Abstract
The regulation of energy balance involves complex processes in the brain, including coordination by hypothalamic neurons that contain pro-opiomelanocortin (POMC). We previously demonstrated that central bone morphogenetic protein (BMP) 7 reduced appetite. Now we show that a type 1 BMP receptor, BMPR1A, is colocalized with POMC neurons and that POMC-BMPR1A-knockout (KO) mice are hyperphagic, revealing physiological involvement of BMP signaling in anorectic POMC neurons in the regulation of appetite. Surprisingly, the hyperphagic POMC-BMPR1A-KO mice exhibited a lack of obesity, even on a 45% high-fat diet. This is because the brown adipose tissue (BAT) of KO animals exhibited increased sympathetic activation and greater thermogenic capacity owing to a reestablishment of energy balance, most likely stemming from a compensatory increase of BMPR1A in the whole hypothalamus of KO mice. Indeed, control animals given central BMP7 displayed increased energy expenditure and a specific increase in sympathetic nerve activity (SNA) in BAT. In these animals, pharmacological blockade of BMPR1A-SMAD signaling blunted the ability of BMP7 to increase energy expenditure or BAT SNA. Together, we demonstrated an important role for hypothalamic BMP signaling in the regulation of energy balance, including BMPR1A-mediated appetite regulation in POMC neurons as well as hypothalamic BMP-SMAD regulation of the sympathetic drive to BAT for thermogenesis.
BMP signaling is important for the hypothalamic regulation of energy balance, and we observed an intriguing reestablishment of energy balance with loss of POMC neuron BMPR1A despite hyperphagia.
An imbalance between energy intake and energy expenditure results in obesity. Energy intake is controlled by appetite-regulatory pathways in the central nervous system (CNS), including homeostatic regulation of food intake by the hypothalamic melanocortin pathway. Pro-opiomelanocortin (POMC) neurons in the hypothalamus are the main anorectic, or appetite-reducing, neurons of the hypothalamic arcuate nucleus (ARC). POMC gene products include α-melanocyte-stimulating hormone (αMSH), which binds melanocortin receptors (such as MC4R) to reduce appetite and increase energy expenditure in response to signals that activate POMC neurons, such as leptin (1, 2). Other hypothalamic and nonhypothalamic CNS pathways are also involved in the coordination of energy balance, but the POMC-αMSH-MC4R (melanocortin) pathway is believed to be the main anorectic pathway regulating homeostatic appetite control in the hypothalamus.
The bone morphogenetic proteins (BMPs) are members of the transforming growth factor β superfamily of developmental regulators and are involved in many aspects of morphogenesis and growth. Recently, BMP and transforming growth factor β pathways have also been implicated in the regulation of metabolism (3–9). Polymorphisms of BMP ligand and receptors, including the type 1 BMP receptor (BMPR)1A, have been associated with human obesity (7, 10). BMPs and BMP receptors are involved in CNS development (11, 12); however, in the adult brain their expression is more restricted. BMP receptors signal through a canonical SMAD1/5/8 pathway and commonly through the p38-MAPK pathway; however, additional BMP signaling pathways have been described, such as STAT3, mTOR-p70S6K, and JNK (5).
We and others have previously shown that BMP7 is able to induce brown adipogenesis of progenitor and uncommitted precursor cells, increase whole-body energy expenditure, and reverse obesity (8, 13–17). We have further demonstrated that central delivery of BMP7 is able to reduce appetite, mainly through the rapamycin-sensitive mTOR-p70S6K signaling pathway (14).
The complex mechanisms by which CNS pathways regulate the control of energy balance are just beginning to become clear. For example, given the surprising finding that the deletion of leptin receptors from POMC neurons had no effect on food intake (18), we now know that POMC neurons represent a heterogeneous population in terms of response to leptin and insulin, as well as utilization of serotonergic signals (19–21). Therefore, identifying novel signaling pathways acting in subsets of POMC neurons that affect appetite is an important goal.
Here, we demonstrated coexpression of the type 1 BMP receptor BMPR1A in a subset of POMC neurons in the hypothalamus. Given the involvement of BMPR1A in human obesity (7, 10) and the known ability of intracerebroventricular (i.c.v.) BMP7 to reduce food intake in mice (14), we predicted that POMC BMPR1A signaling is responsible for the anorectic effects of central BMP7. Unexpectedly, although deletion of BMPR1A in POMC neurons [the POMC-BMPR1A-knockout (KO)] resulted in the expected hyperphagic phenotype, it did not lead to obesity even on a 45% high-fat diet (HFD). We found that this surprising reestablishment of homeostatic energy balance was most likely due to a compensatory upregulation of BMPR1A in non-POMC neurons of the hypothalamus as a whole in KO animals. Furthermore, we identified a mechanism by which hypothalamic BMP signaling was able to increase energy expenditure by driving increased sympathetic activation of brown adipose tissue (BAT). These studies establish the ablation of a BMPR family member in a distinct region of the mouse brain and provide information about the involvement of BMP signaling in the hypothalamus, which regulates both arms of energy balance.
Materials and Methods
Mice and metabolic phenotyping
POMC-Cre mice (catalog no. 005965; The Jackson Laboratory, Bar Harbor, ME) were mated to BMPR1A-flox mice, a kind gift of Prof. Yuji Mishina at the University of Michigan (22). These mice are on a mixed genetic background, and Lox littermates were used as control animals for all studies. All studies included six to eight mice per group unless otherwise indicated, and mice were around 10 to 16 weeks old at the time of data collection. Early data indicated no differences between males and females, so males were used for this publication. POMC-GFP and Rosa-YFP reporter mice were also obtained from The Jackson Laboratory for use in a subset of experiments. Mice were fed either a chow diet or a commercial HFD from Research Diets (45% or 60% of kilocalories from fat; New Brunswick, NJ). Metabolic analyses were conducted in a Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) and by dual-energy x-ray absorptiometry (DEXA) analysis to determine lean and fat mass composition, as described previously (14). CLAMS and DEXA as well as blood pressure and heart rate were measured in the Joslin Diabetes Research Center (DRC) Physiology Core (additional details in the Supplemental Materials (49.5KB, doc) ). Body temperature was measured by a rectal probe (Sable Systems, Las Vegas, NV) during the cold-exposure study, which was conducted in a cold room at 4°C. Serum thyroid hormone (triiodothyronine; T3) level was measured by enzyme-linked immunosorbent assay in the DRC Assay Core at Joslin. All animal work for this manuscript was approved by the Institutional Animal Care and Use Committees at Joslin Diabetes Center and the University of Maine and was conducted in accordance with accepted standards of humane animal care.
BAT denervation
Surgical denervation was conducted as described previously (13, 23), using a method that surgically denervates all neuronal input to the BAT pad while leaving vascular input intact. Under isoflurane anesthetic, the bilateral nerve bundles entering the rostral portion of the interscapular BAT were cut using a dissecting microscope to remove a 1-cm section, thereby reducing the potential for nerve regrowth. Skin incisions were closed using VetBond surgical glue from 3M. Food intake was monitored after recovery from surgery for a period of 2 weeks.
Immunostaining, western blotting, and assessment of lipid droplet size
Immunostaining (utilizing transcardial perfusion-fixed brains with Amresco fixative) and western blotting were conducted as described previously (14). Briefly, for immunofluorescence, autofluorescence was blocked by Sudan black, and antibodies were first tested on western blot to determine specificity. Immunostaining images were obtained on a confocal microscope to confirm colocalization in a single cell. No primary and no antibody controls were run for all experiments. Antibodies for immunostaining or western blots included BMPR1A (catalog no. 38-6000; Invitrogen), tyrosine hydroxylase (TH; catalog no. MAB318; Millipore), and phospho-p70S6K (catalog no. 9204; Cell Signaling Technology). Lipid droplet size was analyzed by perilipin staining of lipid droplets (catalog no. P1873; Sigma). Immunostaining, western blots, and quantification of droplet size were all done using ImageJ software [National Institutes of Health (NIH); Bethesda, MD]. β-tubulin loading control antibody was also from Cell Signaling Technology (catalog no. 2146) (Table 1).
Table 1.
Antibodies Used
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| BMPR1A | Invitrogen, 38-6000 | Rabbit; polyclonal | 1:50 (IF) | AB_2533377 | ||
| Tyrosine hydroxylase | Millipore, MAB318 | Mouse; monoclonal | 1:1000 (WB) | AB_2201528 | ||
| β-Tubulin | Cell Signaling Technology, 2146 | Rabbit; polyclonal | 1:1000 (WB) | AB_2210545 | ||
| Perilipin | Sigma, P1873 | Mouse; polyclonal | 3 μg/mL | AB_532267 | ||
| Phospho-p70S6K | Phospho-p70 S6 Kinase (Thr421/Ser424) | Cell Signaling Technology, 9204 | Rabbit; polyclonal | 1:1000 (WB) | AB_2265913 |
Abbreviation: IF, immunofluorescence; RRID, Research Resource Identifier; WB, western blot.
Measurement of BAT mitochondrial activity
Small, uniformly sized pieces of BAT were excised using a biopsy punch and immediately placed in specially designed “islet plates” in unbuffered media for analysis of basal respiration in a Seahorse Bioanalyzer (Seahorse Bioscience, North Billerica, MA) that measured oxygen consumption rate (pmol/min). Data were not normalized. Up to 5 punches were run for at least two mice in each group, and nonrespiring/dead tissue punches were discarded from calculations for both groups.
Statistical analysis
All plots represent mean ± standard error of the mean. Statistical calculations were carried out in Excel or StatView programs, utilizing the analysis of variance or Student t test as indications of significance. For all figures, *P < 0.05, **P < 0.01, and ***P < 0.001. CLAMS waveform statistical analysis is described in the Physiology section.
Other methods can be found in the Supplemental Materials (49.5KB, doc) .
Results
BMPR1A was expressed in hypothalamic POMC neurons and acted there to regulate appetite
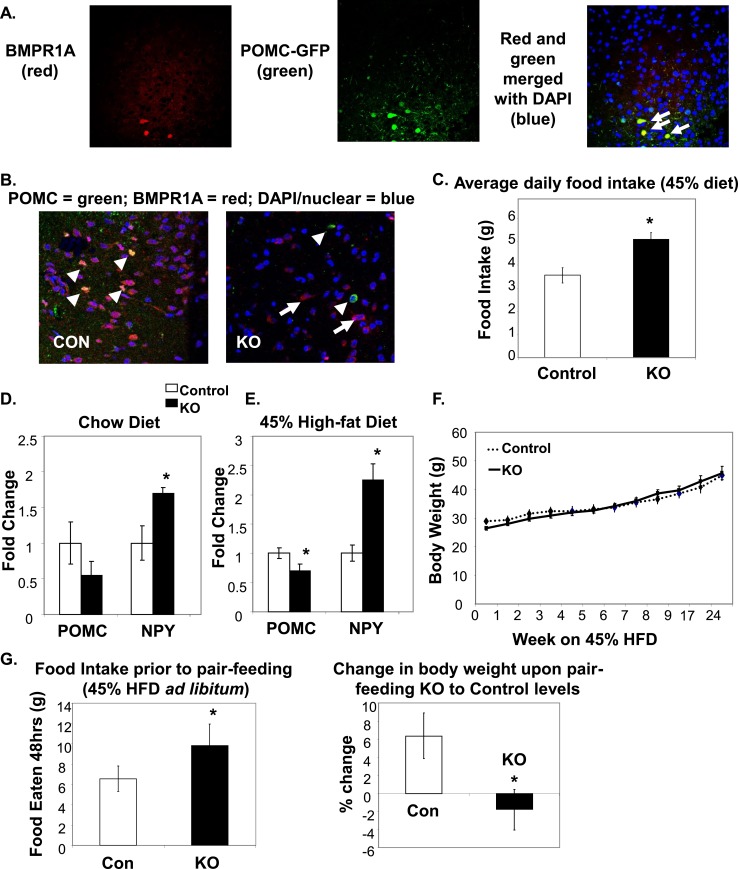
Our previous findings demonstrated that central i.c.v. delivery of BMP7 was able to reduce food intake as well as increase hypothalamic expression of POMC and αMSH (14). Therefore, we sought to determine whether BMP receptors are expressed in POMC neurons, which may mediate the anorectic effects of activating central BMP signaling. By utilizing immunofluorescent colocalization of BMPR1A in a POMC-GFP reporter mouse, we found that BMPR1A was expressed in a subpopulation of hypothalamic POMC neurons in the ARC (Fig. 1A). To determine the role of BMPR1A in POMC neurons, we specifically knocked out BMPR1A by mating POMC-Cre mice with BMPR1A-floxed mice, hereafter designated POMC/BMPR1A-KO. (Supplemental Fig. 1A (500KB, ppt) ). The ablation of BMPR1A in POMC neurons was also confirmed in triple transgenic mice with the Rosa26-YFP reporter, where colocalization of BMPR1A with POMC-Rosa26-YFP neurons was observed only in the Lox/control mice and not in the KO mice (Fig. 1B). This wasnot due to a reduction in POMC neuron number, as may have occurred during development (Supplemental Fig. 1B (500KB, ppt) ), indicating that BMPR1A is not necessarily required for POMC neuron development or survival. It was interesting that the density of BMPR1A-positive cells in the arcuate appeared slightly different between the wild-type C57BL/6 animals in Fig. 1A and the mixed-genetic background Cre-Lox animals in Fig. 1B, which may be due to differences caused by mouse genetics.
Figure 1.
BMPR1A in hypothalamic POMC neurons regulated food intake. (A) Immunofluorescent colocalization shows that POMC neurons in the ARC region of a C57BL/6 mouse hypothalamus were positive for BMPR1A [arrows in far right panel (merged image) point to yellow cells that colocalize]. Red staining indicates BMPR1A (left panel, Invitrogen; catalog no. 38-6000), and green staining indicates GFP-positive neurons in a POMC-GFP reporter mouse (middle panel). Far right panel shows merged staining with a nuclear stain (DAPI/blue). Images were taken on a confocal microscope at ×60 magnification. (B) The hypothalamic colocalization of BMPR1A in POMC neurons (seen in control mice in the left panel, where arrowheads indicate costaining of red BMPR1A with green POMC) is no longer seen in POMC/BMPR1A-KO mice (right panel, where arrowheads indicate green POMC neurons and full arrows indicate remaining red BMPR1A neurons). Images were taken on a confocal microscope at ×60 magnification. (C) Daily food intake measurements of mice on a 45% HFD, showing KO mice were hyperphagic (N = 6 to 8 mice per group). (D and E) POMC/BMPR1A-KO mice displayed increased orexigenic NPY and decreased anorexigenic POMC (D) on chow and (E) on a 45% HFD (N = 6 to 8 mice per group). (F) Despite hyperphagia, POMC/BMPR1A-KO mice did not show a difference in body weight on a 45% HFD, as measured up to 24 weeks (N = 6 to 8 mice per group). (G) A pair-feeding study showed that although KO mice were hyperphagic when fed ad libitum 45% HFD before pair-feeding (left panel), they lost weight when pair-fed to the levels of control mouse food intake (right panel; % change in body weight since start of pair-feeding) (N = 6 mice per group). *Indicates significance where P < 0.05. Error bars represent standard error of the mean. Con, control; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; NPY, neuropeptide Y.
As expected, given the role of centrally delivered BMP7 to reduce food intake (14), mice with POMC-neuron deletion of BMPR1A were hyperphagic on a 45% HFD (containing 45% calories from fat) (Fig. 1C). On a chow diet, the KO mice displayed a trend for hyperphagia, although it did not reach statistical significance (Supplemental Fig. 1D (500KB, ppt) ).
Accordingly, POMC/BMPR1A-KO mice displayed increased messenger RNA (mRNA) expression of orexigenic neuropeptide Y (Fig. 1D and 1E) and decreased anorexigenic POMC mRNA expression in the hypothalamus of mice in both the chow-fed and 45% HFD state compared with their littermate controls (Fig. 1D and 1E). Surprisingly, despite the hyperphagic phenotype of the POMC/BMPR1A-KO mice on a 45% HFD (Fig. 1C), there were no differences in body weight compared with control mice as measured up to 24 weeks on the HFD (Fig. 1F). Upon pair-feeding, where POMC/BMPR1A-KO mice were fed the same amount of food as control mice over a 4-week period, the KO mice displayed a reduction in weight gain (Fig. 1G), indicating a potential contribution of increased energy expenditure to their metabolic phenotype.
Hyperphagic KO mice on an HFD did not increase body weight because of increased energy expenditure and BAT thermogenesis
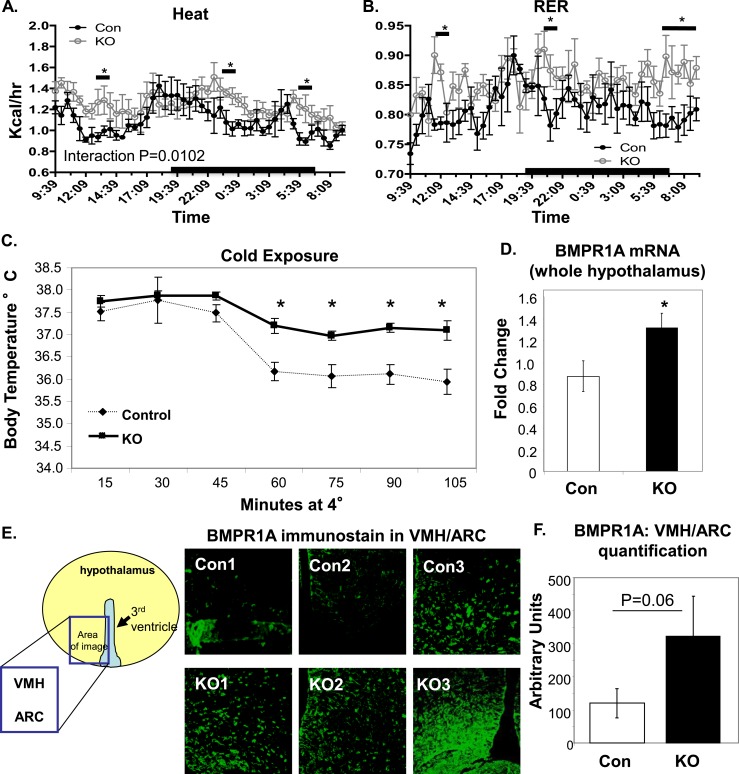
In accordance with the pair-feeding data, when the mice were metabolically phenotyped in a CLAMS, it was revealed that POMC/BMPR1A-KO mice exhibited a significant increase in energy expenditure, calculated as heat [from volume of oxygen consumed (Vo2) volume of carbon dioxide emitted (Vco2) measurements] (Fig. 2A). This was not accompanied by any significant differences in respiratory exchange ratio (RER; a quotient of Vco2/Vo2 that indicates fuel utilization in which values closer to 1 indicate more utilization of carbohydrate and values closer to 1 indicate greater utilization of fats as fuel) (Fig. 2B), although transient periods of increased RER indicate the KO mice may have used more carbohydrates as fuel. When exposed to an acute cold challenge, POMC/BMPR1A-KO mice were better able to maintain their body temperature (Fig. 2C), indicating greater thermogenic activity in the BAT of KO mice. Other parameters that influence energy balance, such as intestinal nutrient absorption and physical activity, were not significantly altered in the KO mice (Supplemental Fig. 1E and 1F (500KB, ppt) ). In addition, there were no differences in lean or adipose mass (measured by DEXA scan; Supplemental Fig. 1G and 1H (500KB, ppt) ) between control and KO mice. Similarly, blood pressure and heart rate were not altered in the KO animals (Supplemental Fig. 2A and 2B (274KB, ppt) ). These data revealed that the POMC/BMPR1A-KO mice displayed an overall upward shift in energy balance, in which increased energy intake caused by hyperphagia was counteracted by elevated energy expenditure.
Figure 2.
Increased energy expenditure in POMC/BMPR1A-KO mice. (A and B) POMC/BMPR1A-KO mice had higher energy expenditure as measured in CLAMS while on a 45% HFD, including (A) transiently increased heat production (calculated from Vo2 and Vco2) and (B) RER. Interaction statistics gave a P value for heat of 0.0102 (significant), and for RER, it was 0.0888 (not significant). Mice undergoing CLAMS analysis were at the same body weight, but data were normalized to lean body mass as measured by DEXA (see Supplemental Material (49.5KB, doc) ), which was also unchanged. N = 6 mice per group; data presented as wave-forms (see Supplemental Material (49.5KB, doc) ). (C) During cold exposure after a 45% HFD, POMC/BMPR1A-KO mice were better able to regulate their body temperature than control mice, as measured by rectal temperature taken every 15 minutes at 4°C (N = 6 to 8 mice per group). (D−F) Despite POMC-neuron ablation, POMC/BMPR1A-KO mice expressed more total hypothalamic BMPR1A, as measured at the (D) mRNA and (E and F) protein levels. BMPR1A immunostaining in (E) indicates greater BMPR1A expression in multiple hypothalamic regions, including the ventromedial hypothalamus (VMH) and ARC. Fluorescent BMPR1A signal from (E) is quantified in (F) from three animals. Despite interindividual variation, there was a trend for increased BMPR1A in POMC/BMPR1A-KO hypothalami of all hypothalamic regions analyzed. The cartoons demonstrate where in the hypothalamus the confocal photomicrographs were taken. All animals were on a 45% HFD. *Indicates significance where P < 0.05. Error bars represent standard error of the mean. Con, control; RER, respiratory exchange ratio.
The increase in energy expenditure as measured in the CLAMS system was also observed in POMC/BMPR1A-KO mice fed a higher-calorie 60% HFD, as well as transient significant increases in heat and RER during the onset of the dark phase (Supplemental Fig. 2C (274KB, ppt) ). The mice on the 60% HFD were also hyperphagic, as observed on the 45% HFD (Supplemental Fig. 2D (274KB, ppt) ), but the increased energy expenditure was not sufficient to overcome the caloric density of the 60% HFD; in addition, the POMC/BMPR1A-KO mice did display greater weight gain on this diet, as originally hypothesized for this mouse model (Supplemental Fig. 2E (274KB, ppt) ). This was accompanied by an increase in white adipose tissue mass (Supplemental Fig. 2F (274KB, ppt) ).
The increased energy expenditure in POMC/BMPR1A-KO mice was surprising given the known ability of BMP7 ligand to increase energy expenditure via systemic actions in the periphery and by directly targeting energy-dissipating BAT (8, 14, 15). Interestingly, it was observed that although we obtained a POMC-neuron KO of BMPR1A as evidenced by the Cre recombination (Supplemental Fig. 1A (500KB, ppt) ) and immunostaining (Fig. 1B), there was also a compensatory increase of BMPR1A expression in the hypothalamus as a whole when measured at the mRNA (Fig. 2D) and protein (Fig. 2E and 2F; Supplemental Fig. 3A (327.5KB, ppt) ) levels across several separate cohorts. Immunostaining revealed an increase in BMPR1A expression in non-POMC neurons (Fig. 2F), including areas known to modulate sympathetic outflow to BAT, such as the ventromedial hypothalamus and ARC (24), which may have contributed to the observed increase in energy expenditure.
In total, at least five separate cohorts displayed increased BMPR1A expression in the hypothalamus as a whole, but this compensatory increase of BMPR1A was not seen in the brainstem, another location of POMC neurons (Supplemental Fig. 3B (327.5KB, ppt) ), and there were also no changes in another type 1 receptor (BMPR1B) as measured by quantitative polymerase chain reaction in the whole hypothalamus or brainstem (Supplemental Fig. 3C (327.5KB, ppt) ). Both BMP7 and BMP8b ligands have been shown to affect CNS energy balance (6, 14). Although levels of BMP8b mRNA were unchanged in the hypothalamus and brainstem, there was a significant increase of BMP7 in the brainstem only (Supplemental Fig. 3D and 3E (327.5KB, ppt) ), suggesting another potential level of compensation in this model. In addition, canonical BMP-SMAD signaling pathway genes (SMAD1, 5, 8/9, and co-SMAD 4) were unchanged in the arcuate of KO mice, and the BMP-SMAD transcriptional target gene Id1 was also unchanged (Supplemental Fig. 4 (966KB, ppt) ). Other appetite pathway receptors, including leptin receptor and melanocortin receptor, were also unchanged in the arcuate of KO mice (Supplemental Fig. 4 (966KB, ppt) ), indicating that the BMP-mTOR-p70S6K pathway was likely the appetite pathway implicated in this model, which we previously demonstrated to be important for the anorectic effects of BMP7 (14).
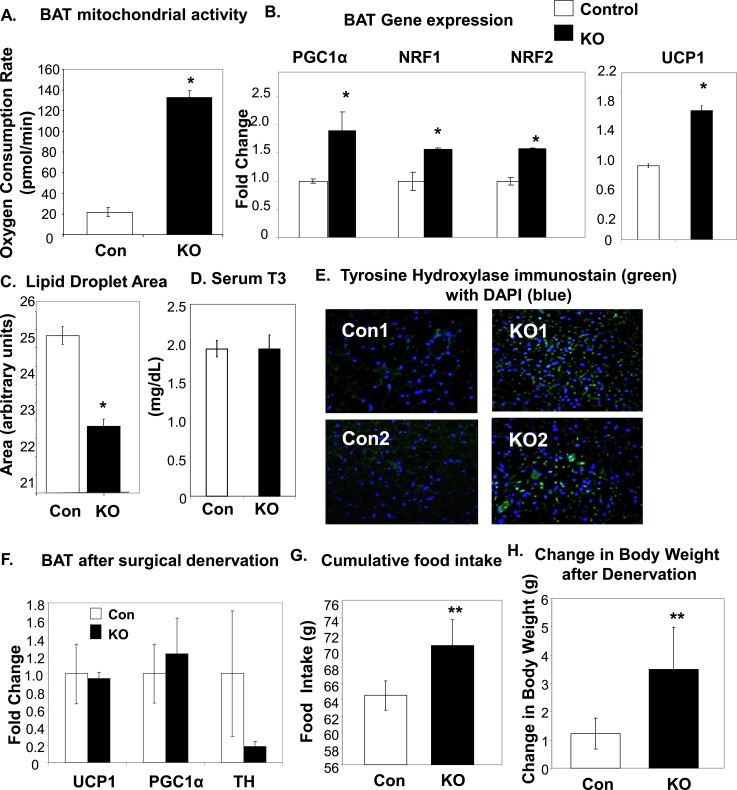
To determine whether the increase in energy expenditure was indeed due to increased BAT activity, we measured mitochondrial activity of the tissue ex vivo in a bioenergetic respirometer. It was found that POMC/BMPR1A-KO BAT had a significantly higher oxygen consumption rate in whole-tissue biopsies (Fig. 3A), indicative of greater mitochondrial activity. Similarly, examination of the expression of thermogenic genes in the interscapular BAT revealed an increase in gene expression of mitochondrial markers such as PPARγ coactivator 1α (PGC1α) and nuclear respiratory factor (NRF) 1 and 2, as well as an increase in thermogenic uncoupling protein 1 (UCP1) (Fig. 3B). These measurements of increased mitochondrial activity were accompanied by a significant decrease in lipid droplet area (Fig. 3C), indicative of a decrease in the lipids stored as fuel in the POMC/BMPR1A-KO BAT. Together, these data revealed that the POMC/BMPR1A-KO mice had acquired an increased propensity for BAT thermogenesis.
Figure 3.
Increased indicators of BAT activation in POMC/BMPR1A-KO mice. (A) Respirometry revealed increased basal oxygen consumption rate (in pmol/min) for ex vivo BAT biopsy punches collected from POMC/BMPR1A-KO mice on a 45% HFD, as measured with a Seahorse respirometer. (B) BAT of POMC/BMPR1A-KO mice on a 45% HFD displayed increased gene expression of mitochondrial markers PGC1α, NRF1, and NRF2 (left) as well as increased expression of UCP1 (right) (N = 6 to 8 mice per group). (C) The size of lipid droplets in the BAT of POMC/BMPR1A-KO mice on a 45% HFD was reduced, as quantified from perilipin staining using Image J and indicating less stored lipid in KO BAT. (D) There was no difference between groups in circulating levels of thyroid hormone T3, as measured by enzyme-linked immunosorbent assay. (E) Immunostaining for TH (AB152; Millipore) revealed increased sympathetic activation of BAT in POMC/BMPR1A-KO mice (green stain) on a 45% HFD. Confocal images of BAT where blue (DAPI) represents cell nuclei. (F−H) After surgical denervation of the interscapular BAT pad of the mice, (F) there were no longer any differences in gene expression between groups, and (G) the POMC/BMPR1A-KO mice remained hyperphagic on a 45% HFD; (H) however, with denervation, the POMC/BMPR1A-KO mice now displayed an increase in body weight (N = 6 to 8 mice per group). *Indicates significance where P < 0.05. Error bars represent standard error of the mean. Con, control; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride; NRF, nuclear respiratory factor; PGC1α, PPARγ coactivator 1α; UCP1, uncoupling protein 1.
The classic pathway to activate BAT fatty acid oxidation and thermogenesis is through increased activation by the sympathetic nervous system (SNS). Sympathetic nerves innervating brown fat release the catecholamine neurotransmitter norepinephrine, which acts on adrenergic receptors on brown adipocytes to increase thermogenesis, mitochondrial activity, and brown adipogenesis (25, 26). Other pathways known to activate BAT, such as thyroid hormone, were not changed in this model [serum levels of thyroid hormone T3 (Fig. 3D) and thyroid hormone receptor target genes (Supplemental Fig. 4 (966KB, ppt) )]. However, in agreement with increased sympathetic activity, we observed an increase in protein immunostaining in the BAT of POMC/BMPR1A-KO mice for TH, the rate-limiting enzyme for the synthesis of norepinephrine and a commonly used marker of activated sympathetic nerves (Fig. 3E). Furthermore, although it is known that sympathetic input is required for UCP1 activation in mitochondria of brown adipocytes, activation of this tissue is not expected to increase electron transport production of adenosine triphosphate. In line with this, we also observed an intriguing reduction in electron transport genes NADP and ATP Synthase in the BAT of KO animals that displayed increased SNS input (Supplemental Fig. 4 (966KB, ppt) ).
Interestingly, our data indicated that subcutaneous, but not perigonadal, white fat also received increased sympathetic activation, leading to increased expression of genes involved in mitochondrial energy utilization. This was shown by increased gene expression of PGC1α, NRF1, and UCP1 in subcutaneous fat (Supplemental Fig. 4A (966KB, ppt) ). Other brown/beige fat markers, such as Cidea and Tbx1, were not changed (Supplemental Fig. 4A (966KB, ppt) ). Along with signs of increased subcutaneous white adipose tissue activation, there was a decrease in cell size but no indications of multilocularity (not shown), suggesting an atypical “browning” event.
Neuronal input to BAT was required for POMC/BMPR1A-KO mice to maintain body weight on an HFD
To determine whether the increased activity of BAT is required for the maintenance of body weight in the hyperphagic POMC/BMPR1A-KO mice, we surgically denervated the interscapular brown fat pad, followed by measurement of food intake and body weight. Seventeen days after surgical denervation of all nerves innervating BAT (but leaving vascular input intact), elevated levels of UCP1 and PGC1α were normalized (Fig. 3F) in the POMC/BMPR1A-KO mice, and TH expression was decreased (Fig. 3F), indicative of a reduction in sympathetic nerve input to the BAT. The POMC/BMPR1A-KO mice with BAT denervation continued to be hyperphagic on a 45% HFD (Fig. 3G), but instead of maintenance of body weight, the lack of enhanced energy expenditure via neuronal activation of BAT resulted in weight gain (Fig. 3H). Together, these data indicated that although POMC neuron deletion of BMPR1A was the anticipated cause of the hyperphagic phenotype, the compensatory increases in BMPR1A expression in non-POMC neurons led to increased energy expenditure phenotype through direct SNS activation of BAT.
Central activation of BMP signaling acutely increased energy expenditure and sympathetic nerve activity in BAT
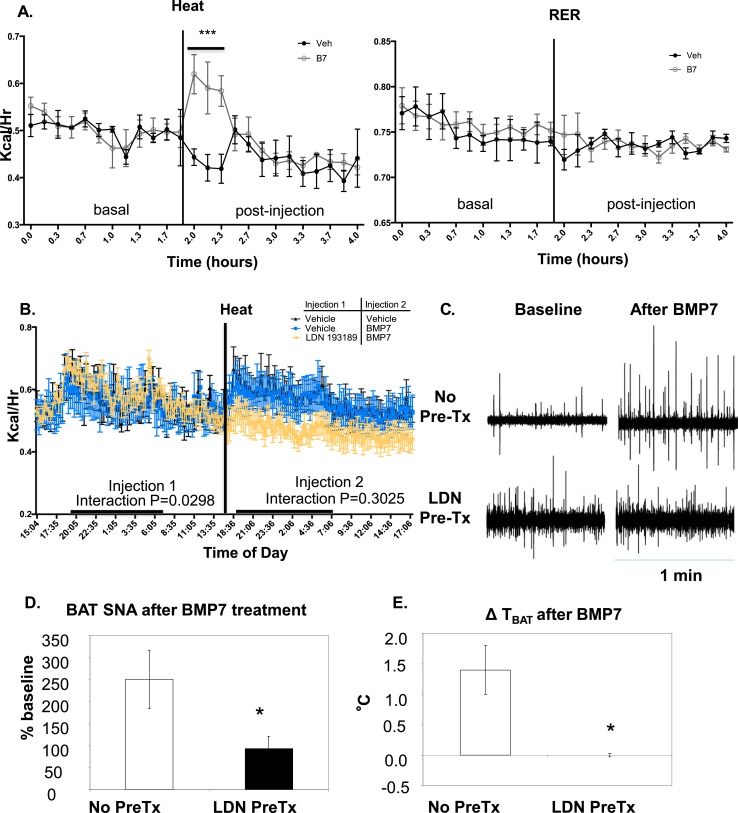
To determine the mechanism for increased energy expenditure in the KO mice displaying higher levels of hypothalamic BMPR1A and more sympathetic activation of BAT, we first acutely measured changes in energy expenditure in wild-type mice receiving i.c.v. delivery of BMP7 ligand. Mice receiving BMP7 displayed an acute increase in heat but not RER (Fig. 4A), as well as increases in both Vo2 and Vco2 (Supplemental Fig. 6A (830KB, ppt) ) vs mice receiving i.c.v. vehicle, an effect that lasted for approximately 30 minutes. In a separate cohort, mice were pretreated with either vehicle or the type 1 BMP-receptor inhibitor LDN193189 (LDN), which blocks the kinase domain of type 1 BMP receptors and inhibits signaling through pSMAD1/5/8 (27), followed by delivery of i.c.v. BMP7. In mice with LDN pretreatment, the ability of BMP7 to cause an acute increase in energy expenditure was blunted (Fig. 4B) and RER was significantly reduced (Supplemental Fig. 5B (242KB, ppt) ), indicating that central BMP signaling through type 1 receptors such as BMPR1A is capable of mediating energy expenditure changes.
Figure 4.
Signaling through type 1 BMP receptors is required for the ability of central BMP7 to increase energy expenditure and drive sympathetic nerve activity in BAT. (A) In control C57BL/6 mice acutely treated with 2 μg in 2 μL of BMP7 by the i.c.v. route, there was a transient and significant increase in energy expenditure above basal levels, as measured by heat (left) in CLAMS metabolic cages. There was no difference in RER between groups (right) (N = 6 mice per group.) The vertical line indicates i.c.v. injection time, and all injections were between 8 am and 1 pm in the fed state. (B) When mice were pretreated i.c.v. with LDN, a potent and selective inhibitor of type 1 BMP receptor signaling, 1.5 hours before BMP7, the i.c.v. BMP7 delivery was no longer able to acutely increase Vo2 (N = 3 mice per group). Interaction P value was not significant: P = 0.3025. The vertical line indicates i.c.v. injection time for injection 2. All injections were between 8 am and 1 pm in the fed state. LDN treatment alone could not be run because of space limitations in the metabolic cages. (C) Representative examples of 1-minute segments of BAT SNA in the baseline condition just before administration of BMP7 (10 mg in 10 μL; baseline panels to the left) and 30 minutes after BMP7 (panels to the right), in either naive rats (no pretreatment) or rats pretreated 1 hour before with LDN (10 μL, 100 mM, i.c.v.). (D) Mean ± standard error of the mean (SEM) of the BAT SNA 30 minutes after BMP7 administration (10 mg in 10 μL, i.c.v.), as a percentage of the baseline activity in rats receiving no pretreatment (N = 5) and in rats pretreated with LDN (10 μL, 100mM, i.c.v.) 1 hour before the BMP7 administration (N = 4). (E) Mean ± SEM of the change in interscapular BAT temperature (TBAT) evoked by i.c.v. administration of BMP7 in rats receiving no pretreatment or in rats pretreated with LDN (10 μL, 100 mM, i.c.v.) 1 hour before the BMP7 administration. *Indicates significance where P < 0.05. Error bars represent standard error of the mean. B7, BMP7; LDN, LDN193189; Pre-Tx, pretreatment; SNA, sympathetic nerve activity; Veh, vehicle.
To determine whether central BMP signaling could directly regulate BAT activity via the SNS, we moved to a rat model for measurement of BAT sympathetic nerve activity (SNA). Rat models benefit from larger brains, larger nerve bundles innervating organs, and larger BAT depots. Together, these enable a very tightly controlled experimental setup to measure real-time acute changes in BAT SNA in response to centrally delivered substances, along with concurrent measurements of energy expenditure (Vco2), BAT and skin temperatures, and blood pressure (sample raw electrophysiological traces in Supplemental Fig. 6B (830KB, ppt) ). In response to an i.c.v. injection of BMP7, there was an increase of BAT SNA to 250% ± 66% of the baseline activity at 30 minutes after the injection, resulting in an increase of BAT temperature (TBAT) by 1.4°C ± 0.4°C. These increases in BAT SNA and TBAT were sustained for at least 40 minutes and in some cases for longer than 1 hour. By contrast, when i.c.v. injection of LDN preceded that of BMP7, the sustained increases in BAT SNA and TBAT were blunted (BAT SNA: 93% ± 28% of the baseline activity 30 minutes after the injection; TBAT: +0.0°C ± 0.3°C) (Fig. 4C–4E; Supplemental Fig. 6C and 6D (830KB, ppt) ). Therefore, inhibition of type 1 BMP receptor activity by LDN prevented the ability of central BMP7 to directly activate sympathetic nerves innervating BAT.
Discussion
The regulation of appetite and energy expenditure involves complex communication within various regions of the CNS and communication between the CNS and peripheral tissues, including adipose tissue. Although some molecular pathways, such as the leptin-melanocortin pathway in the hypothalamus, have been implicated in these processes, thus far the picture appears incomplete and much remains unknown about the role of novel signaling pathways in the hypothalamus that are capable of regulating both appetite and sympathetic activation of adipose tissues. The BMPs are important developmental factors that have only recently been implicated in the regulation of energy balance. For example, we previously showed that systemic delivery of BMP7 is able to reduce appetite and increase energy expenditure to reverse diet- or genetic-induced obesity (14). Although BMP7 plays a role in the CNS in regulating appetite (14), it has also been shown that BMP8b, another member of the BMP family of ligands, is able to further regulate thermogenesis by acting in the CNS (6). However, the role of BMP signaling pathways in specific brain regions and their involvement in metabolic regulation is only beginning to be understood. Here, we provided evidence that a CNS-specific KO of a BMP family member in a subset of POMC neurons resulted in reestablishment of energy balance with hyperphagia as well as increased energy expenditure due to greater sympathetic activation of BAT thermogenesis (see model in Fig. 5).
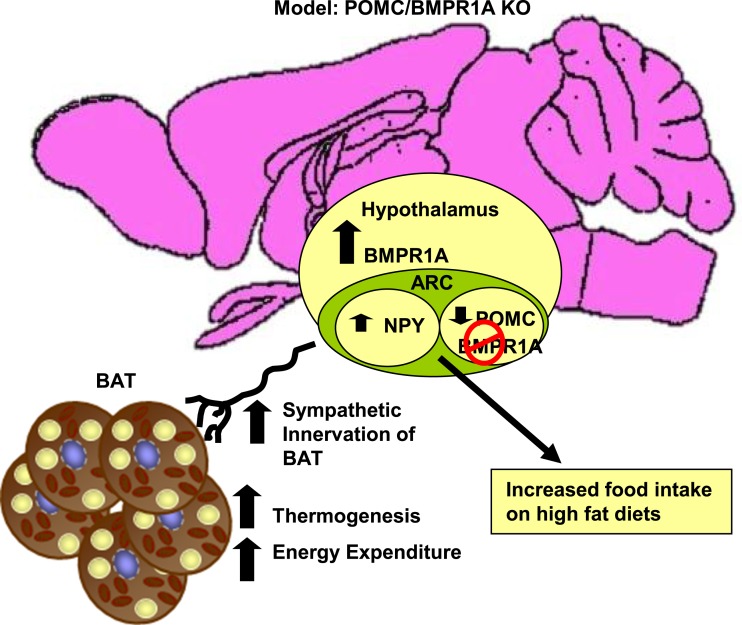
Figure 5.
Model representing the POMC/BMPR1A-KO mice. Deletion of BMPR1A in POMC neurons led to a hyperphagic phenotype of hypothalamic neuropeptide expression, a compensatory increase of BMPR1A in the hypothalamus as a whole, and increased sympathetic activation of BAT, leading to increased thermogenesis and energy expenditure and protection from diet-induced obesity.
The BMPs signal through a receptor complex composed of a type 1 and a type 2 receptor heterodimer. We showed previously that a BMP type 2 receptor, BMPR2, is expressed ubiquitously throughout the hypothalamus, whereas BMPR1A is expressed in a more specific pattern, which we now show includes a subset of POMC neurons. BMP7 is found circulating in the cerebrospinal fluid (28, 29) and is most likely accessible to most brain regions, including the hypothalamus, where BMP7 ligand is also expressed in a subset of neurons (14). Therefore, the anorectic effects of central BMP7 may be via type 1 BMP receptors in the hypothalamus; however, this had not been shown directly. By deleting BMPR1A specifically in POMC neurons, we were able to observe the expected increase of food intake on HFDs, which was accompanied by a decrease in anorectic POMC expression and an increase in orexigenic neuropeptide Y expression. Surprisingly, despite their hyperphagia, the POMC/BMPR1A-KO mice did not gain more weight on a 45% HFD. This was attributable to increased energy expenditure due to increased sympathetic activation of brown fat as a result of a reestablishment of homeostasis through upregulation of non-POMC hypothalamic BMPR1A. However, a higher 60% HFD was able to overcome this enhanced energy expenditure in this model.
Although at first glance these findings appear similar to those of some other models of POMC-Cre mediated deletions (such as suppressor of cytokine signaling 3 [SOCS3 (30)], protein tyrosine phosphatase 1b [PTP1b (31)], and sirtuin 1 [SIRT1 (32)], which exhibited increased Vo2, increased activation of BAT, and increased sympathetic activation and browning of white adipose, respectively), our observed effects on BAT and energy expenditure are unlikely to be directly due to the POMC-neuron ablation of BMPR1A. Our data clearly indicate that deletion of BMPR1A from POMC neurons led to a compensatory increase in non-POMC neurons of the hypothalamus, which then had an effect on reestablishing metabolic homeostasis. This represents an intriguing aspect of biology, whereby receptor systems exhibit flexibility in response to changing signals by altering levels of the same receptor in nearby areas. This type of compensation underscores the importance of the BMP system for regulating energy balance in the brain. Our observed inverse alteration of BMPR1A levels in POMC and non-POMC neurons may have occurred early in brain development, when POMC expression, and thus Cre, turned on. Given the role of BMPs in organogenesis and brain development, the possibility exists that BMPR1A deletion in POMC neurons may have caused remodeling of hypothalamic pathways early in life. This remodeling could have also contributed to the unexpected increase of energy expenditure observed in the POMC/BMPR1A-KO mice and may have led to increased BMP7 expression in the brainstem. This leaves open the possibility that BMP pathways in the brainstem may also contribute to the SNA of BAT. Given our data utilizing a specific BMP-SMAD inhibitor (LDN), we believe that BMP effects on energy expenditure through hypothalamic pathways are most likely via SMAD signaling. By contrast, as we have shown previously, BMP-mTOR-p70S6K signaling is most likely the main pathway responsible for appetite reduction and is not affected by LDN treatment.
Although the subcutaneous, but not perigonadal, white fat also displayed some markers of increased sympathetic activation, it is not clear to what extent increased energy expenditure in this depot may contribute to the overall phenotype. Indeed, denervation of brown fat appeared to be sufficient to reduce energy expenditure, allowing the hyperphagia to lead to weight gain and indicating that white fat is not necessarily a strong contributor to the increased energy expenditure phenotype in this mouse model. Interestingly, placing the mice on a higher-calorie 60% HFD also led to weight gain, presumably by overriding the level of increased energy expenditure, which was observed regardless of diet. Together, these findings may have revealed a kilocalorie maximum derived from food intake that was overcome by the BAT thermogenic energy expenditure; however, this nonetheless strongly indicates that activation of BAT can be sufficient to prevent diet-induced weight gain, at least in mice.
Taken together, these findings provide evidence for a complex regulatory system connecting central and peripheral pathways in the regulation of energy balance. In addition, they provide evidence for the involvement of type 1 BMP receptors in the hypothalamus in the regulation of appetite and sympathetic activation of adipose depots. Given the current global pandemic of obesity and the urgent search for new therapeutic options to treat obesity and its many comorbidities, it is essential to discover and understand all potential pathways regulating energy balance in ways that have favorable metabolic effects. These data indicate that central BMP signaling serves as an important novel target for adjusting whole body energy balance and provides an option for the treatment of obesity and metabolic syndrome.
Acknowledgments
The authors thank Marla Diakow, Tian Lian Huang, Estelle Roger, Allen Clermont, Maura Mulvey, Bethany Miles, and the Joslin DRC animal physiology, histology, and specialized assay cores for technical assistance. We also thank Profs. Eric Widmaier and Thomas Kunz of Boston University for use of the bomb calorimeter. We thank Tim J. Schulz and Young Mi Kwon for a critical reading of the manuscript and Alan Rosenwasser and Matthew Hartman of the University of Maine for assistance with waveform analysis of CLAMS data. The authors thank Stryker Regenerative Medicine (Hopkinton, MA) for the generous gift of recombinant BMP7. Elements of some figures were produced using Servier Medical Art.
Financial Support: This work was supported in part by NIH grants R01 DK077097 and R01DK102898 (to Y.-H.T.) and the Joslin Diabetes Center’s DRC (P30 DK036836 from the National Institute of Diabetes and Digestive and Kidney Diseases), a research grant from the American Diabetes Foundation (7-12-BS-191), and funding from the Harvard Stem Cell Institute (to Y.-H.T.). C.J.M. was funded by a Basic Science Award from the American Diabetes Association, and S.F.M. was funded by NIH (National Institute of Neurological Disorders and Stroke) grant NS40987. K.L.T was funded by grants NIH T32-DK007260-33 and NIH F32-DK091996 and a Junior Faculty Award from the American Diabetes Association (1-14-JF-55).
Author Contributions: K.L.T. wrote the manuscript and designed, conducted, and analyzed the experiments. C.J.M., D.T., and S.F.M. conducted and analyzed the rat sympathetic nerve activity measurements. L.M. assisted with some of the genotyping A extractions, quantitative polymerase chain reaction, and dissections. M.B. assisted with mouse studies, genotyping, and histology. M.D.L. conducted the bomb calorimetry. Y.M. provided the BMPR1A-floxed mice and BMPR1A in situ probes. P.Y. provided the LDN compound and advice. Y.-H.T. wrote the manuscript and designed the experiments. K.L.T. and Y.-H.T. are the guarantors of the work in this manuscript.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- arcuate nucleus
- BAT
- brown adipose tissue
- BMP
- bone morphogenetic protein
- CLAMS
- Comprehensive Laboratory Animal Monitoring System
- CNS
- central nervous system
- DEXA
- dual-energy x-ray absorptiometry
- DRC
- Diabetes Research Center
- HFD
- high-fat diet
- i.c.v.
- intracerebroventricular
- KO
- knockout
- LDN
- LDN193189
- mRNA
- messenger RNA
- NIH
- National Institutes of Health
- NRF
- nuclear respiratory factor
- PGC1α
- PPARγ coactivator 1α
- POMC
- pro-opiomelanocortin
- RER
- respiratory exchange ratio
- SNA
- sympathetic nerve activity
- SNS
- sympathetic nervous system
- T3
- triiodothyronine
- TBAT
- brown adipose tissue temperature
- TH
- tyrosine hydroxylase
- UCP1
- uncoupling protein 1
- αMSH
- α-melanocyte-stimulating hormone
- VO2
- volume of oxygen consumed
- VCO2
- volume of carbon dioxide emitted.
References
- 1.Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93(11, Suppl 1):s37–s50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villanueva EC, Myers MG Jr. Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes. 2008;32(Suppl 7):S8–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawant A, Chanda D, Isayeva T, Tsuladze G, Garvey WT, Ponnazhagan S. Noggin is novel inducer of mesenchymal stem cell adipogenesis: implications for bone health and obesity. J Biol Chem. 2012;287(15):12241–12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamani N, Brown CW. Emerging roles for the transforming growth factor-beta superfamily in regulating adiposity and energy expenditure. Endocr Rev. 2011;32(3):387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulz TJ, Tseng YH. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev. 2009;20(5-6):523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, Dale M, Virtue S, Villarroya F, Cannon B, Rahmouni K, López M, Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149(4):871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Böttcher Y, Unbehauen H, Klöting N, Ruschke K, Körner A, Schleinitz D, Tönjes A, Enigk B, Wolf S, Dietrich K, Koriath M, Scholz GH, Tseng YH, Dietrich A, Schön MR, Kiess W, Stumvoll M, Blüher M, Kovacs P. Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes. 2009;58(9):2119–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend KL, An D, Lynes MD, Huang TL, Zhang H, Goodyear LJ, Tseng YH. Increased mitochondrial activity in BMP7-treated brown adipocytes, due to increased CPT1- and CD36-mediated fatty acid uptake. Antioxid Redox Signal. 2012;19(3):243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav H, Rane SG. TGF-β/Smad3 signaling regulates brown adipocyte induction in white adipose tissue. Front Endocrinol (Lausanne). 2012;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schleinitz D, Klöting N, Böttcher Y, Wolf S, Dietrich K, Tönjes A, Breitfeld J, Enigk B, Halbritter J, Körner A, Schön MR, Jenkner J, Tseng YH, Lohmann T, Dressler M, Stumvoll M, Blüher M, Kovacs P. Genetic and evolutionary analyses of the human bone morphogenetic protein receptor 2 (BMPR2) in the pathophysiology of obesity. PLoS One. 2011;6(2):e16155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bond AM, Bhalala OG, Kessler JA. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev Neurobiol. 2012;72(7):1068–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng CY, Mukhopadhyay A, Jarrett JC, Yoshikawa K, Kessler JA. BMP receptor 1A regulates development of hypothalamic circuits critical for feeding behavior. J Neurosci. 2012;32(48):17211–17224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng YH. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495(7441):379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend KL, Suzuki R, Huang TL, Jing E, Schulz TJ, Lee K, Taniguchi CM, Espinoza DO, McDougall LE, Zhang H, He TC, Kokkotou E, Tseng YH. Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. FASEB J. 2012;26(5):2187–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454(7207):1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, Schrier D, Falb D, Kirkland JL, Wagers AJ, Tseng YH. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci USA. 2011;108(1):143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 2011;14(4):478–490. [DOI] [PubMed] [Google Scholar]
- 18.Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42(6):983–991. [DOI] [PubMed] [Google Scholar]
- 19.Hentges ST, Nishiyama M, Overstreet LS, Stenzel-Poore M, Williams JT, Low MJ. GABA release from proopiomelanocortin neurons. J Neurosci. 2004;24(7):1578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30(7):2472–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sohn JW, Williams KW. Functional heterogeneity of arcuate nucleus pro-opiomelanocortin neurons: implications for diverging melanocortin pathways. Mol Neurobiol. 2012;45(2):225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32(2):69–72. [DOI] [PubMed] [Google Scholar]
- 23.Liew CW, Boucher J, Cheong JK, Vernochet C, Koh HJ, Mallol C, Townsend K, Langin D, Kawamori D, Hu J, Tseng YH, Hellerstein MK, Farmer SR, Goodyear L, Doria A, Blüher M, Hsu SI, Kulkarni RN. Ablation of TRIP-Br2, a regulator of fat lipolysis, thermogenesis and oxidative metabolism, prevents diet-induced obesity and insulin resistance. Nat Med. 2013;19(2):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19(5):741–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins S, Cao W, Robidoux J. Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol. 2004;18(9):2123–2131. [DOI] [PubMed] [Google Scholar]
- 26.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 27.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic ossification [published correction appears in Nat Med. 2009;15:117] Nat Med. 2008;14(12):1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charytoniuk DA, Traiffort E, Pinard E, Issertial O, Seylaz J, Ruat M. Distribution of bone morphogenetic protein and bone morphogenetic protein receptor transcripts in the rodent nervous system and up-regulation of bone morphogenetic protein receptor type II in hippocampal dentate gyrus in a rat model of global cerebral ischemia. Neuroscience. 2000;100(1):33–43. [DOI] [PubMed] [Google Scholar]
- 29.Dattatreyamurty B, Roux E, Horbinski C, Kaplan PL, Robak LA, Beck HN, Lein P, Higgins D, Chandrasekaran V. Cerebrospinal fluid contains biologically active bone morphogenetic protein-7. Exp Neurol. 2001;172(2):273–281. [DOI] [PubMed] [Google Scholar]
- 30.Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4(2):123–132. [DOI] [PubMed] [Google Scholar]
- 31.De Jonghe BC, Hayes MR, Banno R, Skibicka KP, Zimmer DJ, Bowen KA, Leichner TM, Alhadeff AL, Kanoski SE, Cyr NE, Nillni EA, Grill HJ, Bence KK. Deficiency of PTP1B in POMC neurons leads to alterations in energy balance and homeostatic response to cold exposure. Am J Physiol Endocrinol Metab. 2011;300(6):E1002–E1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12(1):78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]