their ER membrane through multiple mechanisms. However, this advancement also came with a cost in that chronically activating the UPR virtually ensures that already-stressed cells become even more significantly challenged. In some cases, this could theoretically cause cell death by contributing to mitochondrial exhaustion and the activation of cytochrome C–mediated apoptotic pathways.
The third and final arm of the UPR pathway is defined by the molecular sensor PERK, which, like IRE1, is an ER-resident transmembrane kinase (13, 16). When activated upon sensing misfolded proteins, PERK oligomerizes and phosphorylates itself as well as eukaryotic translation initiation factor 2A (EIF2A). Phosphorylation of EIF2A inactivates this protein, thereby inhibiting the translation of many mRNA transcripts. As a result, PERK plays an essential role in decreasing ER stress through reducing the influx of nascent proteins. Importantly, mRNAs that contain short open reading frames in their 5′ untranslated regions are preferentially translated when EIF2A levels are reduced, including the transcription factor ATF4. This is important because ATF4’s target genes include several proteins essential for amino acid transport, antioxidant defenses, and the biosynthesis of lipids. Notably, ATF4 also promotes the production of the CCAAT/enhancer-binding protein homologous protein transcription factor, which drives the expression of numerous apoptosis genes. Thus, PERK activation not only serves a protective role by reducing protein translation but also is capable of inducing deleterious effects on cells through its ability to cause death in situations when ER stress is prolonged or severe.
ER Stress in IPF
Arguably, one of the most important recent discoveries in the field of IPF is that mutations causing the misfolding of surfactant proteins have been linked to the development of disease. This association was first reported in 2001, when Nogee and colleagues identified a mutation in the C-terminal region of surfactant protein C (SPC), which tracked to the development of disease in several members of the same family (18, 19). This mutation was found to cause the misfolding of SPC, thereby reducing export of the protein to the secretory pathway and causing retention within the ER lumen of AEII cells. Subsequently, other groups identified different SPC mutations as well as mutations in surfactant protein A2 that also resulted in protein misfolding and retention in the ER (20). The identification of these different mutant proteins not only suggested that epithelial dysfunction might play an inciting role in this disease but also highlighted the fact that IPF shares pathological features with other age-related degenerative disorders, such as Parkinson’s disease, Alzheimer’s disease, and atherosclerosis, in which misfolded proteins are known to accumulate (21, 22). Although it is now appreciated that mutations in surfactant proteins are found in only a small subset of patients with IPF, it is also appreciated that markers of ER stress are up-regulated in the alveolar epithelium of most, if not all, patients with the disease (7–9).
The Alveolar Epithelium in IPF Is a Challenging Environment for Maintaining ER Proteostasis
To date, in cases in which mutations in surfactant proteins have not been identified, the cause of ER stress in IPF remains unknown. Although it is possible that genetic mutations not yet identified contribute to protein misfolding in some cases, it seems equally plausible that protein aggregation results from other factors that compromise the ability of AEII cells to fold or unfold proteins. Recently, some groups have suggested that environmental factors such as viruses, cigarette smoke, and inhaled particulates might contribute to ER stress by activating inflammatory pathways that overwhelm the capacity of the alveolar epithelium to fold proteins. However, the fact that these common environmental insults do not lead to disease more frequently suggests, at the very least, that the ability of AEII cells to handle greater demand on their protein-folding machinery is compromised in the IPF lung.
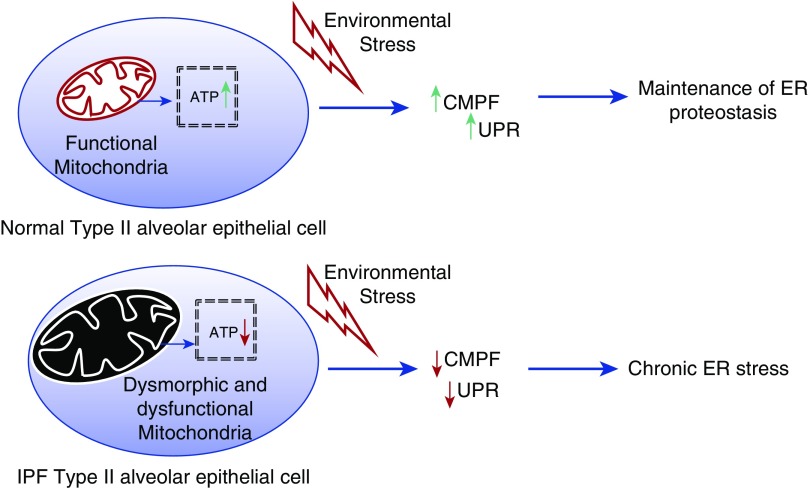
Because age is a major risk factor for IPF, it has been suggested by various groups that aging might contribute to functional decline in the ability of AEII cells to maintain ER proteostasis. For example, it is known that production of some chaperone proteins declines with age in many tissues, suggesting that nonspecific hydrophobic interactions might be more likely to occur under these scenarios. Consistent with this, levels of the chaperone protein HSP70 have recently been shown to be reduced in the lungs of patients with IPF (23). Alternatively, one other potential mechanism by which aging might contribute to protein accumulation is through a decline in metabolic health of AEII cells. This concept has recently been bolstered by data in various laboratories showing that mitochondrial function is impaired in the IPF lung. For example, Bueno and colleagues showed that large dysmorphic mitochondria accumulate in the lungs of patients with IPF (3). Furthermore, they went on to demonstrate that morphological and functional characteristics of mitochondria were also altered in the lungs of old mice and that these cellular changes in the mouse lung, and possibly the human IPF lung, resulted from a decrease in the expression of the mitophagy-associated protein phosphatase and tensin homolog–induced putative kinase 1, or PINK1 (3). Relevant to ER stress, mitochondrial dysfunction due to PINK1 deficiency was associated with an up-regulation in expression of ER stress markers, supporting the hypothesis that mitochondrial dysfunction can contribute directly to loss of proteostasis in the alveolar epithelium in some cases. Figure 1 shows a simplified cartoon illustrating how mitochondrial dysfunction could theoretically contribute to the development of ER stress.
Figure 1.
Endoplasmic reticulum (ER) proteostasis is dependent on the production of ATP. Conditions causing cellular depletion of ATP (i.e., mitochondrial dysfunction) impair chaperone-mediated protein folding (CMPF) and activation of the unfolded protein response (UPR). ER proteostasis is further compromised by insults that up-regulate protein synthesis (i.e., viruses, cigarette smoke). IPF = idiopathic pulmonary fibrosis.
Questions and Future Directions
Many questions remain unanswered regarding the origin of ER stress in the IPF lung and its contribution to the onset and progression of disease. Although genetic mutations clearly link ER stress to early stages of the disease in some cases (e.g., surfactant mutations), it remains unclear whether ER stress develops early or late in most other patients. The concept that mitochondrial disturbances can contribute to ER stress in some scenarios suggests that ER stress might be a consequence rather than a cause of disease in some IPF cases. Future studies determining whether mitochondria-directed therapies can reduce ER stress in the fibrotic lung will be important to improve understanding of the pathogenesis of IPF. That said, it is also plausible that dual approaches that act to enhance protein folding (e.g., delivery of chaperone proteins) and augment mitochondrial function will be necessary to restore proteostasis in the alveolar epithelium in IPF. Last, it remains unclear how ER stress or mitochondrial dysfunction ultimately drives fibrotic responses in the lung. However, emerging evidence indicates that mitochondrial dysfunction alone can drive fibrotic responses in some tissues by triggering cells to adopt a secretory senescence phenotype. This phenotype, also known as the mitochondrial dysfunction-associated senescence phenotype (24), has been shown to produce high concentrations of transforming growth factor-β1, suggesting that, in the context of IPF mitochondrial dysfunction, it could mechanistically link ER stress and fibrotic responses in the lung.
Conclusions
The regulation of ER proteostasis is essential for the proper functioning of cells. In the lung, AEII cells appear to be particularly vulnerable to developing ER stress because of their role in producing so many important proteins. Better understanding of the mechanisms by which AEII cells manage their proteome and what aspects of protein folding and the UPR are most vulnerable to failing will ultimately be important not only for understanding lung biology but also for developing new therapies for lung diseases such as IPF.
Supplementary Material
Footnotes
Funded by National Institutes of Health grants R01 HL105490 (R.S.) and R01 HL131784 (R.S.).
Author Contributions: F.R. and R.S.: wrote and edited the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Massaro GD, Gail DB, Massaro D. Lung oxygen consumption and mitochondria of alveolar epithelial and endothelial cells. J Appl Physiol. 1975;38:588–592. doi: 10.1152/jappl.1975.38.4.588. [DOI] [PubMed] [Google Scholar]
- 2.Massaro GD, Massaro D. Mitochondria of the pulmonary granular pneumocyte in different species. Am Rev Respir Dis. 1977;115:359–361. doi: 10.1164/arrd.1977.115.2.359. [DOI] [PubMed] [Google Scholar]
- 3.Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 5.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1119–L1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 8.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA. 2011;108:10562–10567. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maitra M, Wang Y, Gerard RD, Mendelson CR, Garcia CK. Surfactant protein A2 mutations associated with pulmonary fibrosis lead to protein instability and endoplasmic reticulum stress. J Biol Chem. 2010;285:22103–22113. doi: 10.1074/jbc.M110.121467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulleid NJ. Protein disulfide-isomerase: role in biosynthesis of secretory proteins. Adv Protein Chem. 1993;44:125–150. doi: 10.1016/s0065-3233(08)60566-5. [DOI] [PubMed] [Google Scholar]
- 11.Xu C, Ng DT. Glycosylation-directed quality control of protein folding. Nat Rev Mol Cell Biol. 2015;16:742–752. doi: 10.1038/nrm4073. [DOI] [PubMed] [Google Scholar]
- 12.Ron D, Harding HP. Protein-folding homeostasis in the endoplasmic reticulum and nutritional regulation. Cold Spring Harb Perspect Biol. 2012;4:a013177. doi: 10.1101/cshperspect.a013177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 14.Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 15.Ellis RJ. The molecular chaperone concept. Semin Cell Biol. 1990;1:1–9. [PubMed] [Google Scholar]
- 16.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 17.Mori K. Signalling pathways in the unfolded protein response: development from yeast to mammals. J Biochem. 2009;146:743–750. doi: 10.1093/jb/mvp166. [DOI] [PubMed] [Google Scholar]
- 18.Nogee LM, Dunbar AE, III, Wert S, Askin F, Hamvas A, Whitsett JA. Mutations in the surfactant protein C gene associated with interstitial lung disease. Chest. 2002;121(3) Suppl:20S–21S. doi: 10.1378/chest.121.3_suppl.20s. [DOI] [PubMed] [Google Scholar]
- 19.Nogee LM, Dunbar AE, III, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 20.Thurm T, Kaltenborn E, Kern S, Griese M, Zarbock R. SFTPC mutations cause SP-C degradation and aggregate formation without increasing ER stress. Eur J Clin Invest. 2013;43:791–800. doi: 10.1111/eci.12107. [DOI] [PubMed] [Google Scholar]
- 21.Chung CY, Khurana V, Auluck PK, Tardiff DF, Mazzulli JR, Soldner F, Baru V, Lou Y, Freyzon Y, Cho S, et al. Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons. Science. 2013;342:983–987. doi: 10.1126/science.1245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danzer KM, Ruf WP, Putcha P, Joyner D, Hashimoto T, Glabe C, Hyman BT, McLean PJ. Heat-shock protein 70 modulates toxic extracellular α-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 2011;25:326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aquino-Gálvez A, González-Ávila G, Pérez-Rodríguez M, Partida-Rodríguez O, Nieves-Ramírez M, Piña-Ramírez I, Ramírez-Martínez G, Castillejos-López M, Checa M, Ruiz V, et al. Analysis of heat shock protein 70 gene polymorphisms Mexican patients with idiopathic pulmonary fibrosis. BMC Pulm Med. 2015;15:129. doi: 10.1186/s12890-015-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab. 2016;23:303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.