Abstract
Rationale: Strong evidence supports use of noninvasive ventilation (NIV) for patients with respiratory distress from chronic obstructive pulmonary disease and heart failure (strong evidence conditions [SECs]). Despite unclear benefits of NIV for other causes of acute respiratory failure, utilization for conditions with weaker evidence is increasing, despite evidence demonstrating higher mortality for patients who suffer NIV failure (progression from NIV to invasive mechanical ventilation [IMV])) compared with being treated initially with IMV.
Objectives: To determine the association of hospital variation in evidence-based utilization of NIV with patient outcomes.
Methods: Using the California State Inpatient Database 2011, we identified adult patients who received NIV. Patients were considered to have an SEC for NIV if they had an acute exacerbation of chronic obstructive pulmonary disease or heart failure. We used multivariable hierarchical logistic regression to determine the association between hospital rates of NIV use for SECs and patient risk of NIV failure (need for IMV after NIV).
Results: Among 22,706 hospitalizations with NIV as the initial ventilatory strategy, 6,820 (30.0%) had SECs. Patients with SECs had lower risk of NIV failure than patients with weak evidence conditions (8.1 vs. 18.2%, P < 0.0001). Regardless of underlying diagnosis, patients admitted to hospitals with greater use of NIV for SECs had lower risk of NIV failure (Quartile 4 vs. Quartile 1 adjusted odds ratio = 0.62; 95% CI = 0.49–0.80). Even patients without an SEC benefited from admission to hospitals that used NIV more often for patients with SECs (Quartile 4 vs. Quartile 1 adjusted odds ratio for NIV failure = 0.68; 95% CI = 0.52–0.88).
Conclusions: Most patients who received NIV did not have conditions with strong supporting evidence for its use with wide institutional variation in patient selection for NIV. Surprisingly, we found that all patients, even those without an SEC, benefited from admission to hospitals with greater evidence-based utilization of NIV, suggesting a “hospital effect” that is synergistic with patient selection.
Keywords: noninvasive ventilation, invasive mechanical ventilation, chronic obstructive pulmonary disease, heart failure
Noninvasive ventilation (NIV) is a cornerstone of treatment for patients with acute hypercarbic respiratory failure and heart failure (HF). Multiple randomized controlled trials have shown that patients with acute respiratory failure due to chronic obstructive pulmonary disease (COPD) or HF have lower intubation rates, hospital length of stay, and mortality with early use of NIV (1–7). However, NIV has not consistently shown benefits for patients with conditions such as hypoxemic respiratory failure, respiratory failure in the immunocompromised population, or asthma (8–16), and some studies have suggested harm when NIV was used for hypoxic respiratory failure (17, 18).
Harm may arise from progression of respiratory failure, despite NIV necessitating eventual intubation and invasive mechanical ventilation (IMV); this failure of NIV is strongly associated with increased mortality compared with patients treated initially with IMV (19–21). Despite potential harm from overuse of NIV among patients who are unlikely to benefit, use of NIV for conditions without strong supporting evidence has dramatically increased in the last 2 decades in the United States (20). However, whether clinical practice regarding optimal patient selection for the initiation of NIV at an institutional level, and whether practice variation is associated with meaningful differences in outcomes, is unknown.
We sought to characterize between-hospital variation in NIV utilization for strong evidence-based conditions (COPD or HF) and evaluate associations between hospital rates of evidence-based NIV use and clinical outcomes. We hypothesize that the majority of NIV would be used in strong evidence–based conditions, and that hospitals with more evidence-based utilization of NIV would experience better rates of clinical outcomes. However, we hypothesize that patient selection based on cause of respiratory failure drives patient outcomes, and that admission to hospitals with more evidence-based utilization is not associated with individual patient outcomes.
Methods
Please refer to the Methods in the online supplement for full study details.
Patients
Using the Healthcare Cost and Utilization Project’s California State Inpatient Database (CA SID) (22, 23), we conducted a retrospective cohort study on adult patients (≥18 yr of age) who required ventilatory support. The CA SID contains administrative discharge data for 100% of discharges from nonfederal hospitals in the state of California and, uniquely, includes information about patient early do-not-resuscitate (DNR) status (within 24 h of admission) (24, 25). Our primary patient cohort consisted of patients who received initial ventilatory treatment with NIV identified by previously validated International Classification of Disease, 9th Edition, Clinical Modification (ICD9-CM) billing code 93.90 (26). Patients were considered to have a strong evidence condition (SEC) for NIV if they were admitted for an acute exacerbation of COPD or HF (see Table E1 in the online supplement for ICD9-CM codes to identify disease cohorts) (26–28). We excluded patients with a DNR order, who could be started on NIV, but may ineligible for IMV; patients transferred to or from another acute care hospital; patients with obstructive sleep apnea; and patients admitted to hospitals that treated fewer than 25 patients with NIV during 2011 (Figure E1).
Exposures and Outcomes
The primary exposure was the hospital-level rate of NIV for SECs (NIV-SEC), calculated as the number of patients who received NIV for SECs divided by the total number of patients treated with NIV. Because of nonlinear association between NIV-SEC rates and outcomes, hospital NIV-SEC rate was divided into quartiles. The primary outcome was NIV failure [initial treatment with NIV followed by treatment with IMV (ICD9-CM code 96.7x [28, 29]). NIV failure was selected as the primary outcome, because patients who suffer NIV failure have a higher risk of death compared with those treated initially with IMV (19–21). NIV failure was identified as hospitalizations where IMV was initiated on the same day or after NIV was started, as previously described (19–21). In-hospital mortality for patients receiving NIV was a secondary outcome.
Statistical Analysis
We compared unadjusted continuous variables using Student’s t test, Wilcoxon Rank sum test, and linear regression as appropriate and categorical variables with chi-square and Cochrane Armitage tests for trends. We describe hospital variation in evidence-based patient selection for NIV using the median odds ratio (OR) (30). To evaluate the association between hospital NIV-SEC rates and patient outcomes, we used multivariable hierarchical logistic regression (31) with hospitals as random intercepts to: (1) determine the association of patient diagnosis (SEC or not) with patient outcomes (NIV failure and NIV in-hospital mortality) (patient-level outcome and patient-level exposure); (2) calculate hospital risk-adjusted NIV failure and hospital risk-adjusted NIV mortality rates from random effects output; and (3) determine the association between patient outcomes and hospital NIV-SEC rates (patient-level outcome and hospital-level exposure). These models were adjusted for patient demographics, individual Elixhauser comorbidities (32, 33), acute organ failures (Table E2) (34, 35) that were present on admission (24, 36), and patient indication for NIV (COPD, HF, pneumonia, nonpneumonia sepsis, asthma, and “other”; Table E3). To determine the association between hospital NIV-SEC rates and hospital risk-adjusted NIV failure and mortality rates, we used Spearman’s correlation test with Penalized B-spline regression (37) to visualize the relationship.
Exploratory and Sensitivity Analyses
Controversy exists about the benefits of NIV for immunocompromised patients with acute respiratory failure (14, 16, 38–40). As such, we conducted a sensitivity analysis in which immunocompromised patients were defined as having an SEC for NIV use. In addition, given previous work demonstrating an inverse relationship between NIV case volume and outcomes (21), we conducted an exploratory analysis investigating the relationship between hospital evidence-based NIV case selection, NIV case–volume, and NIV outcomes.
All statistical testing was two-tailed and performed at a critical α of 0.05 and conducted with SAS v9.4 (SAS Institute, Cary, NC). The study was deemed to be exempt from review by the National Jewish Health Institutional Review Board (Denver, CO).
Results
We identified 22,706 hospitalizations with NIV as the initial ventilatory treatment within 212 hospitals in California during 2011. The mean age of patients receiving NIV was 68.9 years (SD = 15.1), with 52.9% female and 57.3% white. SECs were documented for 30.0% of NIV cases. The most common diagnosis associated with NIV use was pneumonia, which was not categorized as an SEC (Table 1).
Table 1.
Etiology of respiratory failure treated with noninvasive ventilation
| Condition | Patients Receiving NIV (n = 22,706) % |
|---|---|
| Pneumonia | 26.1 |
| COPD | 15.0 |
| HF | 15.0 |
| Nonpneumonia sepsis | 4.6 |
| Asthma | 3.6 |
| Other* | 35.6 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; HF = heart failure; NIV = noninvasive ventilation.
Most common diagnoses that constitute “other” include acute or chronic respiratory failure not otherwise specified, acute or chronic myocardial ischemia, cardiac arrhythmia, acute kidney injury, and opioid overdose.
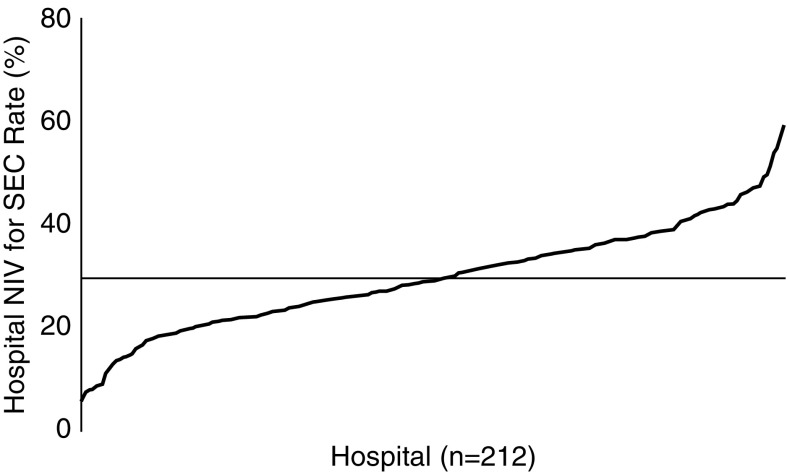
At the hospital level, the average hospital NIV-SEC rate was 29.6% (SD = 10.1), with wide institutional variation in the proportion of patients receiving NIV who had SECs (median OR = 1.43; 95% CI = 1.37–1.52; Figure 1). Patients undergoing NIV admitted to hospitals that used NIV more often for SECs were less likely to be white and more likely to be black/Hispanic, more likely to have Medicaid as a primary payer, and more likely to live in lower-income neighborhoods compared with patients admitted to hospitals that were less likely to use NIV for SECs (Table 2).
Figure 1.
Hospital variation in indications for noninvasive ventilation (NIV). The average hospital NIV–strong evidence condition (SEC) rate was 29.6% (x-axis; SD = 10.1), but ranged from 6.1 to 58.9%.
Table 2.
Characteristics of patients receiving noninvasive ventilation by strong evidence condition hospital quartile
| Hospital Quartile 1 (n = 4,553) | Hospital Quartile 2 (n = 7,144) | Hospital Quartile 3 (n = 6,626) | Hospital Quartile 4 (n = 4,383) | P Value* | |
|---|---|---|---|---|---|
| No. of Hospitals |
53 |
54 |
52 |
53 |
|
| NIV-SEC rate, % |
18.6 |
26.8 |
33.5 |
42.0 |
|
| Age, mean (SD), yr | 68.8 (15.4) | 69.1 (15.4) | 69.5 (14.7) | 68.0 (14.9) | 0.07 |
| Female, % | 51.1 | 53.2 | 53.5 | 53.4 | 0.06 |
| Race/ethnicity, % | <0.0001 | ||||
| White | 62.7 | 58.3 | 55.7 | 52.2 | |
| Black | 8.2 | 9.4 | 11.3 | 21.0 | |
| Hispanic | 13.3 | 17.2 | 18.3 | 15.9 | |
| Other† | 15.8 | 15.0 | 14.7 | 10.9 | |
| Primary payer, % | <0.0001 | ||||
| Medicare | 66.3 | 65.0 | 69.0 | 66.8 | |
| Medicaid | 11.3 | 15.5 | 15.9 | 19.3 | |
| Private Insurance | 17.2 | 14.1 | 9.4 | 8.9 | |
| Other† | 5.3 | 5.4 | 5.7 | 5.0 | |
| Median income of patient ZIP code, % | <0.0001 | ||||
| Low | 18.1 | 22.9 | 34.2 | 47.2 | |
| Low middle | 22.4 | 26.8 | 30.3 | 17.9 | |
| Upper middle | 25.3 | 24.1 | 21.5 | 22.1 | |
| High | 31.5 | 24.7 | 12.1 | 11.0 | |
| Other† | 2.8 | 1.5 | 1.9 | 1.8 | |
| Elixhauser comorbidity score, mean (SD)‡ | 9.3 (8.2) | 9.2 (8.1) | 9.2 (8.1) | 8.6 (8.1) | <0.0001 |
| Shock POA, % | 11.2 | 12.7 | 11.3 | 9.5 | 0.0014 |
| Acute respiratory failure POA, % | 29.3 | 37.0 | 38.5 | 41.2 | <0.0001 |
| Acute renal failure POA, % | 19.9 | 21.3 | 20.2 | 20.3 | 0.87 |
| Acute neurologic failure POA, % | 8.0 | 8.0 | 7.6 | 6.6 | 0.01 |
| Acute hematologic failure POA, % | 8.4 | 8.3 | 7.8 | 6.7 | 0.0019 |
| Acute hepatic failure POA, % | 2.0 | 1.8 | 1.7 | 1.7 | 0.23 |
| Acute metabolic failure POA, % | 12.0 | 11.5 | 12.4 | 11.6 | 0.97 |
Definition of abbreviations: NIV = noninvasive ventilation; POA = present on admission; SEC = strong evidence condition.
Mantel-Haenszel chi-square and Cochrane-Armitage tests for trends were used for categorical variables, and linear regression to test for trends across quartiles was used for continuous variables.
Includes patients with missing data.
Calculated without cardiac arrhythmia comorbidity per HCUP (Healthcare Cost and Utilization Project) software (33).
Patient Outcomes
Overall, 15.2% of patients failed initial treatment with NIV and subsequently required IMV. Patients with SECs had significantly lower risks for NIV failure (8.1 vs. 18.2%; adjusted OR [aOR] = 0.21; 95% CI = 0.14–0.32). A patient’s risk of NIV failure was lower when admitted to hospitals with higher NIV-SEC rates (Quartile 4 vs. Quartile 1 aOR = 0.65; 95% CI = 0.50–0.83). In a subgroup analysis, patients without an SEC also had lower risk of NIV failure when admitted to hospitals with higher NIV-SEC rates (Quartile 4 vs. Quartile 1 aOR = 0.68; 95% CI = 0.52–0.88).
Mortality among patients who suffered NIV failure was higher than those treated initially with IMV (39.4 vs. 31.0%; aOR = 1.49; 95% CI = 1.38–1.62). Among patients who failed initial NIV treatment, those with SECs had lower hospital mortality compared with patients with conditions with weak evidence for NIV use (11.1 vs. 28.7%; aOR = 0.44; 95% CI = 0.29–0.65). Patients who received NIV at hospitals with higher NIV-SEC rates tended to have lower hospital mortality rates than patients receiving NIV at low NIV-SEC rate hospitals (Quartile 4 vs. Quartile 1 aOR = 0.83; 95% CI = 0.68–1.00).
Hospital Outcomes
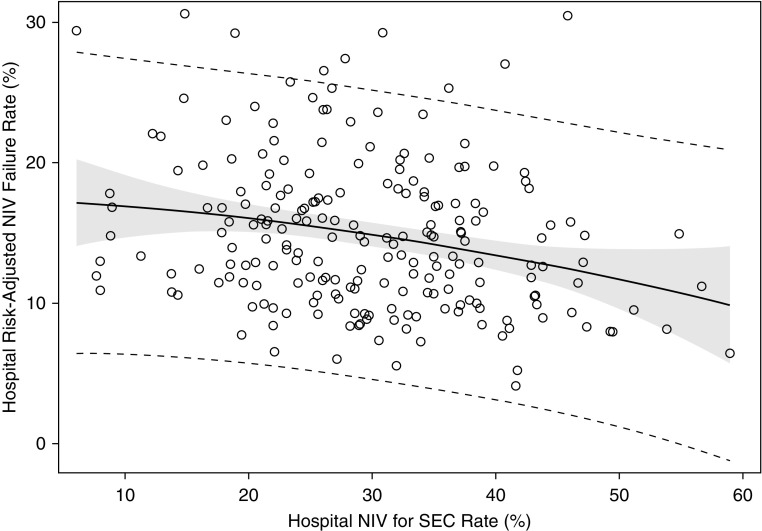
Hospitals with higher rates of NIV-SEC had lower risk-adjusted NIV failure rates (ρ = −0.25, P < 0.0001; Figure 2). We did not observe a significant association between hospital NIV-SEC rates and hospital risk-adjusted mortality rates for patients receiving NIV (ρ = −0.07, P = 0.32).
Figure 2.
Association of hospital noninvasive ventilation (NIV) for strong evidence condition (SEC) rate with hospital risk-adjusted NIV failure rates. Hospitals with higher NIV-SEC rates had lower risk-adjusted NIV failure rates (ρ = −0.27, P < 0.0001).
Exploratory Analysis
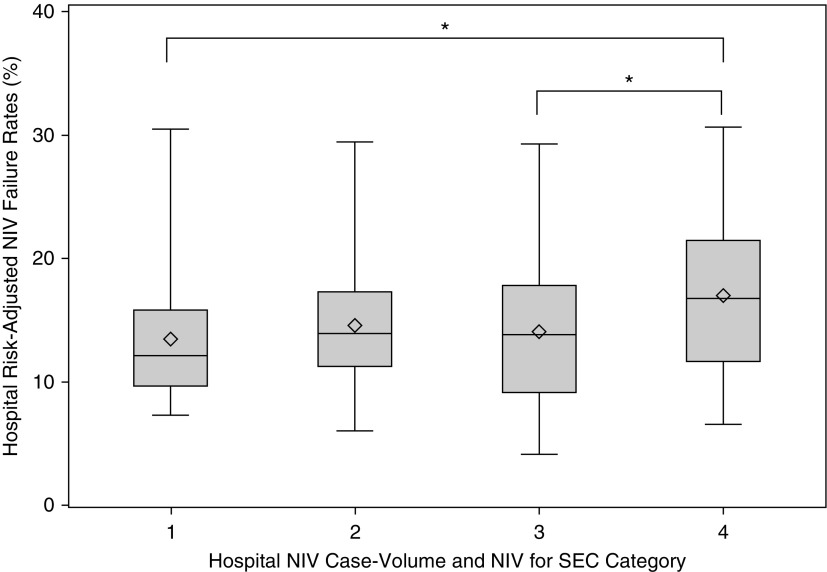
Hospital NIV-SEC rates were not significantly associated with hospital NIV case volume (ρ = −0.05; P = 0.47; Figure E2). At the patient level, patients admitted to a high vs. low NIV case-volume hospital had higher individual risk of NIV failure (Quartile 4 vs. Quartile 1 aOR = 1.34; 95% CI = 1.04–1.73), but, after adjusting for NIV-SEC rate, NIV case volume was no longer associated with the risk of NIV failure (Quartile 4 vs. Quartile 1 aOR = 1.27; 95% CI = 0.98–1.64). When hospitals were grouped by both NIV case volume and NIV-SEC rates, high case-volume and low NIV-SEC rate hospitals tended to have higher risk-adjusted NIV failure rates than other groupings (P = 0.0003 for difference in average risk-adjusted NIV failure rates; Figure 3).
Figure 3.
Association of hospital risk-adjusted noninvasive ventilation (NIV) failure rates with NIV hospital case volume and strong evidence condition (SEC) rate. The data is presented as Whisker plots with the diamonds indicating the mean NIV failure rate. Group 1: low hospital NIV case volume and high hospital NIV-SEC rate (mean NIV failure rate = 13.4% [SD = 4.8%]). Group 2: low hospital NIV case volume and low hospital NIV-SEC rate (mean NIV failure rate = 14.6% [SD = 4.6%]). Group 3: high hospital NIV case volume and high hospital NIV-SEC rate (mean NIV failure rate = 14.0% [SD = 5.4%]). Group 4: high hospital NIV case volume and low hospital NIV-SEC rate (mean NIV failure rate = 17.0% [SD = 5.7%]). P = 0.003 for difference in mean risk-adjusted NIV failure rates across all four groups. *P < 0.05 in pairwise comparison of mean hospital NIV failure rates.
Sensitivity Analysis
When immunocompromised patients were considered to have an SEC for NIV, we observed similar findings to those of the primary analysis. We observed a decreased risk of NIV failure for patients with SECs (aOR = 0.60; 95% CI = 0.45–0.80). Patients admitted to hospitals with higher NIV-SEC rates were at lower risk of NIV failure (Quartile 4 vs. Quartile 1 aOR = 0.64; 95% CI = 0.50–0.82). We also observed that hospitals with higher NIV-SEC rates had lower risk-adjusted NIV failure rates (ρ = −0.25, P = 0.0002).
Discussion
We investigated the association of evidence-based use of NIV with patient and hospital outcomes. In contrast to prior research, we observed that the majority of NIV use in this large and diverse population was for patients without strong evidence, with wide institutional variation in patient selection for NIV. In further contrast to our initial hypotheses, we observed that all patients, regardless of underlying cause of respiratory failure, benefitted from admission to hospitals with greater evidence-based utilization of NIV, suggesting that hospital factors may significantly influence outcomes in addition to underlying patient factors, which has not previously been described to our knowledge. Our findings were robust to sensitivity analysis.
Our study identifies several novel findings not previously described to our knowledge with regard to NIV use and outcomes. COPD was previously identified as the most common condition for which NIV is used in the United States, but with a significant trend toward other diagnoses becoming more common (20). We actually found that COPD is no longer the most common indication for NIV with pneumonia, now accounting for more nearly one-third of all cases of NIV. This shift in conditions treated with NIV is also associated with our observation of double the NIV failure rate among patients with weaker evidence conditions compared with those with COPD or HF. Despite evidence suggesting a lack of survival benefit for patients without SECs, large variation and high rates of NIV use for conditions with high risk of NIV failure suggest a need for further research focused on improving patient selection and minimizing harms associated with overuse of NIV (8–17). In addition, future investigations focused on the biases and decisional heuristics informing clinical decision-making for patients with acute respiratory failure may provide important insights regarding care variations between institutions.
Consistent with previous work, we observed a significantly higher risk of NIV failure associated with weaker evidence conditions as well as higher mortality with NIV failure compared with initial treatment with IMV (19–21). Although the etiology of increased mortality associated with NIV failure has not been fully elucidated, we speculate that patients who fail NIV may experience subtle, but potentially rapid, worsening (e.g., from aspiration or pooling of secretions) that may go unrecognized for a period of time. In addition, recent work suggests that even with NIV, patients with significant respiratory distress still have significantly increased work of breathing, as well as large swings in transpulmonary pressure, that can contribute to worsening lung injury (41). The higher mortality associated with NIV failure for all patients indicates that ongoing studies investigating both patient and hospital factors associated with NIV failure are necessary.
Previous studies investigating NIV failure have mostly focused on patient-level factors, such as hemodynamic instability, metabolic acidosis, impaired mental status, and elevated APACHE II (Acute Physiology and Chronic Health Evaluation II) scores, but few studies have explored hospital-level factors associated with NIV outcomes (21, 26, 40, 42–47). Based on these studies and those suggesting that COPD and HF had the strongest benefits from NIV, we had hypothesized that a patient’s underlying clinical condition and cause of respiratory failure would fully account for NIV-related outcomes. However, contrary to our hypothesis, we found that admission to hospitals with greater evidence-based utilization of NIV was associated with a lower risk of NIV failure (with a trend toward lower mortality). The beneficial effects of being admitted to a hospital with higher use of NIV for patients with SECs persisted even in our subgroup analysis of patients without SECs. For example, our observation would suggest that a patient with pneumonia who receives NIV would have improved outcomes at a hospital that tends to use NIV preferentially for patients with SECs compared with hospitals with liberal use of NIV. This finding suggests a beneficial “hospital effect” that has not previously been described.
We speculate that higher NIV use for SECs may be a marker of a hospital’s practices for selection and/or monitoring of patients for NIV or use of other evidence-based practices that may improve the outcomes for patients with weaker evidence conditions for NIV. Hospitals that are more selective in use of NIV may be more likely to opt for initial treatment with IMV for patients deemed to be at high risk of NIV failure. Such hospitals would have lower rates of NIV failure for patients without SECs given more conservative patient selection practices. Alternatively, higher NIV-SEC rates may correlate with different strategies for monitoring patients receiving NIV (e.g., mandatory admission to an intermediate care unit or intensive care unit), greater respiratory therapist availability, lower nurse-to-patient ratios, et cetera. Hospitals that employ more evidence-based patient selection practices for NIV may also employ other evidence-based practices, such as more rapid administration of antibiotics in pneumonia, rapid fluid resuscitation of patients with sepsis, et cetera. that could contribute to improved NIV outcomes even among patients with conditions with weaker evidence supporting NIV use. Further studies should seek to identify the hospital practice patterns that improve NIV-related outcomes at hospitals that use NIV more often for patients with SECs.
Previous studies have suggested an association between hospital NIV case volume and NIV outcomes (21, 48). We observed that patients admitted to higher NIV case-volume hospitals had an increased risk of NIV failure. However, accounting for rates of NIV for SECs attenuated the association between NIV case volume and outcomes. In fact, we observed a significantly lower risk of NIV failure at high case-volume hospitals that employed greater evidenced-based NIV use compared with high-volume hospitals that had lower evidenced-based NIV utilization. Evaluating the association of NIV case volume alone raises the potential concern that high-volume hospitals may be using NIV on less sick patients and, thus, have better outcomes. Our findings suggest that patient selection practices may be more important than hospital case-volume practices in improving NIV-related outcomes.
In addition, we observed higher rates of racial/ethnic minorities, higher rates of Medicaid as the primary payer, and higher rates of patients living in low-income neighborhoods at hospitals with higher NIV for SEC utilization rates. It is unclear what significance these demographic differences have for NIV-related outcomes. It is possible that higher NIV-SEC-rate hospitals were more likely to be academic medical centers, which often have higher rates of low-income patients with Medicaid as their primary insurance. Academic medical centers may be more likely to employ evidenced-based patient selection practices for NIV. Future studies evaluating differences in hospital characteristics based on evidenced-based utilization patterns are needed.
In addition to those limitations common to retrospective analyses of large datasets, our study has several unique limitations. The CA SID includes a large, diverse population with the unique ability to exclude patients with DNR orders who would not be eligible for NIV failure as they would not want IMV, but may be limited in the generalizability to institutions outside of California. We used ICD9-CM billing code–based algorithms that have previously been validated to identify COPD and HF, but the use of billing codes may increase the possibility of misclassification. The lack of granular patient-level information, such as laboratory values and vital signs, may have introduced unmeasured confounding in our evaluations. Although the CA SID allowed us to exclude patients with an early DNR order (within 24 h of admission) who may have received NIV, but not been eligible for IMV, it does not contain information about later DNR orders. As such, it is possible that some of the patients in our cohort may have had a later DNR order and not been eligible for NIV failure (i.e., progression to IMV). In addition, similar to previous studies, we treated patients who had the same hospital day recorded for both NIV and IMV as having NIV preceding IMV (19, 20, 46). It is possible that a small number of these patients may have first received IMV and been quickly extubated to NIV, as we could not determine the exact time that each form of ventilation was initiated. Finally, we speculate about the association of hospital factors with NIV outcomes, but were unable to evaluate health service delivery components, such as nurse and respiratory therapy staffing, monitoring capabilities, et cetera, that are likely to influence NIV outcomes.
In a population-based study of patients hospitalized in California, we identified that most NIV episodes were initiated for conditions without strong supporting evidence. Patients admitted to hospitals that tended to use NIV for patients with a condition supported by a strong evidence base for improved outcomes with NIV (e.g., COPD and HF exacerbations) had a lower risk of NIV failure. These findings suggest a hospital effect for NIV outcomes, demonstrating that institutional variation in NIV outcomes may partially be explained by institutional practice patterns regarding evidence-based patient selection and clinician decision-making during the initiation of ventilatory support. Future studies will be required to better understand how hospitals and physicians select patients to receive NIV and to identify specific practices that facilitate evidence-based use of NIV and improve NIV outcomes.
Supplementary Material
Footnotes
Supported in part by National Institutes of Health National Heart, Lung, and Blood Institute grant K01HL116768 (A.J.W.) and resources from a Boston University School of Medicine, Department of Medicine Career Investment Award (A.J.W.).
Author Contributions: A.B.M.—study concept, study design, data analysis, data interpretation, manuscript preparation (A.B.M. is also the guarantor of the article and takes responsibility for the integrity of the work from inception to publication); I.S.D.—data interpretation, final manuscript review; A.J.W.—study concept, study oversight, study design, data interpretation, and manuscript preparation.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 2.Plant PK, Owen JL, Elliott MW. Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355:1931–1935. doi: 10.1016/s0140-6736(00)02323-0. [DOI] [PubMed] [Google Scholar]
- 3.Conti G, Antonelli M, Navalesi P, Rocco M, Bufi M, Spadetta G, Meduri GU. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28:1701–1707. doi: 10.1007/s00134-002-1478-0. [DOI] [PubMed] [Google Scholar]
- 4.Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(3):CD004104. doi: 10.1002/14651858.CD004104.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Peter JV, Moran JL, Phillips-Hughes J, Graham P, Bersten AD. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367:1155–1163. doi: 10.1016/S0140-6736(06)68506-1. [DOI] [PubMed] [Google Scholar]
- 6.Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:142–151. doi: 10.1056/NEJMoa0707992. [DOI] [PubMed] [Google Scholar]
- 7.Weng CL, Zhao YT, Liu QH, Fu CJ, Sun F, Ma YL, Chen YW, He QY. Meta-analysis: noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152:590–600. doi: 10.7326/0003-4819-152-9-201005040-00009. [DOI] [PubMed] [Google Scholar]
- 8.Wysocki M, Tric L, Wolff MA, Millet H, Herman B. Noninvasive pressure support ventilation in patients with acute respiratory failure: a randomized comparison with conventional therapy. Chest. 1995;107:761–768. doi: 10.1378/chest.107.3.761. [DOI] [PubMed] [Google Scholar]
- 9.Confalonieri M, Potena A, Carbone G, Porta RD, Tolley EA, Umberto Meduri G. Acute respiratory failure in patients with severe community-acquired pneumonia: a prospective randomized evaluation of noninvasive ventilation. Am J Respir Crit Care Med. 1999;160:1585–1591. doi: 10.1164/ajrccm.160.5.9903015. [DOI] [PubMed] [Google Scholar]
- 10.Martin TJ, Hovis JD, Costantino JP, Bierman MI, Donahoe MP, Rogers RM, Kreit JW, Sciurba FC, Stiller RA, Sanders MH. A randomized, prospective evaluation of noninvasive ventilation for acute respiratory failure. Am J Respir Crit Care Med. 2000;161:807–813. doi: 10.1164/ajrccm.161.3.9808143. [DOI] [PubMed] [Google Scholar]
- 11.Keenan SP, Sinuff T, Cook DJ, Hill NS. Does noninvasive positive pressure ventilation improve outcome in acute hypoxemic respiratory failure? A systematic review. Crit Care Med. 2004;32:2516–2523. doi: 10.1097/01.ccm.0000148011.51681.e2. [DOI] [PubMed] [Google Scholar]
- 12.Keenan SP, Powers C, McCormack DG, Block G. Noninvasive positive-pressure ventilation for postextubation respiratory distress: a randomized controlled trial. JAMA. 2002;287:3238–3244. doi: 10.1001/jama.287.24.3238. [DOI] [PubMed] [Google Scholar]
- 13.Keenan SP, Mehta S. Noninvasive ventilation for patients presenting with acute respiratory failure: the randomized controlled trials. Respir Care. 2009;54:116–126. [PubMed] [Google Scholar]
- 14.Antonelli M, Conti G, Bufi M, Costa MG, Lappa A, Rocco M, Gasparetto A, Meduri GU. Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA. 2000;283:235–241. doi: 10.1001/jama.283.2.235. [DOI] [PubMed] [Google Scholar]
- 15.Lim WJ, Mohammed Akram R, Carson KV, Mysore S, Labiszewski NA, Wedzicha JA, Rowe BH, Smith BJ. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2012;12:CD004360. doi: 10.1002/14651858.CD004360.pub4. [DOI] [PubMed] [Google Scholar]
- 16.Lemiale V, Mokart D, Resche-Rigon M, Pène F, Mayaux J, Faucher E, Nyunga M, Girault C, Perez P, Guitton C, et al. Groupe de Recherche en Réanimation Respiratoire du patient d’Onco-Hématologie (GRRR-OH) Effect of noninvasive ventilation vs oxygen therapy on mortality among immunocompromised patients with acute respiratory failure: a randomized clinical trial. JAMA. 2015;314:1711–1719. doi: 10.1001/jama.2015.12402. [DOI] [PubMed] [Google Scholar]
- 17.Delclaux C, L’Her E, Alberti C, Mancebo J, Abroug F, Conti G, Guérin C, Schortgen F, Lefort Y, Antonelli M, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA. 2000;284:2352–2360. doi: 10.1001/jama.284.18.2352. [DOI] [PubMed] [Google Scholar]
- 18.Bellani G, Laffey JG, Pham T, Madotto F, Fan E, Brochard L, Esteban A, Gattinoni L, Bumbasirevic V, Piquilloud L, et al. LUNG SAFE Investigators and the ESICM Trials Group. Non-invasive ventilation of patients with acute respiratory distress syndrome: insights from the LUNG SAFE Study. Am J Respir Crit Care Med. 2017;195:67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 19.Chandra D, Stamm JA, Taylor B, Ramos RM, Satterwhite L, Krishnan JA, Mannino D, Sciurba FC, Holguín F. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2012;185:152–159. doi: 10.1164/rccm.201106-1094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walkey AJ, Wiener RS. Use of noninvasive ventilation in patients with acute respiratory failure, 2000–2009: a population-based study. Ann Am Thorac Soc. 2013;10:10–17. doi: 10.1513/AnnalsATS.201206-034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta AB, Douglas IS, Walkey AJ. Hospital noninvasive ventilation case volume and outcomes of acute exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13:1752–1759. doi: 10.1513/AnnalsATS.201603-209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agency for Healthcare Research and Quality. doi: 10.1080/15360280802537332. Overview of the state inpatient databases (SID). [Updated 2016 Jan 20; accessed 2016 Feb 2]. Available from: http://www.hcup-us.ahrq.gov/sidoverview.jsp. [DOI] [PubMed]
- 23.Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project. Introduction to the HCUP state inpatient databases. [Updated 2016 Feb; accessed 2016 Jul 16]. Available from: https://www.hcup-us.ahrq.gov/db/state/siddist/Introduction_to_SID.pdf.
- 24.Goldman LE, Chu PW, Prothro C, Osmand D. Office of Statewide Health Planning and Development. Accuracy of condition present on admission, do not resuscitate, and e-codes in California patient discharge data: prepared for the Office of Statewide Health Planning and Development, Healthcare Outcomes Center. Spring 2011 [accessed 2016 Jan 19]. Available from: http://www.oshpd.ca.gov/hid/products/patdischargedata/researchreports/pddvalidation/pdd_validation_study.pdf.
- 25.Goldman LE, Chu PW, Osmond D, Bindman A. Accuracy of do not resuscitate (DNR) in administrative data. Med Care Res Rev. 2013;70:98–112. doi: 10.1177/1077558712458455. [DOI] [PubMed] [Google Scholar]
- 26.Lindenauer PK, Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Hill NS. Outcomes associated with invasive and noninvasive ventilation among patients hospitalized with exacerbations of chronic obstructive pulmonary disease. JAMA Intern Med. 2014;174:1982–1993. doi: 10.1001/jamainternmed.2014.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein BD, Bautista A, Schumock GT, Lee TA, Charbeneau JT, Lauderdale DS, Naureckas ET, Meltzer DO, Krishnan JA. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141:87–93. doi: 10.1378/chest.11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta AB, Syeda SN, Wiener RS, Walkey AJ. Epidemiological trends in invasive mechanical ventilation in the United States: a population-based study. J Crit Care. 2015;30:1217–1221. doi: 10.1016/j.jcrc.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 30.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, Råstam L, Larsen K. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houchens R, Chu B, Steiner C. Hierarchical modeling using HCUP data. HCUP methods series report # 2007-01 online. Online: U.S. Agency for Healthcare Research and Quality; 2007.
- 32.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 34.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 35.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 36.Courtright KR, Raneses E, Harhay MO, Kipnis P, Escobar GJ, Halpern SD, Kerlin MP. External validation of a claims-based ICU risk-adjustment methodology. New York: American Thoracic Society; 2016. p. A3620. [Google Scholar]
- 37.SAS Institute, Inc. SAS Proc Transreg: penalized B-spline. [Updated 2016 Nov 2; accessed 2016 Dec 18]. Available from: http://support.sas.com/documentation/cdl/en/statug/68162/html/default/viewer.htm#statug_transreg_details07.htm.
- 38.Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, Reiffers J, Cardinaud JP. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481–487. doi: 10.1056/NEJM200102153440703. [DOI] [PubMed] [Google Scholar]
- 39.Schnell D, Timsit JF, Darmon M, Vesin A, Goldgran-Toledano D, Dumenil AS, Garrouste-Orgeas M, Adrie C, Bouadma L, Planquette B, et al. Noninvasive mechanical ventilation in acute respiratory failure: trends in use and outcomes. Intensive Care Med. 2014;40:582–591. doi: 10.1007/s00134-014-3222-y. [DOI] [PubMed] [Google Scholar]
- 40.Adda M, Coquet I, Darmon M, Thiery G, Schlemmer B, Azoulay E. Predictors of noninvasive ventilation failure in patients with hematologic malignancy and acute respiratory failure. Crit Care Med. 2008;36:2766–2772. doi: 10.1097/CCM.0b013e31818699f6. [DOI] [PubMed] [Google Scholar]
- 41.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 42.Ko BS, Ahn S, Lim KS, Kim WY, Lee YS, Lee JH. Early failure of noninvasive ventilation in chronic obstructive pulmonary disease with acute hypercapnic respiratory failure. Intern Emerg Med. 2015;10:855–860. doi: 10.1007/s11739-015-1293-6. [DOI] [PubMed] [Google Scholar]
- 43.Confalonieri M, Garuti G, Cattaruzza MS, Osborn JF, Antonelli M, Conti G, Kodric M, Resta O, Marchese S, Gregoretti C, et al. Italian noninvasive positive pressure ventilation (NPPV) study group. A chart of failure risk for noninvasive ventilation in patients with COPD exacerbation. Eur Respir J. 2005;25:348–355. doi: 10.1183/09031936.05.00085304. [DOI] [PubMed] [Google Scholar]
- 44.Corrêa TD, Sanches PR, de Morais LC, Scarin FC, Silva E, Barbas CS. Performance of noninvasive ventilation in acute respiratory failure in critically ill patients: a prospective, observational, cohort study. BMC Pulm Med. 2015;15:144. doi: 10.1186/s12890-015-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stefan MS, Nathanson BH, Higgins TL, Steingrub JS, Lagu T, Rothberg MB, Lindenauer PK. Comparative effectiveness of noninvasive and invasive ventilation in critically ill patients with acute exacerbation of chronic obstructive pulmonary disease. Crit Care Med. 2015;43:1386–1394. doi: 10.1097/CCM.0000000000000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulkarni VT, Kim N, Dai Y, Dharmarajan K, Safavi KC, Bikdeli B, Lindenauer PK, Testani J, Dries DL, Krumholz HM. Hospital variation in noninvasive positive pressure ventilation for acute decompensated heart failure. Circ Heart Fail. 2014;7:427–433. doi: 10.1161/CIRCHEARTFAILURE.113.000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stefan MS, Nathanson BH, Priya A, Pekow PS, Lagu T, Steingrub JS, Hill NS, Goldberg RJ, Kent DM, Lindenauer PK. Hospitals’ patterns of use of noninvasive ventilation in patients with asthma exacerbation. Chest. 2016;149:729–736. doi: 10.1016/j.chest.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dres M, Tran TC, Aegerter P, Rabbat A, Guidet B, Huchon G, Roche N CUB-REA Group. Influence of ICU case-volume on the management and hospital outcomes of acute exacerbations of chronic obstructive pulmonary disease. Crit Care Med. 2013;41:1884–1892. doi: 10.1097/CCM.0b013e31828a2bd8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.