Abstract
Glaucoma after chemical burns represents a posttraumatic glaucoma, usually open-angle glaucoma.
It is a frequent complication of chemical burns, especially with alkali and it can appear in the acute stage or as a late complication.
Because of the complications and scars, the treatment is very difficult. Topical treatment is based on AC inhibitors, β-blockers, α2-agonists. Trabeculectomy, shunts, cyclophotocoagulation, and cryotherapy are the solutions in the late stages.
Glaucoma after irradiation is a closing-angle secondary glaucoma.
The risk factors such as the radiation dose and the volume of the radiated structure are important in the appearance and evolution of this type of glaucoma.
Topical treatment is usually ineffective, the preferable options being laser and surgical treatments.
Although it is not a frequently seen pathology, it is important to know how to diagnose and treat this type of glaucoma. There are various options available for treatment, but choosing one is difficult because of the possible complications.
Keywords: chemical burn, radiation, secondary glaucoma, alkali, IOP
Introduction
Glaucoma after chemical burns and radiation represents secondary, posttraumatic, open angle glaucoma. There are cases in which the mechanism that determines glaucoma is due to an angle closure or a completely closed angle (acute glaucoma) [1].
Glaucoma is a frequently seen complication of chemical burns and it can occur in the acute stage or as a late complication.
Chemical burns
Chemical burns are responsible for 18% of the ocular trauma, approximately 80% of these being due to chemical substances and representing major emergencies. They are the result of work related accidents (60%), domestic accidents, or aggression [2].
Chemical injury ranges in severity from trivial to potentially blinding. The degree of severity depends on the following:
- agent
- type of agent
- quantity
- pH
- duration
- depth of action
- ocular structures affected
- related effects.
Agents that determine chemical burns
These agents can be alkali (alkaline substances), acids, solvents, detergents, irritants (e.g. mace). The burn is most frequently due to alkali [4].
The most frequently alkali involved are the following:
- ammonia
- calcium hydroxide
- sodium hydroxide
- potassium hydroxide
- magnesium hydroxide.
Alkali have a pH between 7 and 14 and they tend to penetrate faster and more deeply than acids. They bind to the membrane of lipid cells and collagen, causing disorganization and determining cellular apoptosis and tissue necrosis. They determine the saponification of fatty acids of the cellular membrane, breaking the intercellular links and easing the depth of penetration on the ocular surface.
Of the ocular tissue, the alkali affects:
- the conjunctiva (vascular necrosis);
- the cornea (saponification of cellular wall lipids, apoptosis of epithelial cells, they penetrate deep in the stroma and destroy the keratocytes);
- the anterior chamber (inflammatory cells, hypopyon);
- the iris (inflammation);
- the ciliary body (inflammation with hyposecretion of aqueous humor or even no secretion at all);
- the lens (hazing - opaque)
- the trabecular meshwork (inflammation, scarring tissue).
The acids involved in the chemical burns are the following:
- sulfuric acid
- hydrofluoric acid: rapidly penetrates the ocular tissues, determining the most severe injuries among the acids
- acetic acid, etc.
Acids have a pH lower than 7. The burns are less severe than those determined by alkali because they coagulate surface proteins from the corneal stroma, forming a protective barrier that does not permit the acid to penetrate more deeply (coagulation necrosis) [5].
The irritants involved in the chemical burns are the following:
- mace (orto-chlorobenzalmalononitrile)
- pepper spray (highly concentrated pepper).
They have a neutral pH; the closing of the eyelids as a result of pain, reduces the quantity and duration of exposure significantly, the only effect being usually the irritation of the conjunctiva, without other injuries and without affecting the visual acuity.
Grading of severity
The acute chemical injuries are graded to plan an appropriate treatment and to establish the prognosis. Grading is performed based on the corneal clarity and the severity of limbal ischemia (Roper-Hall system):
Grade 1: clear cornea (epithelial damage only) and no limbal ischemia (excellent prognosis).
Grade 2: a hazy cornea, but visible iris detail, less than one-third of the limbus being ischemic (good prognosis).
Grade 3: total loss of the corneal epithelium, stromal haze obscuring iris detail and between 1/ 3 and 1/ 2 limbal ischemia (guarded prognosis).
Grade 4: opaque cornea and more than 50% of the limbus showing ischemia (poor prognosis) [3].
Secondary glaucoma appears in grades 3 and 4.
Stages of chemical burns
The acute stage (days 1-7)
- moderate burns: corneal and conjunctival epithelial defects, without injury of the vessels and no limbus ischemia;
- severe burns: major injuries of the epithelial tissue, with limbus ischemia (Fig. 1).
Fig. 1.
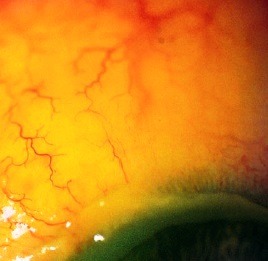
Limbus ischemia after alkali burn
The acute healing stage (1-3 weeks)
This stage involves the possibility of healing and regeneration of the affected ocular structures:
- in the first or second degree burns: corneal and conjunctival epithelium regeneration (by migration of newly formed cells from limbus stem cells), corneal clearing (caused by the corneal regenerating neovascularization and phagocytosis of the affected stromal collagen by the keratocytes); the synthesis of glycosaminoglycans and collagen begins again, with matrix regeneration;
- in the third and fourth degree burns: the regeneration of corneal epithelium progresses very slow and the regeneration of corneal neovascularization is limited; the cornea remains opaque and the corneal endothelium becomes a true retrocorneal membrane.
The late healing stage and the remaining sequelae (scars) (after more than 3 weeks):
- final regeneration and restoration of the affected ocular structures
- the collagenases and the proinflammatory markers work intensely, promoting sequelae formation:
o palpebral sequelae: fornix shortening
o conjunctival sequelae: symblepharon, ankyloblepharon, obstruction of lacrimal canaliculus (Fig. 2)
Fig. 2.

Post chemical burn symblepharon, fornix shortening
corneal sequelae: corneal neovascularization, corneal leucoma, corneal ulcer that can perforate.
Glaucoma secondary to chemical burns
One of the most important complications of ocular chemical burns is secondary glaucoma. It can occur in 25-75% of the cases, depending on the severity of the burn [6]. It can appear in the acute stage or as a late complication. It is more frequent because of chemical burns with alkali [7].
Pathogenesis
In the acute stage of the burn, the glaucoma is transitory and can appear due to the inflammation of the anterior segment (keratocyte coagulation, lysis of mucopolysaccharides), including inflammatory edema of the trabecular meshwork. The rise in the IOP has two picks: the first one due to the compressive effect of the hydrated and shortened collagen fibers, shortening the angle by modifying its architecture; the second pick is due to the defective aqueous humor flow (increase in the uveal and episcleral flow, release of chemical mediators – prostaglandins). The increase in pressure can also be determined by the reduced trabecular outflow (inflammation due to chemical burn) that cannot even face the diminished ciliary production. Between the two picks of the IOP, the pressure can be normal or we can even face hypotonia due to the affected ciliary body that stops producing humor [7].
Usually, this type of glaucoma is an open-angle one, but the inflammatory phenomena are intense (exudates in the angle, goniosynechia, inflammatory trabecular membrane) and we can face closed-angle glaucoma or even an acute glaucoma (Fig. 3)
Fig. 3.
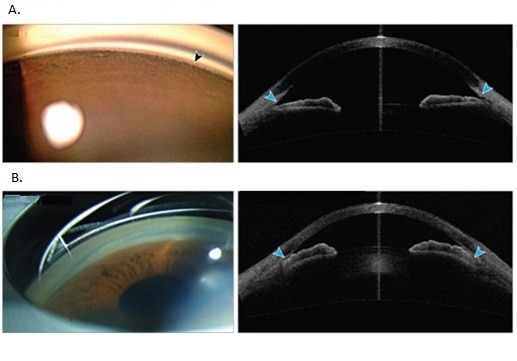
A: Open angle, B: Closed angle (gonioscopic and OCT aspect)
The reduction in the trabecular outflow and the raising IOP can be determined by a direct effect of the substance on the trabecular mesh or by blocking due to the inflammatory cells or detritus. Another mechanism of glaucoma can be the anterior peripheral synechiae that can close the angle, or pupillary block due to posterior synechiae [5,8].
As a late stage complication, glaucoma is due to the closing of the angle by anterior peripheral synechiae or by scars of the trabecular meshwork [5,7].
Diagnosis
When doctors are faced with ocular chemical burns, the IOP is not easily measured because of the affected cornea. It can be estimated digitally or by non-contact measurements.
The examination of the optic fundus, perimetry and other imagistic examination have to wait until the acute stage is resolved and the cornea clears.
Treatment
The complications and scars resulting after the chemical burn make the treatment of glaucoma extremely difficult (Fig. 4) [7].
Fig. 4.
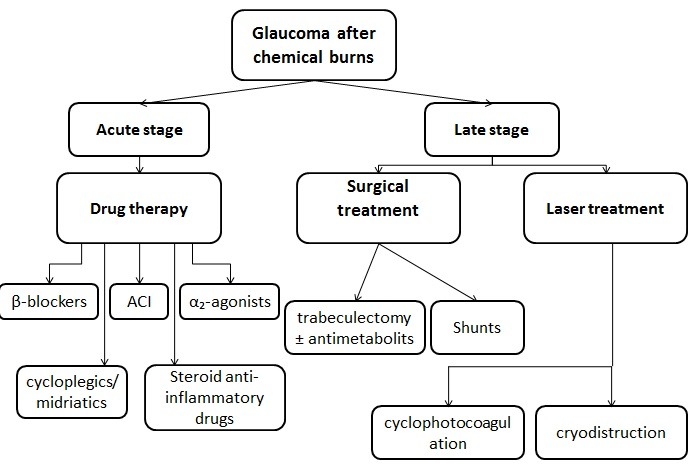
Treatment of glaucoma after chemical burns
In the acute stage, the local treatment is based on AC inhibitors, β-blockers, α2-agonists. Prostaglandin analogues are not indicated because they have a proinflammatory action. When the inflammation is still active, cycloplegics/ mydriatics and steroid anti-inflammatory drugs are useful (in some cases, the IOP rise can be caused by an excessive or insufficient anti-inflammatory treatment).
Anterior chamber paracentesis with aqueous humor aspiration can be another way to decrease the pressure, this maneuver being able to mechanically remove the chemical substance, detritus, and inflammation mediators also from the anterior chamber. As a systemic treatment, hyperosmotic agents and AC inhibitors can be useful. If there is a pupillary block, cycloplegics/ mydriatics can be used, followed by a laser iridotomy [5,8].
In the late stage, treatment is surgical – trabeculectomy (with or without antimetabolites), aqueous humor drainage devices (shunts), cyclophotocoagulation (endoscopic or transscleral) or cryodestruction [9,10,16].
Glaucoma after irradiation
The negative effects of radiation on the ocular structures can be cataracts, glaucoma, optic nerve atrophy, various degrees of radiation palpebral dermatitis.
New techniques are able to measure the exact dose of radiation required and so, the rate of side effects has lowered significantly.
Pathogenesis
Glaucoma after irradiation is a secondary angle-closing glaucoma that can be caused by various mechanisms:
- atrophy and depigmentation of ciliary processes, followed by deposits of pigment in the trabecular mesh, with the obstruction of the outflow;
- trabecular meshwork inflammation;
- damages of the scleral collagen, blocking the drainage through the episcleral veins;
- vascular changes:
o conjunctival telangiectasia;
o obstruction/ thrombosis of ciliary veins/ arteries;
o thrombosis of iris veins/ arteries;
o ischemia or thrombosis of retinal vessels, including central retinal vein obstruction, with the formation of neovessels of the iris and angle (Fig. 5,6), leading to neovascular glaucoma [11,12].
Fig. 5.
Rubeosis iridis (biomicroscopy)
Fig. 6.
Neovessels of the camerular angle (gonioscopy)
Risk factors
The ocular side effects of radiation are directly linked with the dose of radiation and the volume of the radiated structure:
- V50IC (the radiation volume larger than 50 Gray on the iris and ciliary body)
- ODI (optic disc irradiation).
As the radiation volume is increased, the neovascularization of the anterior segment also increases (a 3 times acceleration of neovascularization was seen when radiation was 80 Gy versus 60 Gy) [13].
Treatment
The local treatment (ACI, β-blockers, α2-agonists, cycloplegics, and corticoids) does not usually have the expected efficacy; still, it is important as a stepping-stone in other therapies.
Laser phototherapy is indicated if there are vascular retinal obstructions.
Cryotherapy is the solution if there is a haemophthalmus.
In some situations, posterior vitrectomy with endophotocoagulation is also useful.
Neovascular glaucoma can benefit from the anti-VEGF treatment, intravitreal or in the anterior chamber.
Trabeculectomy with or without antimetabolites is in most cases inefficient. To control IOP, shunts can be used to drain the aqueous humor.
In advanced cases, cyclophotocoagulation or cryodestruction can be options [14,15].
Fig. 7.
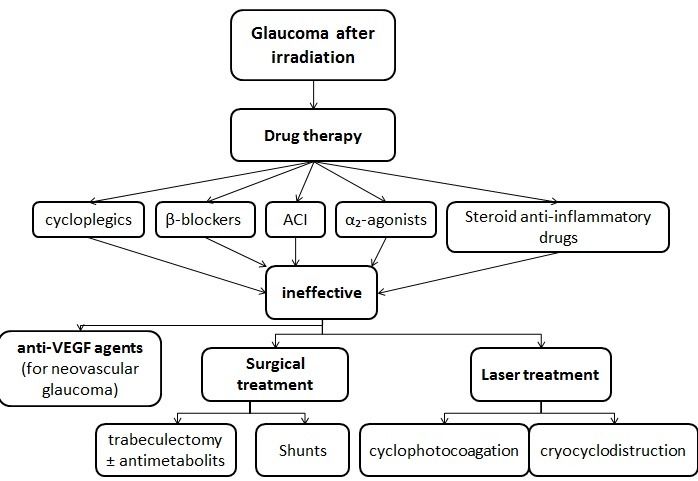
Treatment of glaucoma after irradiation
References
- 1.European Glaucoma Society . Terminology and guidelines for glaucoma. 4th edition. 2014. [DOI] [PubMed] [Google Scholar]
- 2.Kosoko A, Kosoko-Lasaki O. Chemical Ocular Burns: A Case Review. American Journal of Clinical Medicine. 2009;6(3) [Google Scholar]
- 3.Kanski J, Bowling B. Kanski’s Clinical Ophthalmology. A systemic Approach. 8th edition. Elsevier; pp. 881–885. [Google Scholar]
- 4.Gerstenblith AT, Rabinowitz MP. The Wills eye manual. Office and emergency room diagnosis and treatment of eye disease. 6th edition. Lippincott Williams & Wilkins; 2012. pp. 13–15. [Google Scholar]
- 5.Shaarawy TM, Sherwood MB, Hitchings ATRA, Crowston JG. Glaucoma. Medical Diagnosis and Therapy. 2nd edition. Elsevier Saunders; 2015. [Google Scholar]
- 6.Cade F, Grosskreutz CL, Tauber A, Dohlman CH. Glaucoma in eyes with severe chemical burn, before and after keratoprosthesis. Cornea. 2011;30(12):1322–1327. doi: 10.1097/ICO.0b013e31821eead6. [DOI] [PubMed] [Google Scholar]
- 7.Sandeep S, Meredith TA. Clinical ophthalmology. Jaypee-Highlights Medical Publishers Inc; 2010. [Google Scholar]
- 8.Netland PA. Glaucoma Medical Therapy: Principles and Management. Oxford University Press; 2008. pp. 227–228. [Google Scholar]
- 9.Kuckelkorn R, Keller GK, Redbrake C. Glaucoma after extremely severe chemical and thermal eye burns. Surgical possibilities. Der Ophthalmologe. 2008 Jan;98(12):1149–1156. doi: 10.1007/s003470170006. [DOI] [PubMed] [Google Scholar]
- 10.Lin MP, Ekşioğlu Ü, Mudumbai RC, Slabaugh MA, Che PP. Glaucoma in Patients With Ocular Chemical Burns. American Journal of Ophthalmology. 2012 Sep;154(3):481–485. doi: 10.1016/j.ajo.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 11.Tsai JH, Derby E, Holland EJ, Khatana AK. Incidence and prevalence of glaucoma in severe ocular surface disease. Cornea. 2009;25(5):530–532. doi: 10.1097/01.ico.0000220776.93852.d9. [DOI] [PubMed] [Google Scholar]
- 12.Hirasawa N, Tsuji H, Ishikawa H, Koyama-Ito H, Kamada T, Mizoe J-E, Ito Y, Naganawa S, Ohnishi Y, Tsujii H. Risk factors for neovascular glaucoma after carbon ion radiotherapy of choroidal melanoma using dose–volume histogram analysis. 2007 Feb 1;67(2):538–543. doi: 10.1016/j.ijrobp.2006.08.080. [DOI] [PubMed] [Google Scholar]
- 13.Ah Ram C. Radiation-Induced Neovascular Glaucoma: Dose and Volume Issues. Korean J Ophthalmol. 2010 Dec;24(6):384–385. doi: 10.3341/kjo.2010.24.6.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riordan-Eva P, Whitcher JP. Vaughn & Asbury’s General Ophthalmology. 17th edition. 2007. chapter 11. [Google Scholar]
- 15.Yandoff M, Duker J. Ophthalmology. 4th edition. Saunders Elsevier; 2014. pp. 1084–1089. [Google Scholar]
- 16.Fish R, Davidson RS. Management of ocular thermal and chemical injuries, including amniotic membrane therapy. Current Opinion in Ophthalmology. 2010 Jul;21(4):317–321. doi: 10.1097/ICU.0b013e32833a8da2. [DOI] [PubMed] [Google Scholar]