Abstract
OBJECTIVE
Artificial pancreas (AP) systems are best positioned for optimal treatment of type 1 diabetes (T1D) and are currently being tested in outpatient clinical trials. Our consortium developed and tested a novel adaptive AP in an outpatient, single-arm, uncontrolled multicenter clinical trial lasting 12 weeks.
RESEARCH DESIGN AND METHODS
Thirty adults with T1D completed a continuous glucose monitor (CGM)-augmented 1-week sensor-augmented pump (SAP) period. After the AP was started, basal insulin delivery settings used by the AP for initialization were adapted weekly, and carbohydrate ratios were adapted every 4 weeks by an algorithm running on a cloud-based server, with automatic data upload from devices. Adaptations were reviewed by expert study clinicians and patients. The primary end point was change in hemoglobin A1c (HbA1c). Outcomes are reported adhering to consensus recommendations on reporting of AP trials.
RESULTS
Twenty-nine patients completed the trial. HbA1c, 7.0 ± 0.8% at the start of AP use, improved to 6.7 ± 0.6% after 12 weeks (−0.3, 95% CI −0.5 to −0.2, P < 0.001). Compared with the SAP run-in, CGM time spent in the hypoglycemic range improved during the day from 5.0 to 1.9% (−3.1, 95% CI −4.1 to −2.1, P < 0.001) and overnight from 4.1 to 1.1% (−3.1, 95% CI −4.2 to −1.9, P < 0.001). Whereas carbohydrate ratios were adapted to a larger extent initially with minimal changes thereafter, basal insulin was adapted throughout. Approximately 10% of adaptation recommendations were manually overridden. There were no protocol-related serious adverse events.
CONCLUSIONS
Use of our novel adaptive AP yielded significant reductions in HbA1c and hypoglycemia.
Introduction
A number of artificial pancreas (AP) systems have recently been developed to automate basal insulin delivery for individuals with type 1 diabetes (TID) (1). Although initial AP studies were of shorter duration and performed in clinical research centers, the technology has matured enough to permit larger scale and longer outpatient clinical trials culminating in recent U.S. Food and Drug Administration (FDA) approval of the first commercial hybrid AP system in the U.S. that works by automated basal rate modulation (2–4). A limitation of current AP systems is that patients must still input carbohydrate estimations for delivery of meal insulin boluses. Because insulin analogs take ∼50 min to reach peak serum concentration (5), adjustment of the basal rate (reduction/suspension or increase in insulin delivery) is not always enough to compensate for inaccuracies in meal bolus dosing, exercise, illnesses, stress, or other activities that change insulin sensitivity.
As a result, a number of recent AP studies have included a period of clinician-led optimization of open-loop insulin pump settings or continued clinician adjustments to these settings throughout their use, with the goal of letting the AP system function as effectively as possible when basal rates and other settings are already optimized (6–12). To help this process, different degrees of automated adaptation are being developed (13–15). Yet how to best optimize adaptation to safely improve glucose control, in frequency and magnitude of adjustments to the underlying basal insulin and carbohydrate ratio profile, remains to be determined (3,16).
We previously reported on our system that algorithmically optimized open-loop settings to improve AP results (15). We have since improved and expanded this system, and here report the results of a 12-week single-arm, multicenter clinical trial of 24/7 at-home AP in 30 adult patients with T1D. In this trial we performed cloud-based, algorithmic adaptation of basal rate and carbohydrate ratio profiles during AP use, providing a novel automated system for weekly adaptation of insulin delivery settings without the need for clinician or patient involvement for upload or analysis. We used these automated adaptations to initialize the AP system for subsequent weeks in the study to assess long-term improvement in glycemic control by change in hemoglobin A1c (HbA1c) and time in euglycemic target range.
Research Design and Methods
Trial Oversight
This study was an investigator-initiated single-arm, multicenter trial analyzing the effects of weekly adaptations of basal insulin rates and monthly adaptations of carbohydrate ratios during AP use at three clinical sites (William Sansum Diabetes Center, Santa Barbara, CA; Mayo Clinic, Rochester, MN; and University of Virginia, Charlottesville, VA), with engineering support from four sites (Harvard University, Cambridge, MA; University of California, Santa Barbara, Santa Barbara, CA; University of Padova, Padova, Italy; and University of Virginia, Charlottesville, VA). The trial was overseen by a data and safety monitoring board. The full trial protocol was approved by the FDA, the institutional review board at each center, and the data and safety monitoring board. The trial was performed in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent. The protocol was registered on www.clinicaltrials.gov (NCT02705053). The authors assume responsibility for the accuracy and completeness of the data and analysis.
Patients and Study Design
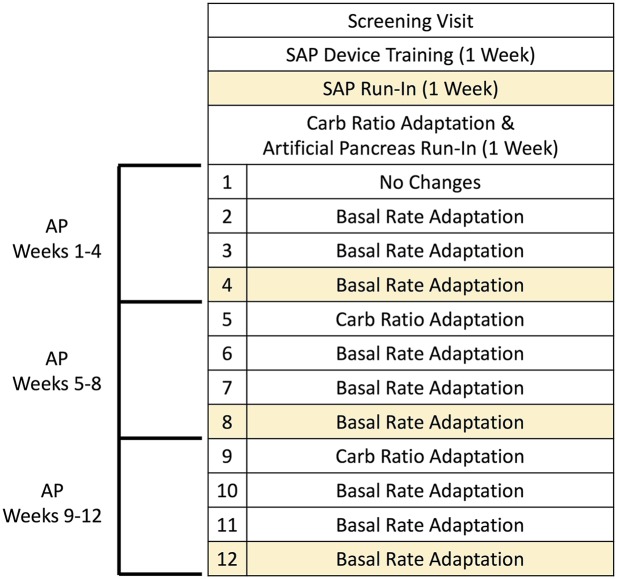
The design of the study is shown in Fig. 1. Adults (aged 21–65 years) with T1D for at least 1 year, using an insulin pump for at least 6 months, and an HbA1c <10% were enrolled. Additional eligibility criteria included baseline screening laboratory tests within normal reference ranges and commitment from a care partner knowledgeable of the participant’s location at all times and available to assist in responding to hypo- or hyperglycemia as needed. Exclusion criteria included pregnancy, diabetic ketoacidosis or severe hypoglycemia in the past 12 months, medical conditions or medication use that increased the risk of hypo- or hyperglycemia, and hypoglycemia unawareness, defined as a score of ≥4 on the Clarke Hypoglycemia Unawareness Questionnaire (17).
Figure 1.
Study design. After a 1-week SAP run-in, patients had an automated carbohydrate (Carb) ratio adaptation and underwent AP training and run-in. They then used the AP for 4 weeks, with automated basal rate adaptations occurring at the start of each week for 3 more weeks. This same pattern (one carbohydrate adaptation followed by 3 weekly basal rate adaptations) was performed again for the second and third months of the study. Prespecified outcome weeks are shaded.
The Accu-Chek Spirit Combo insulin pump (Roche Diabetes Care, Indianapolis, IN) and the G4 Share AP continuous glucose monitor (CGM) with 505 algorithm (Dexcom, San Diego, CA) were connected wirelessly via Bluetooth to the Diabetes Assistant (DiAs) smartphone device (University of Virginia) (Supplementary Fig. 1) (18). The Zone Model Predictive Controller (MPC) (19) AP and the Health Monitoring System (20) hypoglycemia prediction algorithms (University of California, Santa Barbara/Harvard University) both ran on the DiAs. Remote safety monitoring was available to clinical staff, who received short message service–text message alerts for specific conditions where they were instructed to contact a participant or care partner should there be a technical issue or safety concern, both in the sensor-augmented pump (SAP) 1-week run-in and during the 12 weeks of AP use. Study staff reviewed patient data on the remote monitoring site continuously throughout the study according to the FDA mandate. In addition, participants were contacted weekly by study staff and had monthly follow-up visits throughout the study. While using the AP system, patients were instructed to avoid deviating from their regular daily diet and exercise routine and to maintain their usual sleep schedule during the course of the study.
The Zone MPC AP control algorithm modulated insulin delivery every 5 min based on the CGM glucose level, historical glucose measurements, anticipated glucose trends, historical insulin delivery, and patient-specific information such as the basal rate profile. The algorithm strove to maintain CGM glucose levels in the safe euglycemic range (80–140 mg/dL) during the day, with an asymmetric penalty weighted to prevent hypoglycemia (19). Meal boluses were also given by subjects for their meals. Similar to our previous study designs (15,21) and as validated by others (22), the AP system modified the mealtime bolus based on the fingerstick self-monitoring blood glucose (SMBG) value at the time of the meal as follows: for SMBG <120 mg/dL, 80% of the bolus calculated using the patient’s own carbohydrate ratio was delivered; for SMBG between 120 and 150 mg/dL, the full bolus was given; for SMBG >150 mg/dL, the full bolus was given with an additional correction based on the patient’s own correction factor to lower glucose to 150 mg/dL. The correction was limited to 2 units.
Adaptation of Carbohydrate Ratio and Basal Rates
Every 5 min, the DiAs device uploaded CGM, SMBG, meal, and insulin delivery data to a server without any user intervention. At the start of AP use and once every 4 weeks, an automated algorithm running on this server retrospectively reviewed the CGM, insulin delivery, and meal data of the last 7 days to calculate an index of insulin sensitivity relative to each meal, as previously described (23), and recommended changes to the carbohydrate ratio profile. Changes were allowed only if large hypo- or hyperglycemic excursions occurred within the last 7 days. For safety reasons, each carbohydrate ratio value was constrained to lie within the range 4–20 g/unit, with a 0.5 g/unit resolution, and the final recommendation was allowed to deviate from the previous profile by no more than 20% (see Supplementary Data for additional details).
At the start of AP use and three times per month, the basal rate adaptation routine also ran on the cloud, using CGM, SMBG, and insulin delivery data between meals. It computed an “ideal” basal profile, reconciling historical AP insulin delivery over the previous 10 days with the current profile setting and provided an adapted basal profile that deviated from the original by no more than 25% at any time of the day (see Supplementary Data for additional details).
Study physicians reviewed these recommended changes in carbohydrate ratio and basal rate profile as part of the study protocol, with changes made to the insulin delivery profile settings after mutual agreement between the study physician and the patient. A team of study physicians reviewed all overrides.
Outcome Measures
The primary end point was the change in HbA1c from the start of AP use to the end of week 12 of AP use, with HbA1c also measured at 4 and 8 weeks into AP use for repeated measures. Secondary outcome measures included a set of predefined quantifications of the CGM data and data generated about the operation of the adapted AP algorithm. For the CGM data, numerical summaries were calculated at four time points to match the HbA1c assessment periods; the SAP run-in phase week and the last week of each of the 4-week AP periods (Fig. 1). Other CGM-based outcome measures are reported consistent with the recent AP outcomes measures consensus statement (24).
Statistical Analyses
The sample size for this study was based on the change in HbA1c, which was assumed to be similar to recent long-term outpatient AP studies (11). Power analysis showed that at least 26 patients would be required to achieve 95% power at significance level of 0.05 to detect a change of 0.76 SD in HbA1c. To account for attrition during the course of the study, the sample size was adjusted to be 30 overall, with 10 participants per site. For analyses of the primary and CGM-based secondary outcome measures, a mixed model with random subject effect was used to account for the clustering of the four measurements over time per participant. Study time was modeled as a factor variable, and the primary contrast of interest was the change between week 12 of AP and the baseline assessment at the end of the SAP run-in. Additional contrasts comparing the change of the intermediate weeks (i.e., weeks 4 and 8 of AP) with baseline were also estimated. Descriptive statistics, including means ± SDs and n (%), were also computed to describe the sample characteristics and outcome distributions. R 3.3.1 (The R Project for Statistical Computing, Vienna, Austria) and SAS 9.4 (SAS Institute, Cary, NC) were used for statistical programming, including derivation of the CGM summaries. Mixed models were estimated using PROC MIXED in SAS 9.4. Reported P values are two-sided and were not adjusted for multiple testing. All results are expressed as mean ± SD.
Results
Patients
Of 34 patients who were screened, 32 (17 women) enrolled, with 2 withdrawals after device training before the SAP run-in. Baseline HbA1c at the start of AP was 7.0 ± 0.8%. One patient stopped the AP intervention at week 11 owing to an unrelated acute coronary event (see Adverse Events) but was included in all analyses. There were 29 patients who completed the full protocol. Demographic details are listed in Supplementary Table 1.
Glycemic Outcomes
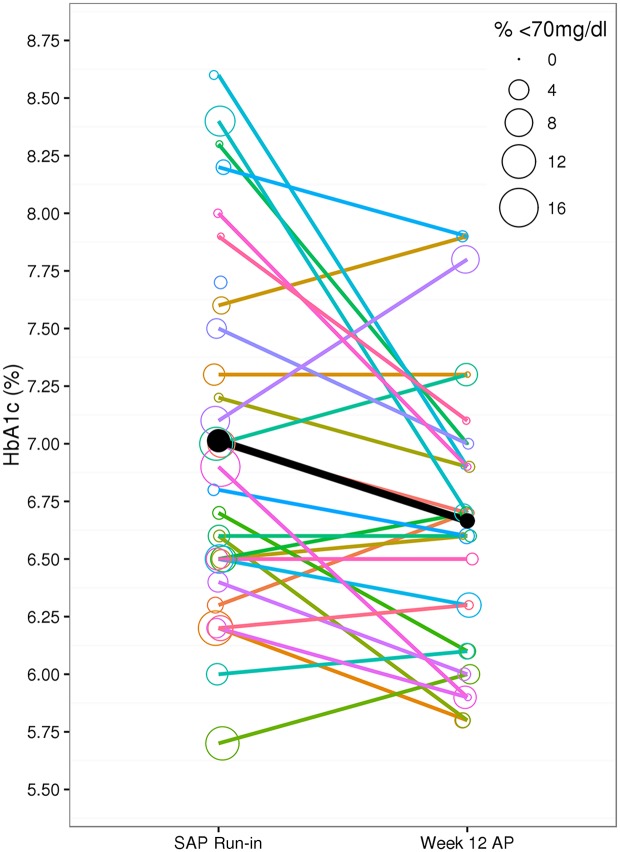
HbA1c, 7.0 ± 0.8% at the start of AP use, improved to 6.7 ± 0.6% after 12 weeks (−0.3, 95% CI −0.5 to −0.2, P < 0.001) (Table 1). Compared with the SAP run-in, the percentage of time CGM glucose was <70 mg/dL improved during the day from 5.0 to 1.9% (−3.1, 95% CI −4.1 to −2.1, P < 0.001) and overnight from 4.1 to 1.1% (−3.1, 95% CI −4.2 to −1.9, P < 0.001) compared with the last week of the AP (Fig. 2 and Table 1). The changes were also reflected in the low blood glucose index.
Table 1.
Summary of clinical metrics
| SAP run-in |
Week 4 AP vs. SAP run-in |
Week 8 AP vs. SAP run-in |
Week 12 AP vs. SAP run-in |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Estimate | 95% CI | P | Mean ± SD | Estimate | 95% CI | P | Mean ± SD | Estimate | 95% CI | P | |
| Primary outcome | |||||||||||||
| HbA1c, % | 7.0 ± 0.8 | 6.8 ± 0.6 | −0.2 | −0.4, −0.1 | 0.008 | 6.7 ± 0.5 | −0.3 | −0.5, −0.2 | <0.001 | 6.7 ± 0.6 | −0.3 | −0.5, −0.2 | <0.001 |
| Secondary outcomes | |||||||||||||
| Overall control | |||||||||||||
| BGRI | 5.9 ± 2.4 | 5.5 ± 2.6 | −0.4 | −1.2, 0.5 | 0.40 | 6.1 ± 3.3 | 0.2 | −0.7, 1.0 | 0.70 | 6.3 ± 3.1 | 0.4 | 0.5, 1.3 | 0.35 |
| Glucose | |||||||||||||
| Mean, mg/dL | 141.9 ± 20.1 | 146.4 ± 17.8 | −0.6 | −1.3, 10.4 | 0.12 | 149.6 ± 21.0 | 7.7 | 1.9, 13.5 | 0.01 | 152.5 ± 20.7 | 10.9 | 5.0, 16.7 | <0.001 |
| SD, mg/dL | 49.2 ± 9.8 | 47.2 ± 11.2 | −2.0 | −5.7, 1.7 | 0.29 | 49.2 ± 14.2 | 0.1 | −3.6, 3.8 | 0.95 | 49.0 ± 13.1 | −0.1 | −3.8, 3.7 | 0.98 |
| CV glucose | 34.6 ± 4.7 | 32.1 ± 4.9 | −2.6 | −4.4, −0.8 | 0.005 | 32.5 ± 6.0 | −2.1 | −3.9, −0.3 | 0.021 | 31.9 ± 5.9 | −2.7 | −4.5, −0.9 | 0.004 |
| % Time | |||||||||||||
| 70–180 mg/dL | 73.9 ± 12.2 | 76.7 ± 10.8 | 2.8 | −1.0, 6.6 | 0.15 | 74.1 ± 13.2 | 0.2 | −3.6, 4.0 | 0.91 | 72.6 ± 13.2 | −1.3 | −5.2, 2.5 | 0.49 |
| 80–140 mg/dL | 46.2 ± 11.0 | 47.1 ± 11.2 | 0.9 | −2.6, 4.5 | 0.60 | 47.4 ± 13.1 | 1.2 | −2.4, 4.7 | 0.51 | 44.2 ± 13.1 | −2.0 | −5.6, 1.6 | 0.26 |
| % Time overnight | |||||||||||||
| 70–180 mg/dL | 73.1 ± 17.9 | 77.9 ± 14.3 | 4.9 | −1.2, 10.9 | 0.116 | 76.3 ± 16.6 | 3.3 | −2.8, 9.3 | 0.29 | 69.5 ± 20.2 | −3.5 | −9.7, 2.6 | 0.26 |
| 80–140 mg/dL | 45.7 ± 16.1 | 46.4 ± 18.6 | 0.7 | −5.7, 7.1 | 0.83 | 48.7 ± 17.6 | 3.1 | −3.3, 9.4 | 0.34 | 40.9 ± 20.4 | −4.6 | −11.0, 1.8 | 0.16 |
| Hypoglycemia | |||||||||||||
| LBGI | 1.3 ± 0.9 | 0.7 ± 0.4 | −0.6 | −0.8, −0.4 | <0.001 | 0.6 ± 0.4 | −0.6 | −0.8, −0.4 | <0.001 | 0.6 ± .4 | −0.7 | −0.9, −0.5 | <0.001 |
| % Time | |||||||||||||
| <50 mg/dL | 0.6 ± 0.8 | 0.2 ± 0.2 | −0.4 | −0.6, −0.2 | <0.001 | 0.2 ± 0.3 | −0.4 | −0.6, −0.2 | <0.001 | 0.2 ± 0.3 | −0.4 | −0.6, −0.2 | <0.001 |
| <60 mg/dL | 2.0 ± 2.2 | 0.7 ± 0.7 | −1.3 | −1.8, −0.8 | <0.001 | 0.7 ± 0.8 | −1.3 | −1.8, −0.8 | <0.001 | 0.7 ± 0.8 | −1.4 | −1.9, −0.8 | <0.001 |
| <70 mg/dL | 5.0 ± 4.2 | 2.0 ± 1.6 | −3.0 | −4.0, −2.0 | <0.001 | 1.9 ± 1.6 | −3.1 | −4.1, −2.1 | <0.001 | 1.9 ± 2.0 | −3.1 | −4.1, −2.1 | <0.001 |
| % Time overnight | |||||||||||||
| <70 mg/dL | 4.1 ± 4.3 | 0.9 ± 1.4 | −3.2 | −4.3, −2.1 | <0.001 | 0.7 ± 1.1 | −3.4 | −4.6, −2.3 | <0.001 | 1.1 ± 1.9 | −3.1 | −4.2, −1.9 | <0.001 |
| Hyperglycemia | |||||||||||||
| HBGRI | 4.7 ± 2.8 | 4.9 ± 2.7 | 0.2 | −0.7, 1.1 | 0.65 | 5.4 ± 3.4 | 0.8 | −0.1, 1.7 | 0.089 | 5.7 ± 3.3 | 1.1 | 0.2, 2.0 | 0.019 |
| % Time | |||||||||||||
| >180 mg/dL | 21.1 ± 13.5 | 21.3 ± 11.3 | 0.2 | −3.8, 4.1 | 0.94 | 24.0 ± 13.5 | 2.9 | −1.0, 6.8 | 0.147 | 25.5 ± 13.7 | 4.5 | 0.5, 8.5 | 0.027 |
| >250 mg/dL | 4.1 ± 4.5 | 4.3 ± 5.7 | 0.2 | −1.8, 2.2 | 0.84 | 5.6 ± 7.7 | 1.6 | −0.4, 3.6 | 0.125 | 5.6 ± 7.2 | 1.6 | −0.4, 3.6 | 0.124 |
| >300 mg/dL | 1.0 ± 1.5 | 1.2 ± 2.9 | 0.2 | −0.9, 1.2 | 0.76 | 1.9 ± 4.2 | 0.9 | −0.2, 1.9 | 0.107 | 1.8 ± 3.1 | 0.7 | −0.3, 1.8 | 0.17 |
Shown are repeated-measures comparisons between the SAP run-in week and the 4th, 8th, and 12th week of AP use. HbA1c was collected at the start of the AP (end of SAP run-in week) and again at the end of the 4th, 8th, and 12th week of AP use. Statistically significant changes are highlighted in bold.
BGRI, blood glucose risk index; CV, coefficient of variation; HBGRI, high blood glucose risk index; LBGI, low blood glucose index.
Figure 2.
Change in HbA1c compared with percentage of time in hypoglycemia. Represented are the individual HbA1c measurements and the percentage of time CGM glucose was <70 mg/dL (radius of the circle) experienced by each patient, comparing the SAP run-in week to the final week of AP use. The mean HbA1c change and reduction in the percentage of time in CGM glucose of <70 mg/dL is shown by the black line and filled in black circles. Changes for individual subjects are shown by the colored lines and colored circles.
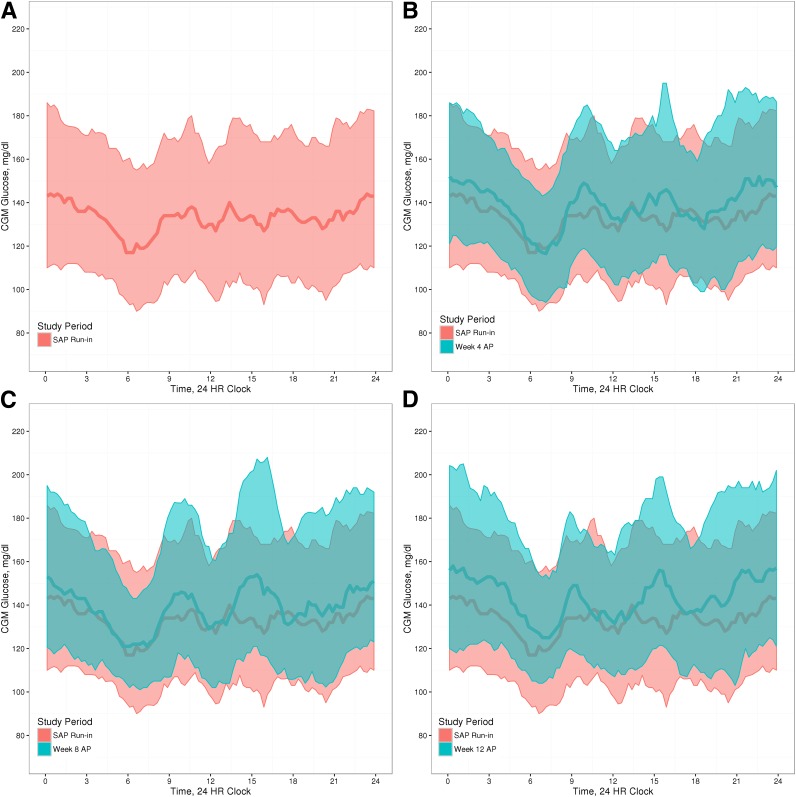
The mean CGM glucose values for the SAP run-in phase, compared with weeks 4, 8, and 12 of AP use, are shown graphically in Fig. 3. The reduced exposure to hypoglycemia throughout the study is reflected in the changes in the blue overlays in Fig. 3, suggesting decreased glycemic variability throughout the study. However, the mean CGM glucose increased from 141.9 ± 20.1 mg/dL at SAP run-in to 149.6 ± 21.0 mg/dL at week 8 and to 152.5 ± 20.7 mg/dL at week 12 (Table 1).
Figure 3.
CGM glucose tracings. Mean glucose measurements (CGM) during the SAP run-in week (A) compared with the 4th (B), 8th (C), and 12th (D) week of AP use. Shaded regions are the interquartile range of the glucose readings for each time period. HR, hour.
Total carbohydrate ratio profile changes showed a larger change with the first adaptation (with some patients changing almost 20%) (Supplementary Fig. 2). After the first month of the AP, there was convergence with minimal changes to carbohydrate ratios.
There was considerable basal rate profile adaptation throughout the study (three times per month) (Supplementary Fig. 3). Total basal profile changed by as much as 2.5 units/day, although the effect of this was difficult to discern given that the AP was adjusting basal rates every 5 min.
The total daily dose of insulin delivered in the prespecified outcome weeks is listed by subject in Supplementary Table 2. The total daily insulin dose during AP increased from a mean of 35.0 ± 15.4 units/day during the SAP run-in to 42.5 ± 21.4 units/day during week 12 of the AP (7.77, 95% CI 4.61 to 10.93, P < 0.001). The total bolus dose did not significantly change, suggesting the increase in TDI was primarily from basal rate changes and controller action during AP use. The total use time of the AP system is also listed in Supplementary Table 2.
Physician Overrides of the Automated Learning System
During the study, the system made a combined 358 recommendations to update basal rate and carbohydrate ratio settings. Study physicians declined the automated recommendation 21 times for the carbohydrate ratio and 14 times for basal rate (90% acceptance rate of the algorithmic adaptations) (Supplementary Table 3). The main reason cited was concern for increasing hyper- or hypoglycemia in the context of patients stating a change to their prior routine (e.g., change in physical activity).
Adverse Events
During the 12-week study period (>60,000 h of AP use), one patient had a hypoglycemic event requiring evaluation and assistance taking oral carbohydrates after a meal bolus but no additional treatment. This event occurred despite the automatic 20% reduction in recommended meal bolus insulin delivery that is built into the AP, which the patient accepted before giving the bolus. Ketones developed in five patients related to infusion set failure, all of which resolved with changing the infusion set. An unrelated acute coronary syndrome developed in one patient during exercise while euglycemic for the past 24 h in the 11th week of the study and was found to have a 90% occlusion of his right coronary artery requiring stent placement. He recovered fully but was withdrawn from the study based on prespecified criteria. All adverse events are summarized in Supplementary Table 4.
Conclusions
The advent of insulin pumps, CGM, and other advanced technology has led to numerous changes in diabetes care. Yet outcomes are still not ideal, with registry data showing a mean HbA1c of 8.4% in persons with T1D, along with up to 6% of T1D Exchange registry participants reporting a seizure or loss of consciousness caused by hypoglycemia in the prior 3 months (25).
AP systems, which adjust up or down preset basal rates on the insulin pump, promise to improve these results. Percentage of time spent in goal glucose range of 70–180 mg/dL is as high as 67.7–79.1% in larger studies of outpatient AP, and the automation involved has increased (3,11,12,15,26). However, a limitation remains on the effectiveness of AP caused by the speed of insulin action, with automated basal rate changes taking time—sometimes several hours—to show full clinical effect (27). Patients who are taking too little or too much basal insulin may find it takes too long to address disturbances in glucose levels.
Automated adaptations to achieve ideal basal insulin delivery settings are clinically important. Having patients on nearly optimal basal rates helps the AP algorithms to be as effective as possible as they work within a given constraint when reducing/suspending or giving extra insulin. The insulin delivery settings for patients starting AP may not be optimized. A well-recognized limitation of current diabetes management is the difficulty in obtaining and analyzing the day-to-day data from diabetes devices, where only a minority of patients ever review their data (28). In fact, during the run-in period of other AP studies, with just the addition of CGM and weekly follow-up for structured education and heightened awareness of their glucose control, patients showed significant improvement in HbA1c over 4 to 6 weeks before use of AP (29).
Some studies have proven the feasibility of using cloud-based diabetes management programs for insulin initiation and titration, although these were not fully automated (30). Our previous efforts have focused on a run-to-run methodology to adapt basal rates and carbohydrate ratios through continuous feedback in the open-loop setting (31–33), followed by application of these results to the AP (15). In this study, we developed a novel, user-friendly (completely automated) cloud computing system to seamlessly integrate data from the AP into our adaptation framework for basal rates and carbohydrate ratios throughout the study, potentially obviating the need for clinician involvement before or during use of the AP. Our approach, adapting open-loop settings that are used by the AP algorithm, is easily understandable and transparent to both patients and physicians.
Our results were similar to other long-term AP trials, with our study showing a mean HbA1c decrease of 0.3% after 12 weeks of use (3,4,12,34). How frequently adaptation should be happening to further improve HbA1c is another aspect of AP systems that is not yet known. Whereas the currently approved 670G system adapts daily (35), our system responds every week.
In this report, the percentage of time glucose was at <70 mg/dL significantly decreased during the day and overnight, but at the same time showed improvement in HbA1c. Despite the mean starting HbA1c of only 7.0%, almost all patients showed an improvement while also reducing time in the hypoglycemic range. No significant change occurred in the percentage of time in the range 70–180 mg/dL, but mean glucose increased from 142 to 153 mg/dL. As is well known, the glucose distribution in patients with T1D is asymmetric; thus, mean glucose provides limited information. The likely explanation for the elevation in mean glucose despite the HbA1c being reduced is that our analyses only account for 4 weeks during this period. The SD remained similar while mean glucose increased, resulting in a decrease of the coefficient of variation. This confirms a decrease in the dispersion of glucose. Finally, the HbA1c values reflect glucose status over 8 to 12 weeks, and glucose analyses for all of the 12 weeks may show lower mean glucose.
It will be important for clinicians and patients to have trust in the recommendations of any automated adaptation system that adjusts insulin delivery settings. For this reason, we allowed study clinicians and patients to override the automated algorithmic adaptations. Less than 10% of the algorithmic insulin delivery profile recommendations were overridden as a result of 1) concern for hyper- or hypoglycemia after meals, 2) changing insulin requirements reported by the patients, and 3) projected changes in activity level for the following week. The largest changes in carbohydrate ratio adaptation were made by the system in the beginning of the study, with subsequent minor changes as the study progressed. Larger controlled studies will be necessary to interpret the true effectiveness of these changes on AP performance.
We recognize limitations in our study. Although we compared AP results to the SAP run-in, this was a single-arm and uncontrolled study for the effects of AP as well as the carbohydrate ratio and basal rate adaptations. The AP period was also much longer than the 1-week SAP run-in. Remote monitoring was used to comply with regulatory requirements in this early feasibility study of an investigational device. In addition, regular study subject contact occurred at least weekly regarding the parameter updates as well as to respond to any technical issues. The number of contacts with study subjects was not tracked; however, no known interventions were required that were not already underway to respond to hypoglycemia. In addition, no care partner was required to intervene on behalf of a study subject. Finally, the starting HbA1c of 7% reflects that the study patients were sophisticated users, implying adherence with diabetes management. Despite this, they showed an improvement in HbA1c and a reduction in hypoglycemia, similar to other studies of an AP with adherent patients (36).
In conclusion, we found that outpatient use of an AP system for 12 weeks, with algorithmic adaptation and eventual optimization of insulin delivery parameters, has the potential to deliver enormous benefits. We have shown significant improvement not only by change in HbA1c but also by reduction of hypoglycemia. Continued study of ways to improve adaptation of AP systems is needed.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge the patients and their families who participated in this clinical trial, Dr. Howard Zisser (Department of Chemical Engineering, University of California, Santa Barbara) for his assistance in designing the study, Benton Mize (Center for Diabetes Technology, University of Virginia), Antoine Roberts (Center for Diabetes Technology, University of Virginia), and Dr. Patrick Keith-Hynes (TypeZero Technologies) for their assistance in programming the DiAs device, and, finally, the staff at the clinical and engineering centers who helped support this project.
Data and Safety Monitoring Board: Dr. Trang T. Ly, (chair) (Division of Pediatric Endocrinology and Diabetes, Department of Pediatrics, Stanford University School of Medicine), Dr. David M. Maahs (Barbara Davis Center, University of Colorado Denver), and Dr. Danielle Gianferante (Type 1 Diabetes Exchange).
Funding. This study was supported by grants from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (DP3-DK-094331) and the National Center for Advancing Translational Science (UL1-TR-000135) and by the Urdang Family Foundation to Mayo Clinic. C.C., C.D.M., and M.S. are partially supported by Italian Ministero dell’Università e della Ricerca Scientifica (Progetto di Ateneo dell’Università di Padova 2014). Roche Diabetes Care (Indianapolis, IN) supplied all insulin pumps (Accu-Chek Spirit Combo) and additional supplies in-kind. Dexcom (San Diego, CA) provided a research discount on pricing on the CGM sensors, transmitters, and receivers (G4 Platinum with Share). Test strips (OneTouch Ultra Blue) were supplied by LifeScan in-kind, through support from the Investigator-Initiated Study Program of LifeScan.
The funders and device manufacturers had no influence on the design or conduct of the trial and were not involved in data collection or analysis, the writing of the manuscript, or the decision to submit it for publication.
Duality of Interest. E.D. has received consulting fees from Animas and Insulet; has received research support from Dexcom, Insulet, Roche, Xeris, and Animas; and receives royalty payments on intellectual property related to the MPC algorithm used. J.E.P. has conducted AP research sponsored by Insulet and Bigfoot and has received product support to his institution from Animas, Lifescan, Roche, and Dexcom. Y.C.K. has performed studies for Medtronic and has received product support from Dexcom, Roche, and Tandem. S.A.B. reports materials support from Roche Diagnostics and Dexcom, grants and material support from Animas Corporation, and grants from Medtronic outside the submitted work. R.G. receives royalty payments on intellectual property related to the MPC algorithm used in this study. S.P. is a shareholder of TypeZero Technologies. S.A. reports support from Dexcom and Roche Diagnostics, grants from Medtronic, personal fees from Senseonics, and grants from InSpark outside the submitted work. A.B. has received research support from Novo Nordisk, Tandem, and AstraZeneca. B.K. has patents and patent applications related to diabetes technology managed by the University of Virginia Licensing and Ventures group; has received grant/research support managed by the University of Virginia from Dexcom, Roche Diabetes Care, Sanofi, and Tandem Diabetes Care; has received advisory board/consultant/speakers' bureau fees for Dexcom, Sanofi, and Senseonics; and cofounded and is a shareholder of TypeZero Technologies. C.C. reports 10 patents and patent applications related to glucose sensors and AP; nonfinancial support from Roche and Dexcom; and research support managed by the University of Padova from Dexcom, Sanofi, and Adocia and is on an advisory panel for Novo Nordisk. F.J.D. has received consulting fees from Mode AGC; has received research support from Dexcom, Insulet, Roche, and Xeris; and receives royalty payments on intellectual property related to the MPC algorithm used in this study. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. E.D., J.E.P., Y.C.K., S.A.B., R.G., C.D.M., S.P., M.S., A.B., B.K., C.C., and F.J.D. helped design the study protocol, contributed to the technology design, ensured the regulatory approval of the CLC system, provided primary technical and clinical support on site during all sessions, analyzed data, and authored the manuscript. E.D., R.G., C.D.M., S.P., M.S., I.D., L.M.H., J.H., D.L., E.S., A.J.L.S., C.C., and F.J.D. contributed to the technology design of the CLC system, aided in data acquisition and analysis, and edited and revised the manuscript. E.D., R.G., I.D., and E.S. helped construct the controller infrastructure, contributed to the discussion, and reviewed and edited the manuscript. J.E.P., Y.C.K., S.A.B., S.P., V.D., M.M.C., W.C.B., J.H., S.A., D.L., E.E., S.K.M.-S., T.J., P.K.B., L.H., and A.B. conducted the clinical trials, researched data, and reviewed and edited the manuscript. R.E.C. performed the statistical analysis and contributed to writing and reviewing of the manuscript. F.J.D. edited and reviewed the manuscript and was the principal investigator of the project. F.J.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 10th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD), Paris, France, 15–18 February 2017.
Footnotes
Clinical trial reg. no. NCT02705053, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1188/-/DC1.
References
- 1.Kovatchev B, Tamborlane WV, Cefalu WT, Cobelli C. The artificial pancreas in 2016: a digital treatment ecosystem for diabetes. Diabetes Care 2016;39:1123–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovatchev BP, Renard E, Cobelli C, et al. . Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care 2014;37:1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thabit H, Tauschmann M, Allen JM, et al.; APCam Consortium and AP@home Consortium. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergenstal RM, Garg S, Weinzimer SA, et al. . Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 5.Home PD, Barriocanal L, Lindholm A. Comparative pharmacokinetics and pharmacodynamics of the novel rapid-acting insulin analogue, insulin aspart, in healthy volunteers. Eur J Clin Pharmacol 1999;55:199–203 [DOI] [PubMed] [Google Scholar]
- 6.Thabit H, Lubina-Solomon A, Stadler M, et al. . Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet Diabetes Endocrinol 2014;2:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leelarathna L, Dellweg S, Mader JK, et al.; AP@home Consortium . Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care 2014;37:1931–1937 [DOI] [PubMed] [Google Scholar]
- 8.Hovorka R, Elleri D, Thabit H, et al. . Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care 2014;37:1204–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nimri R, Muller I, Atlas E, et al. . Night glucose control with MD-Logic artificial pancreas in home setting: a single blind, randomized crossover trial-interim analysis. Pediatr Diabetes 2014;15:91–99 [DOI] [PubMed] [Google Scholar]
- 10.Cameron F, Niemeyer G, Wilson DM, et al. . Inpatient trial of an artificial pancreas based on multiple model probabilistic predictive control with repeated large unannounced meals. Diabetes Technol Ther 2014;16:728–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovatchev B, Cheng P, Anderson SM, et al. . Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther 2017;19:18–24 [DOI] [PubMed] [Google Scholar]
- 12.Garg SK, Weinzimer SA, Tamborlane WV, et al. . Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ly TT, Weinzimer SA, Maahs DM, et al. . Automated hybrid closed-loop control with a proportional-integral-derivative based system in adolescents and adults with type 1 diabetes: individualizing settings for optimal performance. Pediatr Diabetes 2017;18:348–355 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Zhang J, Zeng F, et al. . “Learning” can improve the blood glucose control performance for type 1 diabetes mellitus. Diabetes Technol Ther 2017;19:41–48 [DOI] [PubMed] [Google Scholar]
- 15.Dassau E, Brown SA, Basu A, et al. . Adjustment of open-loop settings to improve closed-loop results in type 1 diabetes: a multicenter randomized trial. J Clin Endocrinol Metab 2015;100:3878–3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell SJ, El-Khatib FH, Sinha M, et al. . Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014;371:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995;18:517–522 [DOI] [PubMed] [Google Scholar]
- 18.Keith-Hynes P, Guerlain S, Mize B, et al. . DiAs user interface: a patient-centric interface for mobile artificial pancreas systems. J Diabetes Sci Technol 2013;7:1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gondhalekar R, Dassau E, Doyle FJ 3rd. Periodic zone-MPC with asymmetric costs for outpatient-ready safety of an artificial pancreas to treat type 1 diabetes. Automatica (Oxf) 2016;71:237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey RA, Dassau E, Zisser H, Seborg DE, Jovanovič L, Doyle FJ III. Design of the health monitoring system for the artificial pancreas: low glucose prediction module. J Diabetes Sci Technol 2012;6:1345–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinsker JE, Lee JB, Dassau E, et al. . Randomized crossover comparison of personalized MPC and PID control algorithms for the artificial pancreas. Diabetes Care 2016;39:1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elleri D, Biagioni M, Allen JM, et al. . Safety, efficacy and glucose turnover of reduced prandial boluses during closed-loop therapy in adolescents with type 1 diabetes: a randomized clinical trial. Diabetes Obes Metab 2015;17:1173–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiavon M, Dalla Man C, Kudva YC, Basu A, Cobelli C. Quantitative estimation of insulin sensitivity in type 1 diabetic subjects wearing a sensor-augmented insulin pump. Diabetes Care 2014;37:1216–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maahs DM, Buckingham BA, Castle JR, et al. . Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care 2016;39:1175–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 26.Anderson SM, Raghinaru D, Pinsker JE, et al.; Control to Range Study Group . Multinational home use of closed-loop control is safe and effective. Diabetes Care 2016;39:1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinemann L, Nosek L, Kapitza C, Schweitzer MA, Krinelke L. Changes in basal insulin infusion rates with subcutaneous insulin infusion: time until a change in metabolic effect is induced in patients with type 1 diabetes. Diabetes Care 2009;32:1437–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong JC, Neinstein AB, Spindler M, Adi S. A minority of patients with type 1 diabetes routinely downloads and retrospectively reviews device data. Diabetes Technol Ther 2015;17:555–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leelarathna L, Thabit H, Hartnell S, et al.; AP@home Consortium . Rapid benefits of structured optimization and sensor-augmented insulin pump therapy in adults with type 1 diabetes. J Diabetes Sci Technol 2017;11:180–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu WC, Lau KH, Huang R, et al. . Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther 2016;18:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zisser H, Palerm CC, Bevier WC, Doyle FJ 3rd, Jovanovic L. Clinical update on optimal prandial insulin dosing using a refined run-to-run control algorithm. J Diabetes Sci Technol 2009;3:487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palerm CC, Zisser H, Jovanovič L, Doyle FJ 3rd. A run-to-run control strategy to adjust basal insulin infusion rates in type 1 diabetes. J Process Contr 2008;18:258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palerm CC, Zisser H, Bevier WC, Jovanovic L, Doyle FJ 3rd. Prandial insulin dosing using run-to-run control: application of clinical data and medical expertise to define a suitable performance metric. Diabetes Care 2007;30:1131–1136 [DOI] [PubMed] [Google Scholar]
- 34.Kropff J, Del Favero S, Place J, et al.; AP@home consortium . 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. Lancet Diabetes Endocrinol 2015;3:939–947 [DOI] [PubMed] [Google Scholar]
- 35.Picazo A. A closer look at the Medtronic 670G: Dr. Bruce Buckingham [article online], 2016. Available from https://carbdm.org/medtronic-670g-bruce-buckingham/. Accessed 13 June 2017
- 36.Bally L, Thabit H, Kojzar H, et al. . Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol 2017;5:261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.