Abstract
OBJECTIVE
Intense exercise is a major challenge to the management of type 1 diabetes (T1D). Closed-loop control (CLC) systems (artificial pancreas) improve glycemic control during limited intensity and short duration of physical activity (PA). However, CLC has not been tested during extended vigorous outdoor exercise common among adolescents.
RESEARCH DESIGN AND METHODS
Skiing presents unique metabolic challenges: intense prolonged PA, cold, altitude, and stress/fear/excitement. In a randomized controlled trial, 32 adolescents with T1D (ages 10–16 years) participated in a 5-day ski camp (∼5 h skiing/day) at two sites: Wintergreen, VA, and Breckenridge, CO. Participants were randomized to the University of Virginia CLC system or remotely monitored sensor-augmented pump (RM-SAP). The CLC and RM-SAP groups were coarsely paired by age and hemoglobin A1c (HbA1c). All subjects were remotely monitored 24 h per day by the study physicians and clinical team.
RESULTS
Compared with physician-monitored open loop, percent time in range (70–180 mg/dL) improved using CLC: 71.3 vs. 64.7% (+6.6% [95% CI 1–12]; P = 0.005), with maximum effect late at night. Hypoglycemia exposure and carbohydrate treatments were improved overall (P = 0.001 and P = 0.007) and during the daytime with strong ski level effects (P = 0.0001 and P = 0.006); ski/snowboard proficiency was balanced between groups but with a very strong site effect: naive in Virginia and experienced in Colorado. There was no adverse event associated with CLC; the participants’ feedback was overwhelmingly positive.
CONCLUSIONS
CLC in adolescents with T1D improved glycemic control and reduced exposure to hypoglycemia during prolonged intensive winter sport activities, despite the added challenges of cold and altitude.
Introduction
Intensive glucose control reduces the complications of type 1 diabetes (T1D) but requires extensive work by patients and their families. Recent data from the T1D Exchange demonstrate that only 30% of patients across their life span achieve the American Diabetes Association hemoglobin A1c (HbA1c) targets and that hypoglycemia continues to be a major complication and impediment to tighter glucose control (1). The highest mean HbA1c occurs during adolescence and young adulthood, indicating that this age-group is a prime target for improved glycemic control (1). As such, closed-loop control (CLC) technology, commonly known as the artificial pancreas (AP), has become a focus of significant research and industrial development effort (2,3). CLC systems involve the pairing of a continuous subcutaneous insulin infusion pump and a continuous glucose monitor (CGM) with a CLC algorithm that automatically adjusts insulin infusion in real time (4). In the past decade, AP studies have advanced from short-term inpatient investigations using algorithm-driven manual control (5) to long-term clinical trials in free-living conditions using portable wireless automated CLC systems (6). Reviews and collections of papers reflecting the progress of the AP field are published regularly (2,3).
The first outpatient AP involved a laptop-based system at the bedside of children in a diabetes camp (7) and then was taken to patients’ homes. In 2011, we introduced the first wearable CLC system, the Diabetes Assistant (DiAs), which was built by using an Android smartphone as a computational hub to run the CLC algorithm and included an AP user interface, a cloud-based remote monitoring, and an automated alert system (8,9). Studies enrolling children and adolescents have shown improved glycemic control while decreasing the rates of hyperglycemia and hypoglycemia in inpatient trials (5,10,11), at diabetes camps (7,12,13), and in outpatient environments (14). In September 2016, the U.S. Food and Drug Administration (FDA) approved the first hybrid CLC system, which is capable of automatically adjusting the pump’s basal rate but still does not automate insulin boluses (15).
Glucose control during exercise, especially during intense exercise, is a particular challenge for people with T1D. Despite the well-defined benefits of physical activity (PA), exercise can still be dangerous in T1D and can result in hypo- or hyperglycemia due to fast-changing insulin sensitivity, increased energy expenditure, and possible counter-regulatory response (16). Thus, it is desirable for patients and health care providers to understand how CLC systems will work in the setting of commonly enjoyed demanding sports. To date, such data are limited, primarily for technological reasons; CLC systems had to evolve and become portable and sufficiently reliable to be tested in intensive sport conditions (17–19).
Using the DiAs system developed at the University of Virginia (UVA), we have conducted clinical trials in summer and winter camp settings where PA was typically much greater than during daily life for adolescents and young adults (13,20). Based on this previous work, we posed the question of whether our CLC system can improve glycemic control during and after demanding exercise. Skiing provides a unique mix of prolonged PA with varied intensities and has metabolic effects compounded by cold, altitude, stress, fear, and excitement. Thus, we embarked on conducting two consecutive ski camp trials in increasingly challenging conditions: the first in Wintergreen, VA, at an elevation of 1,071 m and the second in Breckenridge, CO, at a much higher altitude (3,963 m at the summit and 2,960 m at base camp).
Research Design and Methods
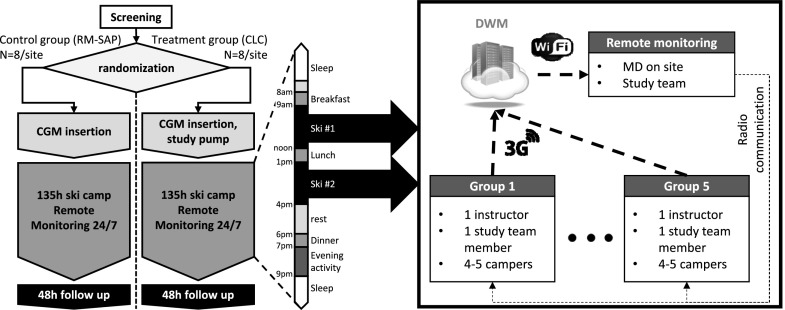
The UVA AP system was tested in a multisite, randomized, controlled clinical trial (clinicaltrials.gov registration NCT02604524) designed to expose the system to large and sustained metabolic as well as environmental disturbances. The research protocol was approved by the FDA (IDE G150221), UVA (IRB-HSR#18529), and the University of Colorado (Colorado Multiple Institutional Review Board protocol 15-2469) institutional review boards. Study subjects (10–25 years old) were recruited by phone and advertisement. Inclusion criteria included insulin-treated T1D (>1 year) and insulin pump use (>3 months); exclusion criteria included recent history of severe hypoglycemia or diabetes ketoacidosis (within the last 6 months), pregnancy, and conditions incompatible with the practice of winter sports in altitude. Subjects and guardians signed consent and assent, and after enrollment (and for each site separately), subjects were coarsely paired by HbA1c (±1%), age (±1 year), and, if possible, sex, and each member was randomly assigned to either remotely monitored sensor-augmented pump (RM-SAP; control arm) or the UVA CLC system (CLC; treatment arm). Subjects then participated in a 6-day ski/snowboard winter camp, with ∼5 h of on-snow activity and evening activity after dinner on each of the 5 full days (Fig. 1). All camp activities (including on-snow activities) were organized by Riding On Insulin (http://www.ridingoninsulin.org), a nonprofit organization specialized in training patients with T1D to ski and snowboard.
Figure 1.
Study design and typical day during camp. While remote monitoring was 24 h per day, its architecture during skiing was adapted to the heightened risk: one study physician and at least one study team member were located at the resort (base camp) and monitored all subjects. Each skiing group of four to five campers was led by one Riding On Insulin instructor and followed by a study team member, charged with all protocol activities, and equipped with replacement material, snacks, water, and emergency treatments. Data were transmitted to base camp via DWM, and all groups and base camp remained in communication using two-way radio.
Devices/systems
Subjects were asked to wear and maintain a CGM (Dexcom G4 with 505 software; Dexcom, San Diego, CA) with minimum calibration before breakfast and dinner (∼7:00 a.m. and 7:00 p.m.), using a study-provided blood glucose meter (BGM) (Contour Next Link; Ascencia Diabetes Care, Parsippany, NJ). RM-SAP subjects used their home insulin pump, whereas treatment subjects were fitted with a study-provided t:AP pump (Tandem, San Diego, CA; used at the Virginia camp) or Roche Accu-CHEK Spirit Combo pump (Roche, Indianapolis, IN; used at the Colorado camp). All subjects used a DiAs system (UVA) (9), linked to their CGM system, which reported data back to the UVA remote monitoring system (DiAs Web Monitoring [DWM]). Furthermore, in the treatment group, DiAs was connected to the pump and controlled all insulin delivery. All subjects’ insulin pump parameters were reviewed by a study physician at the start of the camp prior to randomization and were modified to account for the increased activity. Meal boluses for both groups were computed using personal settings and programmed by the user into either the DiAs’ internal bolus calculator or the personal pump bolus calculator. RM-SAP subjects were allowed to use temporary basal rates to adjust their bolus at will, and systematic changes had to be approved by the study team. Insulin pump insertion sites varied upon the subject’s preference but were systematically changed at least at camp onset and on days 3 and 6. Participants also wore PA trackers (Fitbit Charge HR; Fitbit, San Francisco, CA); PA data were collected after camp completion and not used by the CLC system.
Remote Monitoring and Safety Protocols
During the entire trial, subjects of both groups were remote monitored by the study team, including a study physician 24 h per day, using the DWM system (21) (Fig. 1). The study team intervened if 1) CGM values were <80 mg/dL between 7:00 a.m. and 11:00 p.m. or <70 mg/dL at night, 2) there was a hyper- or hypoglycemia “red light” alarm (CLC predictive alarm when the system judges that it is unable to avoid imminent hypo- or hyperglycemia), and 3) CGM values were >300 mg/L. All interventions consisted of a confirmatory BGM measurement followed, if necessary, by the application of the safety protocol (see protocol in Supplementary Data). In addition, subjects checked glucose before each ski session and 1 h after the start of the session. Any BGM measurement <100 mg/dL triggered graded carbohydrate intakes as follows: 4 g between 100 and 90 mg/dL, 8 g between 90 and 80 mg/dL, 12 g between 80 and 70 mg/dL, 16 g between 70 and 50 mg/dL, and 32 g <50 mg/dL. Whereas two BGM measurements <50 mg/dL was a stopping criterion (participant would leave the study if the criterion was met) during the first camp (Wintergreen, VA), this condition was replaced by two consecutive (within 30 min) BGM measurements <50 mg/dL with carbohydrate treatment in between for the Colorado camp. Loss of remote monitoring or pump disconnection also triggered study team interventions. Loss of remote monitoring for >2 h was a stopping criterion. During skiing, subjects were organized in skiing groups and spread across the mountain. Each group had both a ski instructor and a study team member and was monitored by a study physician at base camp (walkie-talkie and cell phone communication).
Outcomes and Statistical Analysis
All glucose outcomes were computed based on CGM records. Primary outcome was the percent time spent between 70 and 180 mg/dL (22), with secondary outcomes focusing on the quality of glycemic control, including percent time spent between 70 and 140 mg/dL, average CGM, and total insulin used. Secondary outcomes focusing of participant glycemic safety included percent time in hypoglycemia (<70 mg/dL), number of hypoglycemia events (defined as consecutive BGM measurements <70 mg/dL less than 30 min apart each), number of hypoglycemic treatments, amount of said treatments, and finally percent time spent >250 mg/dL. Outcomes were further divided in segments of the day: daytime (7:00 a.m. to 11:00 p.m.), overnight (11:00 p.m. to 7:00 a.m.), and ski (9:00 a.m. to 12:00 p.m. and 1:00 p.m. to 4:00 p.m.). Primary statistical analysis between treatment groups was performed using the univariate ANOVA, with the treatment mode as fixed factor and ski/snowboard level and HbA1c at enrollment as covariates. In addition, we looked at the interaction between treatment mode and ski/snowboard level to identify aspects of control where the impact of the CLC system was modified by the subject’s skiing ability.
Sample size was determined based on a large effect size (0.5) for ANOVA with two covariates and 80% power (G-Power 3.1.9.2). Desired enrollment was 34. Significance level was set at P value <0.05. Data are reported as mean ± SD. The statistical analysis was performed in SPSS 22 (IBM), and data formatting and preparation were executed in Matlab 2016b (Mathwork) and Excel 2016 (Microsoft).
Results
In total, 33 adolescent subjects were enrolled across both sites (16 UVA and 17 Colorado), and 32 participated in the study (16 in RM-SAP group vs. 16 in CLC). Sexes were balanced, with 15 females and 17 males participating in the study (8 of 8 vs. 7 of 9). Age was 13.2 ± 1.7 years (range 10–16, 13.1 ± 1.6 vs. 13.2 ± 1.9), with a slightly better than national average HbA1c: 8.5 ± 1.5% with a range of 6.6–13.2% (8.1 ± 1.1 vs. 8.9 ± 1.9). BMI was 20.5 ± 2.9 (21.1 ± 3.6 vs. 19.9.2 ± 2.0), range 16.1–30, and insulin daily needs varied accordingly 0.9 ± 0.18 units/kg (0.99 ± 0.23 vs. 1.02 ± 0.25), range 0.55–1.15 units/kg. Subjects were all experienced pump users, with pump use of 5.8 ± 2.9 years (5.2 ± 2.6 vs. 4.8 ± 3.3) and T1D duration of 6.9 ± 3.4 years (6.5 ± 3.4 vs. 6.4 ± 3.5). Experience in snow sports (ski/snowboard) was varied, with 14 participants with no experience, 2 beginners, 5 intermediate, and 11 advanced skiers/snowboarders. There was no significant difference in any of these variables between the RM-SAP and CLC groups (Supplementary Table 1), but HbA1c may have been slightly higher in the treatment group (8.86 ± 1.84 vs. 8.1 ± 1.06%, P = 0.2).
We recorded three adverse events: 1) a right distal tibia fracture prior to the start of the experimental study but after the enrollment visit, 2) a wrist fracture during ski activity, and 3) a knee injury (anterior cruciate ligament/medial collateral ligament tear) during ski activity. All were deemed unrelated to the study devices or diabetes control. In addition, one subject was stopped after two treated BGM measurements <50 mg/dL. Data for all four subjects were excluded after the adverse event or leaving the protocol, for, respectively, 135, 33, 24, and 16 h, out of 135 h.
The performance of the CGM systems was lower than previously reported, with a mean absolute relative deviation (MARD) of 18.9% (note that the cold can significantly interfere with BGM measurements, which were used as reference for this computation). CLC systems were able to function 95% of the time.
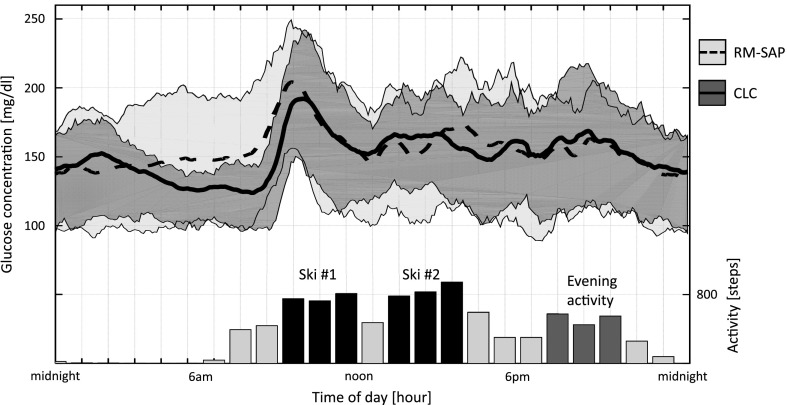
Detailed outcomes can be found in Table 1, and we summarize the main findings below. Percent time spent between 70 and 180 mg/dL over the entire week was 71.3 ± 17.6 vs. 64.7 ± 13.3% (CLC vs. RM-SAP), P = 0.005; i.e., a 7% increase (95% CI 1–12%) or an improvement of 1 h and 40 min in range per day. This difference held during both daytime (67.7 ± 17.5 vs. 62.7 ± 16.5%, P = 0.014) and nighttime (79.3 ± 29.8 vs. 68.8 ± 24.1%, P = 0.01) and was particularly strong in the second half of the night (84.6 ± 13.1 vs. 66.2 ± 26.4%, P = 0.024) (Fig. 2).
Table 1.
Results for RM-SAP and CLC groups
| Mean ± SD |
Quartiles |
Statistical tests |
||||||
|---|---|---|---|---|---|---|---|---|
| RM-SAP | CLC | RM-SAP | CLC | Effect of AP | AP and ski level | |||
| Quality of glucose control |
||||||||
| Percent between 70 and 180 mg/dL |
Overall | 64.7 ± 13.3 | 71.3 ± 17.6 | 52–77.4 | 61.4–83.9 | P = 0.008 | P = 0.740 | |
| Daytime (7:00 a.m. to 11:00 p.m.) | 62.7 ± 16.5 | 67.7 ± 17.5 | 49.1–79 | 52.6–84.2 | P = 0.008 | P = 0.649 | ||
| Overnight (11:00 p.m. to 7:00 a.m.) | 68.8 ± 24.1 | 79.3 ± 29.8 | 47.1–99.2 | 69.8–100 | P = 0.156 | P = 0.932 | ||
| Skiing (9:30 a.m. to noon and 1:00 p.m. to 4:00 p.m.) | 62.8 ± 31.4 | 63.2 ± 31.1 | 43.6–88.3 | 45.5–86.4 | P = 0.604 | P = 0.688 | ||
| Percent between 70 and 140 mg/dL | Overall | 45.4 ± 19.2 | 46.7 ± 20.9 | 26.6–61.4 | 34.9–59.9 | P = 0.086 | P = 0.239 | |
| Daytime (7:00 a.m. to 11:00 p.m.) | 41.9 ± 20.6 | 42.7 ± 18.5 | 24.2–63 | 29–57.8 | P = 0.083 | P = 0.502 | ||
| Overnight (11:00 p.m. to 7:00 a.m.) | 52.3 ± 29.2 | 55.7 ± 38.5 | 14.6–91.1 | 39.6–82.3 | P = 0.332 | P = 0.226 | ||
| Skiing (9:30 a.m. to noon and 1:00 p.m. to 4:00 p.m.) | 43.5 ± 29.4 | 37 ± 22.6 | 16.3–69.7 | 15.5–59.1 | P = 0.685 | P = 0.952 | ||
| Average glycemia (mg/dL) | Overall | 155.9 ± 26.1 | 152.6 ± 24.3 | 130.7–177.1 | 132.5–165.3 | P = 0.133 | P = 0.702 | |
| Daytime (7:00 a.m. to 11:00 p.m.) | 161 ± 29.9 | 157.5 ± 20.1 | 131.6–185.7 | 138.2–173.7 | P = 0.075 | P = 0.678 | ||
| Overnight (11:00 p.m. to 7:00 a.m.) | 145.5 ± 38.2 | 142.2 ± 47.8 | 103.3–178.3 | 113.4–157.3 | P = 0.635 | P = 0.594 | ||
| Skiing (9:30 a.m. to noon and 1:00 p.m. to 4:00 p.m.) | 165 ± 48.2 | 165.1 ± 35.9 | 123.2–200.6 | 136.8–185.9 | P = 0.774 | P = 0.768 | ||
| Total insulin use (units/kg) | Overall | 0.89 ± 0.23 | 0.77 ± 0.32 | 0.7–1.06 | 0.51–1 | P = 0.0001 | P = 0.001 | |
| Daytime (7:00 a.m. to 11:00 p.m.) | 0.76 ± 0.21 | 0.6 ± 0.27 | 0.57–0.94 | 0.42–0.78 | P = 0.0001 | P = 0.014 | ||
| Overnight (11:00 p.m. to 7:00 a.m.) | 0.13 ± 0.05 | 0.14 ± 0.1 | 0.11–0.15 | 0.08–0.18 | P = 0.72 | P = 0.158 | ||
| Skiing (9:30 a.m. to noon and 1:00 p.m. to 4:00 p.m.) | 0.18 ± 0.12 | 0.14 ± 0.11 | 0.11–0.22 | 0.07–0.18 | P = 0.013 | P = 0.729 | ||
| Safety outcomes | ||||||||
| Percent <70 mg/dL | Overall | 3.2 ± 3.1 | 1.8 ± 1.5 | 0.6–5.2 | 0–2.9 | P = 0.0001 | P = 0.002 | |
| Daytime (7:00 a.m. to 11:00 p.m.) | 3.6 ± 3.2 | 1.6 ± 1.9 | 0–4.7 | 0–2.3 | P = 0.0001 | P = 0.164 | ||
| Overnight (11:00 p.m. to 7:00 a.m.) | 2.5 ± 6.5 | 2.2 ± 2.3 | 0–4.2 | 0–1 | P = 0.489 | P = 0.001 | ||
| Skiing (9:30 a.m. to noon and 1:00 p.m. to 4:00 p.m.) | 2.3 ± 6.4 | 1.4 ± 1.6 | 0–1.5 | 0–0 | P = 0.042 | P = 0.273 | ||
| Number of events <70 mg/dL | Overall | 0.7 ± 1 | 0.8 ± 0.6 | 0–1 | 0–1 | P = 0.373 | P = 0.63 | |
| Daytime (7:00 a.m. to 11:00 p.m.) | 0.6 ± 0.9 | 0.7 ± 0.6 | 0–1 | 0–1 | P = 0.391 | P = 0.716 | ||
| Overnight (11:00 p.m. to 7:00 a.m.) | 0.1 ± 0.4 | 0.1 ± 0.3 | 0–0 | 0–0 | P = 0.827 | P = 0.147 | ||
| Skiing (9:30 a.m. to noon and 1:00 p.m. to 4:00 p.m.) | 0.3 ± 0.7 | 0.3 ± 0.4 | 0–1 | 0–0.3 | P = 0.885 | P = 0.773 | ||
| Number of hypoglycemia treatments | Overall | 3.5 ± 3.7 | 3.4 ± 1.8 | 2–5 | 1–5 | P = 0.159 | P = 0.007 | |
| Daytime (7:00 a.m. to 11:00 p.m.) | 2.7 ± 2.8 | 2.6 ± 0.9 | 1–3 | 1–3 | P = 0.133 | P = 0.006 | ||
| Overnight (11:00 p.m. to 7:00 a.m.) | 0.7 ± 1.3 | 0.7 ± 1.4 | 0–1 | 0–1 | P = 0.937 | P = 0.446 | ||
| Skiing (9:30 a.m. to noon and 1:00 p.m. to 4:00 p.m.) | 1.3 ± 1.5 | 1.1 ± 0.7 | 0–2 | 0–2 | P = 0.189 | P = 0.078 | ||
| Amount of CHO treatments (g) | Overall | 49.7 ± 41.9 | 50.6 ± 27.3 | 20.8–68.3 | 16–69.8 | P = 0.319 | P = 0.129 | |
| Daytime (7:00 a.m. to 11:00 p.m.) | 39.9 ± 34.3 | 38.6 ± 18.3 | 15.8–57 | 14.5–52.5 | P = 0.749 | P = 0.954 | ||
| Overnight (11:00 p.m. to 7:00 a.m.) | 9.9 ± 14.8 | 12 ± 21.8 | 0–20 | 0–9 | P = 0.113 | P = 0.001 | ||
| Skiing (9:30 a.m. to noon and 1:00 p.m. to 4:00 p.m.) | 17.4 ± 18.6 | 14 ± 10.3 | 0–30.8 | 0–24.3 | P = 0.428 | P = 0.050 | ||
| Percent >250 mg/dL | Overall | 0.1 ± 0.1 | 0.1 ± 0.1 | 0–0.1 | 0–0.1 | P = 0.048 | P = 0.057 | |
| Daytime (7:00 a.m. to 11:00 p.m.) | 0.1 ± 0.1 | 0.1 ± 0.1 | 0–0.2 | 0–0.1 | P = 0.003 | P = 0.141 | ||
| Overnight (11:00 p.m. to 7:00 a.m.) | 0 ± 0.1 | 0.1 ± 0.1 | 0–0 | 0–0 | P = 0.473 | P = 0.117 | ||
| Skiing (9:30 a.m. to noon and 1:00 p.m. to 4:00 p.m.) | 0.1 ± 0.2 | 0.1 ± 0.2 | 0–0.2 | 0–0.1 | P = 0.125 | P = 0.680 | ||
Mean, SD, and quartiles are reported for both treatments, and P values for the main effect (RM-SAP vs. CLC) and for interaction between AP and ski levels are reported if <0.1. Main effect P values <0.05 show a clear impact of the CLC system, and significant interactions indicate that the impact of CLC is changed by the subject ski level. CHO, carbohydrate. Significance levels <0.05 are presented in bold font.
Figure 2.
Glycemic control as represented by mean (plain and dotted line) and quartiles (gray envelopes) of CGM values during the day for both RM-SAP (light gray and dotted line) and CLC (dark gray and plain line). The glucose traces are aligned with the average hourly step counts (right axis), without group contrast, and planned times of activities (ski in black and evening activity in dark gray).
Time in the tighter glycemic zone of 70–140 mg/dL did not change when using the CLC system (46.7 ± 20.9 vs. 45.4 ± 19.2%, P > 0.1). Average glycemia decreased significantly during daytime (157.5 ± 20.1 vs. 161.0 ± 29.9 mg/dL, P = 0.049) but not overall (152.6 ± 24.3 vs. 155.9 ± 26.1 mg/dL, P = 0.072). These glycemic control results were achieved while using less insulin overall (0.77 ± 0.32 vs. 0.89 ± 0.23 units/kg, P < 0.001), during daytime (0.6 ± 0.27 vs. 0.76 ± 0.21 units/kg, P < 0.001), and while skiing (0.14 ± 0.11 vs. 0.18 ± 0.12 units/kg, P = 0.013).
Percent time <70 mg/dL was almost halved overall (1.8 ± 1.5 vs. 3.2 ± 3.1%, P < 0.001), with a strong ski level effect (P = 0.044) interacting with the CLC impact (P = 0.002, see Conclusions). These results held for the daytime and skiing periods (Fig. 2 and Table 1) but not at night. Whereas the exposure to hypoglycemia was reduced, the number of hypoglycemic events (and amount of rescue carbohydrates) remained unchanged.
Exposure to significant hyperglycemia (percent time >250 mg/dL) was reduced overall (7.0 ± 8.0 vs. 9.3 ± 10.9%, P = 0.047) and during daytime (7.5 ± 8.7 vs. 11.5 ± 13.6%, P = 0.003).
Interaction Between Ski Level and Glycemic Control
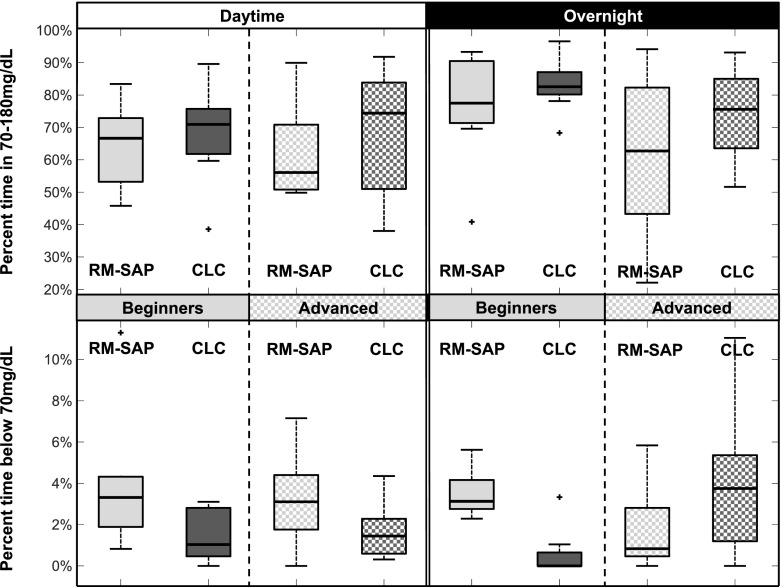
Several outcomes were significantly impacted by the ski experience of the participants (Table 1, last column). Specifically, exposure to hypoglycemia (time <70 mg/dL) was most decreased overall in the beginner group (from 3.5 down to 1.2%) compared with the advanced group (from 2.9 to 2.3%); this difference was particularly apparent overnight, with a potential decrease (3 to 1%) in the beginner group and potential increase (1.9 to 3.2%) in the advanced group. The increase in the advanced group was not significant by itself (P = 0.143) but led to a substantial and not statistically significant increase in needed hypoglycemia treatments 0.8 vs. 0.3 treatments per night (Fig. 3). Of note, this 1.3% increase was accompanied by a 12% increase in time in range (77.1 vs. 65.6%) and a reduction in insulin delivered (5.6 vs. 6.2 units total insulin per night). Of note, glycemia for beginner skiers using RM-SAP was on average 11 mg/dL lower overnight than for advanced skiers using RM-SAP (140.9 vs. 151.6 mg/dL), showing that treatment adjustment by the patients (forbidden in the CLC group) may have played a role (see Conclusions).
Figure 3.
Distribution of the percent time spent in good control (70–180 mg/dL) and percent time spent in hypoglycemia (<70 mg/dL) for all subjects, contrasted by treatment (RM-SAP vs. CLC, light vs. dark gray) and ski/snowboard level (beginner vs. advanced, plain vs. checkered), for the daytime and overnight periods. Crosses mark outliers, filled boxes mark 25th and 75th percentiles, lines mark medians, and error bars mark ranges.
This interaction between ski level and CLC use was also noted in the amount of insulin delivered overall and during the day, with a small reduction in the beginner group using CLC (51.69 vs. 53.67 units) but a 32% reduction in the advanced skier group (30.1 vs. 44.03 units total insulin during the day), which was visible both during the day (beginners 39.19 vs. 45.36 units, advanced 24.50 vs. 37.85 units) and at night (beginners 9.18 vs. 8.31 units, advanced 5.60 vs. 6.18 units).
While skiing, subjects in the CLC groups were less exposed to hypoglycemia regardless of level (beginners 1.4 vs. 2.3%, advanced 1.3 vs. 2.3%) and received less carbohydrates (beginners 15.63 vs. 18.53 g, advanced 12.38 vs. 16.03 g).
Conclusions
Results from this multisite, randomized, controlled clinical trial show the safety and efficacy of the UVA AP system in adolescents even when stressed by prolonged exercise, cold temperatures, and altitude. The system was superior to highly monitored SAP therapy for time in target range during the day and night, and for reducing hypoglycemia overnight when controlling for a patient’s ski level.
Hypoglycemia remains the major concern in pediatric patients with T1D engaging in significant exercise. The use of CLC systems has decreased hypoglycemia fear and burden among children attending summer camp (23). Results from our ski camp study show that the system limited hypoglycemia exposure overall (percent time spent <70 mg/dL), during the day and specifically during skiing, but overnight control was highly dependent on the participant’s ski level, suggesting that more experienced skiers were more adept at managing their risk for hypoglycemia than less experienced skiers (Fig. 3). Significant amounts of additional carbohydrates were still consumed by both groups (∼50 g/day), which can be expected considering the amount of activity and very strict safety protocols; nonetheless, this likely significantly reduced exposure to hypoglycemia (see limitations below).
Clinically, this may suggest that patient knowledge of their diabetes and exercise-related management could affect which aspects of glucose control CLC may benefit: protection against hypoglycemia or tighter control. This effect is clearly visible in the right panel of Fig. 3. For the RM-SAP group, advanced skiers adjusted their settings as per their usual management and were thus able to minimize hypoglycemia even without AP, but at the cost of looser control; in this group, a CLC may provide an automatic tool rather than rely on patients’ calculations. For beginner-level skiers, hypoglycemia was more common and use of a CLC system provided significant hypoglycemia exposure reduction. As indicated above, the heterogeneous patient population, exercise, and environment in this first-of-its-kind study presented formidable challenges for the AP system. These data will allow for future adaptation and potential individualization to more precisely control glucose.
In this trial, the control arm (RM-SAP) consisted of patients using their usual insulin pump, with settings adjusted for exercise as per their usual management, and a CGM with remote monitoring and alerts, which was monitored 24 h per day by a pediatric endocrinologist. This level of supervision, which was deemed necessary for optimal patient safety during a ski camp study, is substantially greater than for patients conducting true control-level diabetes open-loop management in an exercise setting. Despite this bias, percent time in target range was improved in the CLC group compared with the RM-SAP group.
Five other studies have used CLC systems in a camp setting (13,20,23–25). In 2016, Del Favero et al. (24) published results of a 3-day summer camp study in children 5–9 years old comparing single-hormone AP against SAP and found a reduction in time in hypoglycemia in the AP group at the expense of decreased time in target range and with a significant increase in mean glucose. The percent time in the target range 70–180 mg/dL was lower in this study for SAP and AP (56.8 vs. 63.1%) than for either group in our trial (64.7 vs. 71.3%), although the improvement in time in range of ∼6% was similar between the two trials and the patients in the Del Favero trial were significantly younger than in our trial.
In 2016, Russell et al. (25) published results of a dual-hormone (insulin and glucagon) system in children 6–11 years old at summer camp in a crossover study of dual-hormone therapy against SAP therapy and found a significant reduction in mean glucose (167 vs. 136 mg/dL) and percent time <60 mg/dL (2.8 vs. 1.2%) and improvement in time in target range 60–180 mg/dL (57.6 vs. 80.6%). Comparison of the results from this study to our results is limited by the lower threshold for hypoglycemia tolerated in the analysis (60 vs. 70 mg/dL) and the higher glycemic average and lower percent time in target range for the control group in this study. Similarly, Ly et al. (20) obtained a significant reduction in average glycemia, exposure to hypoglycemia, and insulin usage in adolescents and young adults during two 5-day summer and winter camps in 2016. Ly et al. (20) reported 79 vs. 65% time in range, 1.8 vs. 4.2% time <70 mg/dL, and average glycemia of 143 vs. 156 mg/dL. Interestingly this latest trial used an almost identical system as this study, contrasting the UVA CLC system performances in a classic camp environment. None of these studies included the challenges of altitude, cold, and prolonged physical and emotional challenges that skiing presents.
There have also been several older studies investigating the role of overnight glucose control with AP in a camp setting (13,26) that confirmed the trend of reduced hypoglycemia concurrent with improved time in target range observed in our ski camp study.
Exercise in T1D remains one of the most challenging situations for glycemic management regardless of setting. Numerous reports in the last several years have discussed the role of exercise automation in AP technology (27–30). During the ski camp study, heart rate and step counts were collected for the development of future exercise integration systems, but these inputs were not used by the CLC system to detect or modulate insulin delivery during exercise. It appears that the rigor of the clinical trial protocol regarding blood glucose monitoring, testing, and treatment during exercise to assure participant safety had a very strong effect. In both the RM-SAP and CLC groups in this trial, during the ski sessions, average glycemia was very similar between the two groups (165.0 vs. 165.1 mg/dL) as was percent time between 70 and 180 mg/dL (62.8 vs. 63.2%) and percent time <70 mg/dL (2.3 vs. 1.4%). Heart rate–based exercise detection has been shown in a recent study of the UVA CLC system to improve AP performance during exercise with decreased time <70 mg/dL with this augmentation compared with the CLC system without it (0.5 vs. 7.4%) (27). Future work on this project should enable such a system to be used in a ski environment with further expected improvement in performance.
There were several limitations to this study. As noted above, the absence of any other use of AP in similar conditions led to restrictive inclusion/exclusion criteria (e.g., no history of severe hypoglycemia) and strict clinical oversight of both arms, combined with a high level of on-slope supervision. This may have limited the generalizability of the study and biased the results toward the null hypothesis. In addition, the number of interventions directly linked to remote monitoring was not recorded. There were significant differences in glycemic control between patients based on their skiing skill levels and significant site differences in ski level as well as marked difference in altitude between the two clinical sites. In addition, secondary outcomes were analyzed without correction for multiple comparisons. All clinical staff were highly trained in use of the CLC system, having used it in multiple clinical trials, possibly limiting generalizability of these results.
Conclusion
In conclusion, CLC delivered by the UVA CLC system improved glycemic control and reduced the incidence of hypoglycemia during prolonged intensive winter sport activities, despite the added challenges of cold and altitude in adolescents with T1D. Future studies of CLC require longer duration, less supervision, and challenges with activities in which patients engage in their lives.
Supplementary Material
Article Information
Acknowledgments. The authors especially recognize all the volunteers and staff of Riding On Insulin and the Children’s Diabetes Foundation and The Guild, Drs. Stacey Anderson and Sue Brown (UVA), and the research staff of the UVA Center for Diabetes Technology and the University of Colorado Barbara Davis Center.
Funding. This study was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIH-DP3-DK106826), the University of Colorado Foundation, the Children’s Diabetes Foundation, and private donors. Dexcom provided CGMs.
Duality of Interest. M.D.B. reports personal fees from Dexcom, Merck, and The Epsilon Group; grants and personal fees from Roche, Sanofi, and Ascencia; grants from Senseonics; and nonfinancial support from Novo Nordisk and Tandem and is a cofounder and equity holder of TypeZero Technologies, outside the submitted work. In addition, M.D.B. has patents US8562587 and US9430022 issued to TypeZero Technologies, Inc.; patents US20120078067, US20130116649, and US20150018633 pending to TypeZero Technologies, Inc.; and a patent for CGM-based fault detection and mitigation of insulin delivery/monitoring systems via metabolic state tracking pending to TypeZero Technologies, Inc. D.R.C. is the Chief Medical Officer of TypeZero Technologies, Inc. G.P.F. reports personal fees from Abbott Diabetes and conducts research sponsored by Medtronic, Dexcom, Insulet, Tandem, Abbott, Bigfoot, and Novo Nordisk. B.P.K. reports personal fees from Sanofi, personal fees and nonfinancial support from Dexcom, grants and nonfinancial support from Roche, and nonfinancial support from Tandem and is a cofounder and equity holder of TypeZero Technologies, Inc., outside the submitted work. In addition, B.P.K. has patents US8562587 and US20120078067 licensed to TypeZero Technologies, Inc.; patents US20130116649 and US20150018633 pending to TypeZero Technologies, Inc.; patent US9430022 issued to TypeZero Technologies, Inc.; and a patent for CGM-based fault detection and mitigation of insulin delivery/monitoring systems via metabolic state tracking pending to TypeZero Technologies, Inc. D.M.M. reports personal fees from Insulet and Abbott and institutional research support from Dexcom, Medtronic, Roche, and Bigfoot. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.D.B. and G.P.F. researched data and wrote the manuscript. D.R.C., M.D.D., and R.P.W. researched data and revised and edited the manuscript. J.R. researched data. L.H.M. researched data and reviewed the manuscript. B.P.K. contributed to the discussion and revised and edited the manuscript. D.M.M. researched data and reviewed and edited the manuscript. M.D.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT02604524, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-0883/-/DC1.
References
- 1.Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 2.Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia 2016;59:1795–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovatchev B, Tamborlane WV, Cefalu WT, Cobelli C. The artificial pancreas in 2016: a digital treatment ecosystem for diabetes. Diabetes Care 2016;39:1123–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle FJ 3rd, Huyett LM, Lee JB, Zisser HC, Dassau E. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care 2014;37:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hovorka R, Allen JM, Elleri D, et al. . Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–751 [DOI] [PubMed] [Google Scholar]
- 6.Kovatchev BP, Renard E, Cobelli C, et al. . Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care 2014;37:1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson SM, Raghinaru D, Pinsker JE, et al.; Control to Range Study Group . Multinational home use of closed-loop control is safe and effective. Diabetes Care 2016;39:1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keith-Hynes P, Guerlain S, Mize B, et al. . DiAs user interface: a patient-centric interface for mobile artificial pancreas systems. J Diabetes Sci Technol 2013;7:1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keith-Hynes P, Mize B, Robert A, Place J. The Diabetes Assistant: a smartphone-based system for real-time control of blood glucose. Electronics 2014;3:609–623 [Google Scholar]
- 10.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care 2008;31:934–939 [DOI] [PubMed] [Google Scholar]
- 11.Elleri D, Allen JM, Nodale M, et al. . Automated overnight closed-loop glucose control in young children with type 1 diabetes. Diabetes Technol Ther 2011;13:419–424 [DOI] [PubMed] [Google Scholar]
- 12.Russell SJ, El-Khatib FH, Sinha M, et al. . Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med 2014;371:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ly TT, Breton MD, Keith-Hynes P, et al. . Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care 2014;37:2310–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thabit H, Tauschmann M, Allen JM, et al. . Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed]
- 16.Toni S, Reali MF, Barni F, Lenzi L, Festini F. Managing insulin therapy during exercise in type 1 diabetes mellitus. Acta Biomed 2006;77(Suppl. 1):34–40 [PubMed] [Google Scholar]
- 17.Breton MD, Brown SA, Karvetski CH, et al. . Adding heart rate signal to a control-to-range artificial pancreas system improves the protection against hypoglycemia during exercise in type 1 diabetes. Diabetes Technol Ther 2014;16:506–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts AJ, Taplin CE. Exercise in youth with type 1 diabetes. Curr Pediatr Rev 2015;11:120–125 [DOI] [PubMed] [Google Scholar]
- 19.Sherr JL, Cengiz E, Palerm CC, et al. . Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nights with or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care 2013;36:2909–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ly TT, Buckingham BA, DeSalvo DJ, et al. . Day-and-night closed-loop control using the unified safety system in adolescents with type 1 diabetes at camp. Diabetes Care 2016;39:e106–e107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Place J, Robert A, Ben Brahim N, et al. . DiAs web monitoring: a real-time remote monitoring system designed for artificial pancreas outpatient trials. J Diabetes Sci Technol 2013;7:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maahs DM, Buckingham BA, Castle JR, et al. . Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care 2016;39:1175–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weissberg-Benchell J, Hessler D, Polonsky WH, Fisher L. Psychosocial impact of the bionic pancreas during summer camp. J Diabetes Sci Technol 2016;10:840–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Favero S, Boscari F, Messori M, et al. . Randomized summer camp crossover trial in 5- to 9-year-old children: outpatient wearable artificial pancreas is feasible and safe. Diabetes Care 2016;39:1180–1185 [DOI] [PubMed] [Google Scholar]
- 25.Russell SJ, Hillard MA, Balliro C, et al. . Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016;4:233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillip M, Battelino T, Atlas E, et al. . Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368:824–833 [DOI] [PubMed] [Google Scholar]
- 27.DeBoer MD, Cherñavvsky DR, Topchyan K, Kovatchev BP, Francis GL, Breton MD. Heart rate informed artificial pancreas system enhances glycemic control during exercise in adolescents with T1D. Pediatr Diabetes 2017;18:540–546 [DOI] [PubMed] [Google Scholar]
- 28.Patel NS, Van Name MA, Cengiz E, et al. . Mitigating reductions in glucose during exercise on closed-loop insulin delivery: the Ex-Snacks Study. Diabetes Technol Ther 2016;18:794–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taleb N, Emami A, Suppere C, et al. . Efficacy of single-hormone and dual-hormone artificial pancreas during continuous and interval exercise in adult patients with type 1 diabetes: randomised controlled crossover trial. Diabetologia 2016;59:2561–2571 [DOI] [PubMed] [Google Scholar]
- 30.Jacobs PG, El Youssef J, Reddy R, et al. . Randomized trial of a dual-hormone artificial pancreas with dosing adjustment during exercise compared with no adjustment and sensor-augmented pump therapy. Diabetes Obes Metab 2016;18:1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.