Abstract
This article summarizes studies in which the author has been involved over several decades, directed toward providing solutions for the three major limitations to the field of transplantation: (1) drug treatment–related complications; (2) chronic rejection; and (3) the availability of transplantable organs. The first two of these limitations may be overcome by induction of transplantation tolerance, while the third will also require a new source of organs, for which great strides are now being made in xenotransplantation through genetic engineering.
Keywords: transplantation, tolerance, xenotransplantation
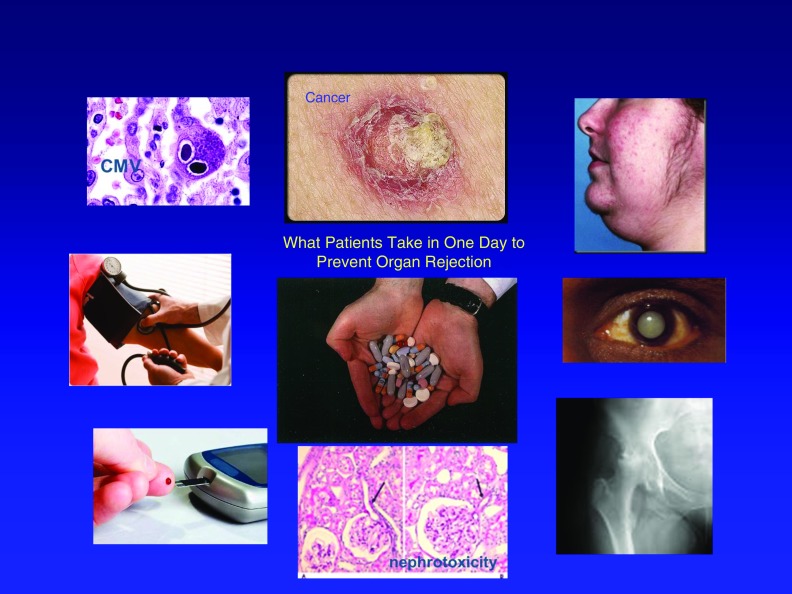
There are currently three major limitations to the field of transplantation: (1) drug treatment–related complications, including an increased incidence of infections, cancer, and diabetes, which often keep patients from seeking a transplant until their underlying disease has become life-threatening (Figure 1); (2) chronic rejection, which causes loss of 5–7% of transplanted organs per year, even in patients who take their immunosuppressive drugs correctly; and (3) the availability of transplantable organs, a limitation that leads to the death of thousands of patients each year, whose lives might have been saved by an organ transplant. The present article summarizes studies in which the author has been involved over several decades, directed toward providing solutions for these three limitations to this field of research. As described in more detail below, the first two of these limitations may be mitigated or even eliminated through the induction of transplantation tolerance. The third limitation, however, will require a new source of transplantable organs, a problem for which the author believes that xenotransplantation offers the most likely near-term solution.
Figure 1.
Problems associated with immunosuppression. Shown are the number of pills a transplant patient may have to take in a single day (center) and some of the most serious side effects of chronic immunosuppression (clockwise from top left): infection, skin cancer, hirsutism, cataracts, osteoporosis, renal dysfunction, diabetes, and hypertension. CMV = cytomegalovirus.
Transplantation Tolerance
Immunologic tolerance is generally defined as the specific absence of a response to a particular antigen in the face of normal immune responses to all other antigens in the environment and the absence of immunosuppression. However, in the field of transplantation, it has become evident over the past several decades that this definition is no longer satisfactory, because tolerance can result both from the loss of a specific immune response (i.e., deletional tolerance) or from an active down-regulatory immune response (i.e., regulatory tolerance). We have therefore proposed a more accurate definition of transplantation tolerance as “the specific absence of a destructive immune response to a transplanted tissue in the absence of immunosuppression.” The key words in this definition are “specific,” because tolerance must be directed only to the transplanted tissue and not to other antigens; “destructive,” to include positive but down-regulatory responses; and “absence of immunosuppression,” indicating that demonstration of tolerance requires withdrawal of all immunosuppressive medications.
It has been known for many years that when a bone marrow transplant is successful, it carries with it tolerance to any other tissue or organ from the donor of the bone marrow. This principle has been established not only from animal experiments, but also from the treatment of patients. Thus, there are now numerous cases in which a bone marrow transplant from a closely matched sibling was performed to treat leukemia or lymphoma in a patient who, later in life, developed renal failure. In many of those cases, the original sibling donor was also willing to give a kidney. Under this circumstance, no immunosuppression was required, because the recipient had become tolerant due to the former bone marrow transplant (1, 2).
Fortunately, for clinical applications, this kind of tolerance does not require a complete bone marrow transplant, but rather requires only that a small percentage of bone marrow–derived cells persist in the recipient at the time of the organ transplant. This state, involving a mixture of bone marrow from recipient and donor, has been called “mixed chimerism” and is the basis of the tolerance induction that we and others have used successfully to free transplant recipients of the requirement for lifelong immunosuppressive drugs (3).
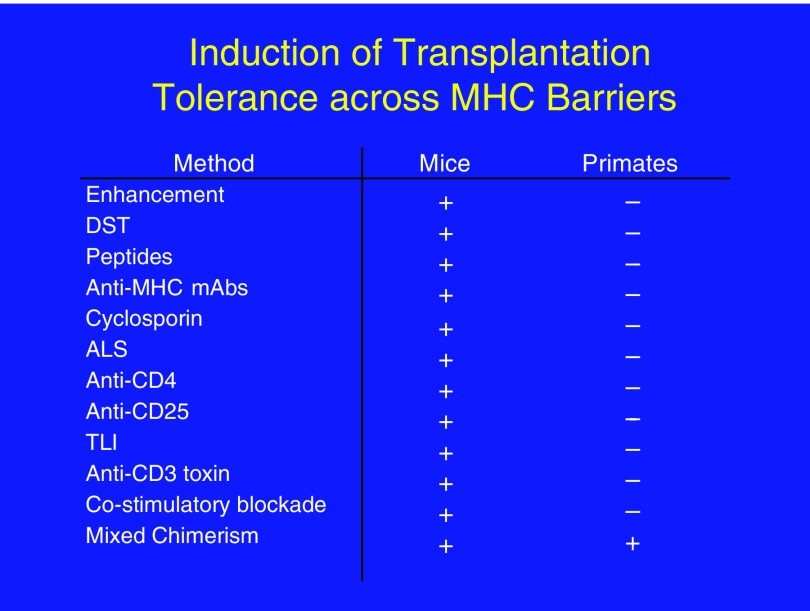
The principle of tolerance through mixed chimerism was demonstrated over a period of 20 years of research, first in mice (4, 5) and then in large animals (6, 7). It was necessary to demonstrate this effectiveness in large animals because, although mouse models are unrivaled in the wealth of strains and reagents available and in the ease of genetic manipulation, it is too easy to induce transplantation tolerance in mice. This need for use of large-animal models in transplantation tolerance research is illustrated in Figure 2, which shows the large number of methodologies that have resulted in transplantation tolerance in mice and the fact that, to date, only hematopoietic chimerism has led to tolerance in nonhuman primates and humans.
Figure 2.
Induction of transplantation tolerance may be too easy in mice. Left: Some of the ways in which tolerance has been induced successfully in mice. Right: Ways in which tolerance has been induced successfully in large animals. ALS = antilymphocytic serum; DST = donor-specific transfusion; mAb = monoclonal antibody; MHC = major histocompatibility complex; TLI = total lymphoid irradiation.
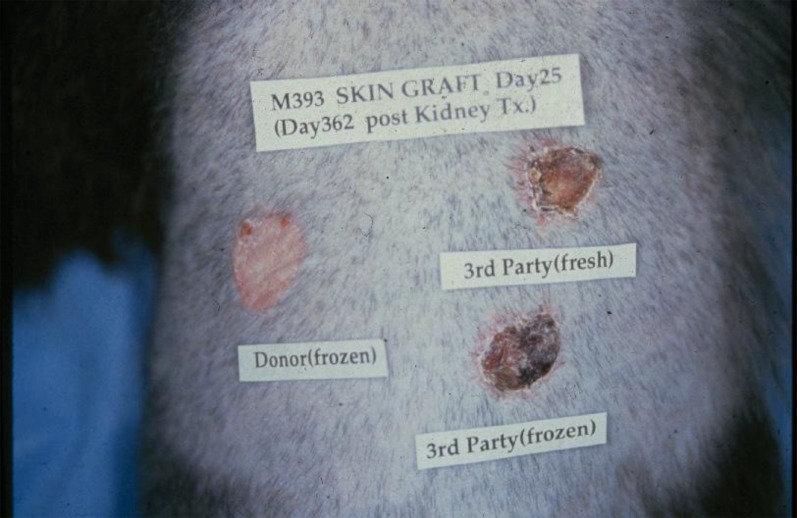
Although mixed chimerism has been effective for the induction of transplantation tolerance in mice (5), pigs (6), monkeys (7) and humans (8), the mechanism of this tolerance is not necessarily the same in each species. Thus, in mice, lifelong mixed chimerism can be achieved, as evidenced by the persistence of a mixture of host and donor lymphohematopoietic elements for the life of the animals (5). In primates, the chimerism achieved has generally been only transient, lasting for weeks to months (7). In this case, there is evidence that the tolerance achieved is likely to involve both deletional and regulatory mechanisms, with long-term tolerance maintained predominantly by regulation rather than deletion (9). We believe that this difference may be attributable, at least in part, to the much greater difficulty with which T cells, especially memory T cells, can be eliminated in large animals as compared with mice (10). Of note, in our primate studies, although all animals lost evidence of mixed chimerism by 2 months, tolerance was maintained as long as a kidney from the bone marrow donor was transplanted while chimerism was still present (11). We therefore hypothesized that the kidney was itself involved in the maintenance of tolerance after loss of chimerism, likely by its ability to induce and/or maintain regulatory mechanisms of tolerance (12). To date, neither the heart nor the lungs have been equally successful in maintaining tolerance in monkeys, using a similar protocol, although combined transplantation of kidney and heart from the same donor has led to long-term acceptance of both organs, again suggesting the importance of the kidney itself in maintaining this tolerance (13–15). Despite the transient nature of the chimerism, the tolerance achieved by bone marrow plus kidney transplantation was systemic, as evidenced by acceptance of donor skin grafts placed almost 1 year later, well after chimerism had disappeared and at a time when third-party skin grafts were promptly rejected (Figure 3). In addition, biopsies of kidneys from long-term tolerant monkeys showed none of the chronic rejection seen routinely in animals bearing kidneys maintained by immunosuppression (11, 16).
Figure 3.
Evidence that skin acceptance was specific and systemic. Tolerance was specific and systemic, even in animals that had lost detectable chimerism by 2 months post-transplantation. Thus, donor skin (frozen at the time of the renal transplantation) was accepted by tolerant animals at 1 year, whereas third-party skin was rejected promptly, whether fresh or frozen.
On the basis of the success achieved in these animal models, studies of kidney allograft tolerance induction in transplant patients were begun in the late 1990s, with the support of the newly established Immune Tolerance Network of the National Institute of Allergy and Infectious Diseases (National Institutes of Health, Bethesda, MD). The first series of transplant recipients included patients with kidney failure due to multiple myeloma, a form of cancer that might also be helped by the tolerance induction procedure. Tolerance of kidney transplants from HLA-matched siblings was achieved in all of these patients, although not all were cured of their myeloma (17, 18). The results were so remarkable that additional trials, also supported by the Immune Tolerance Network, were begun in an attempt to achieve tolerance in patients who did not have any evidence of malignancy and did not have a matched sibling donor to provide the transplant.
The results of these studies have been encouraging. Of the 10 HLA-mismatched patients treated so far by this mixed chimerism approach, 7 were able to be weaned completely from immunosuppressive drugs for at least 5 years (19) and 4 have remained drug-free with stable renal function for 5–13 years (20). The patients all entered the trial with severely compromised renal function, which was restored to normal quickly after transplantation. They all developed multilineage mixed chimerism that was transient, becoming undetectable within 2 weeks. A troublesome complication has been the occurrence of renal dysfunction on about Day 10 post-transplantation, a spontaneously reversible phenomenon that has been called “engraftment syndrome” in recipients of bone marrow transplants (21). However, in the case of bone marrow transplantation in patients with otherwise normal kidneys, this syndrome is much less of a problem than it is in patients who have just undergone kidney transplantation. We are currently exploring changes to the protocol that we hope will avoid this complication in the future. Our results already suggest, however, that tolerance approaches will be capable of avoiding the complications of chronic immunosuppression and may have a salutary impact on the development of chronic rejection, thus ameliorating two of the major limitations to success of transplantation today.
Xenotransplantation
Although the induction of allogeneic tolerance should improve the quality of life of our transplant patients, it will do little if anything to solve the third problem: the shortage of available donor organs. It is for this reason that xenotransplantation, the transplantation of organs from animals to humans, has become the subject of intense investigation over the last few decades, while waiting lists for human organs have continued to increase. One might ask what animal would be the ideal donor for a xenotransplant, and it is clear that if one could use nonhuman primates, the goal would be much easier to achieve. Indeed, even before the advent of modern immunosuppression, the use of a chimpanzee kidney to treat a patient in renal failure was demonstrated to be successful for more than 9 months before rejection (22). In addition, we have successfully induced tolerance of transplanted kidneys from baboons to monkeys, using a mixed chimerism approach similar to that used successfully for allogeneic kidney transplants (23). However, there are a number of compelling arguments against the use of nonhuman primates for this purpose, including the fact that the only nonhuman primates of sufficient size to serve as donors for many organs needed by adult humans would be endangered species (e.g., chimpanzees or great apes). In addition, even the use of more available species, such as baboons, would pose serious ethical questions and concerns about the potential for transmission of viruses, which are more of a problem between closely related than distantly related species. For all of these reasons, most investigators have settled on the pig as a more appropriate potential donor for xenotransplantation of organs to human recipients. Pigs are readily available; have favorable reproductive characteristics; can be bred in controlled, clean environments to avoid pathogens; are amenable to genetic engineering; and are less likely to raise ethical concerns about their use for this purpose because they are accepted as a food source in most modern societies.
In the author’s laboratory, a special strain of pig has been developed through selective breeding over more than 40 years. These pigs are called “miniature swine” because unlike domestic swine, which attain adult weights of greater than 1,000 pounds, the maximum size of these animals is 200–300 pounds. They can therefore be used as organ donors at sizes appropriate for any potential human recipient, from a baby to a large adult. In addition, through selective breeding, it has been possible to control the genetics of these animals and achieve a high coefficient of inbreeding, so that all animals of one strain are essentially identical. This property could be important for the induction of tolerance, because one could potentially use the cells or genes from one animal to induce tolerance of an organ from another animal of the same strain.
Over the past 20 years, extensive research has been performed using pig-to-baboon transplants as a model for potential clinical xenotransplants. Until recently, the major stumbling block to all of these studies was the presence of a large amount of natural antibody in nonhuman primates directed toward the cells of pigs. The majority of these antibodies are directed to a single determinant, α-1,3-galactose, or “Gal knockout” (24). The reason for the presence of these antibodies is that during evolution, at the level of Old World primates, the gene for the enzyme α-1,3-galactosyltransferase (GalT), which puts Gal onto cell surface proteins, became a pseudogene (i.e., lost its function through mutation) (25). Because the Gal antigen is found on bacteria and other environmental antigens, humans and Old World primates make a large amount of antibody against Gal and anti-Gal antibodies cause vigorous rejection.
Using genetic engineering techniques, this enzyme was eliminated from one of our inbred strains of miniature swine by inducing a knockout mutation (26). A similar knockout was produced by another group using standard, nonminiature swine (27). Like humans and Old World primates, these GalT knockout (GalT-KO) pigs do not put Gal on to the surface of their cells. As a result, xenotransplants can now be performed without the powerful rejection previously caused by natural anti-Gal antibodies. The results have been remarkable, with increased survival of both heart and kidney transplants from pigs to baboons (28, 29). Using immunosuppressive drugs, organ survival has been prolonged when using these new GalT-KO pigs as donors, but new antibodies soon appeared, causing rejection (28, 29), and suggesting the need for induction of tolerance.
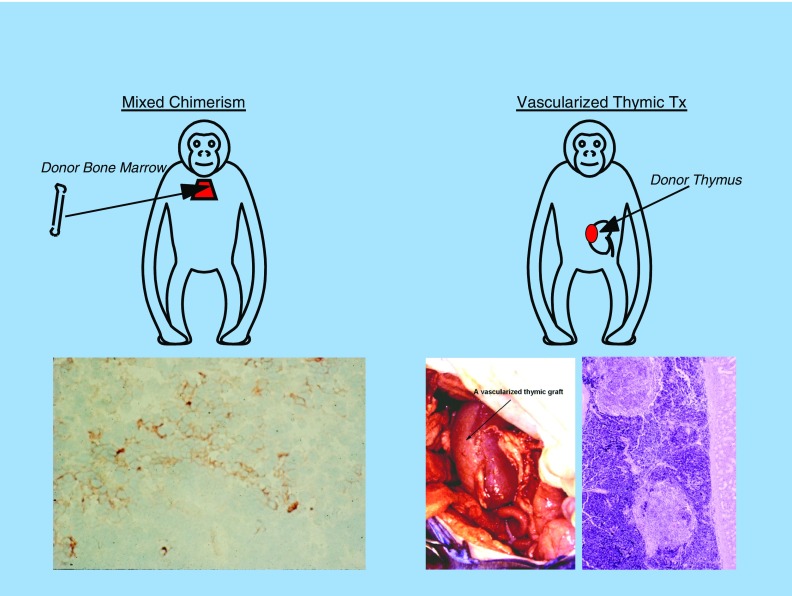
Indeed, as important as induction of tolerance may be for improving the quality of life of transplant patients receiving human organs, we believe that it will be even more important for the success of xenotransplantation, because so much more immunosuppression may be required to prevent rejection across this much stronger histocompatibility barrier. In our laboratory, we have explored two approaches to the induction of xenograft tolerance: (1) mixed chimerism and (2) vascularized thymic transplantation. As illustrated in Figure 4, both of these approaches are based on the premise that the thymus is the center of T cell–mediated transplantation immunity. Thus, in both approaches, mature T cells are eliminated before transplantation. In the case of mixed chimerism, new T cells develop in a host thymus in which both residual host antigen-presenting cells (APCs) and donor APCs, derived from the donor bone marrow inoculum, can participate in negative selection of newly developing T cells, leading to deletional tolerance. Likewise, in the case of thymic transplantation, the host thymus is removed and a vascularized donor thymus is transplanted at the same time as the xenograft organ. As seen in the illustration, the xenograft organ can actually be a composite “thymokidney,” a kidney prepared by transplanting thymic tissue from the donor under the donor animal’s own kidney capsule about 2 months before the xenotransplantation. In this way, both the kidney transplant and vascularized thymic transplant can be performed simultaneously (30). The thymus can also be transplanted as a separate, vascularized thymic lobe (31), which would permit a combined transplant with any other organ. In both cases, new T cells that develop will do so in a thymus in which both host APCs and donor APCs can participate in the negative selection process, leading to deletional tolerance.
Figure 4.
Two approaches to inducing transplantation tolerance. Both approaches involve T-cell depletion and the generation of new T cells in a thymus bearing both host and donor bone marrow–derived elements. Left panel, mixed chimerism (lower: histology) and right panel, vascularized thymic graft tx (lower left: gross appearance; lower right: histology). Tx = treatment.
Both of these approaches are still “work in progress,” but both have produced encouraging results. Until recently, the thymic transplantation approach was pursued most actively (31–33), largely because we had not been able to achieve mixed chimerism lasting more than a few minutes, even using GalT-KO donors. However, by using a new, transgenic GalT-KO donor, produced by introducing the human CD47 gene into our inbred GalT-KO line, we have achieved transient mixed chimerism lasting more than 8 days and allowing a porcine skin graft to survive on the recipient baboon for more than 60 days (34). By combining this technology with intrabone administration of donor bone marrow (35), we hope to extend the level and duration of chimerism. In addition, we are exploring new genetic engineering technologies to produce additional transgenic animals on our inbred GalT-KO background, designed to increase chimerism even further.
Conclusions
Transplantation tolerance has the potential to overcome both drug treatment–related complications and chronic rejection, which represent two of the three major limitations to the field of transplantation today. Induction of mixed lymphohematopoietic chimerism has been demonstrated to be an effective means for inducing such transplantation tolerance both in animal models and in more recent clinical trials. Tolerance alone, however, cannot overcome the shortage of donor organs, which represents the third major limitation to this field. Major strides toward this goal have been made through the use of genetic engineering of swine as potential donors of xenografts. The induction of tolerance to such xenografts is likely to play a major role in ensuring their eventual success.
Supplementary Material
Acknowledgments
Acknowledgment
The author thanks Rebecca Brophy for expert editorial assistance.
Footnotes
The work described in this article was supported by National Institutes of Health grants 5P01AI045897 and R37AI031046.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jacobsen N, Taaning E, Ladefoged J, Kristensen JK, Pedersen FK. Tolerance to an HLA-B,DR disparate kidney allograft after bone-marrow transplantation from same donor. Lancet. 1994;343:800. doi: 10.1016/s0140-6736(94)91881-3. [DOI] [PubMed] [Google Scholar]
- 2.Sayegh MH, Fine NA, Smith JL, Rennke HG, Milford EL, Tilney NL. Immunologic tolerance to renal allografts after bone marrow transplants from the same donors. Ann Intern Med. 1991;114:954–955. doi: 10.7326/0003-4819-114-11-954. [DOI] [PubMed] [Google Scholar]
- 3.Cosimi AB, Sachs DH. Mixed chimerism and transplantation tolerance. Transplantation. 2004;77:943–946. doi: 10.1097/01.tp.0000117779.23431.3f. [DOI] [PubMed] [Google Scholar]
- 4.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 5.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuchimoto Y, Huang CA, Yamada K, Shimizu A, Kitamura H, Colvin RB, Ferrara V, Murphy MC, Sykes M, White-Scharf M, et al. Mixed chimerism and tolerance without whole body irradiation in a large animal model. J Clin Invest. 2000;105:1779–1789. doi: 10.1172/JCI8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs DH. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 8.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreola G, Chittenden M, Shaffer J, Cosimi AB, Kawai T, Cotter P, Locascio SA, Morokata T, Dey BR, Tolkoff-Rubin NT, et al. Mechanisms of donor-specific tolerance in recipients of haploidentical combined bone marrow/kidney transplantation. Am J Transplant. 2011;11:1236–1247. doi: 10.1111/j.1600-6143.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, Sachs DH, Allan J, Madsen JC, Kawai T, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3:86ra51. doi: 10.1126/scitranslmed.3002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Sachs DH, Cosimi AB. Tolerance to vascularized organ allografts in large-animal models. Curr Opin Immunol. 1999;11:516–520. doi: 10.1016/s0952-7915(99)00009-6. [DOI] [PubMed] [Google Scholar]
- 12.Sachs DH, Kawai T, Sykes M. Induction of tolerance through mixed chimerism. Cold Spring Harb Perspect Med. 2014;4:a015529. doi: 10.1101/cshperspect.a015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoyama A, Tonsho M, Ng CY, Lee S, Millington T, Nadazdin O, Wain JC, Cosimi AB, Sachs DH, Smith RN, et al. Long-term lung transplantation in nonhuman primates. Am J Transplant. 2015;15:1415–1420. doi: 10.1111/ajt.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madariaga ML, Shanmugarajah K, Michel SG, Villani V, La Muraglia GM, II, Torabi R, Leonard DA, Randolph MA, Colvin RB, Yamada K, et al. Immunomodulatory strategies directed toward tolerance of vascularized composite allografts. Transplantation. 2015;99:1590–1597. doi: 10.1097/TP.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonsho M, Lee S, Aoyama A, Boskovic S, Nadazdin O, Capetta K, Smith RN, Colvin RB, Sachs DH, Cosimi AB, et al. Tolerance of lung allografts achieved in nonhuman primates via mixed hematopoietic chimerism. Am J Transplant. 2015;15:2231–2239. doi: 10.1111/ajt.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T, Poncelet A, Sachs DH, Mauiyyedi S, Boskovic S, Wee SL, Ko DS, Bartholomew A, Kimikawa M, Hong HZ, et al. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation. 1999;68:1767–1775. doi: 10.1097/00007890-199912150-00022. [DOI] [PubMed] [Google Scholar]
- 17.Bühler LH, Spitzer TR, Sykes M, Sachs DH, Delmonico FL, Tolkoff-Rubin N, Saidman SL, Sackstein R, McAfee S, Dey B, et al. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation. 2002;74:1405–1409. doi: 10.1097/00007890-200211270-00011. [DOI] [PubMed] [Google Scholar]
- 18.Spitzer TR, Delmonico F, Tolkoff-Rubin N, McAfee S, Sackstein R, Saidman S, Colby C, Sykes M, Sachs DH, Cosimi AB. Combined histocompatibility leukocyte antigen–matched donor bone marrow and renal transplantation for multiple myeloma with end stage renal disease: the induction of allograft tolerance through mixed lymphohematopoietic chimerism. Transplantation. 1999;68:480–484. doi: 10.1097/00007890-199908270-00006. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Sachs DH, Sprangers B, Spitzer TR, Saidman SL, Zorn E, Tolkoff-Rubin N, Preffer F, Crisalli K, Gao B, et al. Long-term results in recipients of combined HLA-mismatched kidney and bone marrow transplantation without maintenance immunosuppression. Am J Transplant. 2014;14:1599–1611. doi: 10.1111/ajt.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawai T, Sachs DH, Sykes M, Cosimi AB Immune Tolerance Network. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2013;368:1850–1852. doi: 10.1056/NEJMc1213779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–898. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- 22.Reemtsma K, McCracken BH, Schlegel JU, Pearl MA, Pearce CW, Dewitt CW, Smith PE, Hewitt RL, Flinner RL, Creech O., Jr Renal heterotransplantation in man. Ann Surg. 1964;160:384–410. doi: 10.1097/00000658-196409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartholomew AM, Powelson J, Sachs DH, Bailin M, Boskovic S, Colvin R, Hong HZ, Johnson M, Kimikawa M, LeGuern A, et al. Tolerance in a concordant nonhuman primate model. Transplantation. 1999;68:1708–1716. doi: 10.1097/00007890-199912150-00014. [DOI] [PubMed] [Google Scholar]
- 24.Good AH, Cooper DK, Malcolm AJ, Ippolito RM, Koren E, Neethling FA, Ye Y, Zuhdi N, Lamontagne LR. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24:559–562. [PubMed] [Google Scholar]
- 25.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 26.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 29.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O’Malley P, Nobori S, Vagefi PA, Patience C, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 30.Yamada K, Shimizu A, Ierino FL, Utsugi R, Barth RN, Esnaola N, Colvin RB, Sachs DH. Thymic transplantation in miniature swine. I. Development and function of the “thymokidney.”. Transplantation. 1999;68:1684–1692. doi: 10.1097/00007890-199912150-00011. [DOI] [PubMed] [Google Scholar]
- 31.Yamada K, Griesemer A, Sykes M, Sachs DH. Cotransplantation of vascularized thymus and kidney from GalT-KO pigs to baboons. Xenotransplantation. 2007;14:186–189. [Google Scholar]
- 32.Griesemer AD, Hirakata A, Shimizu A, Moran S, Tena A, Iwaki H, Ishikawa Y, Schule P, Arn JS, Robson SC, et al. Results of Gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009;9:2669–2678. doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekijima M, Waki S, Sahara H, Tasaki M, Wilkinson RA, Villani V, Shimatsu Y, Nakano K, Matsunari H, Nagashima H, et al. Results of life-supporting galactosyltransferase knockout kidneys in cynomolgus monkeys using two different sources of galactosyltransferase knockout swine. Transplantation. 2014;98:419–426. doi: 10.1097/TP.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tena AA, Sachs DH, Mallard C, Yang YG, Tasaki M, Farkash E, Rosales IA, Colvin RB, Leonard DA, Hawley RJ. Prolonged survival of pig skin on baboons after administration of pig cells expressing human CD47. Transplantation. 2017;101:316–321. doi: 10.1097/TP.0000000000001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tasaki M, Wamala I, Tena A, Villani V, Sekijima M, Pathiraja V, Wilkinson RA, Pratts S, Cormack T, Clayman E, et al. High incidence of xenogenic bone marrow engraftment in pig-to-baboon intra-bone bone marrow transplantation. Am J Transplant. 2015;15:974–983. doi: 10.1111/ajt.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.