Abstract
Rationale: Patient participation in medical decision-making is widely advocated, but outcomes are inconsistent.
Objectives: We examined the associations between medical decision-making roles, and patients’ perceptions of their care and knowledge while undergoing pulmonary nodule surveillance.
Methods: The study setting was an academically affiliated Veterans Affairs hospital network in which 121 participants had 319 decision-making encounters. The Control Preferences Scale was used to assess patients’ decision-making roles. Associations between decision-making, including role concordance (i.e., agreement between patients’ preferred and actual roles), shared decision-making (SDM), and perceptions of care and knowledge, were assessed using logistic regression and generalized estimating equations.
Results: Participants had a preferred role in 98% of encounters, and most desired an active role (shared or patient controlled). For some encounters (36%), patients did not report their actual decision-making role, because they did not know what their role was. Role concordance and SDM occurred in 56% and 26% of encounters, respectively. Role concordance was associated with greater satisfaction with medical care (adjusted odds ratio [Adj-OR], 5.39; 95% confidence interval [CI], 1.68–17.26), higher quality of patient-reported care (Adj-OR, 2.86; 95% CI, 1.31–6.27), and more disagreement that care could be better (Adj-OR, 2.16; 95% CI, 1.12–4.16). Role concordance was not associated with improved pulmonary nodule knowledge with respect to lung cancer risk (Adj-OR, 1.12; 95% CI, 0.63–2.00) or nodule information received (Adj-OR, 1.13; 95% CI, 0.31–4.13). SDM was not associated with perceptions of care or knowledge.
Conclusions: Among patients undergoing longitudinal nodule surveillance, a majority had a preference for having active roles in decision-making. Interestingly, during some encounters, patients did not know what their role was or that a decision was being made. Role concordance was associated with greater patient-reported satisfaction and quality of medical care, but not with improved knowledge. Patient participation in decision-making may influence perceptions of care; however, clinicians may need to focus on other communication strategies or domains to improve patient knowledge and health outcomes.
Keywords: decision-making, pulmonary nodule, lung cancer, patient outcome assessment, communication
The Institute of Medicine (1), the Affordable Care Act (2), the U.S. Preventive Services Task Force (3), and the Centers for Medicare & Medicaid Services (CMS) (4) have all called for increased patient participation in medical decision-making to improve health care quality. The majority of patients have role preferences for treatment decisions (5), and increasingly patients desire active involvement (6). Unfortunately, few patients participate in medical decision-making (7–9), and discordance between patients’ preferred and actual roles is common (10, 11). The importance of patient participation in medical decision-making has been associated with measures of adherence, trust, satisfaction with physicians, satisfaction with care, and/or satisfaction with the decisions; however, results across studies are inconsistent (12).
Shared decision-making is a collaborative approach whereby clinicians and the patient take into account the best available evidence as well as the patient’s values and preferences to make medical decisions together. Shared decision-making improves patient knowledge in some clinical contexts, but evidence is mixed regarding its effectiveness to improve patient satisfaction or other important health outcomes (13–15). Among patients with lung and colorectal cancer, physician-controlled decision-making for cancer treatment was associated with lower odds of excellent patient-reported quality, regardless of patients’ preferred role (16). Actual decision-making role was not associated with accurate knowledge about the benefits of chemotherapy among patients with incurable cancers (17). Role concordance (agreement between patients’ preferred and actual roles) in patients with breast cancer was associated with more satisfaction with cancer treatment choices (18). Research examining patient participation in cancer treatment decisions is considerable, whereas patients’ roles in cancer screening decisions are less well studied.
Lung cancer screening is the first adopter of CMS-mandated shared decision-making as part of the initial clinical encounter. The U.S. Preventive Services Task Force envisioned that this process would provide an opportunity for patients and providers to discuss the potential benefits, harms, and uncertainties of screening while potentially minimizing unintended consequences (19). The vast majority of positive lung cancer screening results involve the detection of pulmonary nodules (20). Given the emphasis on patient participation in decision-making and uncertainty regarding its impact in lung cancer screening, we sought to explore the association between decision-making and patients’ perceptions of care and knowledge while undergoing longitudinal incidental pulmonary nodule surveillance. The decisional pathways and unintended consequences in nodule surveillance are similar to those of lung cancer screening. Because widespread implementation of patient participation in decision-making is advocated, it is essential to develop a better understanding of its potential impact. We sought to determine the associations between decision-making and patients’ perceptions of their care and knowledge while undergoing pulmonary nodule surveillance.
Methods
Setting and Participants
We conducted a prospective, repeated-measures cohort study at the Veterans Affairs Portland Health Care System, an academically affiliated hospital network with outlying primary care clinics. The study was conducted from February 2012 through July 2016 with patients with an incidentally detected (i.e., not from lung cancer screening) pulmonary nodule. Patients’ imaging is electronically flagged, and primary care providers are responsible for notifying patients and determining the follow-up plan within this hospital network. Eligible study participants had a newly reported nodule smaller than 3 cm, had not yet obtained follow-up imaging, and were approved for participation by their primary care provider. Participants had study visits after each encounter in which nodule surveillance activities (i.e., imaging or biopsy) were planned or every 6 months if no new surveillance activity was planned, with a maximum of five study visits. The Veterans Affairs Portland Health Care System Internal Review Board approved this study (approval no. 2630), and all participants provided written informed consent. These methods have been described previously (21).
Exposure
The primary exposure was concordance between patients’ preferred and actual decision-making roles regarding initial and longitudinal pulmonary nodule surveillance activities. Preferred and actual decision-making roles were assessed for each encounter using the Control Preferences Scale (CPS) (22). The CPS has been used extensively in decision-making research and measures the control preferences construct, defined as “the degree of control an individual wants to assume when decisions are being made about medical treatment.”22 Roles are measured with a 5-point Likert scale, with items ranging from the individual making the treatment decisions (“patient-controlled”), to the individual making the decisions jointly with the physician (“shared”), to the physician making the decisions (“provider-controlled”). Participants were asked how they wanted the decision to be made (“preferred decision-making role”) and how the final decision actually was made (“actual decision-making role”) for each decision-making encounter. The CPS is an easily administered, valid, and reliable measure of roles in health care decision-making (11, 23, 24), which is clinically relevant across a variety of patient populations and clinical contexts (10, 25, 26).
Outcomes
Patient-reported outcomes included perceptions of care and knowledge. Three different perceptions of care were included:
-
1.
Satisfaction with medical care was measured with a 5-point Likert scale (1 = strongly disagree to 5 = strongly agree) as participants responded to the statement, “I am very satisfied with the medical care I receive.”
-
2.
Quality of medical care was measured with a 0- to 10-point Likert scale (0 = worst to 10 = best) as participants responded to the question, “How would you rate the overall quality of the medical care you are receiving from this provider?”
-
3.
Perception of whether care could be better was measured with a 5-point Likert scale (1 = strongly disagree to 5 = strongly agree) as participants responded to the statement, “There are some things about the medical care I receive that could be better.”
To assess knowledge of their pulmonary nodules, participants were asked the following two questions:
1. At all encounters, they were asked, “What is your likelihood of having lung cancer?” (0–100% by decile). A similar scale for lung cancer risk has been used previously (27).
2. At the initial encounter only, they were asked, “How informed are you about what a pulmonary nodule is?” (0- to 10-point Likert scale with 0 = worst and 10 = best).
Statistical Analysis
Decision-making role was categorized by encounter according to concordance between participants’ preferred and actual roles. Role concordance was defined as exact agreement between participants’ preferred and actual decision-making roles. Three categories of decision-making were stratified (patient controlled, shared, or provider controlled) on the basis of the CPS (11, 21). Additional analyses dichotomized actual decision-making as shared or not shared (patient or provider controlled), by encounter. Outcome variables were dichotomized by median split for analysis: quality of medical care as 0–9 or 10, and satisfaction with medical care and perception that care could be better as at least disagree or at least agree. Patient-reported lung cancer risk was dichotomized by median split per previous analyses as less than or equal to 30% and greater than 30%, and pulmonary nodule information was dichotomized as 0–3 (low) and 4–10 (high) (21, 28).
Exchangeable generalized estimating equations (GEEs) were used to model the within-subject covariance across encounters (29, 30). GEEs are often used to analyze longitudinal and other correlated response data, particularly if responses are binary. Models were adjusted by relevant baseline characteristics selected a priori and included age, sex, race/ethnicity, education, marital status, income, smoking history, and employment status. Exchangeable GEE models were also used to assess trends in decision-making roles (active or passive) and role concordance over time across encounters. Active roles were defined as shared or patient controlled, and passive role was defined as provider controlled. Models were adjusted by the same covariates as the main models. We conducted sensitivity analyses that considered outcome variables as continuous for initial encounters. The findings were similar to those in the primary analysis. All analyses were performed using Stata version 14 (StataCorp LP, College Station, TX), and two-sided statistical significance was defined as a resultant P value less than 0.05.
Results
This study included 121 participants and 319 decision-making encounters. The mean follow-up time for nodule surveillance was 18 months (SD ± 8 mo). Among participants at the first encounter, the mean age was 64 years, 93% of patients were male, 87% were white, 64% had at least some college or vocational school, 47% were former smokers, and the mean number of encounters was 2.3 (SD ± 1.3). Among participants, 45% reported a greater than 30% estimated lung cancer risk, and 57% reported they were not very informed about their nodules (0–3 points) on a 10-point scale (Table 1). According to the Mayo Clinic pulmonary nodule clinical prediction model (31), the mean lung cancer risk was 10% (SD ± 12 %), and it was less than 30% for 95% of participants at the first encounter.
Table 1.
Patient characteristics
| Total Sample | Always Concordant | At Least One Discordant | |
|---|---|---|---|
| Number of patients | 121 | 39 | 67 |
| Age, yr | 64 (±8.7) | 64 (±8.9) | 64 (±9.1) |
| Sex, male | 113 (93) | 38 (97) | 63 (94) |
| Race, white | 101 (87) | 33 (85) | 58 (91) |
| Socioeconomic status/demographics | |||
| Education, at least some college | 77 (64) | 22 (56) | 44 (66) |
| Currently married | 64 (53) | 21 (54) | 37 (56) |
| Annual income, ≥$30,000 | 70 (58) | 25 (69) | 36 (57) |
| Employment, at least part-time | 27 (22) | 7 (18) | 17 (26) |
| Smoking status | |||
| Never | 27 (22) | 11 (28) | 11 (16) |
| Past | 57 (47) | 15 (38) | 33 (49) |
| Current | 37 (31) | 13 (33) | 23 (34) |
| Encounters*, n | 2.3 (±1.3) | 2.3 (±1.3) | 2.4 (±1.3) |
| Total follow-up†, mo | 18 (±8) | 17 (±6.9) | 18 (±8.2) |
| Participant-reported lung cancer risk, scale = 0–100% | |||
| ≤30% | 50 (41) | 13 (39) | 27 (40) |
| >30% | 55 (45) | 20 (51) | 30 (45) |
| Missing | 16 (13) | 6 (15) | 10 (15) |
| Participant-reported nodule information, scale = 0–10 | |||
| 0–3 (low) | 69 (57) | 24 (62) | 34 (51) |
| 4–10 (high) | 48 (40) | 14 (36) | 30 (45) |
| Missing | 4 (3) | 1 (3) | 3 (4) |
Data are presented as count (percent) or mean ± SD. Two participants were missing preferred and actual decision-making roles; 13 participants were missing actual decision-making role.
Thirty-eight percent (121 of 319) were first encounters, 23% (72 of 319) were second encounters, 18% (58 of 319) were third encounters, 13% (42 of 319) were fourth encounters, and 8% (26 of 319) were fifth encounters.
Total time from first to last nodule surveillance activity.
Participant characteristics are presented for the entire cohort and stratified by role concordance, if decision-making roles were always concordant or discordant for at least one encounter (Table 1). Two participants did not answer role questions, and 13 were missing an actual decision-making role for all encounters. Demographics and preferred roles, when available, were similar among participants with and without missing data. Participants expressed a preferred role in decision-making for more than 98% of encounters (314 of 319). The preferred role in decision-making was shared for 56% (176 of 314), provider controlled for 24% (76 of 314), and patient controlled for 20% (62 of 314). Five encounters were missing both (preferred and actual) decision-making roles, and 116 were missing an actual decision-making role only. Among the 116 encounters missing an actual decision-making role, many participants did not know what their role was and/or that a decision was being made. Among encounters where an actual role was described, it was shared for 26% (51 of 198), provider controlled for 65% (129 of 198), and patient controlled for 9% (18 of 198) (Table 2).
Table 2.
Patients’ preferred and actual decision-making roles, based on Control Preferences Scale
| Nodule Decision-making* | Actual Role (n) | Total (n [%]) | ||
|---|---|---|---|---|
| Preferred Role (n) | Patient Controlled | Shared | Provider Controlled | |
| Patient controlled | 13† | 2 | 19 | 34 (17) |
| Shared | 5 | 47† | 59 | 111 (56) |
| Provider controlled | 0 | 2 | 51† | 53 (27) |
| Total, n (%) | 18 (9) | 51 (26) | 129 (65) | 198 (100) |
Five encounters were missing preferred and actual roles; 116 encounters were missing actual role.
Role concordance.
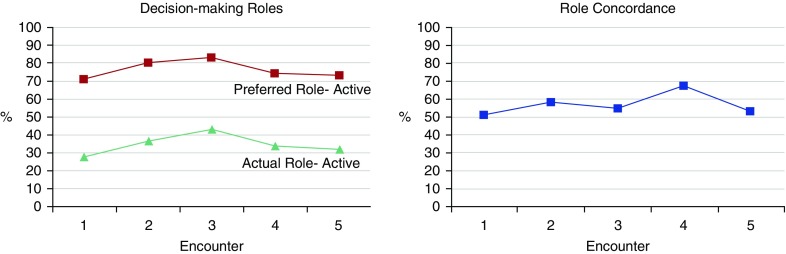
Role concordance (agreement between actual and preferred roles) occurred in 56% (111 of 198) of encounters. When role discordance was present, 92% (80 of 87) of encounters had a more passive role than participants preferred (i.e., shared role was preferred and actual role was provider controlled) (Table 2). Neither participants’ preferred (adjusted odds ratio [Adj-OR], 0.99; 95% confidence interval [CI], 0.81–1.22; P = 0.94) nor their actual (Adj-OR, 1.08; 95% CI, 0.84–1.38; P = 0.57) decision-making role was significantly different across encounters over time. Role concordance increased over the first four encounters; however, the trend was not significant across all encounters (Adj-OR, 1.13; 95% CI, 0.89–1.45; P = 0.31) (Figure 1).
Figure 1.
Decision-making roles (left) and role concordance (right) across encounters.
Encounters with role concordance were associated with greater satisfaction with medical care (Adj-OR, 5.39, 95% CI, 1.68–17.26; P = 0.005) and higher patient-reported quality of medical care (Adj-OR, 2.86; 95% CI, 1.31–6.27; P = 0.009). After encounters with role concordance, patients were more likely to disagree that medical care could be better (Adj-OR, 2.16; 95% CI, 1.12–4.16; P = 0.02) (Table 3). Role concordance was not associated with more accurate pulmonary nodule knowledge with respect to lung cancer risk at encounter 1 (Adj-OR, 0.28; 95% CI, 0.06–1.31; P = 0.11) or at all encounters (Adj-OR, 1.10; 95% CI, 0.62–1.97; P = 0.74), or regarding patient-reported nodule information at encounter 1 (Adj-OR, 1.14; 95% CI, 0.31–4.13; P = 0.85) (Table 4).
Table 3.
Associations between role concordance and patients’ perceptions of medical care
| Outcomes | Decision-making Roles (n [%]) |
Adjusted OR* (95% CI) | P Value | |
|---|---|---|---|---|
| Concordant | Discordant | |||
| Satisfaction with medical care (agree/strongly agree) | 100 (90) | 65 (76) | 5.39 (1.68–17.26) | 0.005 |
| Quality of medical care (10 out of 10) | 40 (56) | 20 (32) | 2.86 (1.31–6.27) | 0.009 |
| Medical care could be better (disagree/strongly disagree/unsure) | 61 (55) | 28 (32) | 2.16 (1.12–4.16) | 0.02 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Adjusted by age, sex, race/ethnicity, education, marital status, income, smoking history, and employment status.
Table 4.
Association between role concordance and patient knowledge
| Perceived Knowledge | Adjusted OR* (95% CI) | P Value |
|---|---|---|
| Lung cancer risk ≤30% | ||
| Encounter 1 | 0.28 (0.06–1.31) | 0.11 |
| All encounters | 1.10 (0.62–1.97) | 0.74 |
| Nodule information, ≥4 out of 10 | ||
| Encounter 1 | 1.14 (0.31–4.13) | 0.85 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
Adjusted by age, sex, race/ethnicity, education, marital status, income, smoking history, and employment status.
In terms of actual decision-making role, patient-reported shared decision-making occurred in 26% (51 of 198) of encounters. Encounters that included shared decision-making were not associated with satisfaction with medical care (Adj-OR, 3.36; 95% CI, 0.74–15.24; P = 0.12), patient-reported quality of medical care (Adj-OR, 0.86; 95% CI, 0.35–2.11; P = 0.73), or perception that care could be better (Adj-OR, 1.58; 95% CI, 0.73–3.41; P = 0.24). Shared decision-making was not associated with pulmonary nodule knowledge with respect to lung cancer risk at encounter 1 (Adj-OR, 1.12; 95% CI, 0.14–9.10; P = 0.91) or at all encounters (Adj-OR, 1.58; 95% CI, 0.72–3.43; P = 0.25) or regarding patient-reported nodule information at encounter 1 (Adj-OR, 4.05; 95% CI, 0.58–28.34; P = 0.16).
Discussion
Among patients undergoing longitudinal pulmonary nodule surveillance, we found that patients overwhelmingly had decision-making role preferences and that most wanted an active role. Interestingly, during some encounters, patients did not know what their actual decision-making role was and/or that a decision was being made. Role concordance occurred only about half the time, and when roles were discordant, the patient usually had a more passive role than they desired. Role concordance was associated with greater satisfaction with medical care, patient-reported higher quality of care, and more disagreement that care could be better. Role concordance was not associated with improved patient perceptions of nodule knowledge. Patient-reported shared decision-making occurred in a minority of encounters and was not associated with perceptions of medical care or knowledge.
Since the term patient-centered care was first coined in 1988 (32), there has been an increasing trend (6) toward incorporating patients’ values and preferences in medical decision-making. However, significant variability exists in patients’ desire for participation (33, 34). Among U.S. adults, the vast majority (96%) want to be asked their opinion and to be offered choices by their clinicians; however, an almost equal proportion favor physician-controlled and patient-controlled decisions (35). Patients’ perceived role can influence their desire to participate because patients who perceive less involvement in decision-making have subsequent preferences for passive decision-making (26). Additionally, patients’ preferences for participation can be influenced by demographic factors, diagnosis, health status, types of decisions, amount of knowledge they possess about their condition, and interactions with their clinicians (36).
For a sizable number of encounters, patients did not know what their actual role in the decision-making process was, even though in the majority of these encounters patients had a preferred role. In some of these encounters, patients reported that they were not aware that a medical decision was actually being made. These results are not entirely surprising, because in a national sample of U.S. adults, for five of six nonsurgical medical decisions, a majority of respondents did not think they were asked their opinion (8). Among patients with cancer (37) and neurological conditions (38), 30% and 72%, respectively, reported being given treatment choices.
This is the first study, to our knowledge, to assess decision-making roles and outcomes in pulmonary nodule surveillance or lung cancer screening. The circumstances surrounding encounters where patients were unaware of their actual role in decision-making deserve more exploration; however, they likely represent a concerning breakdown of communication in the decision-making process. Patient preferences and role concordance may be especially important in scenarios where clinical equipoise exists, such as lung cancer screening. Lung cancer screening can trigger a cascade of serious and stressful interventions (39–41), and different pathways can entail various combinations of therapeutic and adverse effects. Therefore, patients’ preferences should play a major role in decision-making. Patients have demonstrated a willingness to undergo lung cancer screening, and the CMS has mandated patient participation in decision-making (42). However, few studies have demonstrated a willingness of patients to actively participate in the decision-making process or have included examinations of associated outcomes.
Shared decision-making is associated with improved treatment adherence (43) and affective-cognitive outcomes (13) such as patient satisfaction and decisional conflict. However, evidence is lacking for associations with improved health outcomes (13), and results of randomized controlled trials have been mixed (44). In the setting of pulmonary nodule surveillance, neither shared decision-making nor role concordance was associated with patient distress (27). Although shared decision-making is currently espoused as the preferred model in decision-making, we found that role concordance and not shared decision-making per se was important to patients’ perceptions of care.
Role concordance has been associated with reduced anxiety in patients with cancer (45) and with better physical and emotional quality of life in cancer survivors (46). Ensuring role concordance may be a critical step in patient participation in decision-making and may be essential in building a therapeutic alliance between patients and their clinicians. Ideally, clinicians should offer patients an opportunity to participate by first sharing information, asking patients about their values, priorities, and decision-making role preferences, and then engaging in a decision-making process to determine the next diagnostic or treatment steps. The benefits of this strategy are twofold: (1) Clinicians become aware of patients’ decision-making role preferences, and (2) patients have an opportunity to participate as much or as little as they prefer. Our results suggest that a shared and informed model of care that increases patients’ knowledge about treatment options, benefits, and harms depends on more than role congruency or shared decision-making, as neither of these was associated with more accurate patient knowledge. Decision aids deserve more exploration in this population because they have demonstrated an ability to educate patients and provide patients an opportunity to participate in decisions that involve weighing the benefits and harms of treatment options that have scientific uncertainty (47, 48). Patients’ preferences vary on the basis of the type of decision (49), but they do not typically change over time (11, 50, 51). Therefore, if patients indicate an active decision-making preference at the outset, they should be afforded an opportunity to participate throughout the nodule evaluation process.
Limitations
The present study has limitations. The cohort was composed of a mainly white male population from the Pacific Northwest. Participants were consistently surveyed soon after their clinical encounters; however, survey studies are subject to recall bias. A proportion of participants did not know their actual role in decision-making; these encounters had an incomplete CPS and were therefore excluded. Incorporating an objective measure of participants’ actual role in decision-making may have increased role concordance; however, patients’ reliability in reporting decision-making roles has been demonstrated previously (52). When decision-making is measured from the perspective of the patient (as opposed to observers’ or clinicians’ perspectives), regardless of the outcome category, assessments are more likely to result in significant associations (11). Our results are dependent on the dichotomization of decision-making roles based on patient role concordance and do not consider alternative approaches, which is a potential limitation.
Conclusions
Among patients undergoing longitudinal pulmonary nodule surveillance, a majority of patients have preferences about their decision-making roles, and most prefer an active role. For a sizable number of encounters, patients did not know what their actual role in the decision-making process was. Role concordance was associated with improved patient perceptions of care but not with more accurate knowledge. Patient-reported shared decision-making alone had no significant impact on these outcomes. Improving patients’ perceptions of their care may not significantly impact knowledge or other important health outcomes. A process to improve patient-centered health outcomes may need to rely less on ensuring role concordance and focus on other communication domains and strategies, such as effective information exchange or building a therapeutic alliance.
Supplementary Material
Footnotes
Funded by the National Institutes of Health, National Cancer Institute, under award K07 CA190706 (D.R.S.), Department of Veterans Affairs Health Services Research and Development career development awards (CDA 09-025 and CDP 11-227 [C.G.S.]), and resources from the Veterans Affairs Health Care System (D.R.S., S.E.G., L.G., R.S.W., and C.G.S.). The Department of Veterans Affairs did not have a role in the conduct of the study; in the collection, management, analysis, or interpretation of data; or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. government.
Author Contributions: D.R.S., S.E.G., L.G., and C.G.S.: contributed to study conception and design or acquisition of data; D.R.S., R.S.W., K.B.E., and C.G.S.: contributed to analysis and interpretation of data; D.R.S., S.E.G., L.G., R.S.W., K.B.E., and C.G.S.: contributed to the writing of the manuscript; D.R.S., S.E.G., L.G., R.S.W., K.B.E., and C.G.S.: provided final approval of the manuscript version to be published; and D.R.S. and C.G.S.: had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 2.2010. Patient Protection and Affordable Care Act, Public Law 111-148, 42 U.S.C. § 18001 et seq.
- 3.Sheridan SL, Harris RP, Woolf SH Shared Decision-Making Workgroup of the U.S. Preventive Services Task Force. Shared decision making about screening and chemoprevention: a suggested approach from the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26:56–66. doi: 10.1016/j.amepre.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 4.LungCAN. Proposed decision memo for screening for lung cancer with low dose computed tomography (LDCT) [Accessed 2016 Dec 20]. Available from: http://lungcan.org/2014/11/18/proposed-decision-memo-for-screening-for-lung-cancer-with-low-dose-computed-tomography-ldct/
- 5.Tariman JD, Berry DL, Cochrane B, Doorenbos A, Schepp K. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Ann Oncol. 2010;21:1145–1151. doi: 10.1093/annonc/mdp534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86:9–18. doi: 10.1016/j.pec.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CN, Chang Y, Adimorah N, Belkora JK, Moy B, Partridge AH, Ollila DW, Sepucha KR. Decision making about surgery for early-stage breast cancer. J Am Coll Surg. 2012;214:1–10. doi: 10.1016/j.jamcollsurg.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zikmund-Fisher BJ, Couper MP, Singer E, Ubel PA, Ziniel S, Fowler FJ, Jr, Levin CA, Fagerlin A. Deficits and variations in patients’ experience with making 9 common medical decisions: the DECISIONS survey. Med Decis Making. 2010;30(5 Suppl):85S–95S. doi: 10.1177/0272989X10380466. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman RM, Elmore JG, Fairfield KM, Gerstein BS, Levin CA, Pignone MP. Lack of shared decision making in cancer screening discussions: results from a national survey. Am J Prev Med. 2014;47:251–259. doi: 10.1016/j.amepre.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Brom L, Hopmans W, Pasman HRW, Timmermans DRM, Widdershoven GAM, Onwuteaka-Philipsen BD. Congruence between patients’ preferred and perceived participation in medical decision-making: a review of the literature. BMC Med Inform Decis Mak. 2014;14:25. doi: 10.1186/1472-6947-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh JA, Sloan JA, Atherton PJ, Smith T, Hack TF, Huschka MM, Rummans TA, Clark MM, Diekmann B, Degner LF. Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the Control Preferences Scale. Am J Manag Care. 2010;16:688–696. [PMC free article] [PubMed] [Google Scholar]
- 12.Clayman ML, Bylund CL, Chewning B, Makoul G. The impact of patient participation in health decisions within medical encounters: a systematic review. Med Decis Making. 2016;36:427–452. doi: 10.1177/0272989X15613530. [DOI] [PubMed] [Google Scholar]
- 13.Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35:114–131. doi: 10.1177/0272989X14551638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lillie SE, Partin MR, Rice K, Fabbrini AE, Greer NL, Patel SS, MacDonald R, Rutks I, Wilt TJ. The effects of shared decision making on cancer screening – a systematic review: evidence-based synthesis program. Washington, DC: U.S. Department of Veterans Affairs; 2014. [PubMed] [Google Scholar]
- 15.Joosten EA, de Jong CA, de Weert-van Oene GH, Sensky T, van der Staak CP. Shared decision-making reduces drug use and psychiatric severity in substance-dependent patients. Psychother Psychosom. 2009;78:245–253. doi: 10.1159/000219524. [DOI] [PubMed] [Google Scholar]
- 16.Kehl KL, Landrum MB, Arora NK, Ganz PA, van Ryn M, Mack JW, Keating NL. Association of actual and preferred decision roles with patient-reported quality of care: shared decision making in cancer care. JAMA Oncol. 2015;1:50–58. doi: 10.1001/jamaoncol.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weeks JC, Catalano PJ, Cronin A, Finkelman MD, Mack JW, Keating NL, Schrag D. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:1616–1625. doi: 10.1056/NEJMoa1204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keating NL, Guadagnoli E, Landrum MB, Borbas C, Weeks JC. Treatment decision making in early-stage breast cancer: should surgeons match patients’ desired level of involvement? J Clin Oncol. 2002;20:1473–1479. doi: 10.1200/JCO.2002.20.6.1473. [DOI] [PubMed] [Google Scholar]
- 19.Miller YE. Minimizing unintended consequences of detecting lung nodules by computed tomography. Am J Respir Crit Care Med. 2008;178:891–892. doi: 10.1164/rccm.200808-1257ED. [DOI] [PubMed] [Google Scholar]
- 20.National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slatore CG, Wiener RS, Golden SE, Au DH, Ganzini L. Longitudinal assessment of distress among veterans with incidental pulmonary nodules. Ann Am Thorac Soc. 2016;13:1983–1991. doi: 10.1513/AnnalsATS.201607-555OC. [DOI] [PubMed] [Google Scholar]
- 22.Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29:21–43. [PubMed] [Google Scholar]
- 23.Wunderlich T, Cooper G, Divine G, Flocke S, Oja-Tebbe N, Stange K, Lafata JE. Inconsistencies in patient perceptions and observer ratings of shared decision making: the case of colorectal cancer screening. Patient Educ Couns. 2010;80:358–363. doi: 10.1016/j.pec.2010.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasper J, Heesen C, Köpke S, Fulcher G, Geiger F. Patients’ and observers’ perceptions of involvement differ. Validation study on inter-relating measures for shared decision making. PLoS One. 2011;6:e26255. doi: 10.1371/journal.pone.0026255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lavelle K, Sowerbutts AM, Bundred N, Pilling M, Degner L, Stockton C, Todd C. Is lack of surgery for older breast cancer patients in the UK explained by patient choice or poor health? A prospective cohort study. Br J Cancer. 2014;110:573–583. doi: 10.1038/bjc.2013.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez KL, Appelt CJ, Switzer GE, Sonel AF, Arnold RM. Veterans’ decision-making preferences and perceived involvement in care for chronic heart failure. Heart Lung. 2008;37:440–448. doi: 10.1016/j.hrtlng.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Making. 2008;28:917–925. doi: 10.1177/0272989X08322013. [DOI] [PubMed] [Google Scholar]
- 28.Slatore CG, Golden SE, Ganzini L, Wiener RS, Au DH. Distress and patient-centered communication among veterans with incidental (not screen-detected) pulmonary nodules: a cohort study. Ann Am Thorac Soc. 2015;12:184–192. doi: 10.1513/AnnalsATS.201406-283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeger SL, Diggle PJ. Semiparametric models for longitudinal data with application to CD4 cell numbers in HIV seroconverters. Biometrics. 1994;50:689–699. [PubMed] [Google Scholar]
- 30.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 31.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849–855. [PubMed] [Google Scholar]
- 32.Gerteis M, Edgman-Levitan S, Daley J, Delbanco TL, editors. Through the patient’s eyes: understanding and promoting patient-centered care. San Francisco: Jossey-Bass; 1993. [Google Scholar]
- 33.Hubbard G, Kidd L, Donaghy E. Preferences for involvement in treatment decision making of patients with cancer: a review of the literature. Eur J Oncol Nurs. 2008;12:299–318. doi: 10.1016/j.ejon.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Degner LF, Sloan JA. Decision making during serious illness: what role do patients really want to play? J Clin Epidemiol. 1992;45:941–950. doi: 10.1016/0895-4356(92)90110-9. [DOI] [PubMed] [Google Scholar]
- 35.Levinson W, Kao A, Kuby A, Thisted RA. Not all patients want to participate in decision making: a national study of public preferences. J Gen Intern Med. 2005;20:531–535. doi: 10.1111/j.1525-1497.2005.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Say R, Murtagh M, Thomson R. Patients’ preference for involvement in medical decision making: a narrative review. Patient Educ Couns. 2006;60:102–114. doi: 10.1016/j.pec.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Gattellari M, Voigt KJ, Butow PN, Tattersall MHN. When the treatment goal is not cure: are cancer patients equipped to make informed decisions? J Clin Oncol. 2002;20:503–513. doi: 10.1200/JCO.2002.20.2.503. [DOI] [PubMed] [Google Scholar]
- 38.Wiseman H, Chappell P, Toerien M, Shaw R, Duncan R, Reuber M. Do patients want choice? An observational study of neurology consultations. Patient Educ Couns. 2016;99:1170–1178. doi: 10.1016/j.pec.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Croswell JM, Ransohoff DF, Kramer BS. Principles of cancer screening: lessons from history and study design issues. Semin Oncol. 2010;37:202–215. doi: 10.1053/j.seminoncol.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freiman MR, Clark JA, Slatore CG, Gould MK, Woloshin S, Schwartz LM, Wiener RS. Patients’ knowledge, beliefs, and distress associated with detection and evaluation of incidental pulmonary nodules for cancer: results from a multicenter survey. J Thorac Oncol. 2016;11:700–708. doi: 10.1016/j.jtho.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyer VA U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 43.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, Vollmer WM Better Outcomes of Asthma Treatment (BOAT) Study Group. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181:566–577. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77:219–226. doi: 10.1159/000126073. [DOI] [PubMed] [Google Scholar]
- 45.Gattellari M, Butow PN, Tattersall MH. Sharing decisions in cancer care. Soc Sci Med. 2001;52:1865–1878. doi: 10.1016/s0277-9536(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 46.Atherton PJ, Smith T, Singh JA, Huntington J, Diekmann BB, Huschka M, Sloan JA. The relation between cancer patient treatment decision-making roles and quality of life. Cancer. 2013;119:2342–2349. doi: 10.1002/cncr.28046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, Llewellyn-Thomas H, Lyddiatt A, Légaré F, Thomson R. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;(10):CD001431. doi: 10.1002/14651858.CD001431.pub3. [DOI] [PubMed] [Google Scholar]
- 48.Crothers K, Kross EK, Reisch LM, Shahrir S, Slatore C, Zeliadt SB, Triplette M, Meza R, Elmore JG. Patients’ attitudes regarding lung cancer screening and decision aids. a survey and focus group study. Ann Am Thorac Soc. 2016;13:1992–2001. doi: 10.1513/AnnalsATS.201604-289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mansell D, Poses RM, Kazis L, Duefield CA. Clinical factors that influence patients’ desire for participation in decisions about illness. Arch Intern Med. 2000;160:2991–2996. doi: 10.1001/archinte.160.19.2991. [DOI] [PubMed] [Google Scholar]
- 50.Degner LF, Kristjanson LJ, Bowman D, Sloan JA, Carriere KC, O’Neil J, Bilodeau B, Watson P, Mueller B. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277:1485–1492. [PubMed] [Google Scholar]
- 51.Beaver K, Booth K. Information needs and decision-making preferences: comparing findings for gynaecological, breast and colorectal cancer. Eur J Oncol Nurs. 2007;11:409–416. doi: 10.1016/j.ejon.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Pass M, Belkora J, Moore D, Volz S, Sepucha K. Patient and observer ratings of physician shared decision making behaviors in breast cancer consultations. Patient Educ Couns. 2012;88:93–99. doi: 10.1016/j.pec.2012.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.