Abstract
It is known that elevated intraocular pressure (IOP) is the primary risk factor for glaucoma. Recently, more and more evidences have shown that the vascular deficit also plays an important role in the pathogenesis and progressions of glaucomatous optic neuropathy. This issue is backed up by glaucomatous optic neuropathy (GON) cases drug compensated in which the progression of the disease in one or both eyes is ascertained despite a normal and relatively constant IOP.
The present study evaluated the hemodynamic parameters in the retrobulbar circulation in patients with progressive glaucomatous optic neuropathy in one eye, who received compensated medication. The hemodynamic parameters (PSV, EDV, IR) were measured by using color Doppler ultrasound and progression was evaluated by a repeated automated perimetry. The obtained values were statistically analyzed and compared with those obtained for the stable eye.
Keywords: retrobulbar blood flow, color Doppler echography, progressive glaucomatous optic neuropathy, stable glaucomatous optic neuropathy
Introduction
It is known that elevated intraocular pressure (IOP) is the primary risk factor for glaucoma [1,2]. Recently, more and more evidences have shown that the vascular deficit also plays an important role in the pathogenesis and progressions of glaucomatous optic neuropathy (GON) [3-5].
The Thessaloniki Eye Study conducted on a large sample of patients without clinically glaucoma has demonstrated the association between low pulse pressure and the loss of neuro-retinal ring.
The “Barbados Eye Study”, which was mentioned in the European Glaucoma Society guide, 4th edition (2014), confirmed that the low ocular perfusion pressure increases the risk of developing GON.
Morgan et al. recently described the loss of the spontaneous venous pulse at the level of the optical nerve head as a risk factor for glaucoma. The World Glaucoma Society Congress also underlined the role of vascular factors in the pathogenesis of glaucoma in the 2009 WGA.
Several large population-based studies emphasized that although IOP reduction may be beneficial, it may not be sufficient to prevent glaucoma progression [6]:
1. The Ocular Hypertension Study (OHTS) [7]
2. The Early Manifest Glaucoma Trial (EMGT) [8,9]
3. The Collaborative Normal Tension Glaucoma Study (CNTGS) [10]
4. The Advanced Glaucoma Intervention Study (AGIS) [11]
5. The Collaborative Initial Glaucoma Treatment Study (CIGTS) [12]
Glaucomatous optic neuropathy is characterized by morphological changes especially of the optic nerve head (cup-to-disc ratio increased by the loss of retinal ganglion cells and their axons). According to the vascular theory, chronic ischemia contributes to the loss of axons, correlating alterations in the ocular blood flow with the structural changes in the optic nerve [5,6].
The ocular perfusion deficits of the optic nerve head, the retina, choroid, or the retrobulbar vessels are outside the normal range of autoregulation and lead the localized damage (that can be correlated with the visual field defect in glaucoma). This may be the result of systemic dysfunction (vasosclerosis, small vessel disease, vasospasms) or a local abnormality in the ocular blood supply [5].
At present, the lowering of IOP remains the only proven method to prevent the development or slow the progression of GON. Although the cause of the disease progression despite apparent adequate IOP lowering is probably multifactorial, abnormalities in ocular perfusion have become a first consideration [13].
Objectives
To evaluate the hemodynamic parameters of retrobulbar circulation by using color Doppler ultrasound in the patients with progressive GON in one eye, medications compensated, and compare them with those obtained for the stable eye.
Materials and Method
The study lot consisted of 102 patients (202 eyes) with a confirmed diagnosis of GON (normotensive and hypertensive), with specific topical antiglaucoma treatment. The mean IOP was of 15,5mmHg (10,5 - 20,5mmHg) and the mean age was of 66.41 years (sd = 9.57); women: 84 (82.4%) and man: 18 (17.6%).
Patients with progression in one eye were selected from the study group: 48 patients (96 eyes), with a mean age of 68,67 years (sd = 8,54), 37 women, 11 men. The mean IOP = 15,22 mmHg (sd = 1,67) in the progressive eyes and 15,20 mmHg (sd = 1,57) in the stable eyes. The “slope per year” mean for MD was -0,75 dB (sd = 0,37) in the progressive eyes and 0,07dB (sd = 0,55) in the stable eyes. The “slope per year” mean for PD was -2,9 dB (sd = 18) in the progressive eyes and 0,04 dB (sd = 0,35) in the stable eyes.
Selected lot:
- Normal tension glaucoma:10 patients (20,83%); 8 women, 2 men;
- Hypertension glaucoma: 38 patients (79,17%); 27 women, 9 men.
All the patients in the study group underwent color Doppler echography of retrobulbar vessels by using Acuson X300 (Siemens) device. Their systolic (PSV) and diastolic (EDV) blood velocities in the ophthalmic artery (OA), central retinal artery (CRA) and posterior ciliary arteries (PCA) were measured in both eyes by using a linear transducer (VF10-5) with a frequency of 10 MHz.
Resistivity index Pourcelot (IR):
IR = PSV-EDV/ PSV,
it was calculated automatically by the device.
PSV (cm/ s) (peak systolic velocity) = the highest speed of the blood flow during the systolic phase of the cardiac cycle.
EDV (cm/ s) (end diastolic velocity) = the low speed of the blood flow obtained at the end diastolic phase of the cardiac cycle.
IR = characterizes the peripheral vascular resistance of the blood vessels (RI reflects the resistance to blood flow distal to the site of measurement) [14]. Its values ranged from 0 to 1 (higher values indicated a distal increase of vascular resistance) [15,16].
Perimetry was performed with OPTOPOL PTS 910 by using the glaucoma program (Glaucoma Field): 0ᵒ-50ᵒ nasal, 0ᵒ-30ᵒ in rest, combined with the threshold fast strategy (Fast Threshold).
It was considered a base line, the first perimetry being performed at the diagnosis moment or at the time of study entry. The visual fields examination has been repeated, in average, at 6 months. The perimeter modifications were confirmed by repeating the examinations (in the same day, after the visual relaxation of the patient, or at few days interval).
The indices used for the quantitative analysis of the OPTOPOL PTS 910 visual field perimeter were MD (mean defect) and PD (pattern defect) (like numerical indices) and Bebie Curve = curve of cumulative defects (like graphical indices).
MD: the normal interval for PTS-910 system was between -1 and +1dB
MD = 0 = normal
MD positive ( > 0) = supernormal
MD negative ( < 0) = subnormal:
- MD = -1dB: reduction in light sensitivity in the entire VF at half of the norm of the patient’s age
- MD between -1,5 and -2,0 dB and the difference between both the MD of the patient over 1dB = suspect (level of attention)
- MD > -2,0 dB = pathological (level of alarm)
PD values:
- Close to 0 = normal
- Two or more defects at the ring of 10ᵒ = level of attention
- More than 5 defects in the ring of 10ᵒ = level alarm [17].
Following the evolution of the disease it was performed by comparing the modifications of the perimetry, for the PTS-910 system a serial analysis of the alterations of VF (“slope per year” for MD and PSD) existed [17].
The visual field defects were considered significant when:
1. A new defect occurred:
-crowd of 3 or more non peripheral points, each lower value being compared to baseline p < 0.05 in three consecutive visual fields (VF).
- crowd of 2 or more non peripheral points, each lower value being compared to baseline p < 0.02 in 3 consecutive VF.
- crowd of 1 or more peripheral or non peripheral points, each lower value being compared to baseline p < 0.01 in 3 consecutive VF.
2. The deepening of a pre-existing defect:
- crowd of 2 or more points with p < 0.05 falling to 0.02
- crowd of 1 or more points with p < 0.05 falling to 0.01
- crowd of 2 or more points with p < 0.02 falling to 0.01
3. Extension of a preexisting defect:
- crowd of 3 or more non peripheral points each with p < 0.05 in 3 consecutive VF.
- crowd of 2 or more non peripheral points each with p < 0.02 in 3 consecutive VF.
- crowd of 1 or more non peripheral points each with p < 0.01 in 3 consecutive VF [5,18].
Visual field defects in glaucoma become detectable when 40% or more of the axons of the retinal ganglion cell (RGC) are lost [19,20].
The reliable visual field was defined as having a false positive error of less than 33%, a false-negative error of less than 33% and a fixation loss of less than 20% [5,17,21].
All the patients had no other serious eye diseases (e.g., age-related macular degeneration, diabetic retinopathy, and vascular occlusive diseases).
IOP was measured by using the Goldmann applanation tonometry (the same device). In the present study, no significant differences in IOP (registered at the beginning of the study) were observed between glaucoma progression and no-progression patients.
In the cases in which increased IOP values have been found under a medication (in successive measurements) or the occurrence of changes in the VF (progression = a preexisting defect has deepened, it has increased or a new one has appeared), the glaucoma medication was changed.
Data analysis
The data was collected and tested by using SPSS software version 20. Further, the description of the data was assessed with the measures of central tendency for quantitative scales, the mean (the average), standard deviation, maximum, and minimum. After getting an indication of the typical scores in the data set, a normal distribution test was performed by using both a graphical representation and the Kolmogorov-Smirnoff test of Normality. The data sets had non-normal distributions, known in literature as non-parametric distributions.
Moreover, the analyses of the differences between the two conditions for a non-parametric distribution were conducted with the Mann-Whitney U test. The Mann-Whitney test is the non-parametric equivalent of the independent t-test. The Mann-Whitney test was selected due to its distribution-free assumption [22].
ROC curve
The ROC curve is an essential tool for diagnostic test evaluation. More exactly, the true positive rate (Sensitivity) in a ROC curve is plotted according to the false positive rate (1-Specificity) for different cut-off points of a parameter [23].
Results
The values obtained from the measurements of hemodynamic parameters by color Doppler echography and perimeter indices of those 2 groups of patients (with progression and stable) were statistically analyzed.
The values obtained were summarized in two tables:
Table 1 and 2 presented a decrease in the mean values of velocities flow and increased mean values for IR in the progressive glaucoma eyes as compared to stable glaucoma eyes.
Table 1.
Progressive glaucoma
| Ophthalmic artery | Central retinal artery | Posterior ciliary arteries | ||||||
| PSV | EVD | IR | PSV | EVD | IR | PSV | EVD | IR |
| 30.61 | 8.69 | 0.74 | 17.28 | 6.33 | 0 .70 | 18.07 | 6,01 | 0.70 |
| (sd-9.84) | (sd-2.76) | (sd-0.04) | (sd-2.81) | (sd-1.71) | (sd-0.06) | (sd-2.90) | (sd-1.34) | (sd-0.06) |
| PSV = peak systolic velocity; EVD = end diastolic velocity; IR = Resistivity index |
Table 2.
Stable glaucoma
| Ophthalmic artery | Central retinal artery | Posterior ciliary arteries | ||||||
| PSV | EVD | IR | PSV | EVD | IR | PSV | EVD | IR |
| 33,61 | 9,83 | 0.72 | 19.43 | 7.25 | 0.69 | 19.25 | 6.83 | 0.68 |
| (sd-10.04) | (sd-2.88) | (sd-0.05) | (sd-2.14) | (sd-1.61) | (sd-0,06) | (sd-2.27) | (sd-1.43) | (sd-0.06) |
| PSV = peak systolic velocity; EVD = end diastolic velocity; IR = Resistivity index |
The statistically significant differences between the eyes with progressive glaucoma and the eyes with stable glaucoma were registered for the velocities flow values of the PSV (at central retinal artery and posterior ciliary arteries level), EDV (at ophthalmic artery and central retinal artery level), and IR of ophthalmic artery as it can be seen below, in the Mann-Whitney U test.
Mann-Whitney U Test
Table 3.
Ophthalmic artery
| Test Statistics a | Test Statistics a | ||
|---|---|---|---|
| OA EDV | OA IR | ||
| Mann-Whitney U | 842.000 | Mann-Whitney U | 846.000 |
| Asymp. Sig. (2-tailed) | .033 | Asymp. Sig. (2-tailed) | .050 |
The Mann-Whitney U test revealed that there was a statistically significant difference between the eyes with progressive glaucoma and the stable glaucoma registered at the:
• end diastolic velocity (EDV) values: Mann Whitney U index = 842.000, p < 0.03
• resistivity index (RI) values: Mann-Whitney U index = 846.000, p < 0.05
Table 4.
Central retinal artery
| Test Statistics a | Test Statistics a | ||
|---|---|---|---|
| CRA PSV | CRA PSV | ||
| Mann-Whitney U | 623.000 | Mann-Whitney U | 780.000 |
| Asymp. Sig. (2-tailed) | .000 | Asymp. Sig. (2-tailed) | .006 |
The Mann-Whitney U test revealed that there was a statistically significant difference between the progressive eyes and the stable eyes registered at the:
• peak systolic velocity (PSV) values: Mann-Whitney U index = 623.000, p < 0.001
• end diastolic velocity (EDV) values: Mann-Whitney U index = 780.000, p < 0.006
Table 5.
Posterior ciliary arteries
| Test Statistics a | |
|---|---|
| CPA PSV | |
| Mann-Whitney U | 858.500 |
| Asymp. Sig. (2-tailed) | .044 |
The Mann-Whitney U test revealed that there was a statistically significant difference between the progressive eyes and the stable eyes registered only at the peak systolic velocity (PSV) values: Mann-Whitney U index = 858.000, p < 0.04.
ROC Curve
Ophthalmic artery:
Fig. 1 illustrates the ROC curve for EDV in OA. This was statistically significant (p < 0.03) and had the area under the curve of 0.62, which made it a fair but acceptable test of prediction.
Fig. 1.
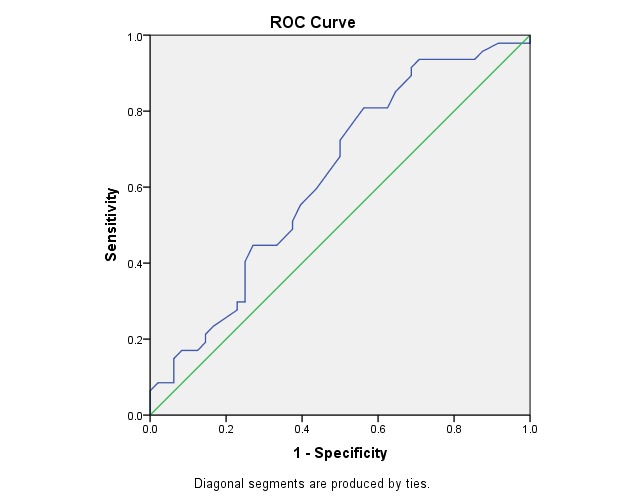
ROC Curve - Variable(s): OA EDV
Area Under the Curve
Table 6.
Test Result Variable(s): OA EDV
| Area | Asymptotic Sig. b | Asymptotic 95% Confidence Interval | |
| Lower Bound | Upper Bound | ||
| .627 | .033 | .514 | .739 |
Table 7.
The cut-off point for sensitivity and specificity at EDV of AO
| CUT OFF POINT | SENSITIVITY | SPECIFICITY |
| > 7.75 | 80% | 44% |
The power to identify the predictive value of the progressive eye by using EDV of OA reached 80% sensitivity at 44% specificity (cut-off point > 7,75).
Fig. 2 illustrates the ROC curve for RI in OA. This was statistically significant (p < 0.05) and had the area under the curve of 0.61, which made it a fair but acceptable test of prediction.
Fig. 2.
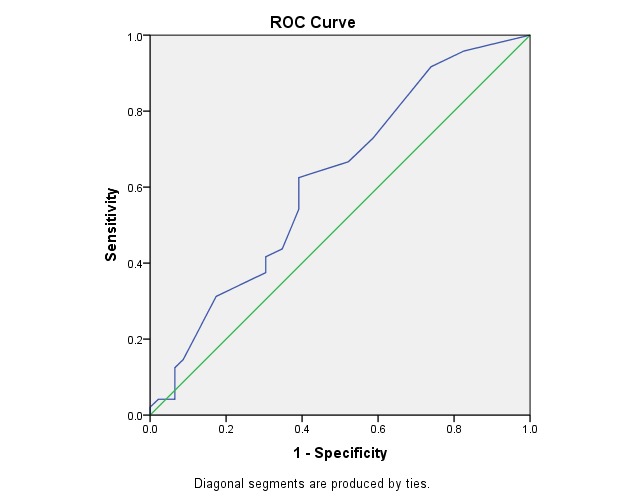
ROC Curve - Variable(s): AO IR
Area Under the Curve
Table 8.
Test Result Variable(s): AO IR
| Area | Asymptotic Sig. b | Asymptotic 95% Confidence Interval | |
| Lower Bound | Upper Bound | ||
| .617 | .051 | .503 | .731 |
Table 9.
The cut-off point for sensitivity and specificity at RI of AO
| CUT OFF POINT | SENSITIVITY | SPECIFICITY |
| > 0.69 | 72% | 42% |
The power to identify the predictive value of the progressive eye by using RI of OA reached 72% sensitivity at 42% specificity (cut-off point > 0,69).
Central retinal artery:
Fig. 3 illustrates the ROC curve for PSV in CRA. This was statistically significant (p < 0.001) and had the area under the curve of 0.73, which made it a good test of prediction.
Fig. 3.
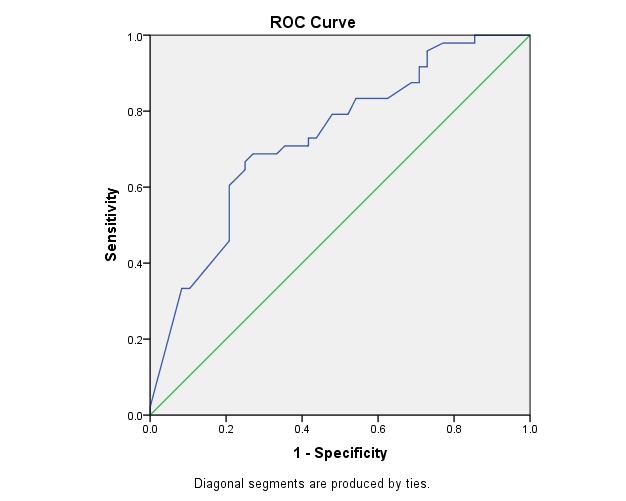
ROC Curve - Variable(s): CRA PSV
Area Under the Curve
Table 10.
Test Result Variable(s): CRA PSV
| Area | Asymptotic Sig. b | Asymptotic 95% Confidence Interval | |
| Lower Bound | Upper Bound | ||
| .730 | .000 | .629 | .830 |
Table 11.
The cut-off point for sensitivity and specificity at PSV of CRA
| CUT OFF POINT | SENSITIVITY | SPECIFICITY |
| > 17.90 | 72% | 59% |
The power to identify the predictive value of the progressive eye by using PSV of CRA reached 72% sensitivity at 59% specificity (cut-off point > 17,90).
Fig. 4 illustrates the ROC curve for EDV in CRA. This was statistically significant (p < 0.006) and had the area under the curve of 0.66, which made it a fair but acceptable test of prediction.
Fig. 4.
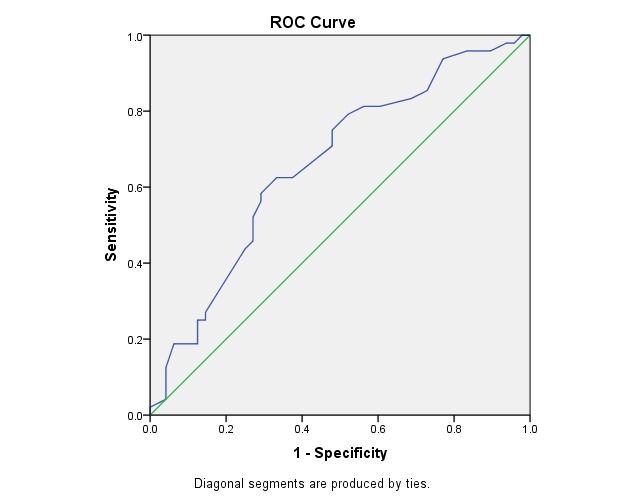
ROC Curve - Variable(s): CRA EDV
Area Under the Curve
Table 12.
Test Result Variable(s): CRA EDV
| Area | Asymptotic Sig. b | Asymptotic 95% Confidence Interval | |
| Lower Bound | Upper Bound | ||
| .661 | .006 | .552 | .771 |
Table 13.
The cut-off point for sensitivity and specificity at EDV of CRA
| CUT OFF POINT | SENSITIVITY | SPECIFICITY |
| > 6,25 | 75% | 53% |
The power to identify the predictive value of the progressive eye by using EDV of CRA reached 75% sensitivity at 53% specificity (cut-off point > 6,25).
Posterior ciliary arteries:
Fig. 5 illustrates the ROC curve for PSV in CPA. This was statistically significant (p < 0.04) and had the area under the curve of 0.61, which made it a fair but acceptable test of prediction.
Fig. 5.
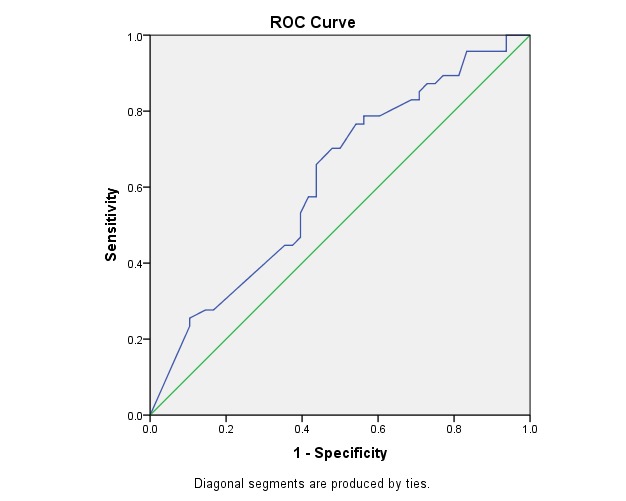
ROC Curve - Variable(s): PCA PSV
Area Under the Curve
Table 14.
Test Result Variable(s): PCA PSV
| Area | Asymptotic Sig. b | Asymptotic 95% Confidence Interval | |
| Lower Bound | Upper Bound | ||
| .619 | .045 | .507 | .732 |
Table 15.
The cut-off point for sensitivity and specificity at PSV of CPA
| CUT OFF POINT | SENSITIVITY | SPECIFICITY |
| > 17,35 | 83% | 30% |
The power to identify the predictive value of the progressive eye by using PSV of CPA reached 83% sensitivity at 30% specificity (cut-off point > 17,35).
Conclusion
Concluding, the present study found that the decrease of the PSV values in CRA (statistically significant p < 0.001, having the area under the curve of 0.73 with 72% sensitivity at 42% specificity - cut-off point > 17,90) were relevant in glaucoma progression.
According to the Pearson values, there were no registered correlations between the perimetry indices (MD and PD) and the hemodynamic parameters because the “p” value was higher than 0.05.
Discussions
Two major factors were involved in the glaucoma disease progression:
- the IOP, which depended on the rate of production of the aqueous humor and its rate of exit through the trabecular meshwork.
- the resistance of the intraocular portion of the optic nerve to the development of the optic atrophy.
Few features indicated that the hemodynamic change involving the optic nerve head played an important role in the pathogenesis and in GON:
- Hayreh et al. have pointed out the importance of perfusion pressure in the optic nerve [24].
- Socci and Anderson have hypothesized that the inhibition of the autoregulation of blood flow to the optic nerve could increase the susceptibility of the disc to pressure-induced ischemia [25].
How the IOP affect the ocular blood flow and the impact of decrease of this needs further investigation [26].
Sung (2009) showed that the fluctuations in the perfusion pressure are particularly important in glaucoma progression, especially in patients with normotensive glaucoma and Gherghel (2004) suggested a correlation between the extent of the autonomic nervous system dysfunction and the severity of the glaucoma disease. In a prospective study, Drance (1995) showed a faster progression in the eye with the more pronounced blood flow impairment [27].
The lower the IOP, due to the occurrence of the damages or progresses, the higher the probability that vascular factors are involved. In terms of progression, a reduced OBF has a similar effect as an increased IOP. However, not all the patients with a low blood pressure will progress. As long as autoregulation functions well, low blood pressure can be tolerated [4].
Only few studies with a limited follow-up were performed to investigate the relation of CDI parameters with a future progression in glaucoma.
Studies with varying participant numbers have found that varying CDI parameters were correlated with the visual field defect progression in POAG and NTG. Nevertheless, the data is still inconclusive.
Kuerten et al. (2015) centralized the trials concerning the correlations between the CDI parameters and glaucoma progression [21]:
1. Schumann et al. (2000) [28] performed a retrospective study concerning POAG with progressive VF defects (VFI mean defect over time): the findings were significant, lower mean blood flow velocities in the OA, higher PSV, and RI in the CRA.
2. Gherghel et al. (2000) [29] performed a retrospective study concerning POAG with progressive VF defects, matched patients with stable disease (deepening of existing scotoma, expansion of existing scotoma, and fresh scotoma): the findings were significant, lower EDV in the CRA.
3. Martínez and Sánchez (2005) [5,30] performed a prospective study concerning POAG with progressive VF defects (deepening of existing scotoma, expansion of existing scotoma and fresh scotoma): the findings were significant, the RI in the OA and SPCAs (the risk of future progression increased with higher RI in the OA and short PCAs; cutoff value of 0.72 for the RI of the OA and 0.65 for the RI of the PCAs was applied).
4. Satilmis et al. (2003) [5,28,31] performed a retrospective study concerning POAG with progressive VF defects (deepening of existing scotoma, expansion of existing scotoma, fresh scotoma and VFI mean defect over time), the findings were significant, EDV in the CRA (EDV of the CRA was inversely correlated with the rate of progression of the visual field MD: lower baseline blood flow velocities and higher baseline resistivity indices (RI) were measured in the central retinal artery of patients with a faster progression of glaucomatous damage, unrelated to the extent of the existing damage. Further, the progression rate of the visual field damage was correlated with the end diastolic velocity (EDV) of the central retinal artery (r = -0.63, p < 0.0037)).
5. Galassi et al. (2003) [5,13,28,32] performed a retrospective study concerning POAG with progressive VF defects (progressive VF defect recorded in at least 3 consecutive examinations), the findings were significant, EDV and RI in the OA (lower EDV and increased RI of the OA compared to patients with stable visual fields; newly diagnosed glaucoma patients were six times more likely to suffer from a progressive disease when their IR of OA was higher than 0.78, compared to patients with an OA index lower than 0.78 (P < .001)).
6. Zeitz et al. (2006) [5,33] performed a prospective study concerning glaucoma patients with progressive VF defects (increase in the cup-disc ratio of the optic disc and an increase in MD), the findings were significant, reducing of PSV and EDV in the PCAs; in the CRA, only the PSV was significantly reduced in the progressive glaucoma group. They reported that the retrobulbar blood flow velocities were altered independently from the IOP and the systemic blood pressure in patients with progressive glaucoma, which could represent a primary risk factor for the disease progression.
7. Calvo et al. (2012) [29,34] performed a prospective study concerning glaucoma suspects with progressive disease (Color coded Moorfields Regression Analysis (MRA) in confocal laser scanning system), the findings were significant, a RI with values > 0.75 in the OA was associated with the development of glaucoma.
8. Jimenez-Aragon et al. (2013) [35] performed a prospective study concerning POAG with progressive VF defects (Color coded Moorfields Regression Analysis (MRA) in confocal laser scanning system), the findings were significant, RI in the OA and CRA.
9. Kuerten et al. (2014) [36] performed a retrospective study concerning NTG (VFI progression per year), the findings were significant, PSV of the CRA, RI of NPCA.
The other studies that had the same theme were the following:
- Mokbel et al. (2010) [5] found that the RI of OA was negatively correlated with the MD values and positively correlated with the PSD values in the POAG patients and the RI of SPCA was negatively correlated with the MD values in the POAG patients. These results revealed that the RI of the OA in the POAG patients might reliably predict the visual field progression.
- Yamazaki and Drance (1997) [33,37] performed a retrospective study concerning the progressive NTG findings lower blood flow velocities of the CRA and PCA compared to patients with stable visual fields.
- Plange et al. (2006) [5,37,38] used the CDI to investigate the inter-ocular differences in the retrobulbar flow velocities in 25 glaucomatous patients with asymmetrical visual field loss; they found higher flow velocities and less RI and PI in the SPCAs in the group of No-Progression; that patients with more severe damages displayed reduced flow velocities and that the asymmetrical visual field loss corresponded to the asymmetrical flow velocities of the CRA and OA. The PSV and EDV of the CRA and the PSV of the OA were significantly decreased in the eyes with more severe glaucomatous visual field loss;
- Sharma and Bangiya (2006) correlated the Color Doppler imaging variables of OA, CRA and PCA with the risk of visual field deterioration in glaucomatous patients and suggesting the major role of vascular factor in the pathogenesis of GON (POAG patients have a negative correlation of field changes with blood flow velocity). They found a statistical significance for PSV and EDV Doppler variables (the severity of the visual field deterioration increased the blood flow velocities decreases), there was positive correlation of the visual field deterioration with RI (increased deterioration of the visual field increase in RI) [39].
- Cellini et al. (1996-97) found a PSV decrease and RI to be significantly increased in patients with greater visual field damage [39].
- Similar results of Cellini et al. were also reported [39] in Renklin et al. (1996) study.
- Suprasanna et al. (2014) found that the RI of OA and the medial posterior ciliary arteries were higher, and the EDV of OA was lower in progressive glaucomatous eyes than in patients with a stable visual field loss. The receiver operating characteristic curve showed the optimal cut-off RI to be of 0.847 [40].
- Alconchel et al. (2012) reported a decrease of IR in OA and PSV in CRA in patients with glaucoma progression [41].
- Popa (2012) found the decrease in EVD of OA and the increase in RI of OA [18].
In the present work, the similarities with some of the studies found were presented above:
- a decrease of EDV and an increase of IR in OA were found, both in the same study, by the Galassi et al. (2003), Suprasanna et al. (2014) and Popa (2012).
- just an increase of IR in OA has been found by Martínez and Sánchez (2005), Calvo et al. (2012), Mokbel et al. (2010).
- decrease of EDV and increase of IR in OA, associated with other hemodynamic parameters change were found by the Suprasanna et al. (2014).
- decrease of PSV and EDV in CRA were found by the Plange et al. (2006), Sharma and Bangiya (2006).
- just a decrease of PSV in CRA associated with other hemodynamic parameters changes (without EDV modification) were found by the Zeitz et al. (2006), Kuerten et al. (2014), Alconchel et al. (2012).
- just a decrease of EDV in CRA (without PSV modification) was found by the Gherghel et al. (2000), Satilmis et al. (2003).
- a decrease of EDV in CPA was found by the Zeitz et al. (2006), associated with other hemodynamic parameters changes.
- Yamazaki and Drance (1997) findings showed lower blood flow velocities of the CRA and PCA in patients with progressive and stable NTG.
However, considering the values obtained at the ROC curve (area under the curve), we could conclude that the present data are in more concordance with the studies of Zeitz et al. (2006), Plange et al. (2006) or Alconchel et al. (2012), who have found a decrease of PSV in CRA, too.
These findings might be restricted by the small sample size and heterogeneity in the manifestation of the disease in the study population. In addition, the variability of the follow-up period, as well as of the number of visual field tests, might confound the results, although the visual field progression index (in dB per year) aims to be comparable between patients [21]. Also, the vessels with statistically significant correlations to the visual field defect progression vary amongst the studies, possibly because of the variability in the measurement technique in the different studies (the small caliber of these vessels avoids individual measurements, and due to their direction, variable insonation angles are usually required for their analysis) and there is no general consensus regarding the best diagnostic tool to identify progression in glaucoma (most authors prefer visual field changes; others prefer optic disc changes via morphometric techniques) [21].
Besides those aspects, numerous others factors may have affected the clinical course of the patients: various therapeutic interventions (topic or systemic medications, laser, and surgical procedures) that patients have undergone during the follow up and individual factors (genetics, life habits, and treatment compliance) might affect the results of this study [39].
Same authors of these studies gave a greater importance to RI involved in the progression of glaucoma:
-Kuerten et al. reported that higher RI values are often recorded in patients suffering from glaucoma and RI values can often be attributed to the diseases’ progression. At the same time, CDI tends to provide the most important parameter in the assessment of disturbed retrobulbar blood flow in glaucoma. A RI value > 0.75 appears to be associated with a higher risk of visual field deterioration [21].
- Sharma et al. reported that RI has the advantage that its value does not depend on the Doppler angle (Contrary to PSV or EDV) as its ratio, hence, its absolute value could be used to compare the results even in different studies which are not suitable for PSV and EDV because their value changes with the change in the Doppler angle [39].
- Galasi and Calvo tried to determine a RI threshold of the OA [21].
Regarding the CDI role in the glaucoma progression, some general conclusions could be drawn:
- retrobulbar hemodynamics and ocular perfusion appeared to play a major role among other factors, some of which are not clearly defined today, in glaucoma progression [21].
- the CDI studies may be important in identifying glaucoma patients (biomarker in glaucoma) that are at a greater risk for progression [27,35,37].
- the CDI measurement’s accuracy and reproducibility are variable [21,42], and due to this reason, it is not yet possible to determine the obvious best parameter that is correlated with the glaucoma progression [21].
- CDI may help to institute a more aggressive clinical management in extremely or with atypical behavior cases with a higher progression risk (prognostic role) [21,35,39,43].
According to the World Glaucoma Association’s consensus on ocular blood flow, these additional investigations should include longitudinal studies, involve a larger number of patients, and use standardized methods to confirm whether blood flow abnormalities precede the visual field defects and correlate with disease severity [6].
Conclusions
As the retrobulbar hemodynamic alteration might represent a risk factor for glaucoma progression even in an early stage, when the visual field is still normal and when a classical risk factor such as IOP is not altered, the orbital CDI would represent an important diagnosis method, whose results could help adopting more or less aggressive therapeutic measures in conflicted cases.
In the present study, we found a decrease in the mean values of velocities flow and increased mean values for IR in the progressive eyes compared to stable eyes. Modifications with statistical significance have been recorded at the RI (p < 0,05) and EDV (p < 0,03) in AO, PSV (p < 0,001) and EDV (p < 0,006) in ACR and PSV (p < 0,04) in CPA for the eyes with progressive glaucoma.
The most important parameter in this study, as it resulted from the ROC curve, was PSV in ACR, which was statistically significant, p < 0.001, and had the area under the curve of 0.73 with 72% sensitivity at 42% specificity (cut-off point > 17,90).
There were indications that reduced the blood flow velocities and/ or the altered resistivity index could be a predictor for glaucoma progression.
Color Doppler Imaging is a good modality for the imaging and the hemodynamic study of the optic nerve vessels, and it may help facilitating the understanding of the pathogenesis of glaucoma.
The introduction of blood flow measurements into the clinical practice for diagnostic and follow up glaucoma disease could help in the care of patients with glaucoma, identifying and introducing innovative vasoprotective therapies to prevent optic nerve damages.
Future clinical studies may establish the abnormal blood flow as a contributor to glaucomatous damage.
References
- 1.European Glaucoma Society . Terminology and guidelines for glaucoma. 3rd Edition. 2008. pp. 95–97. 174 and 4ᵗʰ Edition, 2014, 79-87, 153, 156. [DOI] [PubMed] [Google Scholar]
- 2.Yanagi M, Kawasaki R, Wang MMed JJ, Wong TY, Crowston Franzco J, Kiuchi Y. Vascular risk factors in glaucoma: a review. Clinical and Experimental Ophthalmology. 2011;39:252–258. doi: 10.1111/j.1442-9071.2010.02455.x. doi: 10.1111/j.1442-9071.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- 3.Faridi O, Park SC, Liebmann JM, Ritch R. Glaucoma and obstructive sleep apnoea syndrome. Clinical and Experimental Ophthalmology. 2012;40:408–419. doi: 10.1111/j.1442-9071.2012.02768.x. doi: 10.1111/j.1442-9071.2012.02768.x. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarieh M, Flamer J. Ocular Blood Flow and Glaucomatous Optic Neuropathy. Ed. Springer; 2009. pp. 27–28.pp. 40–44. [Google Scholar]
- 5.Mokbel TH, Ghanem AA. Diagnostic Value of Color Doppler Imaging and Pattern Visual Evoked Potential in Primary Open-Angle Glaucoma. J Clinic Experiment Ophthalmol. 2011;2:127. doi:10.4172/2155-9570.1000127. [Google Scholar]
- 6.Harris A, Rusia D, Moss A, Hopen P, Hopen M, Pernic A, Siesky B, Januleviciene I, Shoshani Y. Ocular Blood Flow in Glaucoma Myths and Reality. Ed. Kugler; 2009. pp. 2–5.pp. 46–52. [Google Scholar]
- 7.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmology. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 8.Leske MC, Heijl A, Hyman L, Bengtsson B. Early Manifest Glaucoma Trial: Design and baseline data. Ophthalmology. 1999;106:2144–2153. doi: 10.1016/s0161-6420(99)90497-9. [DOI] [PubMed] [Google Scholar]
- 9.JoAnn A, Giaconi Simon K, Law Anne L, Coleman JC (Eds.) Pearls of Glaucoma Management. Ed. Springer; 2010. pp. 157–172. [Google Scholar]
- 10.Collaborative Normal-Tension Glaucoma Study Group The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmology. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 11.The Advanced Glaucoma Intervention Study (AGIS) Comparison of treatment outcomes within race. Seven-year results. Am J Ophthalmology. 1998;105:1146–1164. doi: 10.1016/s0161-6420(98)97013-0. [DOI] [PubMed] [Google Scholar]
- 12.Musch D, Gillespie B, Lichter P, et al. Visual field progression in the Collaborative Initial Glaucoma Treatment Study: The impact of treatment and other baseline factors. Am J Ophthalmology. 2009;116:200–207. doi: 10.1016/j.ophtha.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad AA, Yali J, Huang D. Does Blood Flow Measurement Have a Role in Glaucoma Care? Glaucoma Today. 2014 Sep-Oct;:49–52. [Google Scholar]
- 14.Samsudin A, Isaacs N, Tai MLS, Ramli N, Mimiwati Z, Choo MM. Ocular perfusion pressure and ophthalmic artery flow in patients with normal tension glaucoma. BMC Ophthalmol. 2016;16:39. doi: 10.1186/s12886-016-0215-3. doi: 10.1186/s12886-016-0215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiu C, Antochi Fl. Neurosonologie. Ed. Semne; 2006. pp. 23–25. [Google Scholar]
- 16.Gherghel D, Chiselita D. Glaucomul primitiv cu unghi deschis: identificarea factorilor de risc vascular. Oftalmologia. 2001;(Supliment Nr.1):22–25. [PubMed] [Google Scholar]
- 17.Huttmann G. Manual si Atlas de Perimetrie Automatizata in Oftalmologie. Ed. Transilvania Expres; 2006. pp. 100–115.pp. 191–194. [Google Scholar]
- 18.Popa ED. Valorile si limitele Ecografiei Doppler Color in neuropatiile optice ischemice. Ed. Alma Mater; 2012. pp. 46–47.pp. 63–70. [Google Scholar]
- 19.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmology. 1989;107:453–464. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 20.Yanagi M, Ryo Kawasaki R, Wang JJ, Tien YW, Franzco CJ, Kiuchi Y. Vascular risk factors in glaucoma: a review. Clinical and Experimental Ophthalmology. 2011;39:252–258. doi: 10.1111/j.1442-9071.2010.02455.x. doi: 10.1111/j.1442-9071.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuerten D, Fuest M, Koch EC, Koutsonas AN. Retrobulbar Hemodynamics and Visual Field Progression in Normal Tension Glaucoma: A Long-Term Follow-Up Study. BioMed Research International Volume. 2015 doi: 10.1155/2015/158097. http://dx.doi.org/10.1155/2015/158097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brace N, Kemp R, Snelgar R. SPSS for Psychologists. New York: Palgrave Macmillan; 2003. [Google Scholar]
- 23.Karimollah HT. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med. 2013;4(2):627–635. [PMC free article] [PubMed] [Google Scholar]
- 24.Hayreh SS, Revie IHS, Edwards J. Vasogenic origin of visual field defects and optic nerve change in glaucoma. British Journal of Ophthalmology. 1070;54:461. doi: 10.1136/bjo.54.7.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Socci N, Anderson DR. Blockage of axonal transport in optic nerve induced by elevation of intraocular pressure: Effect of arterial hypertension induced by angiotensin 1. Arch Ophthalmology. 1983;101:94. doi: 10.1001/archopht.1983.01040010096017. [DOI] [PubMed] [Google Scholar]
- 26.Chiou HJ, Chou YH, Jui-Ling Liu C, Hsu CC, Tiu CM, Mu-Huo Teng M, Chang CY. Evaluation of Ocular Arterial Changes in Glaucoma with Color Doppler Ultrasonography. J Ultrasound Med. 1999;18:295–302. doi: 10.7863/jum.1999.18.4.295. [DOI] [PubMed] [Google Scholar]
- 27.Rumelt S. Glaucoma - Basic and Clinical Concepts. Ed. InTech; 2011. pp. 225–254. [Google Scholar]
- 28.Schumann J, Orgül S, Gugleta K, Dubler B, Flammer J. Interocular difference in progression of glaucoma correlates with interocular differences in retrobulbar circulation. American Journal of Ophthalmology. 2000;129(6):728–733. doi: 10.1016/s0002-9394(99)00481-x. [DOI] [PubMed] [Google Scholar]
- 29.Gherghel D, Orgül S, Gugleta K, Gekkieva M, Flammer J. Relationship between ocular perfusion pressure and retrobulbar blood flow in patients with glaucoma with progressive damage. American Journal of Ophthalmology. 2000;130(5):597–605. doi: 10.1016/s0002-9394(00)00766-2. [DOI] [PubMed] [Google Scholar]
- 30.Martínez A, Sánchez M. Predictive value of colour Doppler imaging in a prospective study of visual field progression in primary open-angle glaucoma. Acta Ophthalmologica Scandinavica. 2005;83(6):716–722. doi: 10.1111/j.1600-0420.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 31.Satilmis M, Orgül S, Doubler B, Flammer J. Rate of progression of glaucoma correlates with retrobulbar circulation and intraocular pressure. American Journal of Ophthalmology. 2003;135(5):664–669. doi: 10.1016/s0002-9394(02)02156-6. [DOI] [PubMed] [Google Scholar]
- 32.Galassi F, Sodi A, Ucci F, Renieri G, Pieri B, Baccini M. Ocular hemodynamics and glaucoma prognosis: a color Doppler imaging study. Arch of Ophthalmology. 2003;121(12):1711–1715. doi: 10.1001/archopht.121.12.1711. [DOI] [PubMed] [Google Scholar]
- 33.Zeitz O, Galambos P, Wagenfeld L, et al. Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery. British Journal of Ophthalmology. 2006;90(10):1245–1248. doi: 10.1136/bjo.2006.093633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calvo P, Ferreras A, Polo V, et al. Predictive value of retrobulbar blood flow velocities in glaucoma suspects. Investigative Ophthalmology and Visual Science. 2012;53(7):3875–3884. doi: 10.1167/iovs.11-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jimenez-Aragon F, Garcia-Martin E, Larrosa-Lopez R, Artigas-Martin JM, Seral-Moral P, Pablo LE. Role of Color Doppler Imaging in Early Diagnosis and Prediction of Progression in Glaucoma. BioMed Research International. 2013 doi: 10.1155/2013/871689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuerten D, Fuest M, Koch EC, Remky A, Plange N. Long term effect of trabeculectomy on retrobulbar haemodynamics in glaucoma. Ophthalmic and Physiological Optics. 2014;35(2):194–200. doi: 10.1111/opo.12188. [DOI] [PubMed] [Google Scholar]
- 37.Plange N, Kaup M, Weber A, Harris A, Arend KO, Remky A. Performance of color Doppler imaging discriminating normal tension glaucoma from healthy eyes. Eye. 2009;23:164–170. doi: 10.1038/sj.eye.6702943. [DOI] [PubMed] [Google Scholar]
- 38.Plange N, Kaup M, Arend KO, Remky A. Asymmetric visual field loss and retrobulbar haemodynamics in primary open-angle glaucoma. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2006;244(8):978–983. doi: 10.1007/s00417-005-0227-9. [DOI] [PubMed] [Google Scholar]
- 39.Sharma NC, Bangiya D. Comparative study of ocular blood flow parameters by color doppler imaging in healthy and glaucomatous eye. J Radiol Imaging. 2006;16:679–682. [Google Scholar]
- 40.Suprasanna K, Chandrakant M, Charudutt Rajagopal K. Doppler Evaluation of Ocular Vessels in Patients with Primary Open Angle Glaucoma. Journal of Clinical Ultrasound. 2014 Oct;42(8) doi: 10.1002/jcu.22175. [DOI] [PubMed] [Google Scholar]
- 41.Alconchel A, Pablo L, Seral Moral P, Remirez J, Calvo P, Zaragoza Role of ocular Doppler ultrasonography in primary open angle glaucoma. ESR Congress 2012 presentation [Google Scholar]
- 42.Harris A, Williamson TH, Martin B, et al. Test/retest reproducibility of color Doppler imaging assessment of blood flow velocity in orbital vessels. Journal of Glaucoma. 1995;4(4):281–286. [PubMed] [Google Scholar]
- 43.Quaranta L, Harris A, Donato F, et al. Color Doppler Imaging of ophthalmic artery blood flow velocity. Ophthalmology. 1997;104:653–658. doi: 10.1016/s0161-6420(97)30256-5. [DOI] [PubMed] [Google Scholar]


