Abstract
OBJECTIVE
Offspring of pregnancies affected by gestational diabetes mellitus (GDM) are at increased risk of the development of type 2 diabetes. However, the extent to which these dysmetabolic traits may be due to offspring and/or maternal adiposity is unknown. We examined body composition and associated cardiometabolic traits in 561 9- to 16-year-old offspring of mothers with GDM and 597 control offspring.
RESEARCH DESIGN AND METHODS
We measured anthropometric characteristics; puberty status; blood pressure; and fasting glucose, insulin, C-peptide, and lipid levels; and conducted a DEXA scan in a subset of the cohort. Differences in the outcomes between offspring of mothers with GDM and control subjects were examined using linear and logistic regression models.
RESULTS
After adjustment for age and sex, offspring of mothers with GDM displayed higher weight, BMI, waist-to-hip ratio (WHR), systolic blood pressure, and resting heart rate and lower height. Offspring of mothers with GDM had higher total and abdominal fat percentages and lower muscle mass percentages, but these differences disappeared after correction for offspring BMI. The offspring of mothers with GDM displayed higher fasting plasma glucose, insulin, C-peptide, HOMA-insulin resistance (IR), and plasma triglyceride levels, whereas fasting plasma HDL cholesterol levels were decreased. Female offspring of mothers with GDM had an earlier onset of puberty than control offspring. Offspring of mothers with GDM had significantly higher BMI, WHR, fasting glucose, and HOMA-IR levels after adjustment for maternal prepregnancy BMI, and glucose and HOMA-IR remained elevated in the offspring of mothers with GDM after correction for both maternal and offspring BMIs.
CONCLUSIONS
In summary, adolescent offspring of women with GDM show increased adiposity, an adverse cardiometabolic profile, and earlier onset of puberty among girls. Increased fasting glucose and HOMA-IR levels among the offspring of mothers with GDM may be explained by the programming effects of hyperglycemia in pregnancy.
Introduction
Offspring of mothers with gestational diabetes mellitus (GDM) are at increased risk for the development of obesity, insulin resistance (IR), and type 2 diabetes (1–4). Maternal obesity is one of the most important risk factors of both GDM and offspring adiposity, and the extent to which maternal obesity explains offspring adiposity and associated dysmetabolic traits independent of other factors including hyperglycemia in pregnancy is controversial and the particular impact of hyperglycemia per se on offspring metabolic health is debated (5,6). Whereas intensified glucose-lowering treatment of women with GDM reduced macrosomia at birth, no beneficial effect on offspring adiposity or associated cardiometabolic dysfunctions at ages 5–10 years was seen (7,8).
Few studies have examined the impact of GDM on adiposity and IR during adolescence, and the joint influence of these factors on the onset of puberty. Early onset is associated with emotional challenges and can result in short stature, both of which may have influence at the individual and public health levels. One study (9) examined the impact of GDM on adiposity and metabolic variables across five puberty stages and found no difference in puberty development between offspring of mothers with GDM and control subjects but found an increase in body fat percentages during all Tanner stages.
Previous studies of offspring of mothers with GDM have been variable in size (n = 24–1,475 cases) with the largest studies having only a few clinical measurements such as height, weight, and waist-to-hip ratio (WHR) in the offspring (10,11). In the current study, we report clinical and metabolic characteristics including body composition and puberty status in a large cohort of 9- to 16-year-old offspring of women with and without previous GDM who were recruited from the Danish National Birth Cohort (DNBC) (12). Additionally, we examine the association of GDM with offspring metabolic disease and puberty development while accounting for offspring and maternal degree of adiposity.
Research Design and Methods
Subjects
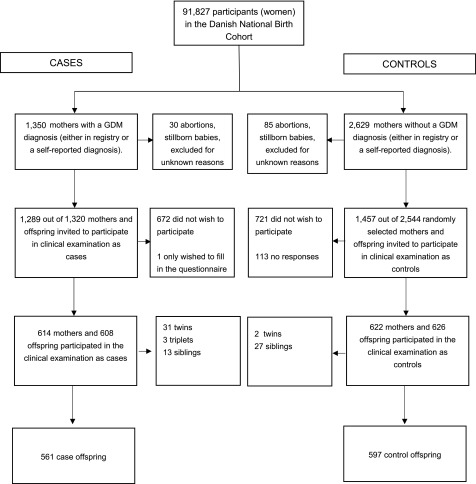
Participants were recruited from the DNBC, which enrolled 91,827 primarily Caucasian women collectively contributing >100,000 pregnancies between January 1996 and October 2002, as described in detail previously (12). Briefly, data collection included four telephone interviews in gestation weeks 12 and 30, and 6 and 18 months postpartum. From the DNBC, we included 1,350 women with a diagnosis of GDM and 2,629 randomly selected control subjects and invited them and their offspring to participate in a clinical follow-up examination during the period March 2012 to April 2014 (13). In total, 608 (44%) case mother-offspring pairs and 626 (28%) control mother-offspring pairs participated. The main reason for nonparticipation was lack of time. We excluded multiple births including 33 twins (31 from mothers with GDM) and 3 triplets (all from mothers with GDM), and only included the first sibling in our analyses (80 siblings in total, 40 kept in analyses) to avoid correlated measures. This left us with 561 offspring of mothers with GDM and 597 control offspring in our analyses (Fig. 1). The offspring were 9–16 years old at the time of examination. The study was approved by the Regional Scientific Ethics Committee for the municipalities of Copenhagen and Frederiksberg (H-4–2011–045 and H-4–2013–129). Consent from both parents was essential for the participation of the child in the study.
Figure 1.
Flowchart of enrollment and examination of women and children in the Diabetes and Women’s Health Study.
Diagnosis of GDM
Initially, we used two different sources to identify women with a history of GDM. Women were classified with a diagnosis of GDM if they had responded positively to a question about GDM in at least one of the interviews conducted in gestation week 30 or 6 months postpartum, respectively. Furthermore, we used the Danish National Patient Register to extract information about diagnoses of GDM (ICD-10 classifications O244 and O249). Women with a self-reported diagnosis of GDM and/or an ICD-10 diagnosis were classified as having had suspected GDM in our main analyses. Additionally, in sensitivity analyses we used an alternative diagnosis of GDM defined as “best clinical judgement.” This diagnosis was based on a thorough review by two clinicians of the hospital records of 96.5% of all GDM case patients classified as described above. The best clinical judgement or verified GDM diagnosis was based on available data such as blood glucose measurements, if available, or on other notes from the doctor indicating GDM. In the sensitivity analyses in the present article, only those with GDM based upon the best clinical judgment were included (n = 332).
In Denmark, a risk factor–based screening for GDM with a 75-g diagnostic oral glucose tolerance test was recommended during 1996–2002. If results from a 75-g 3-h oral glucose tolerance test were available from the hospital records, GDM was diagnosed if two or more glucose values exceeded the mean ± 3 SDs on a curve based on a group of 40 healthy, nonobese, nonpregnant Danish women without a family history of diabetes. However, in a few smaller departments the World Health Organization criteria were used. The diagnosis of the GDM women is described in detail in the study by Olsen et al. (14).
Clinical Examinations
All participating offspring underwent a clinical examination that included anthropometric, metabolic, and body composition measurements. Offspring were weighed without shoes and were lightly dressed. We measured waist circumference at the level of the umbilicus using a soft tape on standing subjects. Hip circumference was measured over the widest part of the gluteal region. After 10 min, the resting blood pressure and heart rate were measured with an Omron blood pressure device with the offspring in the supine position. All measurements were taken twice, and if the differences exceeded 0.5 cm or 0.5 kg for the anthropometric measurements, or 5 mmHg for blood pressure measurements, a third measurement was taken. In all analyses, the mean value of the measurements was used.
Offspring metabolic outcomes were obtained from a fasting blood sample that was taken during the clinical examination, and plasma, serum, buffy coat, and whole blood (PAXgene) were collected. Blood samples for glucose measurements were drawn in K-oxalate-Na-fluoride vials and in lithium-heparin vials for insulin, C-peptide, and lipid traits. All parameters were measured using standard laboratory methods on the Modular P-module (Roche, Mannheim, Germany). Coefficients of variance were 4–5% for glucose, insulin, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides and 8% for C-peptide. HOMA-IR was calculated as follows: ([(fasting plasma insulin (pmol/L) × fasting plasma glucose (mmol/L)/22.5] × 0.144) (15).
In order to determine the puberty status of the study participants, clinical evaluations were made including pubertal staging of breast development and pubic hair for the girls according to the classifications of Marshall and Tanner (16,17). Breast stage ≥B2 or girls at pubic hair stage ≥PH2 was considered to be a marker of pubertal onset. Among the boys, a testicular volume of ≥4 mL, pubic hair stage ≥PH2, or boys genital stage ≥G2 was considered to be a marker of pubertal onset. A total of 238 offspring of mothers with GDM and 256 control offspring agreed to have at least one of the Tanner score examinations performed. Finally, body composition outcomes were evaluated in a subset (n = 637) of the offspring who underwent DEXA scanning (Lunar Prodigy Advanced EnCore, including pediatric software; GE Healthcare).
Statistical Analyses
Normally distributed, continuous outcomes were described using the mean and SD, whereas median and interquartile range were used for skewed, continuous outcomes. Differences in anthropometric, metabolic, and body composition outcomes between offspring exposed to GDM and control offspring were examined using linear regression models. For normally distributed outcome variables, we calculated β-coefficients and 95% CIs to estimate mean differences, whereas skewed variables were log transformed, and for these we calculated the percentage differences and 95% CIs. We used logistic regression models to analyze puberty status and calculated odds ratios and 95% CIs.
A priori, we decided to first adjust for offspring age at the clinical examination and to analyze the age-adjusted estimates separately for boys and girls to investigate potential sex-specific differences. Because we did not see any marked differences between male and female offspring in the effect estimates, we decided to include offspring sex in our minimally adjusted model as a potential confounder rather than to stratify on sex. To examine whether observed cardiometabolic differences were explained by the offspring’s own degree of adiposity, we included one model with adjustment for offspring BMI and one model with additional adjustment for offspring WHR. For those variables that remained statistically significantly different between offspring of mothers with GDM and control offspring after adjustment for offspring BMI and WHR, we subsequently conducted analyses in which we adjusted for maternal prepregnancy BMI (in categories <18.5, 18.5–24.99, 25–29.99, and ≥30 kg/m2). Maternal prepregnancy BMI was obtained from telephone interviews in gestation week 12. We did not adjust for other potential confounding variables, since our main aim was to determine whether any observed differences were related to maternal obesity or hyperglycemia in pregnancy, rather than teasing out the contribution of a range of other potential confounding variables.
In addition, we stratified for maternal prepregnancy BMI in four groups to examine whether there were differences when comparing offspring of mothers with GDM to control subjects across groups of maternal prepregnancy BMI. Additionally, we performed analyses in a subsample of women where GDM (n = 332) was defined according to best clinical judgement (14) compared with control subjects.
Results
Table 1 presents the clinical characteristics of the offspring. There was an even sex distribution among case patient and control offspring, but control offspring were slightly older than offspring of mothers with GDM at the time of follow-up, which was due to a time displacement for the examination time for some of the control offspring. When we adjusted for this age difference, the offspring of mothers with GDM were heavier and had a higher BMI, larger waist and hip circumferences, elevated WHR, as well as increased systolic blood pressure and heart rate compared with control offspring (Table 1). Furthermore, the offspring of mothers with GDM had higher fasting whole-blood and fasting plasma glucose levels, as well as higher fasting plasma insulin, fasting C-peptide, and HOMA-IR levels. In addition, the offspring of mothers with GDM had an unhealthier plasma lipid profile with higher triglyceride and lower HDL levels. Offspring of mothers with GDM also had higher total fat percentages, more abdominal fat, and lower lean body mass percentages than control offspring. Among the female offspring of mothers with GDM, the odds of having reached puberty based on Tanner stage for breast development was almost doubled compared with control offspring. No differences in puberty development were observed between male offspring of mothers with GDM and control offspring (Table 1). After adjustment for puberty status, we still observed significant differences in the IR markers between offspring of mothers with GDM and control offspring (data not shown).
Table 1.
Anthropometric, metabolic, body composition, and puberty characteristics of offspring of mothers with GDM and control subjects at ages 9–16 years
| Crude measurements |
Pa | Age- and sex-adjusted estimates for mean difference (95% CI) or % difference (IQR)* | Pb | ||
|---|---|---|---|---|---|
| Offspring of mothers with GDM | Control offspring | ||||
| Anthropometric characteristics, N | 546–561 | 590–597 | |||
| Age (years) | 12.1 (1.5) | 12.8 (1.5) | <0.001 | ||
| Sex (boys) | 295 (52.6%) | 301 (50.4%) | 0.68 | ||
| Weight (kg) | 48.5 (12.7) | 47.2 (12.1) | 0.08 | 4.66 (3.48, 5.84) | <0.001 |
| Height (cm) | 156.8 (11.4) | 159.5 (11.4) | <0.001 | 1.15 (0.27, 2.03) | 0.01 |
| BMI (kg/m2)* | 18.8 (4.2) | 17.9 (3.4) | <0.001 | 9% (7–11%) | <0.001 |
| Waist (cm) | 73.3 (10.6) | 69.9 (8.4) | <0.001 | 4.92 (3.87, 5.98) | <0.001 |
| Hip circumference (cm) | 83.8 (9.6) | 82.7 (9.3) | 0.07 | 3.47 (2.55, 4.39) | <0.001 |
| WHR | 0.87 (0.06) | 0.85 (0.05) | <0.001 | 0.02 (0.01, 0.03) | <0.001 |
| Head circumference (cm) | 55.8 (2.1) | 55.5 (2.2) | 0.18 | 0.51 (0.28, 0.75) | <0.001 |
| Systolic blood pressure (mmHg) | 109.7 (8.6) | 109.5 (8.6) | 0.75 | 1.04 (0.06, 2.01) | 0.04 |
| Diastolic blood pressure (mmHg) | 62.5 (6.0) | 62.6 (6.1) | 0.84 | −0.20 (−0.92, 0.51) | 0.58 |
| Heart rate (bpm) | 69.7 (2.1) | 68.1 (10.0) | 0.001 | 0.81 (−0.34, 1.96) | 0.17 |
| Metabolic characteristics, N | 468–522 | 508–559 | |||
| Whole-blood fasting glucose (mmol/L)* | 4.7 (0.8) | 4.5 (0.7) | <0.001 | 3% (1–5%) | 0.001 |
| Fasting plasma glucose (mmol/L)* | 5.0 (0.8) | 4.8 (0.6) | <0.001 | 4% (3–6%) | <0.001 |
| Fasting insulin (pmol/L)* | 69.4 (47.3) | 61.3 (34.7) | 0.001 | 17% (10–24%) | <0.001 |
| Fasting C-peptide (pmol/L) | 596 (211) | 575 (189) | 0.05 | 51 (28, 74) | <0.001 |
| HOMA-IR* | 2.2 (1.6) | 1.9 (1.1) | <0.001 | 21% (14–30%) | <0.001 |
| Triglycerides (mmol/L)* | 0.73 (0.4) | 0.70 (0.4) | 0.58 | 5% (1–10%) | 0.04 |
| HDL cholesterol (mmol/L) | 1.5 (0.4) | 1.6 (0.4) | 0.11 | −0.07 (−0.11, −0.02) | 0.004 |
| LDL cholesterol (mmol/L) | 2.4 (0.7) | 2.3 (0.6) | 0.08 | 0.06 (−0.01, 0.14) | 0.11 |
| Total cholesterol (mmol/L) | 4.3 (0.7) | 4.2 (0.7) | 0.22 | 0.03 (−0.05, 0.12) | 0.47 |
| Body composition measured by DEXA, N | 191 | 446 | |||
| Total fat (%) | 31.2 (8.1) | 27.0 (7.0) | <0.001 | 3.45 (2.22, 4.69) | <0.001 |
| Total lean mass (%) | 66.2 (7.5) | 70.1 (6.5) | <0.001 | −3.21 (−4.37, −2.07) | <0.001 |
| Total android tissue (% fat)* | 25.6 (20.1) | 19.4 (13.8) | <0.001 | 22% (12–33%) | <0.001 |
| Total gynoid tissue (% fat) | 35.3 (8.3) | 31.2 (7.8) | <0.001 | 2.98 (1.71, 4.25) | <0.001 |
| Fat distribution (android/gynoid ratio) | 0.73 (0.2) | 0.66 (0.18) | <0.001 | 0.08 (0.05, 0.12) | <0.001 |
| Total bone mass density (mg/cm2) | 0.9 (0.1) | 1.0 (0.1) | <0.001 | 0.02 (0.001, 0.03) | 0.04 |
| Puberty status, N | 192–238† | 176–256† | |||
| Girls breast stage, n yes ≥B2 (%) | 141 (59.2%) | 169 (66.0%) | 0.18 | 1.99 (1.18, 3.34) | 0.01 |
| Girls pubic hair, n yes ≥PH2 (%) | 99 (44.8%) | 133 (56.1%) | 0.06 | 1.51 (0.90, 2.55) | 0.12 |
| Boys testis size, n yes ≥4 mL (%) | 143 (74.5%) | 156 (85.7%) | 0.02 | 0.77 (0.42, 1.41) | 0.40 |
| Boys pubic hair, n yes ≥PH2 (%) | 50 (24.3%) | 60 (29.6%) | 0.02 | 1.74 (0.92, 3.28) | 0.09 |
| Boys genital stage, n yes ≥G2 (%) | 63 (32.6%) | 66 (37.5%) | 0.07 | 1.24 (0.72, 2.14) | 0.45 |
For the crude estimate, data are presented as the mean (SD) or *median (interquartile range [IQR]) for normally and non-normally distributed variables, respectively. When data are adjusted for age and sex, they are presented as either mean difference when the residuals are normally distributed or as % difference when data are log transformed. Android tissue % fat, located in the abdominal area; gynoid tissue fat %, located around the hips.
†Values are n (%) and adjusted estimates are OR (95% CI) and are only adjusted for age.
aP values calculated using Student t test, Kruskal-Wallis test, or χ2 tests.
bP values for age- and sex-adjusted measurements comparing GDM to control offspring.
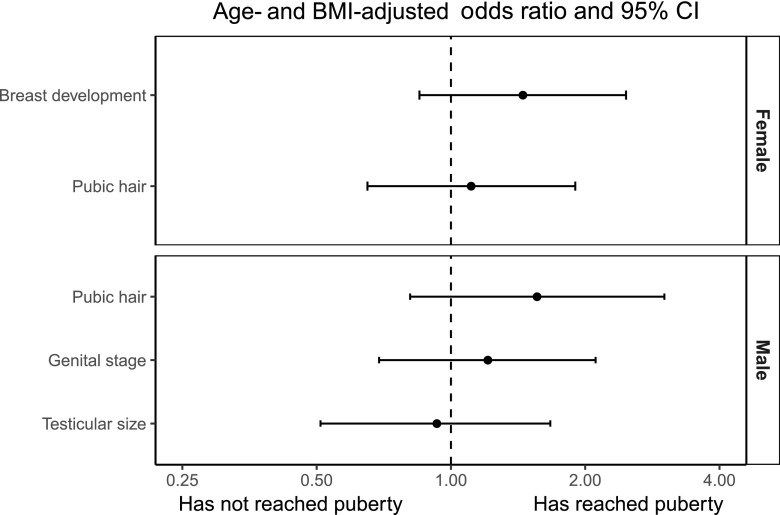
Since adiposity is one of the most important risk factors for IR, type 2 diabetes and cardiovascular disease (18), we examined whether the adverse cardiometabolic profile among offspring of mothers with GDM was explained by their increased adiposity compared with control subjects. When adjusted for offspring BMI, the offspring of mothers with GDM still had significantly higher waist circumference, WHR, fasting plasma glucose levels, fasting insulin levels, and HOMA-IR levels (Table 2). Blood pressure, fasting C-peptide levels, lipid profile, and data on body composition obtained from a DEXA scan were not significantly different between the two groups after adjustment for offspring BMI (Table 2). In addition, the association with earlier onset of puberty among female offspring of mothers with GDM was no longer significant after adjustment for the offspring’s own BMI (Fig. 2). Adjusting for offspring BMI z score instead of BMI did not change our results (data not shown). Additionally, the offspring of mothers with GDM displayed significantly higher fasting glucose levels (OR 1.04 [95% CI 1.02, 1.05]; P < 0.0001) and HOMA-IR (1.09 [1.02, 1.16]; P = 0.006) after further adjustment for WHR.
Table 2.
Differences in offspring anthropometric and metabolic characteristics comparing offspring of mothers with GDM to control subjects after adjustment for offspring age, sex, and BMI and maternal prepregnancy BMI
| Offspring outcomes | Mean difference or % difference* (95% CI) | Pc | Mean difference or % difference* (95% CI) | Pd |
|---|---|---|---|---|
| Anthropometric characteristics | ||||
| BMI (kg/m2)# | 4% (2–6%) | <0.0001 | ||
| Waist circumference (cm) | 0.83 (0.30, 1.35) | 0.002 | 0.52 (−0.06, 1.08) | 0.08 |
| Hip circumference (cm) | 0.01 (−0.48, 0.49) | 0.97 | ||
| WHR | 0.009 (0.003, 0.02) | 0.002 | 0.009 (0.002, 0.02) | 0.01 |
| Head circumference (cm) | 0.17 (−0.05, 0.40) | 0.13 | ||
| Systolic blood pressure (mmHg) | 0.30 (−0.70, 1.30) | 0.55 | ||
| Diastolic blood pressure (mmHg) | −0.63 (−1.26, 0.11) | 0.09 | ||
| Heart rate (bpm) | 0.82 (−0.37, 2.02) | 0.17 | ||
| Metabolic characteristics | ||||
| Whole-blood fasting glucose (mmol/L)* | 2% (1–4%) | 0.02 | 2% (1–4%) | 0.02 |
| Fasting plasma glucose (mmol/L)* | 4% (1–5%) | <0.0001 | 4% (2–5%) | <0.0001 |
| Fasting insulin (pmol/L)* | 7% (1–13%) | 0.04 | 4% (−2 to 11%) | 0.18 |
| C-peptide (pmol/L) | 8.7 (−13, 30) | 0.43 | ||
| HOMA-IR* | 11% (4–18%) | 0.002 | 8% (1–16%) | 0.02 |
| Triglycerides (mmol/L)* | 0% (−5 to 5%) | 0.92 | ||
| HDL cholesterol (mmol/L) | −0.02 (−0.07, 0.02) | 0.30 | ||
| LDL cholesterol (mmol/L) | 0.003 (−0.07, 0.08) | 0.93 | ||
| Total cholesterol (mmol/L) | −0.002 (−0.09, 0.09) | 0.96 | ||
| Body composition measured by DEXA | ||||
| Total fat (%) | 0.72 (−0.17, 1.61) | 0.11 | ||
| Total lean mass (%) | −0.70 (−1.54, 0.14) | 0.10 | ||
| Total android tissue (% fat)* | 2% (−4 to 8%) | 0.50 | ||
| Total gynoid tissue (% fat) | 0.56 (−0.48, 1.59) | 0.29 | ||
| Fat distribution (android/gynoid ratio) | 0.005 (−0.02, 0.03) | 0.66 | ||
| Total bone mass density (mg/cm2) | −0.007 (−0.02, 0.007) | 0.30 |
*Non-normally distributed variables
cP adjusted for age, sex, and offspring BMI.
dP adjusted for age, sex, offspring BMI, and maternal prepregnancy BMI. Only variables that were significant after adjustment for offspring BMI were further adjusted for maternal prepregnancy BMI.
#Adjusted for age, sex, and maternal prepregnancy BMI.
Figure 2.
Differences in offspring puberty status comparing the offspring of mothers with GDM with control offspring. Odds ratios are adjusted for offspring BMI.
As adiposity is highly heritable (19,20), we subsequently examined whether the increased BMI, waist circumference, and WHR together with the adverse metabolic profile among the offspring of mothers with GDM could be explained by maternal obesity before pregnancy. After adjustment for maternal prepregnancy BMI, the offspring of mothers with GDM still had higher BMI (Table 2). WHR, waist circumference, fasting glucose level, and HOMA-IR were all significantly higher in the offspring of mothers with GDM after further adjustment for both offspring BMI and maternal prepregnancy BMI (Table 2). Interestingly, the difference in the anthropometric, metabolic, and body composition outcomes between the offspring of mothers with GDM and control subjects appeared to be stronger among offspring whose mothers were of normal weight in pregnancy (BMI >18.5 and <25 kg/m2) compared with overweight (BMI 25–30 kg/m2) or obese (BMI >30 kg/m2) (Table 3). Among obese mothers, no differences were observed between the offspring of mothers with GDM and control subjects.
Table 3.
Age- and sex-adjusted mean differences or percentage differences (95% CI) for offspring characteristics across groups of maternal prepregnancy BMI comparing offspring of mothers with GDM to control subjects
| Maternal prepregnancy BMI <18.5 |
Maternal prepregnancy BMI 18.5-<25 |
Maternal prepregnancy BMI 25-<30 |
Maternal prepregnancy BMI ≥30 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean difference or % difference* (95% CI) | P | N | Mean difference or % difference* (95% CI) | P | N | Mean difference or % difference* (95% CI) | P | N | Mean difference or % difference* (95% CI) | P | |
| Anthropometric characteristics | ||||||||||||
| Weight (kg) | 44 | −2.04 (−7.64, 3.55) | 0.46 | 646 | 3.24 (1.79, 4.70) | <0.001 | 233 | 1.34 (−1.63, 4.31) | 0.37 | 180 | 1.43 (−2.86, 5.73) | 0.51 |
| Height (cm) | 44 | −2.63 (−9.01, 3.75) | 0.41 | 646 | 1.56 (0.36, 2.76) | 0.01 | 234 | −0.63 (−2.78, 1.52) | 0.57 | 180 | −0.08 (−2.97, 2.80) | 0.95 |
| BMI (kg/m2)* | 44 | −2% (−12 to 9%) | 0.72 | 646 | 5% (3–7%) | <0.001 | 234 | 4% (−1 to 9%) | 0.12 | 180 | 3% (−3 to 10%) | 0.34 |
| Waist (cm) | 44 | 1.34 (−4.32, 7.00) | 0.63 | 645 | 2.66 (1.51, 3.82) | <0.001 | 232 | 3.00 (0.22, 5.78) | 0.03 | 180 | 2.25 (−1.86, 6.37) | 0.28 |
| Hip circumference (cm) | 44 | −1.74 (−5.90, 2.41) | 0.40 | 646 | 1.80 (0.66, 2.94) | 0.002 | 233 | 1.18 (−1.07, 3.43) | 0.30 | 179 | 1.68 (−1.61, 4.97) | 0.32 |
| Head circumference (cm) | 44 | −1.14 (−2.43, 0.15) | 0.08 | 644 | 0.42 (0.08, 0.76) | 0.01 | 230 | 0.53 (0.03, 1.03) | 0.04 | 179 | −0.22 (−0.98, 0.55) | 0.58 |
| Systolic blood pressure (mmHg) | 44 | −0.29 (−7.62, 7.04) | 0.94 | 645 | 1.56 (0.16, 2.97) | 0.03 | 231 | −0.54 (−2.74, 1.65) | 0.63 | 178 | −0.75 (−3.80, 2.30) | 0.63 |
| Diastolic blood pressure (mmHg) | 44 | 0.68 (−4.00, 5.36) | 0.77 | 644 | −0.34 (−1.37, 0.70) | 0.53 | 231 | 0.06 (−1.50, 1.62) | 0.94 | 178 | −1.22 (−3.54, 1.09) | 0.30 |
| Heart rate (bpm) | 44 | 8.87 (−1.48, 19.22) | 0.09 | 640 | 0.33 (−1.27, 1.93) | 0.69 | 227 | 1.94 (−0.74, 4.62) | 0.16 | 174 | −1.26 (−4.87, 2.34) | 0.49 |
| Metabolic characteristics | ||||||||||||
| Whole-blood fasting glucose (mmol/L)* | 44 | 2% (−2 to 16%) | 0.79 | 609 | 2% (1–5%) | 0.07 | 222 | 4% (1–8%) | 0.02 | 165 | 1% (−3 to 6%) | 0.57 |
| Plasma glucose (mmol/L)* | 41 | −3% (−12 to 6%) | 0.46 | 574 | 4% (2–6%) | 0.0001 | 210 | 4% (1–7%) | 0.01 | 156 | 1% (−3 to 5%) | 0.64 |
| C-peptide (pmol/L) | 40 | 155 (−4, 313) | 0.06 | 572 | 26 (−5, 57) | 0.10 | 213 | −5 (−62, 52) | 0.87 | 154 | 43 (−33, 119) | 0.26 |
| Insulin (pmol/L)* | 39 | 10% (−14 to 59%) | 0.60 | 559 | 11% (2–21%) | 0.02 | 204 | −1% (−13 to 13%) | 0.99 | 150 | 19% (−1 to 43%) | 0.06 |
| Triglycerides (mmol/L)* | 40 | 5% (−17 to 51%) | 0.79 | 573 | 5% (−1 to 11%) | 0.12 | 214 | −6% (−17 to 6%) | 0.29 | 154 | 8% (−8 to 27%) | 0.36 |
| HOMA-IR* | 39 | 9% (−27 to 62%) | 0.68 | 551 | 15% (5–26%) | 0.002 | 201 | 3% (−11 to 19%) | 0.68 | 148 | 22% (−1 to 50%) | 0.06 |
| HDL cholesterol (mmol/L) | 40 | −0.10 (−0.41, 0.20) | 0.50 | 573 | −0.03 (−0.10, 0.03) | 0.30 | 214 | −0.03 (−0.14, 0.08) | 0.57 | 154 | −0.01 (−0.15, 0.12) | 0.84 |
| LDL cholesterol (mmol/L) | 40 | 0.02 (−0.51, 0.55) | 0.94 | 573 | 0.02 (−0.09, 0.12) | 0.76 | 214 | 0.13 (−0.07, 0.33) | 0.19 | 154 | 0.03 (−0.23, 0.28) | 0.84 |
| Total cholesterol (mmol/L) | 40 | −0.01 (−0.64, 0.61) | 0.97 | 573 | 0.001 (−0.12, 0.12) | 0.98 | 214 | 0.15 (−0.07, 0.36) | 0.18 | 154 | −0.01 (−0.29, 0.26) | 0.93 |
| Body composition | ||||||||||||
| Total fat (%) | 36 | 1.11 (−7.29, 9.50) | 0.79 | 393 | 2.03 (0.42, 3.64) | 0.01 | 113 | 1.34 (−1.63, 4.30) | 0.37 | 69 | 0.43 (−3.58, 4.43) | 0.83 |
| Lean mass (%) | 36 | −0.98 (−8.73, 6.77) | 0.80 | 393 | −1.91 (−3.40, −0.41) | 0.01 | 113 | −1.26 (−4.01, 1.50) | 0.37 | 69 | −0.47 (−4.19, 3.25) | 0.80 |
| Total android tissue (% fat)* | 36 | 6% (−43 to 97%) | 0.85 | 393 | 12% (1–26%) | 0.06 | 113 | 3% (−14 to 24%) | 0.74 | 69 | 1% (−19 to 26%) | 0.94 |
| Total gynoid tissue (% fat) | 36 | 0.69 (−8.02, 9.41) | 0.87 | 393 | 1.69 (−0.07, 3.45) | 0.06 | 113 | 0.72 (−2.19, 3.63) | 0.63 | 69 | 0.20 (−3.50, 3.90) | 0.92 |
| Fat distribution (android/gynoid ratio) | 36 | 0.06 (−0.17, 0.28) | 0.60 | 393 | 0.05 (−0.004, 0.09) | 0.05 | 113 | 0.02 (−0.06, 0.10) | 0.57 | 69 | 0.004 (−0.09, 0.10) | 0.94 |
| Total bone mass density (mg/cm2) | 36 | −0.04 (−0.14, 0.06) | 0.44 | 393 | 0.01 (−0.01, 0.03) | 0.45 | 113 | 0.001 (−0.03, 0.04) | 0.96 | 69 | 0.01 (−0.04, 0.05) | 0.79 |
Age- and sex-adjusted mean differences or *% differences (95% CI) for offspring characteristics across groups of prepregnancy BMI comparing offspring of mothers with GDM to control offspring.
Finally, defining the GDM diagnosis using best clinical judgement and only including offspring with hospital records indicating a GDM diagnosis resulted in similar or stronger associations, supporting our findings in all cases (Supplementary Table 1).
Conclusions
We found that 9- to 16-year-old offspring of GDM mothers had the following characteristics: 1) higher BMI, WHR, and fat percentages and lower lean mass percentages; 2) increased fasting glucose, insulin, C-peptide, HOMA-IR, systolic blood pressure, and triglyceride levels as well as reduced HDL cholesterol levels; and 3) female offspring had an earlier onset of puberty. Interestingly, after adjustment for the offspring’s own BMI and maternal prepregnancy BMI, the offspring of mothers with GDM still had significantly higher fasting glucose and HOMA-IR levels and WHR, but differences in the onset of puberty disappeared.
Previous studies have shown that exposure to GDM was associated with higher BMI and waist circumferences, increased subscapula-to-triceps skinfold ratio (21), and increased fat mass and central adiposity among male offspring of mothers with GDM (22) and an increased risk of overweight and obesity among offspring of mothers with GDM (11). In these studies, the associations between maternal hyperglycemia and offspring adiposity were attenuated but still significant after adjustment for maternal prepregnancy BMI. In adult offspring born to mothers with GDM, BMI was on average 0.94 kg/m2 greater than in their brothers born before the mother received a diagnosis of diabetes, suggesting that it is most likely due to intrauterine mechanisms and not to familial confounding (10). A meta-analysis by Philipps et al. (6) concluded that maternal diabetes was associated with increased offspring BMI z score but that this was no longer significant after adjustment for maternal prepregnancy BMI. However, only five studies had data available on maternal prepregnancy BMI, and four of these were based on small sample sizes; the adjusted analyses were made based on all pregnancies of women with diabetes and not only on pregnancies affected by GDM. Others studies (23,24) have found increased total fat percentages and increased lean mass measured by DEXA scans in prepubertal offspring of mothers with GDM; however, in these studies no adjustment for offspring BMI or maternal prepregnancy BMI was made. Taken together, our study highlights the importance of adjusting for maternal prepregnancy BMI when analyzing the impact of maternal hyperglycemia in pregnancy on an offspring’s degree of obesity. We a priori chose not to include birth weight in our analyses, since we considered birth weight to be a mediator of the association between GDM and later risk of obesity among the offspring. However, if we adjusted for birth weight, there was still a significant difference between offspring of mothers with GDM and control offspring with regard to adiposity measurements such as BMI, waist circumference, total fat percentages, and gynoid and android fat, whereas birth weight per se independently was associated with offspring BMI and waist circumference but not with DEXA scan results (data not shown).
Our finding of higher systolic blood pressure in offspring of mothers with GDM was also observed in 3-year-old offspring in whom, after adjustment for the offspring’s skinfold thicknesses, the association between GDM and increased systolic blood pressure was no longer significant (25). Similar to our findings, a meta-analysis showed that offspring of mothers with GDM had higher systolic blood pressure but no difference in diastolic blood pressure compared with control offspring. However, all these studies had smaller samples of offspring of mothers with GDM and no adjustment for offspring BMI, and no significant association between offspring systolic blood pressure and maternal prepregnancy BMI was found in five of the studies where data on maternal prepregnancy BMI was available (26). In contrast, in 3- to 17-year-old offspring of mothers with GDM no association between GDM and offspring blood pressure was found (27). However, the latter study included a smaller sample size and the GDM diagnosis was based on one single question on a questionnaire.
Our findings of multiple early disturbances in the cardiometabolic system in offspring of GDM pregnancies are in line with previous smaller studies. However, not all previous studies adjusted the disturbances in the cardiometabolic traits for the offspring’s own degree of adiposity (1,3,28). Similar to our results, other studies adjusted for offspring BMI and reported an attenuation, but still a significant association, of the impact of GDM on offspring insulin insensitivity and risk of future type 2 diabetes (29,30). One study (2) showed that adult offspring born to women with GDM had reduced insulin sensitivity compared with offspring from the background population, also after adjusting for sex and overweight. Holder et al. (31) followed obese adolescents for an average of 2.8 years and found that insulin sensitivity at follow-up was significantly lower in the group that had been exposed to GDM (n = 45) in utero after adjusting for offspring BMI. Additionally, others have found (32) that greater maternal glucose concentration in pregnancy was associated with reduced insulin sensitivity and greater static β-cell response after a meal tolerance test in 21 prepubertal children, independent of the children’s own fat percentages measured by DEXA scanning. In the current study, we found that the C-peptide levels were no longer significantly different between GDM and control offspring after adjustment for offspring BMI, whereas fasting insulin levels remained significant. We speculate that the difference in levels of statistical significance represents variations of the assays used rather than biologically important differences in insulin and C-peptide kinetics. Indeed, the CI for C-peptide was substantially larger than the CI for insulin. Alternatively, the relatively increased plasma insulin levels compared with plasma C-peptide levels among offspring of mothers with GDM could reflect lower insulin clearance as a result of IR. C-peptide is cleared by the kidneys and therefore is not influenced by IR (33).
We found that the probability of having reached puberty assessed by breast development, which is considered to be the gold standard for evaluating puberty onset and development among girls (34), was doubled among the offspring of mothers with GDM. In contrast, others showed (9) no differences in Tanner stages among offspring of mothers with GDM and control offspring in analyses when boys and girls were analyzed together. One study (35) showed that GDM was associated with a 2-month earlier transition to Tanner stage >2 examined by pubic hair development among boys. However, these studies did not adjust for offspring BMI. There is a general agreement that a higher fat mass or higher BMI among girls is associated with an earlier onset of puberty (36,37), which supports our results that the earlier onset of puberty among female offspring of mothers with GDM is mainly driven by the offspring’s BMI and emphasizes the importance of adjusting for offspring adiposity when addressing the impact of hyperglycemia on puberty development.
Our results on stratifying maternal prepregnancy BMI suggest that hyperglycemia may be more relevant in the absence of severe maternal adiposity (i.e., the impact of hyperglycemia on an offspring’s body composition may be overruled by severe obesity in the mother). This is in accordance with the results of a recent study (38), which was also based on the DNBC, showing that the effect of maternal fasting plasma glucose levels in pregnancy on offspring obesity at 7 years appeared more pronounced among nonobese women with GDM compared with obese women with GDM. However, more studies are needed to understand the separate role and the combined potential superimposing effect of maternal hyperglycemia and maternal obesity during pregnancy on offspring metabolic health.
Besides maternal obesity and hyperglycemia, other factors such as paternal obesity influence the offspring’s level of obesity and adiposity. The strengths of this study included a large sample of pregnancies in women with GDM and good statistical power to examine the long-term consequences of intrauterine hyperglycemia in this longitudinal study with >10 years of follow-up. Detailed data were available on body composition, cardiometabolic factors, and clinical assessments of puberty onset in the offspring, allowing for more precise phenotypical characterization. Since puberty is, among other factors, characterized by IR (39), it is an enormous strength in the current study that we can take the stage of pubertal development into account.
The current study had some limitations. The GDM group contained women with both confirmed and suspected cases of GDM and may therefore have included women without GDM. Nonetheless, a sensitivity analysis of groupings based on the clinician’s best judgement in a subsample of the women did not alter the results. Another limitation is that a few smaller departments used the World Health Organization criteria and not the commonly used Danish criteria for GDM. Although detailed clinical data were available for the offspring, comparable information was not available for mothers or fathers for the relevant time period. For example, no detailed measures of maternal body composition were available for the prepregnancy period, and no data on paternal health were available to account for any genetic predisposition. However, the explained genetic variance for most complex diseases is ≤10%, and thus the impact on our results may not have been substantial. Misclassification of reported maternal BMI is likely to have been more prevalent at higher BMI values, and may have underestimated the frequencies in the overweight and obese categories. This would have led to residual confounding in the models adjusting for maternal BMI. We also lacked data on postnatal environment and on possible shared social and familial obesogenic risk factors such as diet. Dietary information for both parents and offspring was not available until the teen years. Although parental and offspring diets during this time period were only weakly correlated (A.A. Bjerregaard, unpublished observations), we cannot exclude the possibility that stronger correlations existed earlier in childhood. Our data on puberty may also be subjective to selection bias, since the boys and girls who did not want to participate in the puberty examination were often also those that were older and had attained puberty. Since the DEXA scanning was only available at the Copenhagen University Hospital, it was only offspring examined at this location who were offered a DEXA scan. This may have caused some bias, since the mothers of offspring with a DEXA scan were older, had higher socioeconomic status, and lower prepregnancy BMI. However, these differences were similar for the two exposure groups, which suggests that any present bias may be minor. Furthermore, we cannot exclude that our results to some extent may be due to other confounding factors such as socioeconomic status or breastfeeding duration, rather than hyperglycemia and maternal obesity per se.
In conclusion, we demonstrated that 9- to 16-year-old offspring of mothers with GDM had higher BMI and WHR, and higher fat percentages with more abdominal obesity, higher systolic blood pressure, fasting glucose level, insulin and C-peptide levels, higher HOMA-IR, and an earlier onset of puberty among girls. The association with higher blood pressure, higher fasting C-peptide levels, adverse lipid profile, and earlier onset of puberty seemed driven by offspring BMI, whereas the offspring of mothers with GDM still had higher WHR, fasting glucose levels, and HOMA-IR even after adjustment for both the offspring’s own BMI and maternal prepregnancy BMI. This supports an independent role of hyperglycemia in pregnancy programming body composition as well as IR among adolescent offspring.
Supplementary Material
Article Information
Acknowledgments. The authors thank all the mother-child dyads who participated in this study.
Funding. The Innovation Fund Denmark (09-067124 and 11-115923), The Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract #HHSN275201000020C), Rigshospitalet, Copenhagen University Hospital.
Duality of Interest. P.D. has participated in a multicenter, multinational study by Novo Nordisk A/S as investigator on the use of insulins in pregnancy in women with type 1 and type 2 diabetes. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. L.G.G. contributed to the study concept and design, took part in the planning and conduct of the clinical examinations, conducted the statistical analyses, and drafted the manuscript. S.H. took part in the planning and conduct of the clinical examinations, conducted the statistical analyses, and contributed critical advice about and revisions to the manuscript. L.H., C.M.M., F.B.K., A.C.B.T., and M.S. took part in the planning and conduct of the clinical examinations and contributed critical advice about and revisions to the manuscript. C.G. conducted the statistical analyses. E.M. and P.D. contributed critical advice and revisions to the manuscript. R.F.-S. took part in the planning and conduct of the clinical examinations. J.E.C., F.B.H., and A.V. contributed to the study concept and design, and contributed critical advice about and revisions to the manuscript. S.F.O. proposed the basic design of the current study and contributed to the study concept and design; the establishing of the DNBC; and critical advice about and revisions to the manuscript. All authors read and approved the final manuscript. L.G.G. and A.V. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-0514/-/DC1.
References
- 1.Boerschmann H, Pflüger M, Henneberger L, Ziegler AG, Hummel S. Prevalence and predictors of overweight and insulin resistance in offspring of mothers with gestational diabetes mellitus. Diabetes Care 2010;33:1845–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelstrup L, Damm P, Mathiesen ER, et al. Insulin resistance and impaired pancreatic β-cell function in adult offspring of women with diabetes in pregnancy. J Clin Endocrinol Metab 2013;98:3793–3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egeland GM, Meltzer SJ. Following in mother’s footsteps? Mother-daughter risks for insulin resistance and cardiovascular disease 15 years after gestational diabetes. Diabet Med 2010;27:257–265 [DOI] [PubMed] [Google Scholar]
- 4.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol 2007;50:972–979 [DOI] [PubMed] [Google Scholar]
- 5.Donovan LE, Cundy T. Does exposure to hyperglycaemia in utero increase the risk of obesity and diabetes in the offspring? A critical reappraisal. Diabet Med 2015;32:295–304 [DOI] [PubMed] [Google Scholar]
- 6.Philipps LH, Santhakumaran S, Gale C, et al. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia 2011;54:1957–1966 [DOI] [PubMed] [Google Scholar]
- 7.Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 2010;33:964–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landon MB, Rice MM, Varner MW, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network . Mild gestational diabetes mellitus and long-term child health. Diabetes Care 2015;38:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis JN, Gunderson EP, Gyllenhammer LE, Goran MI. Impact of gestational diabetes mellitus on pubertal changes in adiposity and metabolic profiles in Latino offspring. J Pediatr 2013;162:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawlor DA, Lichtenstein P, Långström N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation 2011;123:258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nehring I, Chmitorz A, Reulen H, von Kries R, Ensenauer R. Gestational diabetes predicts the risk of childhood overweight and abdominal circumference independent of maternal obesity. Diabet Med 2013;30:1449–1456 [DOI] [PubMed] [Google Scholar]
- 12.Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort---its background, structure and aim. Scand J Public Health 2001;29:300–307 [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Hu FB, Olsen SF, et al.; DWH study team . Rationale, design, and method of the Diabetes & Women’s Health study--a study of long-term health implications of glucose intolerance in pregnancy and their determinants. Acta Obstet Gynecol Scand 2014;93:1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen SF, Houshmand-Oeregaard A, Granstrom C, et al. Diagnosing gestational diabetes mellitus in the Danish National Birth Cohort. Acta Obstet Gynecol Scand 2017;96:563–569 [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 16.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res 2004;59:207–223 [DOI] [PubMed] [Google Scholar]
- 19.Allison DB, Kaprio J, Korkeila M, Koskenvuo M, Neale MC, Hayakawa K. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int J Obes Relat Metab Disord 1996;20:501–506 [PubMed] [Google Scholar]
- 20.Malis C, Rasmussen EL, Poulsen P, et al. Total and regional fat distribution is strongly influenced by genetic factors in young and elderly twins. Obes Res 2005;13:2139–2145 [DOI] [PubMed] [Google Scholar]
- 21.Crume TL, Ogden L, West NA, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia 2011;54:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regnault N, Gillman MW, Rifas-Shiman SL, Eggleston E, Oken E. Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care 2013;36:3045–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandler-Laney PC, Bush NC, Rouse DJ, Mancuso MS, Gower BA. Maternal glucose concentration during pregnancy predicts fat and lean mass of prepubertal offspring. Diabetes Care 2011;34:741–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandler-Laney PC, Bush NC, Granger WM, Rouse DJ, Mancuso MS, Gower BA. Overweight status and intrauterine exposure to gestational diabetes are associated with children’s metabolic health. Pediatr Obes 2012;7:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens 2009;22:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aceti A, Santhakumaran S, Logan KM, et al. The diabetic pregnancy and offspring blood pressure in childhood: a systematic review and meta-analysis. Diabetologia 2012;55:3114–3127 [DOI] [PubMed] [Google Scholar]
- 27.Beyerlein A, Nehring I, Rosario AS, von Kries R. Gestational diabetes and cardiovascular risk factors in the offspring: results from a cross-sectional study. Diabet Med 2012;29:378–384 [DOI] [PubMed] [Google Scholar]
- 28.Krishnaveni GV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care 2010;33:402–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 2008;31:340–346 [DOI] [PubMed] [Google Scholar]
- 30.Vääräsmäki M, Pouta A, Elliot P, et al. Adolescent manifestations of metabolic syndrome among children born to women with gestational diabetes in a general-population birth cohort. Am J Epidemiol 2009;169:1209–1215 [DOI] [PubMed] [Google Scholar]
- 31.Holder T, Giannini C, Santoro N, et al. A low disposition index in adolescent offspring of mothers with gestational diabetes: a risk marker for the development of impaired glucose tolerance in youth. Diabetologia 2014;57:2413–2420 [DOI] [PubMed] [Google Scholar]
- 32.Bush NC, Chandler-Laney PC, Rouse DJ, Granger WM, Oster RA, Gower BA. Higher maternal gestational glucose concentration is associated with lower offspring insulin sensitivity and altered beta-cell function. J Clin Endocrinol Metab 2011;96:E803–E809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CC, Haffner SM, Wagenknecht LE, et al. Insulin clearance and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes Care 2013;36:901–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanner JM, Whitehouse RH. Standards for subcutaneous fat in British children. Percentiles for thickness of skinfolds over triceps and below scapula. BMJ 1962;1:446–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monteilh C, Kieszak S, Flanders WD, et al. Timing of maturation and predictors of Tanner stage transitions in boys enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol 2011;25:75–87 [DOI] [PubMed] [Google Scholar]
- 36.Aksglaede L, Juul A, Olsen LW, Sørensen TI. Age at puberty and the emerging obesity epidemic. PLoS One 2009;4:e8450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crocker MK, Stern EA, Sedaka NM, et al. Sexual dimorphisms in the associations of BMI and body fat with indices of pubertal development in girls and boys. J Clin Endocrinol Metab 2014;99:E1519–E1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Olsen SF, Mendola P, et al. Growth and obesity through the first 7 y of life in association with levels of maternal glycemia during pregnancy: a prospective cohort study. Am J Clin Nutr 2016;103:794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly LA, Lane CJ, Weigensberg MJ, Toledo-Corral CM, Goran MI. Pubertal changes of insulin sensitivity, acute insulin response, and β-cell function in overweight Latino youth. J Pediatr 2011;158:442–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.