Abstract
OBJECTIVE
Intensive treatment (INT) of type 1 diabetes reduces the incidence of cardiovascular disease (CVD) events compared with conventional treatment (CONV), but it also results in more weight gain. Our objective was to examine whether excessive weight gain from INT of type 1 diabetes is independently associated with subsequent CVD events.
RESEARCH DESIGN AND METHODS
Quartiles (Q) of weight gain in 1,213 participants aged 18 years and older at enrollment in the Diabetes Control and Complications Trial (DCCT) were determined within randomized treatment groups (INT vs. CONV) using change in BMI from baseline to the closeout DCCT visits. Effects of this weight gain on CVD risk factors and outcomes during an additional 20 years of observational follow-up were then determined.
RESULTS
The Q4 INT group experienced greater proportional weight gain (median change in BMI, 6.08 kg/m2), increases in CVD risk factors, and need for medications for hypertension and lipids compared with the Q1–3 INT and comparable CONV groups. Over a mean of 26 years of follow-up, the numbers of major and total CVD events were not statistically different in Q4 compared with Q1–3 of either the INT or CONV group. By year 14, however, the incident CVD event curve became significantly higher in the Q4 INT group than in the Q1–3 INT groups (P = 0.024) and was similar to that for the CONV group.
CONCLUSIONS
For the first 13 years after DCCT, INT for type 1 diabetes reduced macrovascular events compared with CONV, even when excessive weight gain occurred. After this, total CVD events significantly increased in the Q4 INT group, becoming equivalent to those in the CONV group. Longer follow-up is needed to determine whether this trend continues and results in more major CVD events.
Introduction
The Diabetes Control and Complications Trial (DCCT) and its observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) study, demonstrated that intensive diabetes therapy achieving near-normal glucose control reduces long-term macrovascular complications in type 1 diabetes compared with conventional treatment (1,2). However, during an average of 6.5 years in the DCCT, approximately 25% of participants randomized to receive intensive therapy experienced weight gain that resulted in obesity (mean BMI, ≥30 kg/m2) and was considerably more than the conventionally treated group. We termed this “excessive” weight gain because not only was the total amount greater, it also was characterized by a more central distribution and was accompanied by insulin resistance and worsened cardiac risk factors compared with participants with less weight gain in both the intensive and conventional diabetes therapy treatment groups (3)—differences that persisted during the EDIC observational phase of the study (4). Reports from several observational studies of type 1 diabetes have found associations between obesity, subclinical cardiovascular disease (CVD) (4–6), and mortality rates (7) within this population, although not all remained significant after adjusting for obesity-related CVD risk factors. Nevertheless, our studies and these reports raise concerns that excessive weight gain with intensive diabetes management might abrogate the benefits of that management on macrovascular disease outcomes.
The overall aims of this analysis were to examine the association between quartile change in BMI during DCCT and subsequent cardiovascular events during the EDIC follow-up and to assess whether excessive weight gain (i.e., fourth quartile of weight gain) with intensive therapy during DCCT affects the long-term benefits of intensive therapy on CVD risk. Our hypothesis was that CVD event rates would be higher in participants who gained excessive weight than in those who gained only a minimal amount of weight with intensive therapy and that the higher rate of CVD events would be largely attributable to the worsening of cardiac risk factors.
Research Design and Methods
The DCCT/EDIC study has been previously described in detail (8–10). Briefly, 1,441 subjects with type 1 diabetes were enrolled in the DCCT between 1983 and 1989. Subjects were randomly assigned to receive either intensive therapy (INT), with a goal of maintaining glycemic levels as close to the nondiabetic range as safely possible, or conventional therapy (CONV), with a goal of clinical well-being and freedom from symptoms of both hyperglycemia and hypoglycemia. The DCCT was stopped after a mean of 6.5 years of follow-up, and all participants were encouraged to implement or continue intensive treatment (instruction provided), with subsequent diabetes care administered by their own health care providers. In 1994, 96% of the surviving DCCT cohort enrolled in the EDIC observational study. After an additional 20 years in the EDIC study, 1,251 participants (94% of survivors) continue to be followed. A total of 1,213 subjects were included in the following analysis after excluding participants <18 years of age at DCCT enrollment.
DCCT/EDIC Evaluations
Follow-up visits occurred quarterly during the DCCT and annually throughout the EDIC study. A detailed medical history was taken at each visit, including demographics, behavioral risk factors, and medical outcomes, as was a physical examination that included measurements of height, weight, and blood pressure. BMI was calculated by dividing weight (kilograms) by height (meters) squared. Waist circumference at the iliac crest was measured at the DCCT closeout and annually during the EDIC study.
Blood samples were assayed centrally for hemoglobin A1c (HbA1c), using high-performance ion-exchange liquid chromatography (8). Fasting lipids and albumin excretion rate (AER) were measured in the DCCT central laboratory annually during DCCT and in alternate years during the EDIC study. Sustained albuminuria was defined as an AER of at least 30 mg/24 h at two consecutive visits. End-stage renal disease was defined as the development of an estimated glomerular filtration rate <15 mL/min/1.73 m2 using the Chronic Kidney Disease Epidemiology Collaboration equation (11).
In addition to the current HbA1c value, the DCCT updated mean was used to reflect the cumulative glycemic exposure from baseline up to and including the HbA1c at each visit throughout the DCCT. The DCCT/EDIC study time-weighted arithmetic means were calculated using the quarterly DCCT and annual EDIC study values weighted by 3 and 12 months, respectively.
Cardiovascular Outcomes
The primary outcome was the time to the first of any of the following CVD events: nonfatal myocardial infarction (MI) or stroke; death judged to be secondary to CVD; subclinical (“silent”) MI detected on an annual electrocardiogram; angina confirmed by ischemic changes with exercise tolerance testing or by clinically significant obstruction on coronary angiography; congestive heart failure with paroxysmal nocturnal dyspnea, orthopnea, or marked limitation of physical activity caused by heart disease; or revascularization with angioplasty and/or coronary artery bypass. Major adverse cardiovascular events (MACE) were defined as nonfatal MI or stroke, or CVD-related death. Medical records were obtained and all CVD events were centrally adjudicated by a mortality and morbidity review committee masked to DCCT treatment assignment, HbA1c, and glucose levels.
Statistical Analyses
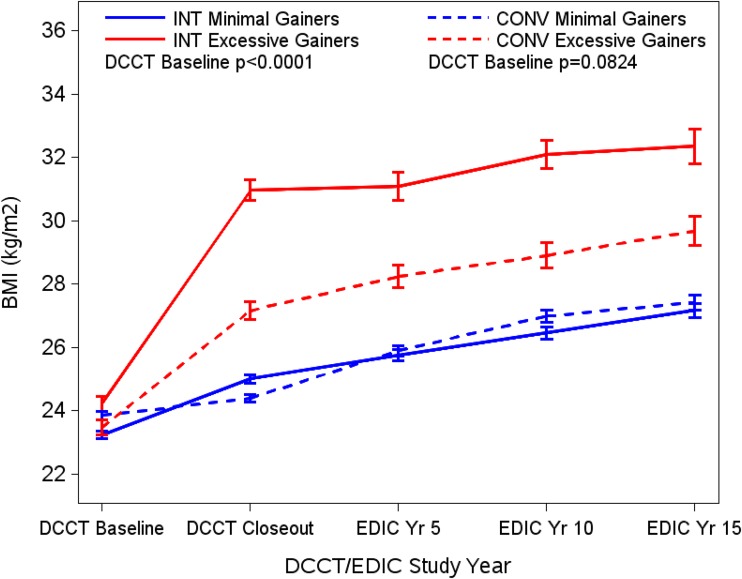
Quartiles of weight gain were defined separately within the two treatment groups as the change in BMI from DCCT baseline to DCCT closeout (3). In each treatment group, the fourth quartile of weight gain was used to define excessive weight gain compared with minimal weight gain defined by the first three quartiles (3) (Supplementary Table 1). The separation in weights by quartile group during DCCT was maintained during the observational follow-up in the EDIC study (Fig. 1).
Figure 1.
BMI of participants at the DCCT baseline, DCCT closeout, and EDIC years 5, 10, and 15 by quartile of weight gain between the DCCT baseline and closeout (excessive = fourth quartile of weight gain [red lines]; minimal = first through third quartiles combined [blue lines]) in both the INT and CONV groups. Data are mean ± SE.
Clinical characteristics were compared between weight gain groups (excessive vs. minimal) using the Wilcoxon rank sum test for quantitative variables and the χ2 test for categorical variables. The Kaplan-Meier method was used to estimate the cumulative incidence of the first occurrence of any CVD event (12). The Cox proportional hazards model was used to estimate the effect of weight gain during DCCT (excessive vs. minimal) on subsequent CVD risk during the EDIC study (13). Adjustments were made for fixed characteristics (e.g., sex), and covariates measured repeatedly over time entered the models as time-dependent covariates. All analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC). Two-sided P ≤ 0.05 was considered statistically significant.
Results
At DCCT baseline, participants in the INT group who experienced excessive weight gain were slightly older and more likely to be female than participants in the minimal weight gain group (Supplementary Table 2). At the DCCT closeout visit, those in the excess weight gain group had worse levels of a number of cardiac risk factors than the minimal weight gain group, including higher systolic and diastolic blood pressures; greater total, LDL, and non-HDL cholesterol; higher triglyceride levels; and lower HDL cholesterol levels. In addition, participants with excessive weight gain had larger waist circumferences and slightly higher current HbA1c levels despite taking larger doses of insulin, indicating more insulin resistance. In the CONV group, the difference in BMI between the excessive and minimal weight gain groups at DCCT closeout was roughly 50% that of the difference observed in the INT group (3 vs. 6 kg/m2). Nevertheless, participants in the excessive weight gain group (fourth quartile) in the CONV group also required more insulin and had worse levels of some cardiac risk factors than those in the minimal weight gain group (first through third) quartiles, including higher systolic blood pressure, total and non-HDL cholesterols, and triglycerides. However, these marginally significant differences were not as large as the ones observed in the INT group (Supplementary Table 2).
After DCCT closeout, the differences with regard to BMI, waist circumference, and cardiac risk factors persisted between the excessive and minimal weight gain groups despite slight worsening of glucose control in the INT group (Fig. 1 and Table 1). In addition, at years 10 and 15 of follow-up during the EDIC study, the excess gain group reported using medications to treat hypertension and lower lipids at a higher frequency than the minimal gain group. Aspirin use and the frequency of postmenopausal hormonal replacement therapy were comparable between the groups. Similar to the INT group, those assigned to CONV in the DCCT who experienced the greatest weight gain (quartile 4) remained heavier and had a more central distribution of the weight than those in the minimal gain group during the observational follow-up period in the EDIC study (Fig. 1 and Supplementary Table 3). By 15 years of EDIC follow-up, however, the significant differences in CVD risk factors between the weight gain groups noted at DCCT closeout had disappeared, and the frequencies of using medication for hypertension and lipids, and of taking aspirin, were similar (Supplementary Table 3).
Table 1.
Clinical characteristics of DCCT/EDIC participants treated with INT during EDIC years 5, 10, and 15, by quartiles of weight gain
| EDIC year 5 | EDIC year 10 |
EDIC year 15 |
||||
|---|---|---|---|---|---|---|
| Characteristics | Minimal gain group | Excessive gain group | Minimal gain group | Excessive gain group | Minimal gain group | Excessive gain group |
| Subjects at risk for CVD (n) | 446 | 149 | 437 | 147 | 426 | 138 |
| Any medication use (%) | ||||||
| Antihypertensive medication† | 10 | 16 | 31 | 46* | 45 | 66* |
| Lipid-lowering medication | 5 | 13* | 26 | 39* | 48 | 59 |
| Aspirin | 10 | 12 | 30 | 38 | 43 | 52 |
| Postmenopausal HRT | 6 | 10 | 5 | 14* | 4 | 6 |
| DCCT/EDIC time-weighted means | ||||||
| Weight (kg) | 74 ± 12 | 85 ± 14* | 76 ± 13 | 87 ± 15* | 77 ± 12 | 88 ± 14* |
| BMI (kg/m2) | 25 ± 3 | 29 ± 4* | 25 ± 3 | 30 ± 4* | 26 ± 3 | 30 ± 4* |
| EDIC waist circumference (cm) | 83 ± 10 | 94 ± 14* | 85 ± 10 | 96 ± 12* | 86 ± 11 | 97 ± 12* |
| Systolic blood pressure (mmHg) | 116 ± 8 | 118 ± 8* | 117 ± 8 | 120 ± 8* | 118 ± 8 | 121 ± 8* |
| Diastolic blood pressure (mmHg) | 75 ± 6 | 76 ± 5* | 75 ± 5 | 76 ± 5* | 74 ± 5 | 76 ± 5* |
| Cholesterol (mg/dL [mmol/L]) | ||||||
| HDL | 55 ± 13 (1.4 ± 0.3) | 52 ± 11 (1.3 ± 0.3) | 56 ± 13 (1.4 ± 0.3) | 53 ± 11 (1.4 ± 0.3) | 57 ± 14 (1.5 ± 0.4) | 54 ± 11 (1.4 ± 0.3) |
| LDL | 112 ± 24 (2.9 ± 0.6) | 122 ± 24 (3.2 ± 0.6)* | 111 ± 23 (2.9 ± 0.6) | 121 ± 22 (3.1 ± 0.6)* | 109 ± 20 (2.8 ± 0.5) | 116 ± 21 (3.0 ± 0.5)* |
| Total | 183 ± 27 (4.7 ± 0.7) | 194 ± 27 (5.0 ± 0.7)* | 183 ± 25 (4.7 ± 0.6) | 193 ± 25 (5.0 ± 0.6)* | 181 ± 23 (4.7 ± 0.6) | 189 ± 22 (4.9 ± 0.6)* |
| Triglycerides | 77 ± 36 (0.9 ± 0.4) | 96 ± 47 (1.1 ± 0.5)* | 79 ± 38 (0.9 ± 0.4) | 97 ± 47 (1.1 ± 0.5)* | 77 ± 34 (0.9 ± 0.4) | 96 ± 39 (1.1 ± 0.4)* |
| HbA1c (% [mmol/mol]) | ||||||
| DCCT | 7.1 ± 0.8 (54 ± 9) | 7.2 ± 0.7 (55 ± 8) | 7.1 ± 0.8 (54 ± 9) | 7.1 ± 0.7 (54 ± 8) | 7.1 ± 0.8 (54 ± 9) | 7.1 ± 0.7 (54 ± 8) |
| EDIC | 8.0 ± 1.2 (64 ± 13) | 8.4 ± 1.3 (68 ± 14)* | 7.8 ± 1.1 (62 ± 12) | 8.3 ± 1.0 (67 ± 11)* | 7.8 ± 1.0 (62 ± 11) | 8.2 ± 0.9 (66 ± 10)* |
| DCCT/EDIC | 7.5 ± 0.9 (58 ± 10) | 7.7 ± 0.9 (61 ± 10)* | 7.5 ± 0.9 (58 ± 10) | 7.8 ± 0.8 (62 ± 9)* | 7.6 ± 0.9 (60 ± 10) | 7.9 ± 0.8 (63 ± 9)* |
Data are mean ± SD unless otherwise indicated. See statistical analyses for definition of minimal and excess weight gain groups. HRT, hormone replacement therapy.
*P < 0.01, minimal vs. excessive gain groups (Wilcoxon rank sum test or χ2 test).
†Includes ACE inhibitors, angiotensin receptor blockers, β-blockers, and calcium channel blockers.
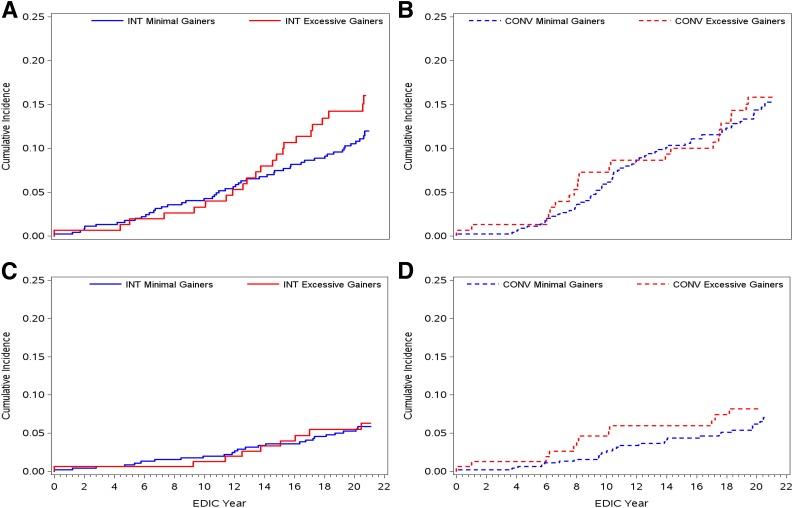
During a mean of 26 years’ EDIC follow-up, 86 participants in the CONV group experienced 171 events (Supplementary Table 4). Of these, 63 participants (14%) with minimal weight gain experienced 107 CVD events compared with 23 participants (15%) with excessive weight gain who experienced 64 events (unadjusted hazard ratio [HR] 1.06; 95% CI 0.66–1.71) (Fig. 2 and Supplementary Table 4). No specific CVD events, including MACE, appeared to occur more frequently in one group than the other. In the INT group, 133 adjudicated CVD events (one or more per person) occurred in 73 participants (Supplementary Table 5). Of 151 participants in the excessive weight gain group, 23 (15%) had 40 events, compared with 50 of 453 participants (11%) in the minimal weight gain group who had 93 total events (unadjusted HR 1.35; 95% CI 0.83–2.22) (Fig. 2 and Supplementary Table 5). This small difference was primarily driven by more frequent revascularization procedures in the excessive weight gain group. The HR (1.14; 95% CI 0.68–1.91) was attenuated after adjustment for non–weight-related covariates (age, sex, smoking, renal function, aspirin and hormone replacement therapy use, and HbA1c) and nearly completely attenuated (HR 1.02; 95% CI 0.60–1.75) when adjustments also included weight-related CVD risk factors (blood pressure, cholesterol and triglyceride levels, and antihypertensive and lipid-lowering medication use) (Supplementary Table 5). HRs for MACE were also not significant both before and after adjustments for non–weight-related and weight-related CVD events (Supplementary Table 5).
Figure 2.
Cumulative incidence during the EDIC study of the first occurrence of any CVD event with INT (P = 0.23, log-rank test) (A) or CONV (P = 0.81, log-rank test) (B) for type 1 diabetes and of nonfatal MI, stroke, or death from CVD (MACE) with INT (P = 0.93, log-rank test) (C) or CONV (P = 0.52, log-rank test) (D) for type 1 diabetes therapy. Excessive = fourth quartile of weight gain (red lines); minimal = first through third quartiles of weight gain combined (blue lines).
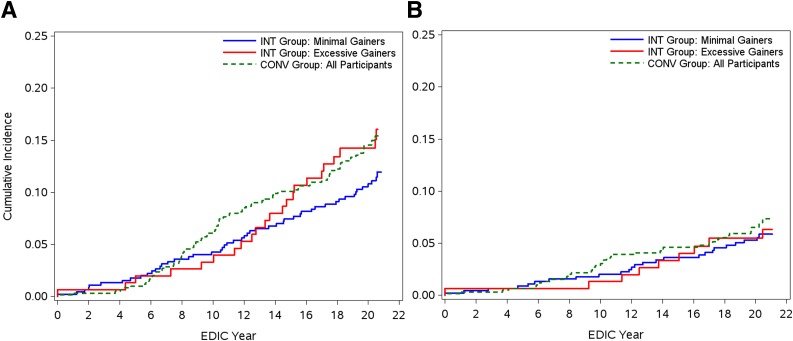
Because a separation in CVD event rates in the INT group became apparent after 14 years of EDIC follow-up (Fig. 2), an exploratory analysis was conducted to test the difference between these groups and the CONV group (as a whole) after EDIC year 14 (Fig. 3 and Table 2). Using a Cox proportional hazards model, we evaluated the interaction between weight gain groups (excessive vs. minimal) and an indicator variable for study year, which was defined as “0” for the first 13 years of EDIC and “1” thereafter. Incident CVD events in the excessive weight gain group (quartile 4) with INT became significantly higher than the minimal gain group (quartiles 1–3) with INT after EDIC year 14 (unadjusted HR 1.99; 95% CI 1.12–3.63) and were comparable to incident CVD in the CONV group (Fig. 3). With further adjustment for weight-related CVD risk factors, however, this HR was no longer significant (Table 2). The HRs for MACE were not different between the weight gain with INT groups before and after year 14 (Fig. 3 and Table 2).
Figure 3.
Cumulative incidence during the EDIC study of the first occurrence of any CVD (P = 0.21, log-rank test) (A) and nonfatal MI, stroke, or death from CVD (MACE) (B), comparing the group with excessive weight gain group (fourth quartile of weight gain, n = 152 [red solid lines]) with the group with minimal weight gain (first through third quartiles combined, n = 457 [blue solid lines]) in the INT group and with all participants in the CONV group (n = 609 [green dotted lines]). Using a Cox proportional hazards model, the unadjusted difference in hazards after year 14 of EDIC follow-up between the excessive and minimal weight gain groups receiving INT was significant (P = 0.024).
Table 2.
Association in the DCCT INT group between excessive (quartile 4) and minimal (quartiles 1–3 combined) weight gain during DCCT and subsequent cardiovascular events after 14 years of EDIC follow-up
| Unadjusted | Partially adjusted | Fully adjusted | |
|---|---|---|---|
| Any CVD event | 1.99 (1.12–3.53) | 1.62 (0.90–2.92) | 1.57 (0.86–2.86) |
| MACE | 1.04 (0.48–2.22) | 0.91 (0.41–2.00) | 0.79 (0.35–1.76) |
Data are hazard ratios (95% CI). Cox proportional hazard regression models were unadjusted; partially adjusted for age at DCCT closeout, sex, and the following time-dependent covariates: smoking, sustained AER ≥30 mg/24 h or end-stage renal disease, estimate glomerular filtration rate <15 mL/min/1.73 m2 or end-stage renal disease, aspirin use, hormone replacement therapy use, and DCCT/EDIC time-weighted mean HbA1c; and fully adjusted to include also the following time-dependent covariates: systolic and diastolic blood pressures, antihypertensive medication use, total cholesterol, triglycerides, and lipid-lowering medication use. MACE include nonfatal myocardial infarction or stroke, or CVD-related death.
Conclusions
We previously showed that excessive weight gain leading to obesity with intensive treatment of type 1 diabetes was associated with increased CVD risk factors and subclinical atherosclerosis in the DCCT/EDIC study (3,4). Consistent with previous observational cohorts, we expected to find that expression of obesity during intensive treatment of type 1 diabetes would also be associated with higher rates of macrovascular disease (5,6) and mortality (7). Instead, we found that, over 13 years of observational follow-up in the EDIC study, rates of CVD events, including MACE, were not different in participants who had gained excessive weight and became obese compared with those who gained minimal amounts of weight with INT. However, beginning at approximately year 14 of the EDIC study, the event rate curves in the participants in the INT group began to diverge, with higher total CVD event rates (but not MACE rates) reported in the excessive weight gain group compared with the minimal weight gain group, such that by EDIC year 20 the incident rate of total CVD events in the excessive weight gain group treated intensively approximated that of the CONV group (Fig. 3).
One possible contribution to the delayed divergence of the total CVD event curves in the INT group could have been the additional nutritional counseling provided by dietitians to DCCT participants who were gaining weight rapidly, a prevention strategy implemented when unwanted weight gain accompanying intensive management became apparent during pilot and feasibility testing before the start of the DCCT (14,15). Another potential explanation is that the group with excessive weight gain reported taking medications for blood pressure and to lower lipids at higher rates than the group with minimal gain during EDIC follow-up. Indeed, we found that adjusting for traditional CVD risk factors and the medications used to treat them (e.g., antihypertensives and lipid-lowering medications) eliminated the excess risk found between the two groups before year 14 (the unadjusted HR fell from 1.35 to 1.02 with full adjustment). This, and the lack of impact of excessive weight gain on MACE, parallels findings in the U.S. populace; several decades’ worth of population weight gain (16) has coincided with an overall reduction in CVD mortality rates (17), in part because of better medical management of CVD risk factors (18). In addition, the revascularization rate in the Q4 INT (excessive gain) was nearly twice that of the Q1–3 INT (minimal gain) group, which might have acted as a “preventive” measure (similar to blood pressure and lipid-lowering medications) to reduce progression to MACE.
After EDIC year 14, the attenuation effect of adjusting for CVD risk factors and medication use was somewhat reduced; HRs became nonsignificant but fell only from 1.99 to 1.62 and 1.57. If this increase in adjusted HR persists and becomes significant during longer follow-up, it would suggest that nontraditional obesity-related CVD risk factors make a delayed contribution to the expression of atherosclerotic events. Examples include increased insulin resistance, cholesterol accumulation in VLDL and remnant particles, and increased prothrombotic and proinflammatory factors that are thought to contribute to the 50–60% “residual risk” for cardiac events despite optimal LDL cholesterol lowering reported in statin trials (19).
This analysis has several limitations. While the DCCT was a randomized controlled trial, the EDIC study was observational. However, despite the loss of randomized treatment group assignment, the continued beneficial effect of intensive management begun during DCCT and carried forward into the EDIC study was consistent for both microvascular and macrovascular disease events. Nevertheless, the failure of the INT group to maintain target glucose control during the EDIC study limits our ability to determine the beneficial effects of persistent near normalization of glucose control, despite excessive weight gain, on CVD events in patients with type 1 diabetes.
In summary, although excessive weight gain with INT of type 1 diabetes worsens obesity-related CVD risk factors, rates of total CVD events and MACE during the first 13 years of observational follow-up in that group were not significantly different from the rates in those who experienced only minimal weight gain. However, this might have been due to CVD risk mitigation though the greater use of lipid-lowering and blood pressure medications by the excessive weight gain group. A significant divergence of CVD event rate curves after 14 years of follow-up in the EDIC study suggests that more revascularization procedures eventually are needed to delay or prevent MACE. Further follow-up of this well-characterized population with type 1 diabetes will determine whether excessive weight gain with INT eventually results in increased (“breakthrough”) MACE.
Supplementary Material
Article Information
Acknowledgments. This article is dedicated to the memory of John D. Brunzell, MD (1937–2015), who inspired and guided us both personally and professionally.
Funding. The DCCT/EDIC has been supported by cooperative agreement grants (1982–1993, 2012–2017) and contracts (1982–2012) with the Division of Diabetes, Endocrinology, & Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (grants U01 DK094176 and U01 DK094157) and through support from the National Eye Institute, the National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and Clinical Translational Science Center Program (2006–present), Bethesda, MD.
Duality of Interest. J.Q.P. serves on an advisory board for Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Industry contributors had no role in the DCCT/EDIC study but provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (Westchester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), LifeScan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Ft. Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater, NJ).
Author Contributions. J.Q.P. designed the study, analyzed data, and prepared the manuscript. B.H.B. conducted the statistical analyses and wrote sections of the manuscript. B.Z., R.A.G.-K., W.S., J.P.B., G.Z., and P.A.C. reviewed the manuscript and helped prepare the final manuscript. J.D.B. designed the study and planned data analysis. B.H.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. nos. NCT00360815 and NCT00360893, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-2523/-/DC1.
Deceased.
A complete list of participants in the DCCT/EDIC Research Group can be found at http://www.nejm.org/doi/suppl/10.1056/NEJMoa1409463/suppl_file/nejmoa1409463_appendix.pdf.
Contributor Information
Collaborators: DCCT/EDIC Research Group, D.M. Nathan, B. Zinman, O. Crofford, S. Genuth, J. Brown-Friday, J. Crandall, H. Engel, S. Engel, H. Martinez, M. Phillips, M. Reid, H. Shamoon, J. Sheindlin, R. Gubitosi-Klug, L. Mayer, S. Pendegast, H. Zegarra, D. Miller, L. Singerman, S. Smith-Brewer, M. Novak, J. Quin, Saul Genuth, M. Palmert, E. Brown, J. McConnell, P. Pugsley, P. Crawford, W. Dahms, N.S. Gregory, M.E. Lackaye, S. Kiss, R. Chan, A. Orlin, M. Rubin, D. Brillon, V. Reppucci, T. Lee, M. Heinemann, S. Chang, B. Levy, L. Jovanovic, M. Richardson, B. Bosco, A. Dwoskin, R. Hanna, S. Barron, R. Campbell, A. Bhan, D. Kruger, J.K. Jones, P.A. Edwards, A. Bhan, J.D. Carey, E. Angus, A. Thomas, A. Galprin, M. McLellan, F. Whitehouse, R. Bergenstal, M. Johnson, K. Gunyou, L. Thomas, J. Laechelt, P. Hollander, M. Spencer, D. Kendall, R. Cuddihy, P. Callahan, S. List, J. Gott, N. Rude, B. Olson, M. Franz, G. Castle, R. Birk, J. Nelson, D. Freking, L. Gill, W. Mestrezat, D. Etzwiler, K. Morgan, L.P. Aiello, E. Golden, P. Arrigg, V. Asuquo, R. Beaser, L. Bestourous, J. Cavallerano, R. Cavicchi, O. Ganda, O. Hamdy, R. Kirby, T. Murtha, D. Schlossman, S. Shah, G. Sharuk, P. Silva, P. Silver, M. Stockman, J. Sun, E. Weimann, H. Wolpert, L.M. Aiello, A. Jacobson, L. Rand, J. Rosenzwieg, D.M. Nathan, M.E. Larkin, M. Christofi, K. Folino, J. Godine, P. Lou, C. Stevens, E. Anderson, H. Bode, S. Brink, C. Cornish, D. Cros, L. Delahanty, A. deManbey, C. Haggan, J. Lynch, C. McKitrick, D. Norman, D. Moore, M. Ong, C. Taylor, D. Zimbler, S. Crowell, S. Fritz, K. Hansen, C. Gauthier-Kelly, F.J. Service, G. Ziegler, A. Barkmeier, L. Schmidt, B. French, R. Woodwick, R. Rizza, W.F. Schwenk, M. Haymond, J. Pach, J. Mortenson, B. Zimmerman, A. Lucas, R. Colligan, L. Luttrell, M. Lopes-Virella, S. Caulder, C. Pittman, N. Patel, K. Lee, M. Nutaitis, J. Fernandes, K. Hermayer, S. Kwon, A. Blevins, J. Parker, J. Colwell, D. Lee, J. Soule, P. Lindsey, M. Bracey, A. Farr, S. Elsing, T. Thompson, J. Selby, T. Lyons, S. Yacoub-Wasef, M. Szpiech, D. Wood, R. Mayfield, M. Molitch, D. Adelman, S. Colson, L. Jampol, A. Lyon, M. Gill, Z. Strugula, L. Kaminski, R. Mirza, E. Simjanoski, D. Ryan, C. Johnson, A. Wallia, S. Ajroud-Driss, P. Astelford, N. Leloudes, A. Degillio, B. Schaefer, S. Mudaliar, G. Lorenzi, M. Goldbaum, K. Jones, M. Prince, M. Swenson, I. Grant, R. Reed, R. Lyon, O. Kolterman, M. Giotta, T. Clark, G. Friedenberg, W.I. Sivitz, B. Vittetoe, J. Kramer, M. Bayless, R. Zeitler, H. Schrott, N. Olson, L. Snetselaar, R. Hoffman, J. MacIndoe, T. Weingeist, C. Fountain, R. Miller, S. Johnsonbaugh, M. Patronas, M. Carney, S. Mendley, P. Salemi, R. Liss, M. Hebdon, D. Counts, T. Donner, J. Gordon, R. Hemady, A. Kowarski, D. Ostrowski, S. Steidl, B. Jones, W.H. Herman, C.L. Martin, R. Pop-Busui, D.A. Greene, M.J. Stevens, N. Burkhart, T. Sandford, J. Floyd, J. Bantle, N. Flaherty, J. Terry, D. Koozekanani, S. Montezuma, N. Wimmergren, B. Rogness, M. Mech, T. Strand, J. Olson, L. McKenzie, C. Kwong, F. Goetz, R. Warhol, D. Hainsworth, D. Goldstein, S. Hitt, J. Giangiacomo, D.S. Schade, J.L. Canady, M.R. Burge, A. Das, R.B. Avery, L.H. Ketai, J.E. Chapin, M.L Schluter, J. Rich, C. Johannes, D. Hornbeck, M. Schutta, P.A. Bourne, A. Brucker, S. Braunstein, S. Schwartz, B.J. Maschak-Carey, L. Baker, T. Orchard, L. Cimino, T. Songer, B. Doft, S. Olson, D. Becker, D. Rubinstein, R.L. Bergren, J. Fruit, R. Hyre, C. Palmer, N. Silvers, L. Lobes, P. Paczan Rath, P.W. Conrad, S. Yalamanchi, J. Wesche, M. Bratkowksi, S. Arslanian, J. Rinkoff, J. Warnicki, D. Curtin, D. Steinberg, G. Vagstad, R. Harris, L. Steranchak, J. Arch, K. Kelly, P. Ostrosaka, M. Guiliani, M. Good, T. Williams, K. Olsen, A. Campbell, C. Shipe, R. Conwit, D. Finegold, M. Zaucha, A. Drash, A. Morrison, J.I. Malone, M.L. Bernal, P.R. Pavan, N. Grove, E.A. Tanaka, D. McMillan, J. Vaccaro-Kish, L. Babbione, H. Solc, T.J. DeClue, S. Dagogo-Jack, C. Wigley, H. Ricks, A. Kitabchi, E. Chaum, M.B. Murphy, S. Moser, D. Meyer, A. Iannacone, S. Yoser, M. Bryer-Ash, S. Schussler, H. Lambeth, P. Raskin, S. Strowig, M. Basco, S. Cercone, B. Zinman, A. Barnie, R. Devenyi, M. Mandelcorn, M. Brent, S. Rogers, A. Gordon, N. Bakshi, B. Perkins, L. Tuason, F. Perdikaris, R. Ehrlich, D. Daneman, K. Perlman, S. Ferguson, J. Palmer, R. Fahlstrom, I.H.de Boer, J. Kinyoun, L. Van Ottingham, S. Catton, J. Ginsberg, C. McDonald, J. Harth, M. Driscoll, T. Sheidow, J. Mahon, C. Canny, D. Nicolle, P. Colby, J. Dupre, I. Hramiak, N.W. Rodger, M. Jenner, T. Smith, W. Brown, M. May, J. Lipps Hagan, A. Agarwal, T. Adkins, R. Lorenz, S. Feman, L. Survant, N.H. White, L. Levandoski, G. Grand, M. Thomas, D. Joseph, K. Blinder, G. Shah, D. Burgess, I. Boniuk, J. Santiago, W. Tamborlane, P. Gatcomb, K. Stoessel, P. Ramos, K. Fong, P. Ossorio, J. Ahern, R. Gubitosi-Klug, L. Meadema-Mayer, C. Beck, K. Farrell, S. Genuth, J. Quin, P. Gaston, M. Palmert, R. Trail, W. Dahms, J. Lachin, J. Backlund, I. Bebu, B. Braffett, L. Diminick, X. Gao, W. Hsu, K. Klumpp, H. Pan, V. Trapani, P. Cleary, P. McGee, W. Sun, S. Villavicencio, K. Anderson, L. Dews, Naji Younes, B. Rutledge, K. Chan, D. Rosenberg, B. Petty, A. Determan, D. Kenny, C. Williams, C. Cowie, C. Siebert, M. Steffes, V. Arends, J. Bucksa, M. Nowicki, B. Chavers, D. O’Leary, J. Polak, A. Harrington, L. Funk, R. Crow, B. Gloeb, S. Thomas, C. O’Donnell, E.Z. Soliman, Z.M. Zhang, Y. Li, C. Campbell, L. Keasler, S. Hensley, J. Hu, M. Barr, T. Taylor, R. Prineas, E.L. Feldman, J.W. Albers, P. Low, C. Sommer, K. Nickander, T. Speigelberg, M. Pfiefer, M. Schumer, M. Moran, J. Farquhar, C. Ryan, D. Sandstrom, T. Williams, M. Geckle, E. Cupelli, F. Thoma, B. Burzuk, T. Woodfill, R. Danis, B. Blodi, D. Lawrence, H. Wabers, S. Gangaputra, S. Neill, M. Burger, J. Dingledine, V. Gama, R. Sussman, M. Davis, L. Hubbard, M. Budoff, S. Darabian, P. Rezaeian, N. Wong, M. Fox, R. Oudiz, L. Kim, R. Detrano, K. Cruickshanks, D. Dalton, K. Bainbridg, J. Lima, D. Bluemke, E. Turkbey, R. J. van der Geest, C. Liu, A. Malayeri, A. Jain, C. Miao, H. Chahal, R. Jarboe, D.M. Nathan, V. Monnier, D. Sell, C. Strauch, S. Hazen, A. Pratt, W. Tang, J. Brunzell, J. Purnell, R. Natarajan, F. Miao, L. Zhang, Z. Chen, A. Paterson, A. Boright, S. Bull, L. Sun, S. Scherer, M. Lopes-Virella, T.J. Lyons, A. Jenkins, R. Klein, G. Virella, A. Jaffa, R. Carter, J. Stoner, W.T. Garvey, D. Lackland, M. Brabham, D. McGee, D. Zheng, R. K. Mayfield, J. Maynard, H. Wessells, A. Sarma, A. Jacobson, R. Dunn, S. Holt, J. Hotaling, C. Kim, Q. Clemens, J. Brown, and K. McVary
References
- 1.Nathan DM, Cleary PA, Backlund JY, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group Intensive diabetes treatment and cardiovascular outcomes in type 1 diabetes: the DCCT/EDIC study 30-year follow-up. Diabetes Care 2016;39:686–69326861924 [Google Scholar]
- 3.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. JAMA 1998;280:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purnell JQ, Zinman B, Brunzell JD; DCCT/EDIC Research Group . The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation 2013;127:180–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conway B, Miller RG, Costacou T, et al. . Double-edged relationship between adiposity and coronary artery calcification in type 1 diabetes. Diab Vasc Dis Res 2007;4:332–339 [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues TC, Veyna AM, Haarhues MD, Kinney GL, Rewers M, Snell-Bergeon JK. Obesity and coronary artery calcium in diabetes: the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study. Diabetes Technol Ther 2011;13:991–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway B, Miller RG, Costacou T, et al. . Adiposity and mortality in type 1 diabetes. Int J Obes 2009;33:796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The DCCT Research Group The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 1986;35:530–545 [PubMed] [Google Scholar]
- 9.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 10.The DCCT/EDIC Research Group Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snedecor GW, Cochran WG. Statistical Methods. 8th ed. Ames, Iowa State University Press, 1989 [Google Scholar]
- 13.Lachin J. Biostatistical Methods: The Assessment of Relative Risks. 2nd ed. Hoboken, NJ, Wiley, 2011 [Google Scholar]
- 14.The DCCT Research Group Weight gain associated with intensive therapy in the diabetes control and complications trial. Diabetes Care 1988;11:567–573 [DOI] [PubMed] [Google Scholar]
- 15.Anderson EJ, Richardson M, Castle G, et al.; The DCCT Research Group . Nutrition interventions for intensive therapy in the Diabetes Control and Complications Trial. J Am Diet Assoc 1993;93:768–772 [DOI] [PubMed] [Google Scholar]
- 16.Ogden CL, Carroll MD. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960–1962 through 2007–2008. NCHS Health E-Stat, June 2010. Available from https://www.cdc.gov/nchs/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf. Accessed 21 September 2017
- 17.Mozaffarian D, Benjamin EJ, Go AS, et al.; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016;133:e38–e360 [DOI] [PubMed] [Google Scholar]
- 18.Schenkeveld L, Magro M, Oemrawsingh RM, et al. . The influence of optimal medical treatment on the ‘obesity paradox’, body mass index and long-term mortality in patients treated with percutaneous coronary intervention: a prospective cohort study. BMJ Open 2012;2:e000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep 2012;14:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.