Abstract
OBJECTIVE
Severe hypoglycemia is a rare but important complication of type 2 diabetes. Few studies have examined the epidemiology of hypoglycemia in a community-based population.
RESEARCH DESIGN AND METHODS
We included 1,206 Atherosclerosis Risk in Communities (ARIC) Study participants with diagnosed diabetes (baseline: 1996–1998). Severe hypoglycemic events were identified through 2013 by ICD-9 codes from claims for hospitalizations, emergency department visits, and ambulance use. We used Cox regression to evaluate risk factors for severe hypoglycemia.
RESULTS
The mean age of participants was 64 years, 32% were black, and 54% were female. During a median follow-up period of 15.2 years, there were 185 severe hypoglycemic events. Important risk factors after multivariable adjustment were as follows: age (per 5 years: hazard ratio [HR] 1.24; 95% CI 1.07–1.43), black race (HR 1.39; 95% CI 1.02–1.88), diabetes medications (any insulin use vs. no medications: HR 3.00; 95% CI 1.71–5.28; oral medications only vs. no medications: HR 2.20; 95% CI 1.28–3.76), glycemic control (moderate vs. good: HR 1.78; 95% CI 1.11–2.83; poor vs. good: HR 2.62; 95% CI 1.67–4.10), macroalbuminuria (HR 1.95; 95% CI 1.23–3.07), and poor cognitive function (Digit Symbol Substitution Test z score: HR 1.57; 95% CI 1.33–1.84). In an analysis of nontraditional risk factors, low 1,5-anhydroglucitol, difficulty with activities of daily living, Medicaid insurance, and antidepressant use were positively associated with severe hypoglycemia after multivariate adjustment.
CONCLUSIONS
Poor glycemic control, glycemic variability as captured by 1,5-anhydroglucitol, kidney damage, and measures of cognitive and functional impairments were strongly associated with increased risk of severe hypoglycemia. These factors should be considered in hypoglycemia risk assessments when individualizing diabetes care for older adults.
Introduction
Hypoglycemia is an important complication of type 2 diabetes that can have a major impact on quality of life and health outcomes (1–3). Severe hypoglycemia is more common in older age, approximately doubling with each decade of life after age 60 years (4). Because of the increased risk of hypoglycemia associated with tight glycemic control, clinical guidelines typically recommend individualizing glycemic targets for older adults based on a personalized assessment of risk for hypoglycemia and expected benefit from tight glycemic control (5). The 2017 Standards of Medical Care highlight “renal insufficiency” and cognitive dysfunction as important risk factors for hypoglycemia, but other risk factors remain less well documented. For instance, there is conflicting evidence that female sex or cardiovascular disease increases the risk of hypoglycemia (2,6,7). Advancing our understanding of risk factors for hypoglycemia can lead to improvements in the clinical assessment of hypoglycemia risk and contribute to personalized and safe diabetes care.
There are also substantial racial disparities in rates of hypoglycemia (8–10). Blacks have approximately twofold higher rates of severe hypoglycemia, and this excess risk persists after multivariate adjustment, suggesting that other factors may contribute to hypoglycemia risk in blacks (6). To date, previous studies have not specifically examined whether hypoglycemia risk factors may be different in blacks compared with whites.
Most prior studies of risk factors for hypoglycemia have been conducted either as post hoc analyses of randomized clinical trials or as retrospective studies of routinely collected clinical or administrative data. In clinical trials, patients are selected based on strict criteria and thus are unlikely to be representative of typical older adults with diabetes (11). Studies of administrative data may be more representative but lack standardized clinical assessments and are often missing important clinical characteristics, such as duration of diabetes. Epidemiologic cohort studies can fill a crucial gap in the literature, as they are typically more representative and have detailed demographic and clinical characteristics collected in a standardized fashion.
Our study aims were 1) to characterize the incidence rates of severe hypoglycemia in a community-based epidemiologic cohort of older black and white adults with type 2 diabetes, 2) to rigorously evaluate traditional and nontraditional risk factors for severe hypoglycemia in older adults with diabetes, and 3) to determine whether risk factor associations for hypoglycemia differ in blacks compared with whites.
Research Design and Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is an ongoing prospective cohort study that recruited participants in 1987–1989 from four U.S. communities: Jackson, MS; Forsyth, NC; Washington County, MD; and the suburbs of Minneapolis, MN (12). During the first 12 years of the study, study visits with physical examinations and detailed questionnaires occurred every 3 years. The fourth study visit occurred in 1996–1998 and is the baseline for the present analysis. At visit 4, there were 1,511 individuals with self-reported physician-diagnosed diabetes or currently taking diabetes medications. For the present analysis, we excluded four participants who self-identified as being of a race other than black or white. For the main analysis of traditional risk factors, we excluded individuals who were missing any risk factors of interest (n = 301), leaving a final sample size of 1,206. For the analysis of nontraditional risk factors, we additionally excluded individuals who were missing any of the additional variables of interest (n = 62), leaving a sample size of 1,144.
Risk Factors for Severe Hypoglycemia
Based on the existing literature, we classified risk factors as either “traditional” or “nontraditional.” Traditional risk factors were those where there was existing evidence of an association with hypoglycemia in the literature and strong biologic plausibility, as follows: age, sex, race, BMI, duration of diabetes, glucose-lowering medication use, glycemic control, kidney function, albuminuria, and cognition. BMI was calculated from measured height and weight at visit 4. Because the exact date of diabetes diagnosis was not available, we calculated diabetes duration based on the time since the participant first reported a diagnosis or medication use. Diabetes duration was categorized as ≥9 years (reported at the first ARIC Study visit in 1987–1989) or <9 years. For assessment of medication use, participants brought in all medications taken in the past 2 weeks to each study visit. Diabetes medication use was classified as “no medication use,” “oral medications only,” and “any insulin use” at the baseline visit (1996–1998). Since the vast majority of participants in the oral medication–only category were receiving treatment with sulfonylureas, it was not possible to examine the association of other types of oral medications with severe hypoglycemia because of small sample size. Because HbA1c was not measured at visit 4, we used fructosamine (categorized into tertiles) as a proxy for glycemic control. Reduced kidney function was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, using the Chronic Kidney Disease Epidemiology Collaboration equation for serum creatinine (13). Albuminuria was categorized by the albumin-to-creatinine ratio (ACR): <30, 30 to <300, and ≥300 mg/g. Cognitive function was assessed using the Digit Symbol Substitution Test (DSST), a neuropsychological test of executive function that has previously been associated with hypoglycemia (14). Because of large differences in the mean values between blacks and whites, race-specific z scores were used in all analyses.
“Nontraditional” risk factors were items that were plausible but untested or factors that had more limited biological plausibility but had been shown in one or two studies to be associated with severe hypoglycemia. Nontraditional risk factors included functional disabilities, self-reported health, history of cardiovascular disease, and a number of different biomarkers. Disability was based on the assessment of any difficulty with activities of daily living (ADLs) (eating, dressing, getting out of bed, or walking between rooms) and any difficulty with instrumental ADLs (IADLs) (managing money, preparing meals, or vacuuming/other light housework). We included two measures of general health asked via telephone within 12 months prior to visit 4: self-rated health (poor or fair vs. good or excellent) and ≥10 lb of unintentional weight loss. We created a count of comorbidities, based on adjudicated coronary heart disease or stroke, heart failure hospitalization, and self-report of the following: lung disease, liver cirrhosis, Parkinson disease, cancer within the past 5 years, recent spine or hip fracture, arthritis, or a blood clot in lung or legs. The count of comorbidities was categorized into zero, one, two, and three or more. Since previous studies have reported that a history of cardiovascular disease was associated with severe hypoglycemia, we also looked individually at prevalent coronary heart disease and stroke (2,7). Additional nontraditional risk factors included family history of diabetes, education, Medicaid insurance, use of antidepressant agents, and use of β-blockers (15–17). We also considered a family history of diabetes, because it may indicate genetic risk of reduced insulin secretion and more labile diabetes that may predispose individuals to hypoglycemia (18).
Finally, we examined biomarkers that are plausible contributors to hypoglycemia risk but have not been rigorously examined in prior studies. This included the liver enzymes alanine transaminase, aspartate transaminase, and γ-glutamyl transferase, since hepatic glucogenolysis and gluconeogenesis are crucial autoregulatory responses when blood glucose levels are low (19). We also examined N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hs-cTnT) as markers of poor prognosis and because hs-cTnT is more likely to be elevated in those with a history of severe hypoglycemia (20). Finally, since glycemic variability has been associated with hypoglycemia (21,22), we hypothesized that low levels of 1,5-anhydroglucitol (1,5-AG), a biomarker of glucose excursions, would be associated with increased risk of hypoglycemia independent of average glycemia.
Severe Hypoglycemia
We assessed severe hypoglycemia using a validated algorithm with ICD-9 codes in the primary position from hospitalizations, emergency department visits, and ambulance calls through 31 December 2013 (23). Records of hospitalizations are obtained for all ARIC Study participants through surveillance of local community hospital records and through medical records requests for hospitalizations that occurred outside of the ARIC hospital surveillance system (12). For participants enrolled in Medicare, linkage to Centers for Medicare and Medicaid Services (CMS) provided claims for hospitalizations, emergency department visits, and ambulance use from 1991 through 2013.
Statistical Analysis
We used Poisson regression with robust SEs to calculate incidence rates and incidence rate ratios (IRRs) by race, age, and sex. We used Cox proportional hazards regression models to evaluate the associations of traditional and nontraditional risk factors with severe hypoglycemia. Model 1 was adjusted for age, sex, and race. Model 2 included all variables in model 1 plus all traditional risk factors (obesity, duration of diabetes, diabetes medication use, glycemic control, low eGFR, albuminuria, and cognitive function). Nontraditional risk factors were evaluated individually in models 1 and 2. We evaluated the proportional hazards assumption by visually examining the log-log survival plots. We tested for effect modification by race using the likelihood ratio test.
We conducted a number of sensitivity analyses to examine the robustness of the results. First, we repeated our analyses in the subgroup of participants ≥65 years of age with CMS Fee-for-Service Part B coverage. In this analysis, we censored participants if they died or changed to a different type of insurance. We also examined associations after excluding participants with possible type 1 diabetes as defined by exclusive insulin use at all four ARIC Study visits.
Results
At baseline (visit 4, 1996–1998), the mean age was 64 years, 54% of participants were female, and 55% of participants were obese (BMI ≥30 kg/m2) (Table 1). Overall, 42% of participants had diabetes for at least 9 years, 46% were receiving treatment with oral medications only, and 28% used insulin. Kidney function was relatively preserved, as follows: only 11% of participants had an eGFR <60 mL/min/1.73 m2, and 7.5% had an ACR ≥300 mg/g. There were substantial racial differences in baseline characteristics. Reflective of the overall ARIC Study population, blacks were more likely to be female and to be obese. Blacks were also more likely to be receiving therapy with insulin and to have poorer glycemic control and albuminuria.
Table 1.
Baseline characteristics of black and white ARIC Study participants with diagnosed diabetes (visit 4, 1996–1998)
| Overall (n = 1,206) | Blacks (n = 391) | Whites (n = 815) | |
|---|---|---|---|
| Age | 63.7 (5.66) | 62.7 (5.74) | 64.2 (5.56) |
| Female sex | 651 (54.0) | 275 (70.3) | 376 (46.1) |
| BMI category | |||
| Overweight | 413 (34.4) | 126 (32.2) | 290 (35.6) |
| Obese | 657 (54.8) | 239 (61.1) | 421 (51.7) |
| Diabetes duration ≥9 years | 508 (42.1) | 178 (45.5) | 330 (40.5) |
| Diabetes medication use | |||
| No medication | 323 (26.8) | 82 (21.0) | 241 (29.6) |
| Oral medications only | 550 (45.6) | 151 (38.6) | 399 (49.0) |
| Any insulin | 333 (27.6) | 158 (40.4) | 175 (21.5) |
| Fructosamine | |||
| Middle tertile (296–350 μmol/L) | 397 (32.9) | 127 (32.5) | 270 (33.1) |
| Highest tertile (>350 μmol/L) | 499 (33.1) | 168 (43.0) | 231 (28.3) |
| eGFR <60 mL/min/1.73 m2 | 138 (11.4) | 49 (12.5) | 89 (10.9) |
| ACR | |||
| 30 to <300 mg/g | 176 (14.6) | 65 (16.6) | 111 (13.6) |
| ≥300 mg/g | 90 (7.5) | 41 (10.5) | 49 (6.0) |
| DSST | 38.0 (13.8) | 28.0 (12.9) | 42.8 (11.5) |
Age and DSST values are mean (SD); all other values are n (%).
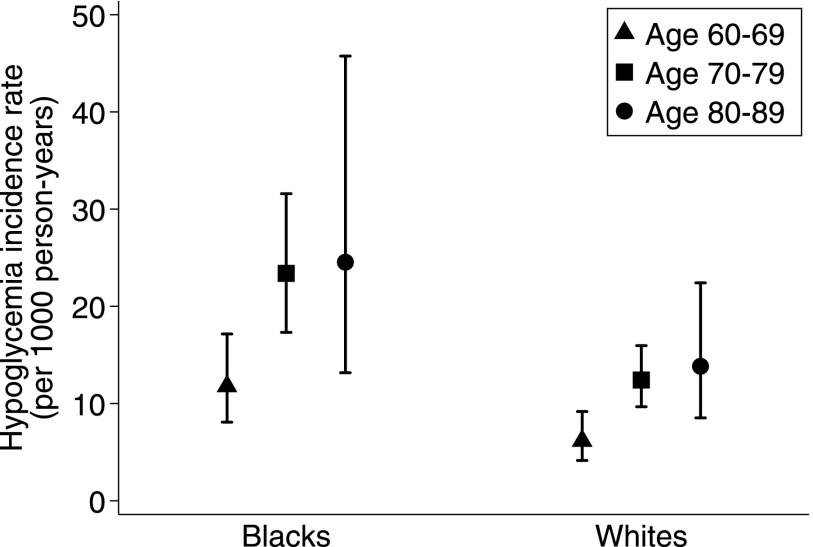
There were 185 severe hypoglycemia events during a median time of 15.2 years of follow-up in the 1,206 participants. The sex-adjusted incidence rates ranged from 6.2/1,000 person-years for whites 60–69 years of age to 24.5/1,000 person-years for blacks 80–89 years of age (Fig. 1). The incidence rate of severe hypoglycemia was almost two times greater for blacks compared with whites (age- and sex-adjusted IRR for black race 1.88; 95% CI 1.39–2.53). There was no difference in incidence rates by sex (age- and race-adjusted IRR for female sex 1.00; P = 0.99).
Figure 1.
Incidence rates of severe hypoglycemia by age (years) and race, adjusted for sex.
All traditional risk factors, with the exception of sex, were significantly associated with severe hypoglycemia after adjustment for age, sex, and race (Table 2 model 1). Patterns of association were similar but attenuated after additional adjustment for all traditional risk factors (Table 2, model 1). Risk factors that remained significant in model 2 were age, black race, fructosamine, medication use, ACR ≥300 mg/g, and race-specific SD lower DSST score. We did not find robust evidence for any interactions by race (Supplementary Table 1).
Table 2.
Adjusted HRs and 95% CIs for traditional risk factors for severe hypoglycemia (n = 1,206; 185 people with hypoglycemia)
| Model 1 | Model 2 | |
|---|---|---|
| Age, per 5 years | 1.42 (1.25–1.62) | 1.24 (1.07–1.43) |
| Female sex | 0.96 (0.71–1.30) | 1.09 (0.80–1.48) |
| Black race | 1.92 (1.42–2.60) | 1.39 (1.02–1.88) |
| Obese* | 1.46 (1.07–1.97) | 1.31 (0.96–1.78) |
| Fructosamine (vs. lowest tertile) | ||
| Middle tertile (296–350 μmol/L) | 2.30 (1.46–3.62) | 1.78 (1.11–2.83) |
| Highest tertile (>350 μmol/L) | 4.04 (2.62–6.21) | 2.62 (1.67–4.10) |
| Diabetes duration ≥9 years | 1.75 (1.31–2.35) | 1.19 (0.86–1.65) |
| Diabetes medication (vs. none) | ||
| Oral only | 3.01 (1.78–5.07) | 2.20 (1.28–3.76) |
| Any insulin use | 5.51 (3.25–9.34) | 3.00 (1.71–5.28) |
| eGFR <60 mL/min/1.73 m2 (creatinine) | 2.00 (1.35–2.97) | 1.40 (0.92–2.13) |
| ACR | ||
| 30 to <300 mg/g | 1.51 (1.02–2.24) | 1.16 (0.78–1.74) |
| ≥300 mg/g | 3.07 (2.00–4.72) | 1.95 (1.23–3.07) |
| DSST, per 1 lower race-specific SD | 1.67 (1.42–1.96) | 1.57 (1.33–1.84) |
Values are reported as HR (95% CI). Model 1 included age, sex, and race. Model 2 included all variables in model 1 plus all covariates listed in the table.
*Overweight and normal weight were collapsed into one reference group because of the small numbers of normal weight participants.
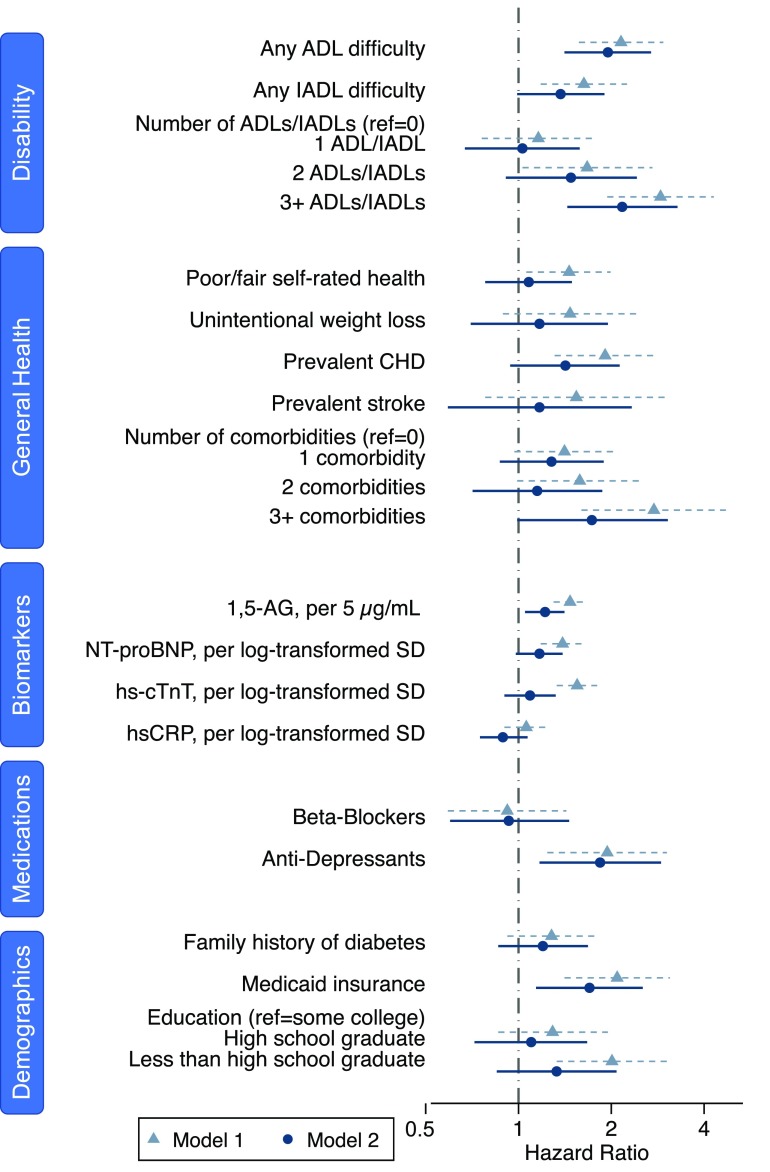
In our analysis of nontraditional risk factors, we found that measures of disability were associated with severe hypoglycemia but other general health metrics were not. Difficulty with ADLs was strongly associated with hypoglycemia (hazard ratio [HR] 1.95; 95% CI 1.41–2.69), whereas difficulty with IADLs was marginally not statistically significant (HR 1.37; 95% CI 0.99–1.90) (Fig. 2, model 2, and Supplementary Table 2, numeric results). There appeared to be a dose relationship with the number of ADLs or IADLs and increasing risk of hypoglycemia. Several other metrics of general health, including unintentional weight loss, fair or poor self-rated health, prevalent coronary heart disease, and prevalent stroke, were not associated with hypoglycemia. The number of comorbidities was associated with hypoglycemia only when the count reached three or more comorbidities (HR for ≥3 vs. 0 comorbidities 2.17; 95% CI 1.44–3.28) (Fig. 2, model 2).
Figure 2.
HRs and 95% CIs for nontraditional risk factors for hypoglycemia (n = 1,144; 169 people with hypoglycemia). CHD, coronary heart disease.
Family history of diabetes was weakly but not statistically significantly associated with hypoglycemia (Fig. 2, model 2). Similarly, although not statistically significant, those participants with less education appeared to have a higher risk of hypoglycemia. Having Medicaid insurance was strongly associated with hypoglycemia (HR 1.70; 95% CI 1.14–2.53) (model 2).
After adjustment for traditional risk factors, 1,5-AG level was linearly associated with severe hypoglycemia (HR per 5 μg/mL 1.22; 95% CI 1.05–1.41) (Fig. 2, model 2). For the cardiac biomarkers, both hs-cTnT and NT-proBNP were associated with severe hypoglycemia in the model adjusted only for age, sex, and race, but the HRs were substantially attenuated and were no longer statistically significant after adjustment for traditional risk factors. There was no association with hs-CRP. The findings for the liver enzymes alanine transaminase, aspartate transaminase, and γ-glutamyl transferase were also null (Supplementary Fig. 1).
Antidepressant use was strongly associated with hypoglycemia (HR 1.83; 95% CI 1.11–3.04) (model 2). In a post hoc analysis looking at the type of antidepressant, tricyclics were more strongly associated with risk of severe hypoglycemia than selective serotonin reuptake inhibitors (tricylic agents HR 2.08; [95% CI 1.18–3.66]; selective serotonin reuptake inhibitor HR 1.28; [95% CI 0.59–2.75]) (model 2). Use of β-blockers was not associated with hypoglycemia.
In the sensitivity analyses restricted to participants ≥65 years of age with CMS Fee-For-Service Part B insurance coverage, estimates for the traditional and nontraditional risk factors were similar but had much wider CIs, likely owing to the much smaller sample size (n = 463; 76 hypoglycemic events) (Supplementary Fig. 2 and Supplementary Tables 3 and 4). After the exclusion of 68 participants with possible type 1 diabetes, results for traditional risk factors were largely similar, although obesity became significantly associated with hypoglycemia (HR 1.62; 95% CI 1.15–2.15) (model 2) (Supplementary Table 5).
Conclusions
The incidence rates of severe hypoglycemia in our study (range in demographic groups 6–25/1,000 person-years) are similar to other studies of persons with type 2 diabetes and attest to the high burden of hypoglycemia in the community (4,10,14,24). Our results extend the evidence for risk factors previously identified in the literature. We found that older age, black race, poor glycemic control, glucose-lowering medication use, kidney damage, and poor cognition were all independently associated with the risk of severe hypoglycemia. We also identified several novel risk factors, as follows: low 1,5-AG concentration, antidepressant use, difficulty with any ADL, and Medicaid insurance.
To our knowledge, our study is the first to examine 1,5-AG as a risk factor for hypoglycemia. We observed a strong association between 1,5-AG concentration and severe hypoglycemia independent of average glucose level. This finding is consistent with the biology of 1,5-AG as a biomarker of glucose excursions, and the variability captured by low 1,5-AG concentration may indicate greater insulin deficiency. Measuring 1,5-AG together with average glucose level may identify a subgroup of patients with diabetes who had high glycemic instability and were at high risk of future hypoglycemia.
Our finding that poor glycemic control was strongly associated with severe hypoglycemia extends the existing epidemiologic literature in type 2 diabetes (25). Although this may seem counter to the findings of clinical trials that have found higher rates of hypoglycemia associated with intensive glucose-lowering interventions, it is in concordance with an analysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, which found that hypoglycemic events primarily occurred in those with high HbA1c values during the trial who were unable to attain the glycemic target despite intensive treatment (2,6,26). Combined with the findings for 1,5-AG, our results suggest that concern about hypoglycemia should be greatest in those participants with high HbA1c values and glycemic variability, rather than in those with well-controlled HbA1c levels.
Our study is also the first to provide rigorous evidence on the association of disability with severe hypoglycemia, in the form of difficulty with either ADLs or IADLs. Difficulty with these tasks, such as eating, dressing, and preparing meals, is easily assessed with a few questions. Combined with the strong influence of cognitive score on hypoglycemia risk, these findings highlight that difficulty with diabetes self-care can arise due to either mental or physical incapacities.
Our finding that blacks are at higher risk of hypoglycemia is consistent with those of prior studies (6,8–10,27) demonstrating major racial disparities in hypoglycemia risk. In sensitivity analyses, we found that the observed racial disparities were not entirely explained by the available metrics of socioeconomic status. Indeed, neither education nor income was strongly associated with severe hypoglycemia risk in our study population after adjustment for traditional risk factors. However, we identified Medicaid insurance status as an important and robust predictor of hypoglycemia, which may reflect both low socioeconomic status and disability. Unmeasured health care access and use, medication adherence, and other socioeconomic and geographic disparities may play a major role in the racial differences in hypoglycemia risk. Future studies are needed to better identify those modifiable factors that can help ameliorate racial disparities in hypoglycemia risk and improve health outcomes in blacks.
We observed an increased risk of severe hypoglycemia among individuals using antidepressant medications. Prior studies of antidepressants (16,17) have found that the association with hypoglycemia varied either by the duration of use or by the type of medication. With respect to depression and depressive symptoms, several cross-sectional studies have shown that a history of severe hypoglycemia is associated with the severity of depressive symptoms, but the directionality of causation is unclear: depressive symptoms such as lack of appetite could lead to increased risk of hypoglycemia or previous episodes of hypoglycemia could lead to fear of hypoglycemia and withdrawal from daily activities, leading to depressive symptoms (28,29). Prospective cohort studies (15,26,30,31) looking at depression and subsequent hypoglycemia have been mixed, with several null and one positive association.
Contrary to other studies (6,15), we did not observe an association of β-blocker use with severe hypoglycemia. Although β-blockers are known to suppress symptoms of hypoglycemia and also may interfere with hepatic glycogenolysis and gluconeogenesis, there is some evidence that the use of nonselective β-blockers is more strongly associated with hypoglycemia than the use of cardioselective β-blockers (32,33). In our study, 82% of participants taking β-blockers were using cardioselective β-blockers, and in a post hoc analysis with assessment by type of β-blocker, the HR for nonselective β-blockers was 1.25 (95% CI 0.50–3.09) and for cardioselective β-blockers it was 0.93 (95% CI 0.58–1.49).
Previous studies of BMI and hypoglycemia have been mixed. In our minimally adjusted analyses, obesity appeared to be associated with increased risk of hypoglycemia, but after adjustment this association was no longer statistically significant. Because several prior studies (2,6,27) have shown increased risk of hypoglycemia among normal-weight persons rather than obese persons, we conducted a post hoc analysis examining waist circumference and waist-to-hip ratio. Neither of these alternative measures was associated with hypoglycemia. Ultimately, we did not observe a clear link between adiposity and hypoglycemia.
Kidney disease is a well-known risk factor for hypoglycemia because of its role in gluconeogenesis and drug clearance. We found a robust association of albuminuria with hypoglycemia, similar to those found in previous studies (6,34,35). Although eGFR was associated with hypoglycemia prior to multivariable adjustment, the association remained positive but was attenuated and was no longer significant after adjustment. It is likely that our study had limited power to detect a moderate association with reduced kidney function; indeed, there were few people with very poor kidney function (only 45 participants with eGFR <45 mL/min/1.73 m2). Other studies (27,35) have found a strong dose-response relationship between lower eGFR and increased risk of severe hypoglycemia.
There are several limitations of our study that are important to consider in the interpretation of these results. First, we had <200 severe hypoglycemic events, which limited our power to detect small to moderate associations. However, this was a similar or greater number of severe hypoglycemic events compared with other studies (7,14,30,31,34,36). Second, we were not able to look in detail at medication class and risk of hypoglycemia, since at the baseline examination for our study (1996–1998) many newer classes of medication were not yet available. Third, we did not have HbA1c data at baseline, but we were able to account for glycemic control using fructosamine. Fourth, within the ARIC Study, race and geographic location are conflated: blacks and whites were recruited at different study sites in the ARIC Study, with overlap only at the Forsyth County study site. Thus, although we cannot be certain that the difference between blacks and whites is due to racial disparities and not geographic differences, other studies (6,8–10) have found similarly higher rates among African Americans compared with whites. Fifth, we only had single baseline measurements of the risk factors examined here and cannot evaluate how changes in risk factors, including diabetes medications, during follow-up may have affected hypoglycemia risk. Sixth, as with all epidemiologic studies, underascertainment of hypoglycemia is a general concern, and it is possible that we may have missed some severe hypoglycemia events, especially those treated by ambulance or in the emergency department among participants who were not covered by CMS Fee-For-Service Part B. In analyses restricted to participants with CMS coverage at baseline, our results were generally similar.
The strengths of our study included the well-characterized epidemiological cohort with rigorous measurements of both traditional and nontraditional risk factors. The large percentage of black participants (32%) allowed us to examine black-white disparities and evaluate potential effect modification by race. Last, the linkage to CMS claims allowed us to include hypoglycemic events that were treated by ambulance or in the emergency department and not just events treated in the hospital. This may have resulted in better identification of risk factors for hypoglycemia itself rather than factors that would lead a person with hypoglycemia to be hospitalized.
Greater awareness of the risk of hypoglycemia is sorely needed. Numerous studies have documented the use of sulfonylureas and insulin in patients with chronic kidney disease, dementia, and/or low HbA1c values among older adults (37,38). In a recent survey in the Veterans Affairs Health System (39), 45% of primary care providers did not see any potential harm in continuing to treat a 77-year-old man with an eGFR of 26 mL/min/1.73 m2 with sulfonylureas. In contrast, patients’ choices about diabetes medication are strongly influenced by the risk of hypoglycemia, followed by long-term HbA1c (40). As the calls for shared decision-making in diabetes care grow louder, there is a greater need for accurate characterization of patients’ risk of hypoglycemia (41).
In conclusion, our results add to the paucity of data on incidence rates and risk factors for severe hypoglycemia. Given the aging population and increasing burden of diabetes, the importance of hypoglycemia and the risks and benefits from glycemic control will continue to be of great significance.
Supplementary Material
Article Information
Acknowledgments. The authors thank the staff and participants of the ARIC Study for their important contributions.
Funding. The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This research was supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants K24-DK-106414 and R01-DK-089174 to E.S. A.K.L. was supported by NIH/NHLBI grant T32HL007024. C.J.L. was supported by NIH/NIDDK grant 1K23-DK-107921. E.S.H. was supported by NIH/NIDDK grants K24-DK-105340 and P30-DK-092949 and by the Agency for Healthcare Research and Quality (AHRQ) grant R01-HS-018542.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.K.L. conceived and designed the study, conducted the statistical analyses, and wrote the manuscript. C.J.L., E.S.H., A.R.S., and J.C. made critical revisions to the manuscript for important intellectual content. E.S. helped to conceive and design the study, provided guidance for the statistical analysis, and made critical revisions to the manuscript for important intellectual content. E.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Heart Association EPI|Lifestyle Scientific Sessions 2017, Portland, OR, 7–10 March 2017.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-0819/-/DC1.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Laiteerapong N, Karter AJ, Liu JY, et al. Correlates of quality of life in older adults with diabetes: the diabetes & aging study. Diabetes Care 2011;34:1749–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoungas S, Patel A, Chalmers J, et al.; ADVANCE Collaborative Group . Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 3.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med 2014;174:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association Standards of medical care in diabetes–2017. Diabetes Care 2017;40(Suppl. 1):S1–S131 [DOI] [PubMed] [Google Scholar]
- 6.Miller ME, Bonds DE, Gerstein HC, et al.; ACCORD Investigators . The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 2010;340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun JS, Ko SH, Ko SH, et al. Cardiovascular disease predicts severe hypoglycemia in patients with type 2 diabetes. Diabetes Metab J 2015;39:498–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipska KJ, Ross JS, Wang Y, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med 2014;174:1116–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karter AJ, Laiteerapong N, Chin MH, et al. Ethnic differences in geriatric conditions and diabetes complications among older, insured adults with diabetes: the Diabetes and Aging Study. J Aging Health 2015;27:894–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karter AJ, Lipska KJ, O’Connor PJ, et al.; SUPREME-DM Study Group . High rates of severe hypoglycemia among African American patients with diabetes: the surveillance, prevention, and Management of Diabetes Mellitus (SUPREME-DM) network. J Diabetes Complications 2017;31:869–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Carpena-Ruiz M, Montero-Errasquín B, Sánchez-Castellano C, Sánchez-García E. Exclusion of older adults from ongoing clinical trials about type 2 diabetes mellitus. J Am Geriatr Soc 2013;61:734–738 [DOI] [PubMed] [Google Scholar]
- 12.ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Punthakee Z, Miller ME, Launer LJ, et al.; ACCORD Group of Investigators; ACCORD-MIND Investigators . Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 2012;35:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathak R, Schroeder EB, Seaquist ER, et al. Severe hypoglycemia requiring medical intervention in a large cohort of adults with diabetes receiving care in U.S. integrated health care delivery systems: 2005-2011. Diabetes Care 2016;39:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derijks HJ, Heerdink ER, De Koning FH, Janknegt R, Klungel OH, Egberts AC. The association between antidepressant use and hypoglycaemia in diabetic patients: a nested case-control study. Pharmacoepidemiol Drug Saf 2008;17:336–344 [DOI] [PubMed] [Google Scholar]
- 17.McIntyre RS, Soczynska JK, Konarski JZ, Kennedy SH. The effect of antidepressants on glucose homeostasis and insulin sensitivity: synthesis and mechanisms. Expert Opin Drug Saf 2006;5:157–168 [DOI] [PubMed] [Google Scholar]
- 18.Gerich JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev 1998;19:491–503 [DOI] [PubMed] [Google Scholar]
- 19.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008;57:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee AK, McEvoy JW, Hoogeveen RC, Ballantyne CM, Selvin E. Severe hypoglycemia and elevated high-sensitivity cardiac troponin T in older adults with diabetes: the ARIC Study. J Am Coll Cardiol 2016;68:1370–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klimontov VV, Myakina NE. Glucose variability indices predict the episodes of nocturnal hypoglycemia in elderly type 2 diabetic patients treated with insulin. Diabetes Metab Syndr 2017;11:119–124 [DOI] [PubMed] [Google Scholar]
- 22.Qu Y, Jacober SJ, Zhang Q, Wolka LL, DeVries JH. Rate of hypoglycemia in insulin-treated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol Ther 2012;14:1008–1012 [DOI] [PubMed] [Google Scholar]
- 23.Ginde AA, Blanc PG, Lieberman RM, Camargo CA Jr. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leese GP, Wang J, Broomhall J, et al.; DARTS/MEMO Collaboration . Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care 2003;26:1176–1180 [DOI] [PubMed] [Google Scholar]
- 25.Lipska KJ, Warton EM, Huang ES, et al. HbA1c and risk of severe hypoglycemia in type 2 diabetes: the Diabetes and Aging Study. Diabetes Care 2013;36:3535–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ORIGIN Trial Investigators Predictors of nonsevere and severe hypoglycemia during glucose-lowering treatment with insulin glargine or standard drugs in the ORIGIN trial. Diabetes Care 2015;38:22–28 [DOI] [PubMed] [Google Scholar]
- 27.Cahn A, Raz I, Mosenzon O, et al. Predisposing factors for any and major hypoglycemia with saxagliptin versus placebo and overall: analysis from the SAVOR-TIMI 53 Trial. Diabetes Care 2016;39:1329–1337 [DOI] [PubMed] [Google Scholar]
- 28.Green AJ, Fox KM, Grandy S; SHIELD Study Group . Self-reported hypoglycemia and impact on quality of life and depression among adults with type 2 diabetes mellitus. Diabetes Res Clin Pract 2012;96:313–318 [DOI] [PubMed] [Google Scholar]
- 29.Kikuchi Y, Iwase M, Fujii H, et al. Association of severe hypoglycemia with depressive symptoms in patients with type 2 diabetes: the Fukuoka Diabetes Registry. BMJ Open Diabetes Res Care 2015;3:e000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruce DG, Davis WA, Casey GP, et al. Severe hypoglycaemia and cognitive impairment in older patients with diabetes: the Fremantle Diabetes Study. Diabetologia 2009;52:1808–1815 [DOI] [PubMed] [Google Scholar]
- 31.Yaffe K, Falvey CM, Hamilton N, et al.; Health ABC Study . Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med 2013;173:1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawicki PT, Siebenhofer A. Betablocker treatment in diabetes mellitus. J Intern Med 2001;250:11–17 [DOI] [PubMed] [Google Scholar]
- 33.ter Braak EW, Appelman AM, van de Laak M, Stolk RP, van Haeften TW, Erkelens DW. Clinical characteristics of type 1 diabetic patients with and without severe hypoglycemia. Diabetes Care 2000;23:1467–1471 [DOI] [PubMed] [Google Scholar]
- 34.Yun JS, Ko SH, Ko SH, et al. Presence of macroalbuminuria predicts severe hypoglycemia in patients with type 2 diabetes: a 10-year follow-up study. Diabetes Care 2013;36:1283–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodge M, McArthur E, Garg AX, Tangri N, Clemens KK. Hypoglycemia incidence in older adults by estimated GFR. Am J Kidney Dis 2017;70:59–68 [DOI] [PubMed] [Google Scholar]
- 36.Davis TME, Brown SGA, Jacobs IG, Bulsara M, Bruce DG, Davis WA. Determinants of severe hypoglycemia complicating type 2 diabetes: the Fremantle diabetes study. J Clin Endocrinol Metab 2010;95:2240–2247 [DOI] [PubMed] [Google Scholar]
- 37.Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorpe CT, Gellad WF, Good CB, et al. Tight glycemic control and use of hypoglycemic medications in older veterans with type 2 diabetes and comorbid dementia. Diabetes Care 2015;38:588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caverly TJ, Fagerlin A, Zikmund-Fisher BJ, et al. Appropriate prescribing for patients with diabetes at high risk for hypoglycemia: national survey of Veterans Affairs Health Care Professionals. JAMA Intern Med 2015;175:1994–1996 [DOI] [PubMed] [Google Scholar]
- 40.Mühlbacher A, Bethge S. What matters in type 2 diabetes mellitus oral treatment? A discrete choice experiment to evaluate patient preferences. Eur J Health Econ 2016;17:1125–1140 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Gutierrez R, Gionfriddo MR, Ospina NS, et al. Shared decision making in endocrinology: present and future directions. Lancet Diabetes Endocrinol 2016;4:706–716 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.