Abstract
Mucosal tissues represent surfaces that are exposed to the outside world and provide a conduit for internal and external communication. Tissues such as the intestine and the lung are lined by layer(s) of epithelial cells that, when organized in three dimensions, provide a critical barrier to the flux of luminal contents. This selective barrier is provided through the regulated expression of junctional proteins and mucins. Tissue oxygen metabolism is central to the maintenance of homeostasis in the mucosa. In some organs (e.g., the colon), low baseline Po2 determines tissue metabolism and results in basal expression of the transcription factor, hypoxia-inducible factor (HIF), which is enhanced after ischemia/inflammation. Recent studies have indicated that HIF contributes fundamentally to the expression of barrier-related genes and in the regulation of barrier-adaptive responses within the mucosa. Here, we briefly review recent literature on the topic of hypoxia and HIF regulation of barrier in mucosal health and during disease.
Keywords: metabolism, cell–cell junction, inflammation, creatine, creatine kinase
Surfaces lined by epithelial cells provide a selective barrier between biologic surfaces, preventing the free mixing of luminal antigenic material with the underlying tissues. The maintenance of this selectively permeable barrier occurs through interactions of multiple transmembrane proteins found in select domains of the plasma membrane (e.g., adherens junctions [AJs] and tight junctions [TJs]) and between the epithelium and the extracellular matrix (1). These membrane domains define the three-dimensional structure of the tissue and establish the polarized protein and lipid organization within the plasma membrane (i.e., the “fence” function of the epithelium).
Mucosal tissues also maintain a rich and complex vasculature. The gastrointestinal tract, for example, adapts to profound fluctuations in blood perfusion on a daily basis (2). At baseline, epithelial cells lining the gastrointestinal mucosa experience Po2 levels approaching anoxia, a homeostatic state termed “physiologic hypoxia” (3). Analysis of oxygen exchange within the intestine has revealed that arterial blood–derived O2 diffuses across the villus to parallel venules, resulting in graded regions of significant hypoxia (4). In the colon, a gradient of O2 emanating from the submucosa toward the anaerobic lumen establishes one of the more complex microenvironments found in mammals (5). In these settings, even small perturbations in blood flow can result in relatively large decreases in O2 delivery with resultant ischemia/hypoxia. These changes in blood flow can be particularly profound during injury. The high-energy requirements of the mucosa, in combination with the role of the epithelium in maintaining homeostasis, has driven the evolution of a number of mechanisms to cope with this low oxygen state. Here, we discuss how the mucosa adapts to changes in blood flow in health and disease.
Oxygen Utilization in the Mucosa
A comparison of the various mucosal tissues reveals marked difference in oxygen distribution. The healthy lung alveolus, for example, supports a surface Po2 of 100–110 mm Hg (6). Conversely, the tip of villi in healthy colon exits at a Po2 of less than 10 mm Hg (5, 7). Such differences reflect a combination of O2 sources, the anatomy of blood flow, and the presence of large numbers of commensal microbes in the colon (8). Inflammatory lesions are profoundly hypoxic (or even anoxic), comparable to levels observed in some large tumors (7). Although there are multiple contributing factors (i.e., increased O2 consumption, vasoconstriction, edema) that result in decreased O2 delivery and resultant hypoxia (7), it was recently shown that a major component of deep-tissue hypoxia in active inflammation is derived from nicotinamide adenine dinucleotide phosphate oxidase–dependent O2 consumption by activated leukocytes, particularly neutrophils (9).
Numerous studies have shown that the transcription factor, hypoxia-inducible factor (HIF), regulates the expression of target genes that enable the epithelium to function as an effective barrier (10, 11). The two α isoforms of HIF, namely HIF-1 and HIF-2, represent period–aryl hydrocarbon receptor nuclear translocator–single-minded members of the basic helix-loop-helix family of transcription factors, and function as central regulators of tissue O2 metabolism (12). HIF stabilization in the cytoplasm depends on modifications to the O2-dependent degradation domain expressed on the HIF-1α and HIF-2α subunits with subsequent nuclear localization to form a functional complex with the common β subunit HIF-1β, also called the aryl hydrocarbon receptor nuclear translocator (13). When O2 supply is sufficient, O2- and iron-dependent hydroxylation of two proline motifs within the O2-dependent degradation domain of the α subunit of HIF initiates von Hippel-Lindau tumor suppressor protein–dependent ubiquitylation and degradation by the proteasome (14).
Although the majority of studies in the literature have focused on hypoxia as the key regulator of HIF activity, nonhypoxic HIF stabilizers have also been described. Inflammatory cytokines, such as IL-1 and tumor necrosis factor, have long been known to regulate the activity of HIF-1 in both normoxia and hypoxia (15). Such regulation can occur through increased HIF-1 mRNA, increased protein stability, and increased HIF-1 DNA binding (15). Parallel studies have shown that pathways upstream and downstream of HIF stabilization can impact HIF activity, including phosphoinositide-3-kinase (16), cullin-2 neddylation (17), nuclear factor-κB (18), and sestrin-2 (19). Likewise, microbial-derived products found within the mucosa, including butyrate (20) and siderophores (21, 22), can also stabilize HIF. It is notable that the activation of some intracellular pathways, such as nitric oxide synthase (23), can result in the redistribution of intracellular oxygen to the extent that HIF is stabilized. Thus, it remains to be determined to what extent the nonhypoxic pathways may be mediated by shifts in cellular metabolism that can be “sensed” as hypoxia by the proly hydroxylase enzymes.
HIF Targets of Barrier Regulation
A major function of the mucosa is the provision of a physical barrier between the inside and outside world. This barrier is tightly regulated in the healthy mucosa and barrier dysfunction is associated with a plethora of mucosal diseases. Here, we provide examples of barrier components regulated by HIF.
Mucin Expression and HIF
A number of mucosal surfaces extend the barrier apically through formation of a mucus layer. Goblet cells produce and secrete mucins that form mixture of glycoproteins at the epithelial surface that prevents the direct exposure of the mucosa to luminal contents. Depending on the tissue origin, goblet cells secrete up to 10 distinct surface-localized and gel-forming mucins (24), which, in the healthy mucosa, consist of an adherent mucus layer that is devoid of bacteria and a superficial layer that is many times the depth of the epithelium (25, 26). Hypoxia and HIF regulate several components of the mucus layer. For example, the promoter of mammalian mucin 5AC contains evolutionarily conserved regions proximal to the mRNA coding region that bind functional sma-mothers against decapentaplegic 4 and HIF-1α binding regions (27). In the colon, both mucin-3 and intestinal trefoil factor-3 are prominent HIF-1α target genes that function in concert to provide epithelial protection and to promote wound healing (28, 29).
Mucus also provides a reservoir for secreted epithelial factors, such as antimicrobial peptides (AMPs) (30). Defensins, for example, are a class of cysteine-rich AMPs that possess broad antimicrobial activity (31, 32). Human β defensin-1 (hBD1) is an example of an AMP secreted by the intestinal epithelium in a constitutive manner, as opposed to other defensin-like AMPs that are released only in response to inflammatory mediators (8). Homeostatic expression of hBD1 was demonstrated to depend on HIF-1α signaling in multiple cells, where hBD1 expression correlated with other HIF target genes in human tissues (33). A notable feature of hBD1 is that the full spectrum of its antimicrobial activity is most prominently revealed with reduced disulfide bonds (34). Thus, at multiple levels, hypoxia and HIF appear to provide important regulatory roles for the expression and function of the mucus layer.
Tight Junctions and HIF
TJs form the backbone to the structural integrity of the barrier and provide the physical basis for permeability to solutes and ions (35). TJs also prevent lipid diffusion between apical and basolateral membrane domains, the so-called “fence function” (35). The TJ is composed of both transmembrane and peripheral membrane proteins tightly linked to the actin-based cytoskeleton (36). The assembly of TJ structure and function within the membrane is regulated by a variety of physiological and pathophysiological stimuli (1). Hypoxia, for example, dramatically influences the integrity of TJ, and can result in loss of barrier function. These results have been observed using a number of approaches, including chemical depletion of ATP (37) and in vitro hypoxia (28, 38, 39).
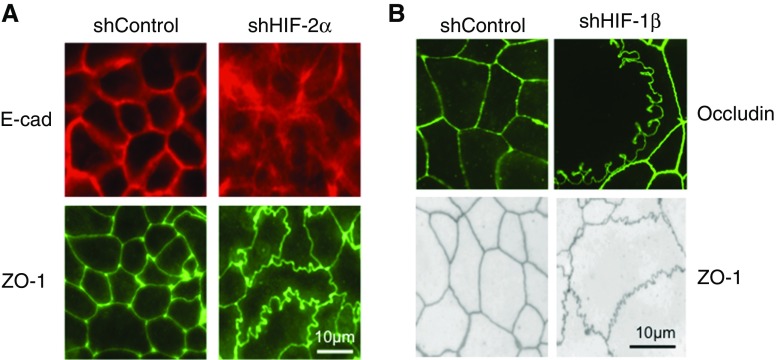
Claudins are a large family of tetraspannin integral membrane proteins that function to provide the selective permeability of TJs (35). Functionally, claudins are categorized as “tight” or “leaky” with regard to their influence on barrier properties (40). Claudin-1 (CLDN1) is a “tight” claudin that has been shown to be dysregulated in a variety of human diseases, including inflammatory bowel disease (35). In a screen of TJ targets, CLDN1 was identified to explain an aberrant junctional morphology of HIF deficient intestinal epithelial cell lines (41) (see Figure 1). Using loss- and gain-of-function approaches, this work showed that HIF signaling maintains CLDN1 expression through binding HIF-responsive element sequences in the gene promoter. The reintroduction of CLDN1 expression in HIF-deficient epithelial cells restored barrier function and reversed the morphologic abnormalities. Furthermore, in vivo analysis revealed an importance for HIF-mediated CLDN1 expression during mucosal insult. These results identify a critical link between HIF and TJ structure/function, providing important insight into mechanisms of HIF-regulated epithelial homeostasis (41).
Figure 1.
Epithelial junctional defects in cells lacking hypoxia-inducible factor (HIF) expression. (A) The localization of the adherens junction protein E-cadherin (E-cad) and the tight junction (TJ) protein zonula occludens (ZO)-1 in epithelial cells deficient in HIF-2α. Cells were depleted of HIF-2α by transduction of lentiviral short hairpin RNA. Shown here is a nontargeting control (shControl) and shHIF-2α. Note defective expression of both E-cad and ZO-1 in shHIF-2α cells. (B) Defective expression of the TJ proteins occludin and ZO-1 in cells lacking the HIF-1 and HIF-2 dimeric partner, HIF-1β. Adapted from References 41 and 47. sh = short hairpin.
Adherens Junctions and HIF
Polarization and intercellular junctions depend, in large part, on cell–cell contact mediated by cadherin–catenin interactions and, subsequent, assembly of the AJ (42). Evidence from both ATP depletion models and ischemia described the dissolution of AJ complexes (43), likely initiated by the hyperphosphorylation of the catenins (44). Because the AJ plays a crucial role for the establishment and maintenance of polarized epithelia, these changes constitute a critical lesion in epithelial hypoxia (45). During restitution, epithelia depend on reassembly and reformation of the AJ proteins. Within the AJ, β-catenin signaling through T cell factor/lymphoid enhancer factor transcription factors regulates barrier restitution during active mucosal inflammation (46). These studies in the lung revealed that neutrophil migration across epithelial monolayers elicits an epithelial gene programming that results in activated β-catenin signaling and the restitution of epithelial barrier function (46).
Original studies using ATP depletion models revealed an important role for high-energy phosphates in the regulation of barrier function (47). These studies have prompted further analysis of hypoxia adaptation to such conditions. Notable is the observation that cytosolic creatine (Cr) kinase genes are HIF-2–selective targets expressed within the AJ of confluent intestinal epithelia. Studies in the mucosa revealed that each of the Cr kinase subunits (i.e., muscle, brain, and mitochondrial) is expressed in cultured intestinal epithelial cell lines, murine colonic epithelia, and in human colonic epithelia (47). During high–energy demand states, Cr and phospho-Cr levels are regulated to near equilibrium, providing a buffering capacity for ATP and ADP, allowing for the proper functioning of a numerous of cellular ATPases (48). Active inflammation represents a high-energy state accounted for by functions such as cell migration, proliferation, and the restitution of epithelial cells after insult. Under such conditions, energy expenditure at epithelial cell–cell junctions is tightly linked to the circumferential F-actin belt (36). Thus, it would appear that Cr:phospho-Cr ratios may serve as functional biomarkers of cellular energy demand that could be targetable in ways to promote tissue barrier function and epithelial wound healing in the mucosa.
Conclusions
Epithelial cells that line mucosal surface normally function in diverse and often harsh conditions. A major function of the epithelium is the provision of a barrier as a selectively permeable membrane that prevents the free mixing of luminal and serosal constituents. The stark differences in tissue O2 tension between mucosal tissues (e.g., compare oxygenation profiles of lung and colon) and the potential shifts in energy requirements during injury have unveiled important lessons about tissue metabolism in health and disease. In particular, HIF-target pathways have revealed potential targets with the tissue barrier that could serve as templates for new therapies. A more precise understanding of the gene targets and functional components of the HIF pathway provides ample opportunities for the development of therapies directed at promoting mucosal wound healing.
Supplementary Material
Footnotes
Supported by National Institutes of Health grants DK50189, DK104713, DK095491, and DK103712, grant BX002182 from the Veterans Administration, and the Crohn’s and Colitis Foundation of America.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Koch S, Nusrat A. The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol. 2012;7:35–60. doi: 10.1146/annurev-pathol-011811-120905. [DOI] [PubMed] [Google Scholar]
- 2.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karhausen J, Haase VH, Colgan SP. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle. 2005;4:256–258. [PubMed] [Google Scholar]
- 4.Shepherd AP. Metabolic control of intestinal oxygenation and blood flow. Fed Proc. 1982;41:2084–2089. [PubMed] [Google Scholar]
- 5.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055–1063.e8. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schaible B, Schaffer K, Taylor CT. Hypoxia, innate immunity and infection in the lung. Respir Physiol Neurobiol. 2010;174:235–243. doi: 10.1016/j.resp.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng L, Kelly CJ, Colgan SP. Physiologic hypoxia and oxygen homeostasis in the healthy intestine: a review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309:C350–C360. doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colgan SP, Curtis VF, Lanis JM, Glover LE. Metabolic regulation of intestinal epithelial barrier during inflammation. Tissue Barriers. 2015;3:e970936. doi: 10.4161/21688362.2014.970936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colgan SP, Campbell EL, Kominsky DJ. Hypoxia and mucosal inflammation. Annu Rev Pathol. 2016;11:77–100. doi: 10.1146/annurev-pathol-012615-044231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb Symp Quant Biol. 2011;76:347–353. doi: 10.1101/sqb.2011.76.010678. [DOI] [PubMed] [Google Scholar]
- 13.Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 14.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19:4298–4309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellwig-Bürgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94:1561–1567. [PubMed] [Google Scholar]
- 16.Stiehl DP, Jelkmann W, Wenger RH, Hellwig-Bürgel T. Normoxic induction of the hypoxia-inducible factor 1alpha by insulin and interleukin-1beta involves the phosphatidylinositol 3-kinase pathway. FEBS Lett. 2002;512:157–162. doi: 10.1016/s0014-5793(02)02247-0. [DOI] [PubMed] [Google Scholar]
- 17.Curtis VF, Ehrentraut SF, Campbell EL, Glover LE, Bayless A, Kelly CJ, Kominsky DJ, Colgan SP. Stabilization of HIF through inhibition of Cullin-2 neddylation is protective in mucosal inflammatory responses. FASEB J. 2015;29:208–215. doi: 10.1096/fj.14-259663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta–mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 19.Olson N, Hristova M, Heintz NH, Lounsbury KM, van der Vliet A. Activation of hypoxia-inducible factor-1 protects airway epithelium against oxidant-induced barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2011;301:L993–L1002. doi: 10.1152/ajplung.00250.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmann H, Eltzschig HK, Wurz H, Hantke K, Rakin A, Yazdi AS, Matteoli G, Bohn E, Autenrieth IB, Karhausen J, et al. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology. 2008;134:756–767. doi: 10.1053/j.gastro.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Holden VI, Lenio S, Kuick R, Ramakrishnan SK, Shah YM, Bachman MA. Bacterial siderophores that evade or overwhelm lipocalin 2 induce hypoxia inducible factor 1α and proinflammatory cytokine secretion in cultured respiratory epithelial cells. Infect Immun. 2014;82:3826–3836. doi: 10.1128/IAI.01849-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- 24.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 26.Strugala V, Allen A, Dettmar PW, Pearson JP. Colonic mucin: methods of measuring mucus thickness. Proc Nutr Soc. 2003;62:237–243. doi: 10.1079/pns2002205. [DOI] [PubMed] [Google Scholar]
- 27.Young HW, Williams OW, Chandra D, Bellinghausen LK, Pérez G, Suárez A, Tuvim MJ, Roy MG, Alexander SN, Moghaddam SJ, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1–dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis NA, Hamilton KE, Canny G, Shekels LL, Ho SB, Colgan SP. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem. 2006;99:1616–1627. doi: 10.1002/jcb.20947. [DOI] [PubMed] [Google Scholar]
- 30.Antoni L, Nuding S, Weller D, Gersemann M, Ott G, Wehkamp J, Stange EF. Human colonic mucus is a reservoir for antimicrobial peptides. J Crohn’s Colitis. 2013;7:e652–e664. doi: 10.1016/j.crohns.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 32.Pazgier M, Hoover DM, Yang D, Lu W, Lubkowski J. Human beta-defensins. Cell Mol Life Sci. 2006;63:1294–1313. doi: 10.1007/s00018-005-5540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly CJ, Glover LE, Campbell EL, Kominsky DJ, Ehrentraut SF, Bowers BE, Bayless AJ, Saeedi BJ, Colgan SP. Fundamental role for HIF-1α in constitutive expression of human β defensin-1. Mucosal Immunol. 2013;6:1110–1118. doi: 10.1038/mi.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schroeder BO, Wu Z, Nuding S, Groscurth S, Marcinowski M, Beisner J, Buchner J, Schaller M, Stange EF, Wehkamp J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature. 2011;469:419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 35.Capaldo CT, Nusrat A. Claudin switching: physiological plasticity of the tight junction. Semin Cell Dev Biol. 2015;42:22–29. doi: 10.1016/j.semcdb.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol. 1999;276:F737–F750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- 38.Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of intestinal epithelial permeability induced by hypoxia: role for basolateral release of tumor necrosis factor alpha. Gastroenterology. 1998;114:657–668. doi: 10.1016/s0016-5085(98)70579-7. [DOI] [PubMed] [Google Scholar]
- 39.Friedman GB, Taylor CT, Parkos CA, Colgan SP. Epithelial permeability induced by neutrophil transmigration is potentiated by hypoxia: role of intracellular cAMP. J Cell Physiol. 1998;176:76–84. doi: 10.1002/(SICI)1097-4652(199807)176:1<76::AID-JCP9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 40.Anderson JM, Van Itallie CM, Fanning AS. Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol. 2004;16:140–145. doi: 10.1016/j.ceb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Saeedi BJ, Kao DJ, Kitzenberg DA, Dobrinskikh E, Schwisow KD, Masterson JC, Kendrick AA, Kelly CJ, Bayless AJ, Kominsky DJ, et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol Biol Cell. 2015;26:2252–2262. doi: 10.1091/mbc.E14-07-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhatt T, Rizvi A, Batta SP, Kataria S, Jamora C. Signaling and mechanical roles of E-cadherin. Cell Commun Adhes. 2013;20:189–199. doi: 10.3109/15419061.2013.854778. [DOI] [PubMed] [Google Scholar]
- 43.Bush KT, Keller SH, Nigam SK. Genesis and reversal of the ischemic phenotype in epithelial cells. J Clin Invest. 2000;106:621–626. doi: 10.1172/JCI10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz JH, Shih T, Menza SA, Lieberthal W. ATP depletion increases tyrosine phosphorylation of beta-catenin and plakoglobin in renal tubular cells. J Am Soc Nephrol. 1999;10:2297–2305. doi: 10.1681/ASN.V10112297. [DOI] [PubMed] [Google Scholar]
- 45.Fish EM, Molitoris BA. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med. 1994;330:1580–1588. doi: 10.1056/NEJM199406023302207. [DOI] [PubMed] [Google Scholar]
- 46.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, Suzuki T, Yang IV, De Langhe S, Reynolds SD, Mason RJ, et al. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci U S A. 2011;108:15990–15995. doi: 10.1073/pnas.1110144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glover LE, Bowers BE, Saeedi B, Ehrentraut SF, Campbell EL, Bayless AJ, Dobrinskikh E, Kendrick AA, Kelly CJ, Burgess A, et al. Control of creatine metabolism by HIF is an endogenous mechanism of barrier regulation in colitis. Proc Natl Acad Sci U S A. 2013;110:19820–19825. doi: 10.1073/pnas.1302840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.