Abstract
OBJECTIVE
Both lifestyle and metformin interventions can delay or prevent progression to type 2 diabetes mellitus (DM) in people with impaired glucose regulation, but there is considerable interindividual variation in the likelihood of receiving benefit. Understanding an individual’s 3-year risk of progressing to DM and regressing to normal glucose regulation (NGR) might facilitate benefit-based tailored treatment.
RESEARCH DESIGN AND METHODS
We used the values of 19 clinical variables measured at the Diabetes Prevention Program (DPP) baseline evaluation and Cox proportional hazards models to assess the 3-year risk of progression to DM and regression to NGR separately for DPP lifestyle, metformin, and placebo participants who were adherent to the interventions. Lifestyle participants who lost ≥5% of their initial body weight at 6 months and metformin and placebo participants who reported taking ≥80% of their prescribed medication at the 6-month follow-up were defined as adherent.
RESULTS
Eleven of 19 clinical variables measured at baseline predicted progression to DM, and 6 of 19 predicted regression to NGR. Compared with adherent placebo participants at lowest risk of developing diabetes, participants at lowest risk of developing diabetes who adhered to a lifestyle intervention had an 8% absolute risk reduction (ARR) of developing diabetes and a 35% greater absolute likelihood of reverting to NGR. Participants at lowest risk of developing diabetes who adhered to a metformin intervention had no reduction in their risk of developing diabetes and a 17% greater absolute likelihood of reverting to NGR. Participants at highest risk of developing DM who adhered to a lifestyle intervention had a 39% ARR of developing diabetes and a 24% greater absolute likelihood of reverting to NGR, whereas those who adhered to the metformin intervention had a 25% ARR of developing diabetes and an 11% greater absolute likelihood of reverting to NGR.
CONCLUSIONS
Unlike our previous analyses that sought to explain population risk, these analyses evaluate individual risk. The models can be used by overweight and obese adults with fasting hyperglycemia and impaired glucose tolerance to facilitate personalized decision-making by allowing them to explicitly weigh the benefits and feasibility of the lifestyle and metformin interventions.
Introduction
The Diabetes Prevention Program (DPP) demonstrated that over 3 years, intensive lifestyle intervention and metformin treatment reduced the incidence of diabetes mellitus (DM) in high-risk participants with impaired glucose regulation. In addition to reporting average benefit by randomized treatment group, the DPP reported treatment effectiveness by age, sex, race/ethnicity, baseline BMI, and baseline measures of glycemia. Lifestyle intervention was effective in all age-groups, both sexes, all racial and ethnic groups, in overweight, obese, and severely obese participants, and in participants with lesser and greater degrees of fasting and postglucose load hyperglycemia (1). In contrast, metformin was more effective in participants <60 years of age, in those with BMI ≥35 kg/m2, and in those with greater degrees of fasting hyperglycemia than in those who were older, less obese, and who had fewer degrees of fasting hyperglycemia (1).
A better understanding of who is more likely to benefit from these effective interventions could inform individual treatment decisions and help make treatment more effective and cost-effective. Although ∼100 risk models and scores for DM have been published (2), few have developed multivariable models to facilitate tailoring preventive interventions to individuals, and none has reported the impact of interventions on both progression to DM and regression to normal glucose regulation (NGR). Recently, Sussman et al. (3) described the use of prediction models to estimate a person’s likelihood of benefit, describing this approach as “benefit-based tailored treatment.” They subsequently argued that treatment decisions should be based on the best estimate of absolute risk reduction (ARR) considering all of the patient and treatment factors that determine an individual patient’s chances of benefiting (4). An individual’s ARR can be calculated as the difference between an individual’s risk without treatment and the risk with treatment. These risks may vary substantially among individuals, even in seemingly homogeneous study populations (4). Although Sussman et al. (3) developed multivariable models to predict progression to DM for individuals in the DPP population with and without interventions, they assessed some variables not routinely assessed in clinical practice (such as waist circumference and waist-to-hip ratio [5]), did not account for treatment adherence, and did not assess the possibility of regression to NGR (3).
The purpose of this article was to use baseline data from the DPP to develop risk equations that use routinely assessed clinical variables and predict progression to DM and regression to NGR among individuals who adhere to the interventions. These equations can be used in clinical practice by patients >25 years of age who are overweight or obese and have fasting hyperglycemia and impaired glucose tolerance (IGT) to answer the question, “What will happen to me over 3 years if I adopt the DPP lifestyle intervention or metformin intervention or do nothing?” The answer should facilitate individual benefit-based tailored treatment and personalized decision-making.
Research Design and Methods
Study Population
The study population included overweight and obese adults with IGT and fasting hyperglycemia enrolled in the DPP, a randomized, controlled clinical trial comparing the impact of intensive lifestyle intervention, metformin, and placebo on the development of DM over an average 3.2 years. Inclusion criteria included age ≥25 years, BMI ≥24 kg/m2 (≥22 kg/m2 in Asian Americans), plasma glucose 2-h after a 75-g oral glucose load (2-h PG) of 140–199 mg/dL, and fasting PG (FPG) of 95–125 mg/dL (no lower limit in the American Indian centers). The DPP was conducted between 1996 and 2001 at 27 sites in the U.S. The design, rationale, and outcomes have been described in detail elsewhere (1,6).
Candidate Predictor Variables
Nineteen routinely assessed clinical variables measured at DPP baseline and known to be associated with progression to DM or regression to NGR were selected as candidate predictor variables. These variables included sociodemographic characteristics (age, sex, race/ethnicity, education, and household income); health behaviors (smoking and self-reported physical activity assessed as minutes per week); selected medications (antihypertensive medications and statins); medical history (polycystic ovarian disease, pregnancy, and gestational diabetes mellitus [GDM]); family history of diabetes (DM in parents, siblings, or children); anthropometric measures (weight, BMI, systolic blood pressure [SBP], and diastolic blood pressure); and laboratory measures of FPG and fasting triglycerides (TG). FPG and BMI were chosen as candidate predictor variables over HbA1c and waist circumference because DPP eligibility was based on FPG and BMI. In the DPP, HbA1c and waist circumference were conditioned on having met glucose and BMI eligibility criteria. As a result, HbA1c and waist circumference assessed in the general population without respect to glucose tolerance or BMI might not confer the same risk for progression to DM or regression to NGR as they did in the DPP. Two-hour postchallenge glucose level was not included as a predictor variable because it is not routinely measured in clinical practice. For ease of interpretation, we created a composite variable that included female sex, history of pregnancy, and history of GDM. Because TG levels were not normally distributed, we used the natural logarithm of TG levels.
Outcome Variables
The outcomes of interest were progression to DM and regression to NGR. Progression to DM was defined as two consecutive measures of FPG ≥126 mg/dL and/or 2-h PG ≥200 mg/dL with the event time defined as the first occurrence subsequently confirmed. Regression to NGR was defined as the first occurrence of FPG <100 mg/dL and 2-h PG <140 mg/dL. HbA1c levels were not used to define progression to DM or regression to NGR. Regression to NGR was not a prespecified outcome in the DPP, and the protocol did not call for confirmation. The NGR event was counted regardless of what happened after the occurrence of NGR, even if the participant ultimately progressed to DM. No participants regressed to NGR after progression to DM, as DM was considered to be an absorbing state.
Model Development
Using the 19 candidate predictor variables, we developed separate Cox proportional hazard models for progression to DM and regression to NGR for the DPP lifestyle, metformin, and placebo intervention groups. Mean follow-up was 3.2 years, and the time horizon for prediction was 3 years. To adjust for the effect of adherence to the interventions, an adherence variable was included in each of the models. Participants adherent to the lifestyle intervention were defined as having lost ≥5% of initial weight by the 6-month visit, and those adherent to the metformin and placebo interventions were defined as reporting taking at least 80% of DPP-prescribed masked medication at the 6-month visit. We chose to define adherence using data from the 6-month visit because adherence at 6 months predicted long-term adherence.
To develop the models and estimate the coefficients, we included both adherent and nonadherent participants. Because our primary goal was to provide physicians and patients with information about the clinical effectiveness of the DPP interventions, we focused our evaluations on adherent participants. Clearly, the results represent best-case scenarios. For completeness, we also present the multivariable models and predicted probabilities of progression to DM and regression to NGR for the entire DPP population regardless of their adherence (Supplementary Tables 1–3). Because only 2.5% of persons initially screened for the DPP, and approximately one-fourth of those who met 2-h PG eligibility criteria were randomized (7), it is difficult to generalize the results of the DPP to the U.S. population with impaired glucose regulation.
To develop each of the models, we first used multiple additive regression trees to assess interactions (8). For continuous predictors such as age, we used cubic splines to assess nonlinearity (9). We excluded extreme covariate values from the analysis if they appeared to be potential leverage points (FPG ≤90 mg/dL and TG ≥1,000 mg/dL). A backward stepwise procedure based on Akaike’s information criteria was used to select the final models (10).
Model Validation
To internally validate the models, we used a cross validation procedure. We first determined which factors entered the models using all of the data. We then randomly divided the data into 20 subsets and estimated the parameters from 95% of the data (omitting 1 of the 20 subsets). We then applied the model to the data from the omitted subset. Harrell C-statistics were computed as measures of discrimination for each of the omitted subsets (11). We then compared the observed and predicted risks of progression and regression for each decile of predicted risk and determined the magnitude of the deviation using the D’Agostino and Nam statistic (12). Nonsignificant P values from this test indicate good model fit. Finally, we used the decision-curve analysis of Vickers and Elkin (13) to identify the range of threshold probabilities in which the models had added value for predicting progression to DM and regression to NGR and the magnitude of benefit.
All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and R 3.0.0 (The R Foundation).
Results
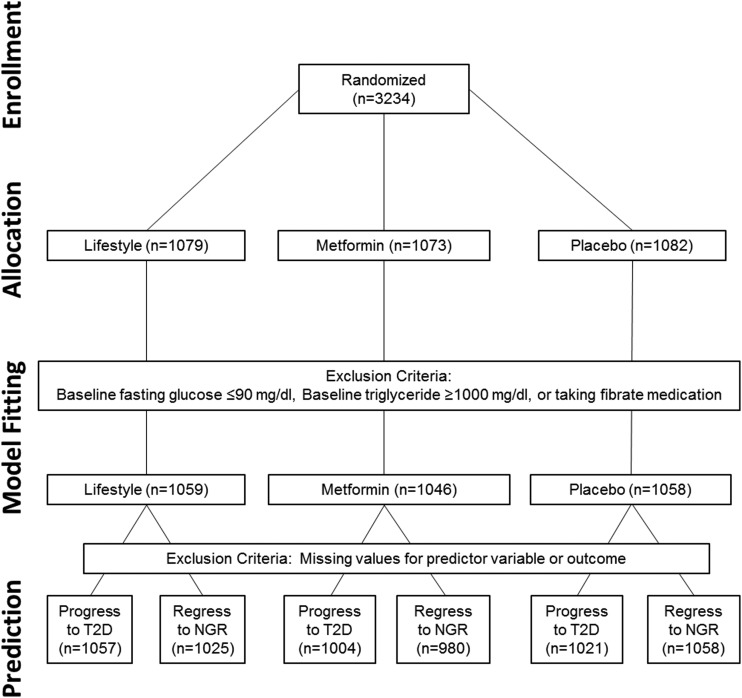
The DPP randomized 3,234 participants: 1,079 to the lifestyle, 1,073 to the metformin, and 1,082 to the placebo intervention. A total of 71 participants (2%) with extreme values of baseline FPG (FPG ≤90 mg/dL) or TG (TG ≥1,000 mg/dL) or who were taking fibrates for hypertriglyceridemia were excluded from the analyses (Fig. 1). Because of missing values for predictor variables and outcomes covariates, a few additional participants were excluded when we developed the prediction models. Figure 1 shows the data sets used for our analyses.
Figure 1.
Consort diagram.
Table 1 shows the baseline characteristics of the study participants by DPP intervention group and adherence. Mean age was ∼50 years, approximately one-third of participants were men, and slightly more than half were white. Almost half of participants were college graduates. Approximately 7% of participants reported current smoking. At baseline, approximately half of participants reported engaging in >150 min/week of moderate physical activity. Approximately 15% were taking medications for hypertension, and <5% were taking statins for hypercholesterolemia. Mean BMI was ∼34 kg/m2, and mean SBP was 124 mmHg. Mean FPG was 107 mg/dL, and median fasting TG was 141 mg/dL.
Table 1.
Baseline characteristics of the DPP population by adherence status
| Lifestyle |
Metformin |
Placebo |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 1,059) | Adherent (N = 661 [62%]) | Nonadherent (N = 398 [38%]) | P value* | Total (N = 1,046) | Adherent (N = 713 [68%]) | Nonadherent (N = 291 [32%]) | P value* | Total (N = 1,058) | Adherent (N = 766 [72%]) | Nonadherent (N = 238 [28%]) | P value* | |
| Sociodemographic characteristics | ||||||||||||
| Mean age ± SD (years) | 50.6 ± 11.3 | 52.0 ± 11.4 | 48.4 ± 10.9 | <0.0001 | 50.9 ± 10.3 | 51.3 ± 10.2 | 50.5 ± 10.4 | 0.2142 | 50.3 ± 10.4 | 51.1 ± 10.4 | 50.5 ± 10.4 | 0.0011 |
| Sex [N (%)] | 0.0641 | 0.1223 | ||||||||||
| Male sex [(N (%)] | 339 | 226 (34) | 113 (28) | 352 | 251 (35) | 85 (29) | 328 | 238 (31) | 75 (32) | 0.7625 | ||
| Female with no history of pregnancy | 111 | 62 (9) | 49 (12) | 102 | 73 (10) | 25 (9) | 97 | 74 (10) | 18 (8) | |||
| Female with history of pregnancy but no GDM | 474 | 298 (45) | 176 (44) | 464 | 306 (43) | 140 (48) | 485 | 347 (45) | 113 (47) | |||
| Female with history of pregnancy and GDM | 104 | 57 (9) | 47 (12) | 103 | 65 (9) | 35 (12) | 111 | 77 (10) | 26 (11) | |||
| Race/ethnicity [N (%)] | <0.0001 | 0.0200 | ||||||||||
| White | 576 | 401 (61) | 175 (44) | 589 | 429 (60) | 142 (50) | 575 | 445 (58) | 102 (43) | 0.0005 | ||
| African American | 202 | 94 (14) | 108 (27) | 218 | 135 (19) | 72 (25) | 218 | 138 (18) | 69 (29) | |||
| Hispanic | 176 | 107 (16) | 69 (17) | 159 | 96 (13) | 53 (18) | 166 | 112 (15) | 43 (18) | |||
| Asian | 55 | 32 (5) | 23 (6) | 35 | 21 (3) | 11 (4) | 47 | 34 (4) | 11 (5) | |||
| American Indian | 50 | 27(4) | 23 (6) | 45 | 32 (4) | 13 (4) | 52 | 37 (5) | 13 (5) | |||
| College graduate [N (%)] | 490 | 318 (48) | 172 (43) | 0.1219 | 490 | 358 (50) | 113 (39) | 0.0010 | 489 | 359 (47) | 108 (45) | 0.6875 |
| Annual household income [N (%)] | 0.3926 | 0.0145 | 0.5807 | |||||||||
| <$30,000 | 144 | 88 (13) | 56 (14) | 134 | 77 (11) | 51 (18) | 156 | 105 (14) | 39 (16) | |||
| $30,000–50,000 | 405 | 244 (37) | 161 (40) | 377 | 263 (37) | 97 (33) | 396 | 290 (38) | 86 (36) | |||
| >$50,000 | 510 | 329 (50) | 181 (45) | 534 | 372 (52) | 143 (49) | 506 | 371 (48) | 113 (47) | |||
| Health behaviors [N (%)] | ||||||||||||
| Current smoking | 70 | 33 (5) | 37 (9) | 0.0063 | 72 | 46 (6) | 23 (8) | 0.4093 | 84 | 64 (8) | 13 (5) | 0.1429 |
| Physical activity | 0.2895 | 0.1190 | 0.6519 | |||||||||
| >150 min/week | 514 | 310 (47) | 204 (51) | 521 | 365 (51) | 145 (50) | 528 | 377 (49) | 122 (51) | |||
| 90–150 min/week | 163 | 101 (15) | 62 (16) | 154 | 110 (15) | 33 (11) | 166 | 125 (16) | 33 (15) | |||
| <90 min/week | 380 | 248 (38) | 132 (33) | 371 | 238 (33) | 113 (39) | 364 | 264 (34) | 83 (35) | |||
| Past history and family history | ||||||||||||
| Polycystic ovary disease (among women) | 18 | 7 (1) | 11 (3) | 0.0376 | 26 | 19 (3) | 6 (2) | 0.5780 | 20 | 12 (2) | 5 (2) | 0.5768 |
| Family history of diabetes | 739 | 448 (68) | 291 (73) | 0.0722 | 719 | 494 (69) | 198 (68) | 0.6993 | 738 | 529 (69) | 172 (72) | 0.3597 |
| Medications | ||||||||||||
| Antihypertensive medication | 177 | 113 (17) | 64 (16) | 0.6681 | 161 | 104 (15) | 52 (18) | 0.1926 | 160 | 129 (17) | 26 (11) | 0.0273 |
| Statin | 31 | 21 (3) | 10 (3) | 0.5344 | 39 | 31 (4) | 7 (2) | 0.1434 | 49 | 41 (5) | 7 (3) | 0.1278 |
| Anthropometric measures (mean ± SD) | ||||||||||||
| Weight (kg) | 94.3 ± 20.8 | 93.8 ± 20.8 | 95.1 ± 20.9 | 0.3361 | 94.4 ± 19.8 | 94.5 ± 19.3 | 93.2 ± 19.1 | 0.3578 | 94.5 ± 20.1 | 94.7 ± 20.1 | 93.8 ± 19.8 | 0.5434 |
| BMI (kg/m2) | 33.9 ± 6.8 | 33.6 ± 6.7 | 34.4 ± 7.0 | 0.0717 | 33.9 ± 6.6 | 33.8 ± 6.3 | 33.8 ± 6.6 | 0.9919 | 34.2 ± 6.7 | 34.3 ± 6.8 | 34.0 ± 6.3 | 0.6131 |
| SBP (mmHg) | 124 ± 15 | 124 ± 14 | 123 ± 16 | 0.0966 | 124 ± 15 | 124 ± 15 | 123 ± 15 | 0.4598 | 124 ± 14 | 124 ± 14 | 123 ± 14 | 0.2004 |
| DBP (mmHg) | 79 ± 9 | 79 ± 9 | 79 ± 9 | 0.5834 | 78 ± 10 | 78 ± 10 | 78 ± 10 | 0.7682 | 78 ± 9 | 78 ± 9 | 78 ± 10 | 0.5321 |
| Laboratory measures | ||||||||||||
| FPG mean ± SD (mg/dL) | 106 ± 8 | 106 ± 8 | 106 ± 8 | 0.8797 | 107 ± 8 | 107 ± 8 | 107 ± 9 | 0.9148 | 107 ± 8 | 107 ± 8 | 107 ± 8 | 0.8123 |
| TG median (IQR) (mg/dL) | 137 (97–198) | 137 (98–197) | 139 (93–200) | 0.9319 | 136 (98–195) | 141 (101–199) | 133 (93–186) | 0.3958 | 147 (104–205) | 149 (105–211) | 136 (101–183) | 0.1727 |
DBP, diastolic blood pressure; IQR, interquartile range.
*P values are calculated from Pearson χ2 test of independence for categorical variables and two-sample t test for continuous variables.
Sixty-two percent of lifestyle participants, 68% of metformin participants, and 72% of placebo participants were adherent to the interventions at 6 months (Table 1). In general, adherent participants were more likely to be older and of white race/ethnicity (Table 1). One-hundred forty-one (14%) adherent lifestyle participants, 218 (21%) adherent metformin participants, and 296 (28%) adherent placebo participants developed DM. Four hundred twenty-three (40%) adherent lifestyle participants, 272 (26%) adherent metformin participants, and 214 (20%) adherent placebo participants regressed to NGR.
Table 2 shows the baseline predictor variables included in the final prediction models for progression to DM and regression to NGR for participants in each of the three treatment groups. All participants, adherent and nonadherent, were used to estimate the coefficients for the models. The coefficients for adherence represent the conditional log hazard ratios for adherent versus nonadherent participants. To calculate the risk for an adherent participant, the coefficient for adherence is set to 1.
Table 2.
Multivariable models
|
A: Multivariable models predicting progression to diabetes at 3 years |
Hazard ratio (95% CI) |
||
|---|---|---|---|
| Predictors | Lifestyle | Metformin | Placebo |
| Age | 1.016 (1.002, 1.029) | ||
| Sex and GDM status | |||
| Female with no history of pregnancy vs. male | 0.745 (0.453, 1.225) | ||
| Female with history of pregnancy but no GDM vs. male | 1.120 (0.847, 1.482) | ||
| Female with history of GDM vs. male | 1.566 (1.080, 2.272) | ||
| College graduate vs. non–college graduate | 1.322 (1.044, 1.673) | ||
| Current smoking: yes vs. no | 1.590 (0.998, 2.531) | ||
| Physical activity >150 min/week: yes vs. no | 1.304 (0.935, 1.818) | ||
| Polycystic ovarian disease: yes vs. no | 1.920 (0.944, 3.905) | ||
| Family history of diabetes: yes vs. no | 1.311 (0.964, 1.783) | ||
| BMI (kg/m2) | 1.050 (1.028, 1.072) | 1.019 (1.002, 1.035) | |
| SBP (mmHg) | 1.009 (1.000, 1.018) | ||
| Fasting glucose (mg/dL) | 1.074 (1.054, 1.094) | 1.057 (1.042, 1.073) | 1.089 (1.075, 1.103) |
| Natural log-transformed TG (mg/dL) | 1.506 (1.104, 2.053) | 1.717 (1.305, 2.260) | 1.279 (1.005, 1.627) |
| Adherence at 6 months: yes vs. no | 0.366 (0.264, 0.507) | 0.819 (0.614, 1.094) | 1.243 (0.928, 1.665) |
|
B: Multivariable models predicting regression to NGR at 3 years |
Hazard ratio (95% CI) |
||
|---|---|---|---|
| Predictors | Lifestyle | Metformin | Placebo |
| Age | 0.989 (0.981, 0.998) | 0.984 (0.971, 0.996) | 0.991 (0.978, 1.004) |
| Sex and GDM status | |||
| Female with no history of pregnancy vs. male | 0.610 (0.388, 0.960) | ||
| Female with history of pregnancy but no GDM vs. male | 0.771 (0.585, 1.016) | ||
| Female with history of GDM vs. male | 0.594 (0.370, 0.955) | ||
| College graduates vs. non–college graduates | 1.301 (1.010, 1.667) | ||
| SBP (mmHg) | 0.992 (0.983, 1.001) | ||
| Fasting glucose (mg/dL) | 0.950 (0.936, 0.963) | 0.945 (0.928, 0.962) | 0.919 (0.898, 0.940) |
| Natural log-transformed TG (mg/dL) | 0.765 (0.634, 0.923) | 0.733 (0.578, 0.929) | 0.727 (0.550, 0.961) |
| Adherence at 6 months: yes vs. no | 1.867 (1.496, 2.331) | 1.093 (0.831, 1.438) | 0.753 (0.556, 1.018) |
Adherence is defined as losing 5% of initial body weight at 6 months in the lifestyle group or reporting taking pills ≥80% of the time in the metformin and placebo groups. For categorical variables, 1 = yes and 0 = no.
Eleven of the 19 variables assessed at baseline remained in any of the three models predicting progression to DM, with little overlap across intervention groups. Only FPG and TG entered all three models (Table 2A). In the lifestyle intervention group, greater physical activity (>150 min/week) at baseline (before lifestyle intervention was implemented), higher BMI, higher FPG, and higher TG predicted greater risk of progression to DM. The seemingly paradoxical finding that greater reported physical activity at baseline was associated with greater risk of progression to DM in the lifestyle intervention group may be explained by the observations that those participants increased the physical activity less and lost less weight than participants who reported less physical activity at baseline. In the metformin intervention group, older age, smoking, history of polycystic ovarian disease, family history of diabetes, higher FPG, and higher TG predicted greater risk of progression to DM. In the placebo group, female sex and having a previous pregnancy with or without GDM, being a college graduate, and higher BMI, SBP, FPG, and TG predicted greater risk of progression to DM.
Table 2B shows the baseline predictor variables associated with regression to NGR. Only 6 of the 19 variables entered any of the 3 NGR prediction models, and only 3 of the 6 (younger age, lower FPG, and lower TG) were common to all 3 models. In both the lifestyle and placebo intervention groups, no other variables predicted regression to NGR, whereas in the metformin intervention group, regression to NGR was also associated with male sex, being a college graduate, and lower SBP.
The 3-year probability of progression to DM and regression to NGR for the three intervention groups was calculated using the equations presented in Table 3A and B, respectively. The Harrell C-statistic was used to assess the discriminative ability of the models. The C-statistic indicates the proportion of all pairs of subjects that can be ordered such that the subject with the higher predicted risk is the one who experiences the outcome earlier. In other words, the C-statistic assesses the ability of the model to correctly distinguish those at higher risk from those at lower risk of progression or regression. Two of the three diabetes progression models had fair discrimination (Harrell C-statistic 0.753 for the lifestyle intervention model and 0.721 for the placebo intervention model). Discrimination of the diabetes progression model for the metformin intervention group was not as good (Harrell C-statistic: 0.652). When the outcome was regression to NGR, Harrell C-statistics were generally lower: 0.673 for the lifestyle model, 0.674 for the metformin model, and 0.681 for the placebo model.
Table 3.
Equations
|
A: Equation to calculate probability of progression to DM at 3 years |
|||
|---|---|---|---|
| Equation |
Probability (progression to DM) = 1 − S0exp(F(X)) |
||
| Treatment | S0 | F(X) | |
| Lifestyle | 0.824 | 0.048 × (BMI − 34) + 0.071 × (fasting glucose − 107) + 0.409 × (log(TG) − 5) + 0.266 × PA − 1.005 × adherence | |
| Metformin | 0.821 | 0.015 × (age − 51) + 0.271 × FH + 0.056 × (fasting glucose − 107) + 0.541 × (log(TG) − 5) + 0.463 × SM + 0.652 × polycystic − 0.199 × adherence | |
| Placebo | 0.825 | −0.294 × FNP + 0.114 × FP + 0.449 × GDM + 0.018 × (BMI − 34) + 0.085 × (fasting glucose − 107) + 0.009 × (SBP − 124) + 0.246 × (log(TG) − 5) + 0.279 × CG + 0.217 × adherence | |
|
B: Equation to calculate probability of regression to NGR at 3 years |
|||
|---|---|---|---|
| Formulas |
Probability (progression to DM) = 1 − S0exp(F(X)) | ||
| Treatment | S0 | F(X) | |
| Lifestyle | 0.737 | −0.011 × age − 0.052 × fasting glucose − 0.268 × log(TG) + 0.624 × adherence | |
| Metformin | 0.729 | −0.017 × age − 0.494 × FNP − 0.260 × FP − 0.521 × GDM − 0.056 × fasting glucose − 0.008 × SBP − 0.311 × log(TG) + 0.263 × CG + 0.089 × adherence | |
| Placebo | 0.775 | −0.009 × age − 0.085 × fasting glucose − 0.319 × log(TG) − 0.284 × adherence | |
CG, 1 if a subject is a college graduate, 0 otherwise; FH, 1 if a subject has family history of diabetes, 0 otherwise; FNP, 1 if a subject is female and has never been pregnant, 0 otherwise; FP, 1 if a subject is female and has been pregnant but no history of GDM, 0 otherwise; GDM, 1 if a subject is female and has had GDM, 0 otherwise; PA, 1 if physical activity >150 min/week, 0 otherwise; polycystic, 1 if a subject is female and has polycystic syndrome, 0 otherwise; S0, 3-year survival probability for a participant with reference covariate pattern (continuous covariates equal the sample average and categorical covariates equal male or no); SM, 1 if a subject is a current smoker, 0 otherwise.
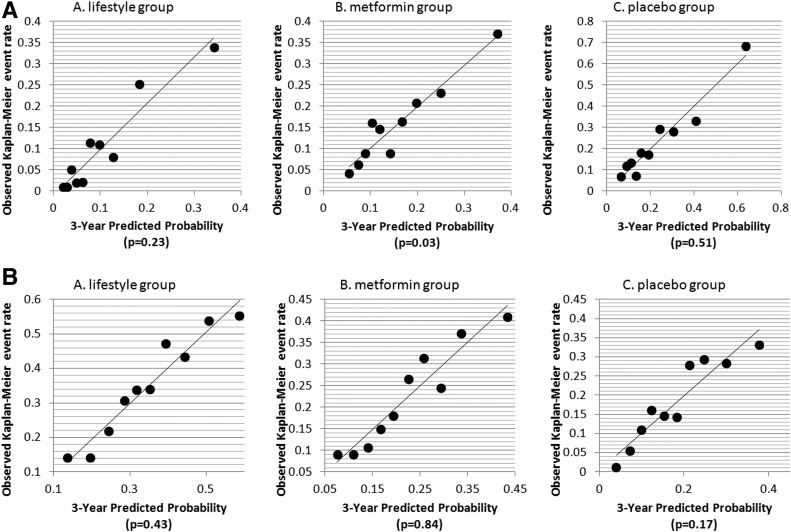
Figure 2A and B shows the calibration curves of the observed versus predicted probabilities of progression to DM and regression to NGR, respectively. A nonsignificant P value from the D’Agostino and Nam (12) χ2 statistic indicates good fit of a model. Five of the six models had nonsignificant P values, indicating that in general, the models had good fit at year 3.
Figure 2.
A: Calibration curves for progression to DM. Black dots represent deciles of risk for progression to DM.. B: Calibration curves for regression to NGR. Black dots represent deciles of risk for regression to NGR. P values are for D’Agostino and Nam (12) χ2 statistics.
Vickers and Elkin (13) decision-curve analyses were used to identify the range of threshold probabilities in which the models added value for predicting progression to DM and regression to NGR. Both models added value for decision-making: the lifestyle model added value for the full range of threshold probabilities, and the metformin model added value for threshold probabilities from ∼10 to 70% (data not shown).
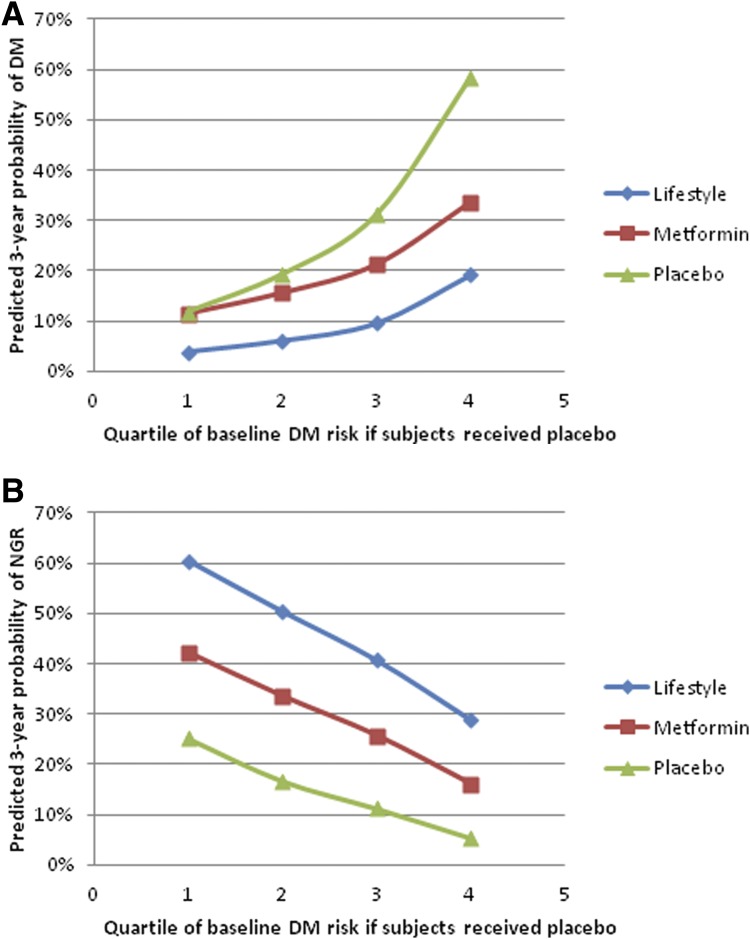
Figure 3A shows the 3-year predicted probabilities of progression to DM in DPP participants who adhered to the lifestyle, metformin, and placebo interventions stratified according to their quartile of risk of progressing to diabetes if they had been assigned to the placebo intervention. For those at lowest risk of progression to DM (quartile 1) who adhered to the lifestyle intervention, the predicted 3-year probability of progression to DM was only 4%. The corresponding probability was 11% for those in the lowest quartile of risk who adhered to the metformin intervention and 12% for those in the lowest quartile of risk who adhered to the placebo intervention. The ARR was thus 8% for low-risk participants who adhered to the lifestyle intervention but 0% for low-risk participants who adhered to the metformin intervention. For those in the highest quartile of risk, the predicted 3-year probabilities of progression to DM were 19% for individuals who adhered to the lifestyle intervention, 34% for those who adhered to the metformin intervention, and 59% for those who adhered to the placebo intervention. The ARR was thus 39% for those at highest risk who adhered to the lifestyle intervention and 25% for those at highest risk who adhered to the metformin intervention. These results indicate that the lifestyle intervention was effective in adherent participants regardless of baseline risk, and substantially more effective than the metformin intervention in those at highest risk, whereas the metformin intervention was only effective in adherent participants who were at higher risk (quartiles 3 and 4).
Figure 3.
A: Predicted 3-year probabilities of progressing to DM in adherent participants by quartile of risk of developing DM if participants had been assigned to the placebo intervention. B: Predicted 3-year probabilities of regressing to NGR in adherent participants by quartile of risk of developing DM if participants had been assigned to the placebo intervention.
Figure 3B shows the 3-year predicted probabilities of regression to NGR by treatment group stratified by the same quartiles of risk of progressing to diabetes if the participants had been assigned to the placebo intervention. For both those with the lowest probability of progressing to DM (quartile 1) and the highest probability of progressing to DM (quartile 4), the probability of regressing to NGR was substantially greater for those who were adherent to the lifestyle and metformin interventions compared with those who were adherent to the placebo intervention. Taken together, these results demonstrate the large variation in individual risk of progression to diabetes and regression to NGR even in this relatively homogeneous study population.
Conclusions
The DPP demonstrated the efficacy of lifestyle and metformin interventions for delaying progression to DM in diverse participants at high-risk for DM. Sussman et al. (3) previously demonstrated that the average benefit for DPP participants randomized to the metformin intervention was distributed unevenly across the population with one-fourth of patients at the highest risk for developing DM receiving a dramatic benefit (22% ARR over 3 years), whereas the remainder of the study population received modest or no benefit. We have extended this approach evaluating “benefit-based tailored treatment” by developing multivariable models to predict individual risk of progression to DM and regression to NGR using variables routinely assessed in clinical practice and accounting for intervention adherence.
Our results demonstrate that compared with participants who adhered to the placebo intervention, the absolute reduction in the predicted 3-year probability of progression to DM is 20% for participants who adhered to the lifestyle intervention and 9% for participants who adhered to the metformin intervention. However, only 62% of lifestyle participants, 68% of metformin participants, and 72% of placebo participants were able to adhere to their randomized intervention assignments at 6 months. Adherence to the lifestyle intervention, defined as achieving ≥5% weight loss at 6 months, was associated with reduced progression to DM across all four quartiles of baseline risk, with the greatest ARR relative to placebo occurring in the quartiles of participants at highest risk. Adherence to the metformin intervention was also associated with reduced risk of progression to DM. However, this benefit was strongly affected by baseline DM risk: in the lowest-risk quartile, the metformin intervention reduced the predicted 3-year probability of progression to DM by <1%, whereas in the group at highest risk, the ARR was 25% with metformin compared with placebo. Absolute risks of progression to DM were consistently lower among adherent lifestyle participants than among adherent metformin participants.
Among those who were adherent to the DPP interventions, lifestyle intervention was also more effective than metformin intervention in promoting regression to NGR (absolute increase in predicted probability of regression to NGR 30% for lifestyle and 14% for metformin). The predicted 3-year probability of regression to NGR by quartile of DM risk was greater with the lifestyle intervention than the metformin intervention, although the benefit of the lifestyle intervention relative to the metformin intervention in increasing the probability of regression to NGR was greatest in the quartile at highest risk of progressing to DM (35 vs. 17% and difference of 18%). In those at lowest risk of progressing to DM, the corresponding rates were 24 and 11% (difference of 13%). Better identification of individuals at low risk of progressing to DM may prevent overtreatment, reduce treatment-related adverse events, and encourage more appropriate resource utilization (14).
Recently, Davidoff (15) pointed out that although randomized trials can demonstrate whether treatments work, the benefits of treatment vary from patient to patient. Focusing solely on aggregate results for the randomized treatment groups may lead to the faulty inference that an effective treatment provides equal benefits to everyone who receives it. Understanding this phenomenon, termed “heterogeneity in treatment effects,” requires knowledge about which participants do and do not benefit from the treatment. Treatment benefits generally increase as an individual’s baseline risk increases, and “failure to explore differences in treatment effects among subgroups with different levels of baseline risk represents incomplete reporting of trial results” (15). Davidoff (15) goes on to recommend that investigators first determine the baseline risk for each trial participant and then develop disease-specific multivariable prognostic models to risk-stratify the population. Then, by assessing outcomes in study participants according to baseline risk, investigators can compare clinical outcomes directly between treated and untreated participants within each baseline risk subgroup.
There are a number of limitations to our analyses. First, it is important to recognize that the equations were developed and validated for individuals living in the U.S. who met DPP eligibility criteria. It is not clear how the equations will perform, for example, in individuals identified with prediabetes based on a risk-screening questionnaire alone. Second, it is important to recognize that individuals who participated in the DPP were likely more highly motivated to prevent diabetes than individuals with prediabetes in the general population and that the 3-year probabilities of progression to DM and regression to NGR that we reported are for adherent participants. To the extent that intervention participants are less motivated, and their adherence is less complete, the outcomes will be less favorable than we report. In addition, to the extent that long-term adherence to a medication intervention is better than long-term adherence to a lifestyle intervention, the apparent benefit of the lifestyle intervention relative to the metformin intervention will be attenuated. Finally, it should be recognized that the diabetes prevention intervention being implemented in community settings today differs from that implemented in the DPP. For example, the National Diabetes Prevention Program lifestyle intervention lasts for only 1 year and is delivered in a group format rather than one-on-one (16). To the extent that these differences reduce the efficacy of the lifestyle intervention, the apparent benefit of the lifestyle intervention will be overestimated.
In the future, we plan to upload the multivariable models for individual risk prediction on the DPP Outcomes Study (DPPOS) website. By entering the values for each individual’s predictor variables, people meeting DPP eligibility criteria and their health care providers can calculate and compare their 3-year risk of progression to DM and likelihood of regression to NGR if they adhere to an intensive lifestyle intervention and lose at least 5% of their initial body weight or adhere to a metformin intervention by taking metformin 850 mg twice daily at least 80% of the time. They can also see the number of individuals who would need to be treated with the lifestyle or metformin intervention for 3 years to prevent one person from progressing to DM or to induce one person to regress to NGR (the numbers needed to treat). Use of these equations will also allow patients and providers to reassess the choice of intervention after 6 months in light of the intervention’s demonstrated feasibility and side effects. For example, if an individual is unable to adhere to the lifestyle intervention after 6 months, the patient and provider may wish to consider metformin therapy. Similarly, if a patient experiences intolerable side effects with metformin therapy, the patient and provider may wish to consider the lifestyle intervention.
For example, a 40-year-old man with IGT, BMI of 27 kg/m2, FPG of 99 mg/dL, and TG of 67 mg/dL is substantially less likely to progress to DM and more likely to regress to NGR than a 40-year-old man with IGT, BMI of 41 kg/m2, FPG of 115 mg/dL, and TG of 245 mg/dL, regardless of which intervention he chooses (Table 4). Because of the substantially lower risk of progression to DM and the lower efficacy of the metformin intervention in a 40-year-old man with IGT and lower BMI, FPG, and TG versus higher BMI, FPG, and TG, 16 men would need to be treated with lifestyle (vs. 4 men) and 82 men would need to be treated with metformin (vs. 7 men) to prevent one case of diabetes over 3 years in the lower-risk compared with the higher-risk men (Table 4). However, in a 60-year-old man, adhering to a lifestyle intervention is as effective as in a younger man, but the metformin intervention is less effective in preventing progression to DM in a man with higher BMI, FPG, and TG and ineffective in a man with lower BMI, FPG, and TG (Table 4). Forty- and 60-year-old women with IGT and histories of pregnancy but no histories of GDM are as likely to benefit from the lifestyle and metformin interventions as 40- and 60-year-old men with the same risk-factor profiles. Compared with men, women are less likely to regress to NGR with the metformin intervention compared with the lifestyle intervention.
Table 4.
Impact of extreme values of predictor variables on 3-year predicted probabilities of progression to type 2 DM and regression to NGR for adherent participants
| Predictor variables |
Progress |
Regress |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3-year predicted probabilities (%) if treated with placebo | Percent ARR (NNT) | 3-year predicted probabilities (%) if treated with placebo | Percent absolute chance increment (NNT) | |||||||
| Age (years) | Sex | BMI (kg/m2) | FG (mg/dL) | TG (mg/dL) | Lifestyle | Metformin | Lifestyle | Metformin | ||
| 40 | M | 27 | 99 | 67 | 8 | 6 (16) | 1 (82) | 57 | 25 (4) | 12 (7) |
| 40 | M | 41 | 115 | 245 | 45 | 26 (4) | 14 (7) | 20 | 22 (4) | 11 (9) |
| 60 | M | 27 | 99 | 67 | 8 | 6 (16) | −2 (—) | 45 | 24 (4) | 7 (11) |
| 60 | M | 41 | 115 | 245 | 45 | 26 (4) | 6 (16) | 15 | 18 (5) | 7 (13) |
| 40 | F | 27 | 99 | 67 | 8 | 6 (14) | 1 (46) | 47 | 25 (4) | 2 (18) |
| 40 | F | 41 | 115 | 245 | 47 | 28 (3) | 16 (5) | 16 | 22 (4) | 7 (14) |
| 60 | F | 27 | 99 | 67 | 8 | 6 (14) | −2 (—) | 37 | 24 (4) | −1 (—) |
| 60 | F | 41 | 115 | 245 | 47 | 28 (3) | 8 (10) | 12 | 18 (5) | 4 (23) |
—, not effective; F, woman with history of pregnancy but no history of GDM; FG, fasting glucose; M, male; NNT, number needed to treat.
Clinical trials assess the efficacy of new treatments and often highlight the effects of an intervention on the relative risk reduction for a prespecified outcome, such as the development of DM. Treatment decisions should, however, be based on the best estimate of the ARR or risk difference associated with alternative courses of action for a particular patient. The purpose of this article was to use baseline data from the DPP to develop risk equations to predict progression to DM and regression to NGR among individuals who adhere to the interventions. These simple equations can be used in clinical practice by patients and their providers to better understand the benefits of the alternative treatments to delay or prevent the development of DM and to induce regression to NGR in order to facilitate individual benefit-based tailored treatment and personalized decision-making.
Supplementary Material
Article Information
Acknowledgments. The DPP Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS.
Funding. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant U01-DK-048489. During the DPP and DPPOS, the NIDDK of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study and collection, management, analysis, and interpretation of the data. The Southwestern American Indian Center was supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center, National Center for Research Resources, and the U.S. Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart, Lung, and Blood Institute, the National Cancer Institute, the Office of Research on Women’s Health, the National Institute on Minority Health and Health Disparities, the Centers for Disease Control and Prevention, and the American Diabetes Association. This research was also supported, in part, by the Intramural Research Program of the NIDDK. Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP, Merck Lipha Health provided medication, and LifeScan, Inc. donated materials during the DPP and DPPOS. LifeScan, Inc., Health O Meter, Hoechst Marion Roussel Inc., Merck-MedcoManaged Care, Merck & Co., Inc., Nike Inc., SlimFast, andQuakerOats Company donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices, Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center.
Duality of Interest. L.P. has received personal fees from Novo Nordisk, AstraZeneca, Janssen, Boehringer Ingelheim, Eli Lilly and Company, Orexigen Therapeutics, Inc., Merck, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
The sponsor of this study was represented on the Steering Committee and played a part in study design, how the study was done, and publication. The funding agency was not represented on the writing group, although all members of the Steering Committee had input into the report’s contents. All authors in the writing group had access to all data. The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies. A complete list of centers, investigators, and staff can be found in the Supplementary Data online. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions. W.H.H., Q.P., and W.Y. researched data, wrote the manuscript, reviewed and edited the manuscript, and contributed to the discussion. S.L.E., K.J.M., L.P., E.B.-C., D.M.D., E.H., S.E.K., W.C.K., C.L., X.P.-S., and E.V. researched data, reviewed and edited the manuscript, and contributed to the discussion. W.H.H. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
Clinical trial reg. no. NCT00004992, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc17-1116/-/DC1.
A complete list of the DPP Research Group Investigators can be found in the Supplementary Data online.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noble D, Mathur R, Dent T, Meads C, Greenhalgh T. Risk models and scores for type 2 diabetes: systematic review. BMJ 2011;343:d7163DOI: 10.1136/bmj.d7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sussman JB, Kent DM, Nelson JP, Hayward RA. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ 2015;350:h454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofer TP, Sussman JB, Hayward RA. New studies do not challenge the American College of Cardiology/American Heart Association lipid guidelines. Ann Intern Med 2016;164:683–684 [DOI] [PubMed] [Google Scholar]
- 5.Smith SC Jr, Haslam D. Abdominal obesity, waist circumference and cardio-metabolic risk: awareness among primary care physicians, the general population and patients at risk--the Shape of the Nations survey. Curr Med Res Opin 2007;23:29–47 [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Prevention Program Design and methods for a clinical trial in the prevention of type 2 diabetes [published correction appears in Diabetes Care 1999;22:1389]. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin RR, Fujimoto WY, Marrero DG, et al.; DPP Research Group . The Diabetes Prevention Program: recruitment methods and results. Control Clin Trials 2002;23:157–171 [DOI] [PubMed] [Google Scholar]
- 8.Friedman JH, Meulman JJ. Multiple additive regression trees with application in epidemiology. Stat Med 2003;22:1365–1381 [DOI] [PubMed] [Google Scholar]
- 9.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, Springer, 2001 [Google Scholar]
- 10.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics 2005;61:92–105 [DOI] [PubMed] [Google Scholar]
- 11.Harrell FE Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med 1984;3:143–152 [DOI] [PubMed] [Google Scholar]
- 12.D’Agostino RB, Nam BH. Evaluation of the performance of survival analysis models: Discrimination and Calibration measures. In: Handbook of Statistics, Survival Methods. Vol 23. Balakrishnan N, Rao CR, Eds. Amsterdam, Elsevier BV, 2004, p 1–25 [Google Scholar]
- 13.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yudkin JS, Montori VM. The epidemic of pre-diabetes: the medicine and the politics. BMJ 2014;349:g4485DOI: 10.1136/bmj.g4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidoff F. Can knowledge about heterogeneity in treatment effects help us choose wisely? Ann Intern Med 2017;166:141–142 [DOI] [PubMed] [Google Scholar]
- 16.Ely EK, Gruss SM, Luman ET, et al. . A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care 2017;40:1331–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.