Abstract
The mechanism that regulates sperm release at spermiation is unknown. Herein, we used an animal model wherein rats were treated with adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide, via oral gavage to induce premature release of elongating/elongated spermatids, followed by round spermatids and spermatocytes. Spermatid release mimicking spermiation occurred within 6 to 12 hours following adjudin treatment and, by 96 hours, virtually all tubules were devoid of elongating/elongated spermatids. Using this model, we tracked the organization of F-actin and microtubules (MTs) by immunofluorescence microscopy, and the association of actin or MT regulatory proteins that either promote or demolish cytoskeletal integrity through changes in the organization of actin microfilaments or MTs by coimmunoprecipitation. Adjudin treatment induced an increase in the association of (1) epidermal growth factor receptor pathway substrate 8 (an actin barbed-end capping and bundling protein) or formin 1 (an actin nucleator) with actin and (2) end-binding protein 1 (an MT stabilizing protein) with MT shortly after adjudin exposure (at 6 hours), in an attempt to maintain spermatid adhesion to the Sertoli cell at the apical ectoplasmic specialization (ES). However, this was followed by a considerable decline of their steady-state protein levels, replacing with an increase in association of (1) actin-related protein 3 (a branched actin nucleator that converts actin filaments into a branched/unbundled network) with actin and (2) MT affinity-regulating kinase 4 (an MT destabilizing protein kinase) with MTs by 12 hours after adjudin treatment. These latter changes thus promoted actin and MT disorganization, leading to apical ES disruption and the release of elongating/elongated spermatids, mimicking spermiation. In summary, spermiation is a cytoskeletal-dependent event, involving regulatory proteins that modify cytoskeletal organization.
The release of sperm at spermiation is a cytoskeletal-dependent event, involving regulatory proteins that modify actin- and microtubule-based cytoskeletal organization.
During spermiogenesis, haploid spermatids derived from spermatocytes through meiosis I/II undergo extensive morphological changes, from steps 1 through 19 in rat testes (vs 1 to 16 and 1 to 6 in the mouse and human testis, respectively), which are transported across the seminiferous epithelium in the adluminal (apical) compartment (1–5). Thus, fully developed spermatids (i.e., spermatozoa) line up at the edge of tubule lumen to prepare for their eventual release at spermiation at stage VIII of the epithelial cycle (1, 6). Studies have shown that step 8 to 19 spermatids in the rat testis, or 8 to 16 spermatids in the mouse, are anchored onto the Sertoli epithelium by the only type of testis-specific and actin-rich anchoring device called apical ectoplasmic specialization (ES) (5, 7–9). At the Sertoli-spermatid (step 8 to 19) interface, the most notably feature of the apical ES is an array of actin filament bundles residing in Sertoli cell that are sandwiched in between the cisternae of the endoplasmic reticulum and the apposing Sertoli cell and spermatid plasma membranes. Furthermore, this array of actin filament bundles is supported by a microtubule (MT) network located nearby based on electron microscopy analysis (10, 11). Thus, the actin filament bundles and the MT network at the apical ES play a crucial role to support spermatid transport and the release of sperm at spermiation (12). On the other hand, ES is also found at the Sertoli cell-cell interface near the basement membrane known as the basal ES, coexisting with the actin-based tight junction and gap junction, which together with the intermediate filament-based desmosome constitute the blood-testis barrier (BTB) (13, 14). The only ultrastructural difference between the apical and basal ES is that the former is composed of only a single array of actin microfilaments found in the Sertoli cell vs two arrays of actin microfilaments at the basal ES found on both sides of adjacent Sertoli cells (5, 13, 15, 16).
In this context, it is of interest to note that although the morphological series of events pertinent to spermiation are known for over five decades (1, 6), the role of actin- and MT-based cytoskeletons and the involving molecules, in particular the regulatory proteins of actin and MT dynamics, in spermiation remain unknown. Studies have shown that treatment of adult rats with a single dose of adjudin at 50 mg/kg body weight (b.w.) by oral gavage induced extensive germ cell exfoliation, initially elongating/elongated spermatids that were readily detectable within 6.5 hours posttreatment, whereas depletion of round spermatids (i.e., step 1 to 8 spermatids) and spermatocytes were not noted until ∼3 and 6 days thereafter (17, 18). These differences regarding the time-dependent release of elongating/elongated spermatids thus illustrate that adjudin-treated rats are a useful model to study the biology of sperm release at spermiation and also spermatid transport during the epithelial cycle. In fact, using this animal model, we have shown that the track-like structures across the seminiferous epithelium conferred by either F-actin or MTs are grossly disrupted following adjudin treatment, thereby failing to support the transport of spermatids and organelles (e.g., residual bodies, phagosomes) (19). Adjudin treatment also perturbs the organization of F-actin at the ES, and this thus impedes spermatid adhesion function (19). But because the basal ES is composed of two arrays of actin microfilament bundles, this makes the basal ES/BTB more resistant to adjudin treatment unless a considerable higher dose of adjudin was used to disrupt the BTB integrity (19, 20). Herein, we hypothesize that changes in F-actin and MT organization across the seminiferous epithelium that support ES function, spermatid transport, and also spermiation are mediated by changes in the association of actin- and MT-based regulatory proteins with the corresponding cytoskeleton. This is the subject of this report.
Materials and Methods
Animals and antibodies
Adult male Sprague-Dawley rats at 250 to 300 g b.w. were purchased from Charles River Laboratories (Kingston, NY). The use of animals and detailed Experimental Protocols used for studies involving laboratory rats reported herein were approved by the Rockefeller University Institutional Animal Care and Use Committee with Protocol Number 15-780-H. Rats were euthanized by CO2 asphyxiation using slow (20% to 30%/min) displacement of chamber air from compressed carbon dioxide in a euthanasia chamber approved by the Rockefeller University Laboratory Safety and Environmental Health. Antibodies were obtained commercially, unless specified, and are listed in Table 1.
Table 1.
Antibodies Used for Different Experiments in This Report
| Antibody | Host Species | Vendor | Catalog Number | RRID |
Working Dilution |
|
|---|---|---|---|---|---|---|
| IB | IF | |||||
| Arp3 | Mouse | Sigma-Aldrich | A5979 | AB_476749 | 1:3000 | 1:200 |
| Actin | Goat | Santa Cruz Biotechnology | sc-1616 | AB_630836 | 1:200 | |
| α-Tubulin | Mouse | Abcam | ab7291 | AB_2241126 | 1:1000 | 1:500 |
| β-Tubulin | Rabbit | Abcam | ab6046 | AB_2210370 | 1:1000 | |
| Bovine anti-goat IgG-HRP | Bovine | Santa Cruz Biotechnology | sc-2350 | AB_634811 | 1:3000 | |
| Bovine anti-mouse IgG-HRP | Bovine | Santa Cruz Biotechnology | sc-2371 | AB_634824 | 1:3000 | |
| Bovine anti-rabbit IgG-HRP | Bovine | Santa Cruz Biotechnology | sc-2370 | AB_634837 | 1:3000 | |
| EB1 | Rabbit | Santa Cruz Biotechnology | sc-15347 | AB_2141629 | 1:200 | 1:300 |
| Eps8 | Mouse | BD Biosciences | 610143 | AB_397544 | 1:5000 | 1:100 |
| Formin 1 | Mouse | Abcam | ab68058 | AB_2105244 | 1:500 | 1:100 |
| MARK4 | Rabbit | Cell Signaling Technology | 4834 | AB_2140610 | 1:500 | |
| MARK4 | Rabbit | Proteintech Group | 20174-1-AP | AB_2636847 | 1:50 | |
| Mouse IgG-Alexa Fluor 488 | Goat | Invitrogen | A11029 | AB_138404 | 1:250 | |
| Mouse IgG-Alexa Fluor 555 | Goat | Invitrogen | A21424 | AB_141780 | 1:250 | |
| Rabbit IgG-Alexa Fluor 555 | Goat | Invitrogen | A21428 | AB_141784 | 1:250 | |
Abbreviations: Arp3, actin-related protein 3; EB1, end-binding protein 1; Eps8, epidermal growth factor receptor pathway substrate 8; HRP, horseradish peroxidase; IB, immunoblotting; IF, immunofluorescence; IgG, immunoglobulin G; MARK, microtubule affinity-regulating kinase; RRID, Research Resource Identifier.
Treatment of rats with adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide
Adult male rats at 250 to 300 g b.w. were treated with a single dose of adjudin at 50 mg/kg b.w. by oral gavage as earlier described (21). In brief, adjudin was prepared as a suspension in 0.05% methylcellulose (0.05 g methylcellulose in 100 mL Milli-Q water) with a final concentration of 20 mg/mL adjudin. At specified time points following drug administration, rats were euthanized by CO2 asphyxiation. Testes were removed immediately and then either snap-frozen in liquid nitrogen (for subsequent analysis using frozen cross-sections of testes) or fixed in modified Davidson’s fixative (30% of a 37% solution of formaldehyde, 15% ethanol, 5% glacial acetic acid, and 50% double-distilled H2O) (22) (for histological analysis, and also for immunofluorescence (IF) analysis of α-tubulin, end-binding protein 1 (EB1), and microtubule affinity-regulating kinase (MARK) 4. Testes fixed in modified Davidson’s fixative were embedded in paraffin. Each time point had n = 5 rats, including controls. All samples within a treatment vs control groups of an experiment were processed simultaneously for hematoxylin and eosin (H&E) staining, IF microscopy, or coimmunoprecipitation (Co-IP) to avoid interexperimental variations. Each experiment was repeated at least five times with n = 5 rats, which yielded similar results excluding pilot experiments to establish optimal experimental conditions. For all experiments, one of the two testes from n = 5 rats were fixed, embedded in paraffin, and used for histological analysis/IF analysis of α-tubulin, EB1, and MARK4; and the other testes (also with n = 5 rats) were snap-frozen in liquid nitrogen and subsequently used for IF and/or dual-labeled IF of F-actin, epidermal growth factor receptor pathway substrate 8 (Eps8), formin 1, and actin-related protein 3 (Arp3). In short, α-tubulin, EB1, and MARK4 were examined in the same testes from n = 5 rats, whereas F-actin, Eps8, formin 1, and Arp3 were also examined in the same testes from the n = 5 rats.
H&E staining
Cross-sections of testes embedded in paraffin at 4 µm (thickness) were obtained using a Microm (Model HM335E, Walldorf, Germany) microtome and mounted on microscopic slides and stained with H&E for histological analysis. In brief, paraffin wax sections were dissolved in xylene, and sections were rehydrated by incubating sections through xylene and decreasing strengths of ethanol (100% to 0%), and then Milli-Q water. After rehydration, sections were stained with Mayer’s hematoxylin, washed with tap water, and then stained with eosin (Richard-Allan Scientific, San Diego, CA). Thereafter, sections were mounted in Aqua-Poly-Mount (Polysciences, Warrington, PA) for microscopic examination. Images were acquired using an Olympus BX61 with a built-in Olympus DP-71 digital camera at 12.5 megapixels using the Olympus MicroSuite Five software package (version 1224; Olympus Corp, Shinjuku, Japan).
Dual-labeled IF and immunohistochemistry
Dual-labeled IF was performed as described (23) using frozen cross-sections of testes at 7 μm (thickness) obtained in a Microm (Model HM500M) cryostat at –22°C. Sections were fixed in either 4% paraformaldehyde in phosphate-buffered saline (PBS) or ice-cold methanol, permeabilized in 0.1% Triton X-100 in PBS (10 mM sodium phosphate, 0.15M NaCl, pH 7.4, at 22°C), and subsequently blocked in 1% bovine serum albumin in PBS (weight-to-volume ratio). Sections were then incubated with corresponding specific primary antibodies (Table 1) at appropriate dilution to be followed by Alexa Fluor–conjugated secondary antibodies (Alexa Fluor 555 for red fluorescence, Alexa Fluor 488 for green fluorescence; Invitrogen). For F-actin staining, sections and cells were incubated with fluorescein isothiocyanate–conjugated phalloidin (green fluorescence; Invitrogen). To visualize cell nuclei, sections or cells were incubated with 4′,6-diamidino-2-phenylindole (DAPI) (50 µg/mL PBS) and then mounted in ProLong Gold Antifade mounting medium (catalog no. P36930, Thermo Fisher Scientific). Immunohistochemistry was performed using Bouin’s-fixed, paraffin-embedded sections as described (24). In brief, sections were deparaffinized, rehydrated, and subjected to antigen retrieval in 10 mM citrate buffer (pH 6.0) for 7 minutes in a microwave. Sections were then blocked in 10% normal serum [in PBS, volume-to-volume ratio (v/v)] of the same species of the primary antibody and then incubated overnight with the primary antibody (Table 1) at 4°C. Thereafter, sections were incubated with the corresponding biotinylated immunoglobulin G (IgG; secondary antibody) to be followed by an incubation with streptavidin-horseradish peroxidase (Invitrogen). Color development was performed using an aminoethyl carbazole substrate kit (Invitrogen). Microscopic slides were mounted with Aqua-Poly/Mount (Polysciences) for microscopy. Fluorescence images were captured using a Nikon Eclipse 90i Fluorescence Microscope system using Nikon NIS Elements 3.2 Imaging Software package (Nikon Instruments Inc.). Immunohistochemical images were acquired using an Olympus BX61 microscope. Images were analyzed using Adobe Photoshop for image overlay. To assess changes in F-actin organization at the basal ES/BTB, the distribution of F-actin at a specific site (e.g., basal ES/BTB) was quantified by measuring its distribution (annotated by white bracket) in control testes vs testes after adjudin treatment (annotated by yellow bracket) at the opposite ends of the cross-section of a tubule. At least 50 tubules were randomly selected from a rat testis for quantification, and a total of n = 5 rat testes from different rats were examined in which the F-actin distribution found in control/normal testes was arbitrarily set at 1 against which adjudin-treated testes were compared.
Protein lysate preparation and immunoblotting
Testis lysates were obtained by suspending testis sections in immunoprecipitation lysis buffer [10 mM tris(hydroxymethyl)aminomethane (pH 7.4 at 22°C) containing 0.15 M NaCl, 2 mM EGTA, 1% Nonidet P-40 (v/v), and 10% glycerol (v/v)] supplemented with protease and phosphatase inhibitors freshly added to the lysis buffer, including 1 mM 4-(2-aminoethyl)benzene sulfonyl fluoride hydrochloride, 1 mM sodium orthovanadate, 0.05 mM bestatin, 0.05 mM sodium EDTA, 15 μM E64, 1 mM pepstatin, 4 mM sodium tartrate dehydrate, 5 mM NaF, and 3 mM β-glycerophosphate disodium salt by sonication as earlier described (23). Immunoblot analysis was performed and images were acquired using a Fujifilm LAS-4000 Mini-Luminiscent Image Analyzer and in-house enhanced chemiluminescence kits as described (25).
Co-IP
To assess the changes in protein-protein interaction between actin and its binding/regulatory proteins (e.g., Arp3, formin 1, Eps8) as well as between MT and its binding/regulatory (e.g., EB1, MARK4) in testes following treatment of rats with adjudin, testes obtained from rats (n = 3 to 4 rats) at 0, 6, 12, 24, and 96 hours were used for Co-IP as earlier described (26). In short, testis lysates (1 mg protein for each time point) were incubated with either anti-actin or anti–α-tubulin to serve as the immunoprecipitating antibody for Co-IP, and the immunocomplexes obtained by Protein A/G Plus (Santa Cruz Biotechnology) were then used for immunoblot analysis to assess changes in association with the corresponding interacting proteins at specified time points after adjudin treatment using corresponding antibodies (Table 1). To eliminate interexperimental variations, all samples within a given experiment (i.e., adjudin-treated samples obtained at different time points vs control testes) were processed simultaneously. These included samples that examined the interactions of actin with the three F-actin regulatory proteins (i.e., Eps8, formin 1, and Arp3) by Co-IP vs their lysates (to quantify their corresponding steady-state protein level without Co-IP). The same approach was used to examine MT-regulatory proteins (i.e., EB1 and MARK4) and their interactions with MTs vs their lysates. In short, for each marker protein (e.g., EB1, Eps8), it was derived from a new gel/blot, but all markers that monitored their interaction with either actin or MT were processed in a single experimental session. These blots were then stripped and reprobed with anti–β-actin or α-tubulin antibody to serve as protein loading control. This experiment was repeated at least three times using n = 3 rats, which yielded similar results.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 6 software (GraphPad Software) using either a Student t test (for two-group comparisons), one-way analysis of variance (ANOVA) (for multigroup comparisons), or two-way ANOVA with Bonferroni post hoc tests. All experiments had n = 3 to 5 rats or samples for analysis.
Results
Adjudin impedes spermatogenesis by disrupting apical ES, leading to the release of elongating/elongated spermatids by mimicking spermiation
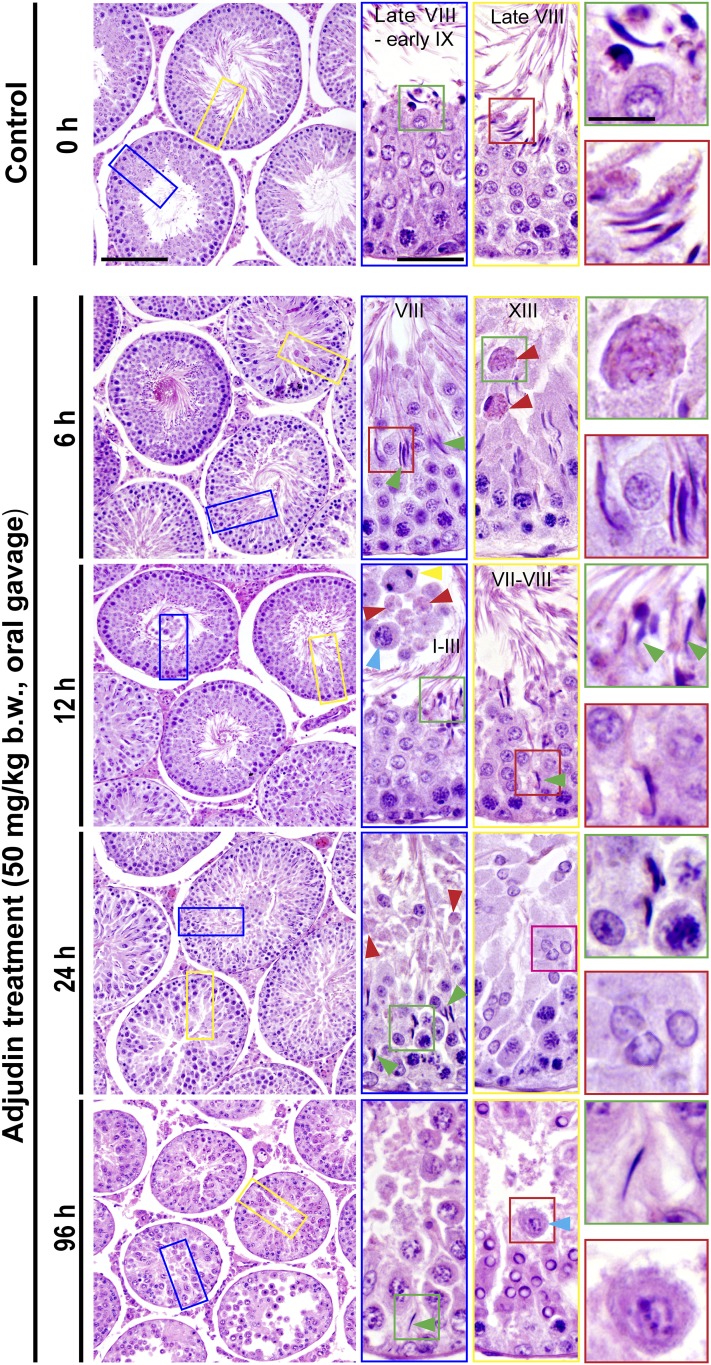
Following treatment of adult rats with adjudin (50 mg/kg b.w., oral gavage), defects in spermatogenesis were noted in the seminiferous epithelium within 6 hours (Fig. 1). For instance, premature release of elongated spermatids as detected in stages VI and VII and early-stage VIII tubules; however, many of these tubules appeared as stage VIII tubules as noted herein due to sperm release, yet some elongated spermatids remained trapped inside the epithelium (Fig. 1). Also, phagosomes were seen in stage XIII tubules near the tubule lumen when they should be detected near the base of the tubule (red arrowheads in Fig. 1). By 12 hours, premature release of elongating spermatids was detected even in stage I to III tubules; and postmeiotic spermatocytes, spermatocytes, and even phagosomes were also found in the tubule lumen; yet some elongating spermatids remained trapped deep inside the seminiferous epithelium (Fig. 1). This entrapment of elongated spermatids inside the epithelium and the failure of proper transport of phagosomes across the epithelium were persistent even by 24 and 96 hours following treatment of adjudin when virtually all elongating/elongated spermatids had undergone premature spermiation (Fig. 1). Herein we sought to examine the molecular mechanism(s) underlying these adjudin-mediated defects in spermiation and spermatid transport and/or detention.
Figure 1.
Adjudin treatment in adult rats rapidly induces defects in spermatogenesis prior to the occurrence of germ cell release from the seminiferous epithelium that mimics spermiation. Adult rats treated with a single dose of adjudin at 50 mg/kg b.w. by oral gavage at time 0 (controls; n = 5 rats). Thereafter, rats (n = 5) were terminated at specified time points at 6, 12, 24, and 96 hours vs 0 hours (control) for histological analysis using paraffin cross-sections of testes and H&E staining. By 6 and 12 hours, defects in spermatogenesis were noted in the testis. For instance, many elongated spermatids were trapped deep inside the epithelium (annotated by green arrowheads) when “unwanted spermiation” had taken place in nonstage VIII tubules but also stage VIII tubules; and phagosomes were found in a XIII tubule, which appeared to be derived from multinucleated round spermatids (annotated by red arrowheads; see also enlarged image in inset at 6 hours). In an apparently stage I to III tubule, a meiotic spermatocyte (annotated by a yellow arrowhead), phagosomes (annotated by red arrowheads), and a pachytene spermatocyte (annotated by a blue arrowhead) were detected in the tubule lumen when steps 15 and 16 spermatids (see green square box and the corresponding inset) were also depleting to the tubule lumen. By 24 hours, few elongating/elongated spermatids were found in the epithelium across the testis sections except those that were trapped deep inside the epithelium (annotated by green arrowheads and the corresponding boxed areas shown in insets), and both round spermatids, spermatocytes, and even some phagosomes were also emptied into the tubule lumen (see inset). At 96 hours (i.e., day 4), obvious defects in spermatogenesis were detected in the testis. For instance, virtually all elongated/elongated spermatids from 98% of tubules examined were not found in the seminiferous epithelium but depleted from the testis, yet some elongated spermatids remain trapped deep inside the epithelium, and this extensive germ cell loss also led to a reduction in tubule diameters. Scale bars = 180 µm in left panel at 0 hours, 70 µm in the blue boxed rectangle, and 30 µm in green boxed square, which apply to corresponding images in the same column or row.
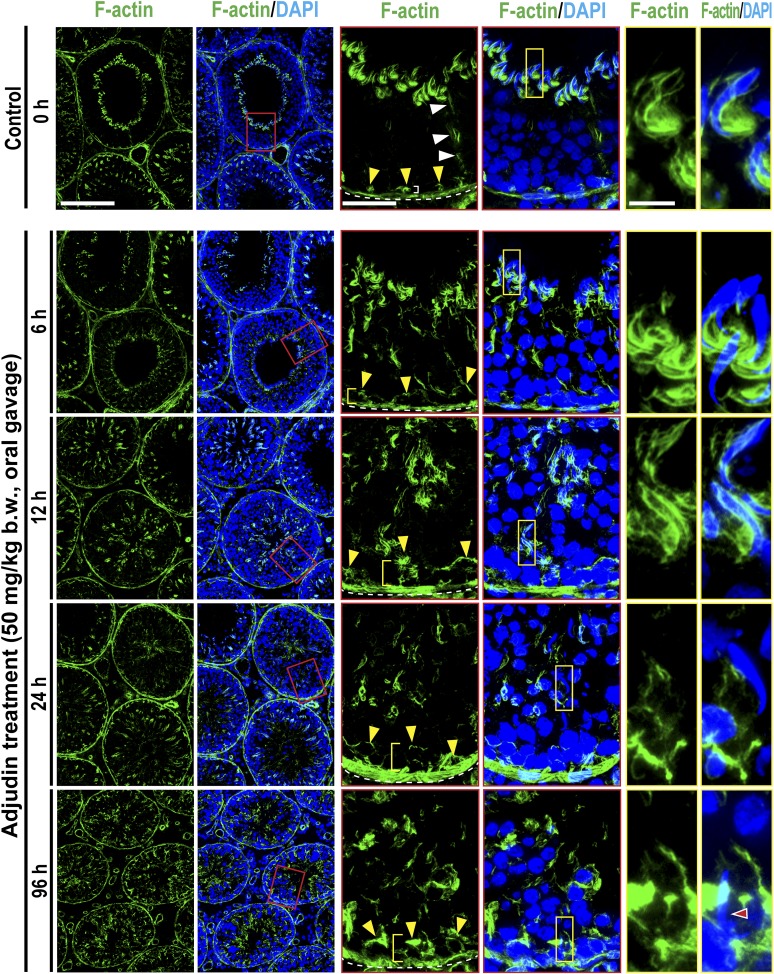
Adjudin rapidly induces defects in apical ES by perturbing Sertoli cell F-actin organization
In stage VIII tubules, F-actin was prominently expressed at the apical ES, mostly at the concave (ventral) side of spermatid heads, appearing as bulb-like ultrastructures (Fig. 2), which eventually would diminish considerably to facilitate the release of sperm at spermiation. In control testes, some track-like structures conferred by F-actin were also noted (see white arrowheads), and F-actin was also detected at the basal ES that constitute the BTB (annotated by yellow arrowheads) with tight junction and gap junction near the basement membrane of the tunica propria (the base of the tunica propria was annotated by a dashed white line) (Fig. 2). Adjudin treatment, by 6 hours and through 96 hours, induced considerable and extensive disruption on F-actin organization, such that in similarly staged tubules, F-actin no longer appeared as the bulb-like ultrastructures seen in control testes, but mislocalized by diffusing away from the spermatid head (Fig. 2). These changes thus failed to support spermatid adhesion, leading to their loss from the seminiferous epithelium, mimicking spermiation because adhesion protein complexes (e.g., nectin/afadin, laminin/integrin) at the apical ES all used F-actin for their attachment. However, at up to 96 hours, changes in the basal ES organization were not considerably disrupted (Fig. 2), possibly due to the presence of two arrays of actin microfilament bundles at the basal ES vs a single array at the apical ES (5, 15), making the basal ES/BTB more resistant to adjudin treatment. This concept is also consistent with a report indicating that it took almost 2 weeks for the BTB to become “leaky,” but reversible, following adjudin treatment at a similar dose unless an acute dose of adjudin was used (20). However, the distribution of F-actin at the basal ES was somewhat affected because F-actin no longer tightly localized at the basal ES/BTB as noted by the white bracket in control testes, but diffused away after adjudin treatment (see yellow brackets) (see also Supplemental Fig. 1 (3.3MB, pdf) ).
Figure 2.
Adjudin treatment induces defects in F-actin organization prior to germ cell release from the seminiferous epithelium that mimics spermiation. Adult rats (∼300 g b.w.) were treated with a single dose of adjudin at 50 mg/kg b.w. by oral gavage at 0 (controls; n = 5 rats). Thereafter, rats (n = 5) were terminated at specified time points at 6, 12, 24, and 96 h (i.e., day 4) for IF microscopy to visualize the organization of F-actin network across the seminiferous epithelium using FITC-conjugated phalloidin (green fluorescence; Invitrogen). Cell nuclei were visualized by DAPI (Invitrogen). A section of the images (boxed in red) on the second column was magnified and shown on the third and fourth columns; and a section of the images (boxed in yellow) on the fourth column was also magnified and shown on the fifth and sixth columns. The relative location of the base of the tunica propria was annotated by a dashed white line in the third column, and the relative location of the BTB was annotated by yellow arrowheads. The track-like structures (annotated by white arrowheads) conferred by F-actin were also shown in control testes. After adjudin treatment, F-actin that supported the BTB was no longer tightly aligned at the BTB (see white bracket in control testes on the third column), but diffusely localized in particular by 12 to 96 hours (see yellow brackets). These changes in F-actin distribution were semiquantified and shown in Supplemental Fig. 1 (3.3MB, pdf) . Also, the track-like structures conferred by F-actin were virtually nondetectable by 12 hours following adjudin treatment. Moreover, F-actin that appeared as bulb-like structures located at the concave side of spermatid heads was extensively mislocalized by 6 hours after adjudin treatment, moving to the concave side of spermatid heads and considerably diminished by 24 and 96 hours. This time-dependent F-actin disorganization at the apical ES induced by adjudin thus led to germ cell release from the epithelium, mimicking spermiation. Scale bars = 180, 70, and 30 µm in the micrograph in the first, third, and fifth column, which apply to corresponding images.
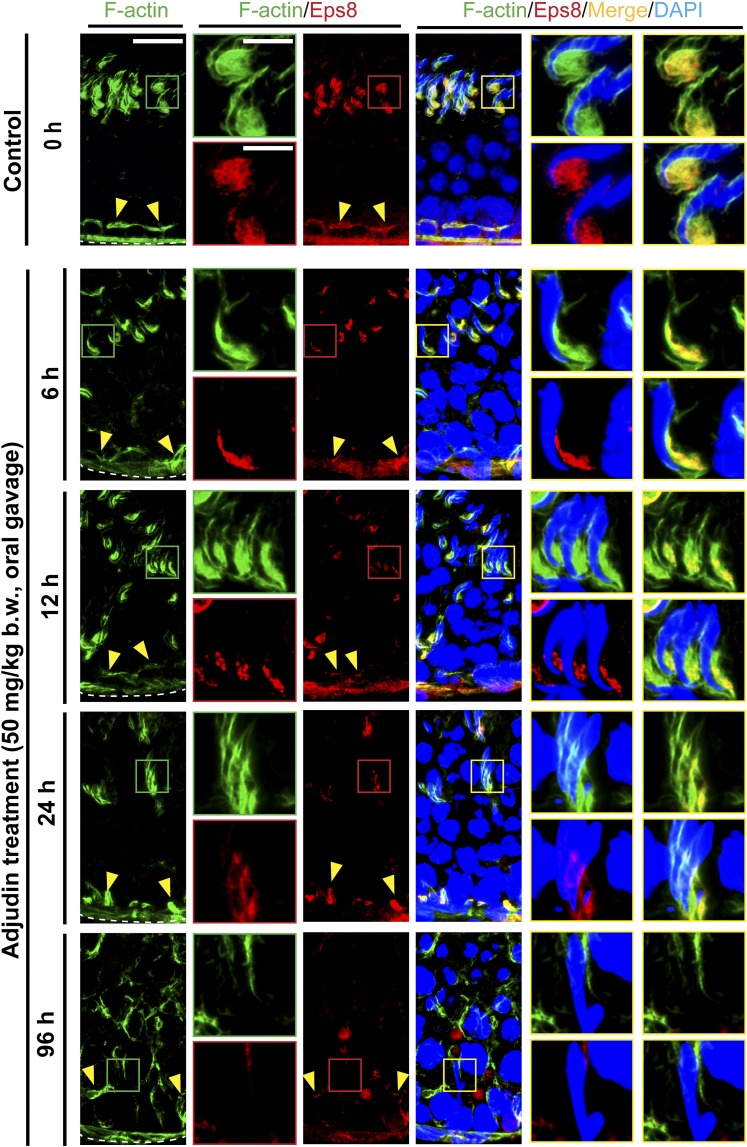
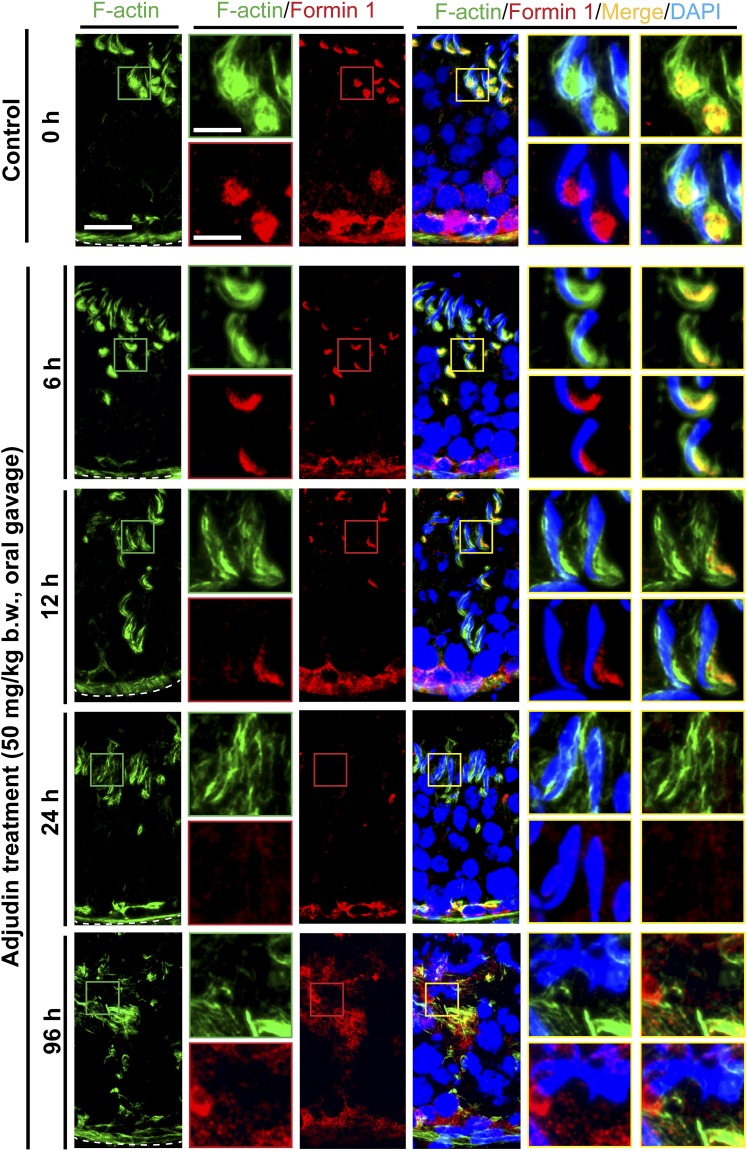
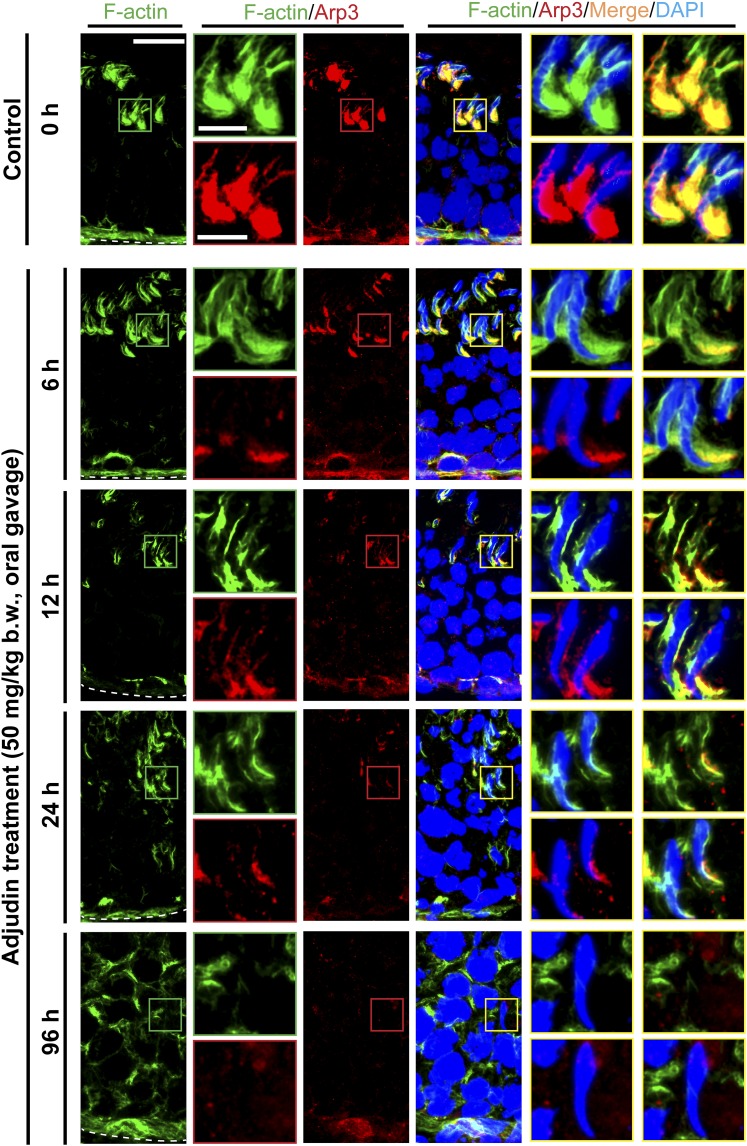
Adjudin-induced premature spermiation is mediated by disruptive spatiotemporal expression of Eps8 and formin 1 vs Arp3 in the seminiferous epithelium, perturbing the organization of actin filament bundles at the ES
Proper organization of F-actin in the seminiferous epithelium, in particular the apical ES, to support spermatogenesis is known to be mediated by the following proteins: (1) actin bundling proteins (e.g., Eps8, an actin barbed-end capping and bundling protein capable of maintaining actin filament bundles at the ES in the rat testis) (27); (2) actin nucleation proteins such as formin, an actin nucleator capable of generating a long stretch of actin microfilaments to support ES function (28) when assembled into bundles at the ES; and (3) branched actin polymerization proteins such as Arp3 in the Arp2/3 complex, a barbed-end nucleation protein complex capable of converting a linear actin filament to a branched configuration in the testis (29). The concerted effects of these proteins thus confer plasticity to the F-actin network by converting actin filaments from a bundled to an unbundled/branched network, and vice versa. Following adjudin treatment, by 6 to 12 hours, Eps8 (Fig. 3; Supplemental Fig. 2 (3.3MB, pdf) ), formin 1 (Fig. 4; Supplemental Fig. 3 (3.3MB, pdf) ), and Arp3 (Fig. 5; Supplemental Fig. 4 (3.3MB, pdf) ) no longer restrictively expressed at the concave side of spermatid heads such as in stage VII tubules as bulb-like ultrastructures, colocalizing with F-actin in control testes (Figs. 3, 4 and 5; Supplemental Figs. 2–4 (3.3MB, pdf) ). Instead, these regulatory proteins were diffusely localized at the apical ES and/or considerably diminished (Figs. 3–5; Supplemental Figs. 2–4 (3.3MB, pdf) ), thereby failing to support the F-actin network to confer adhesion to the adhesion protein complexes at the apical ES. These changes thus led to spermatid release from the seminiferous epithelium, mimicking spermiation.
Figure 3.
Adjudin treatment induces considerable changes in the spatiotemporal expression of Eps8 and its colocalization with F-actin at the ES prior to germ cell release from the seminiferous epithelium that mimics spermiation. Adult rats (∼300 g b.w.) were treated with a single dose of adjudin at 50 mg/kg b.w. by oral gavage at time 0 (controls; n = 3 rats). Thereafter, rats (n = 5) were terminated at specified time points for IF microscopy to visualize changes in the spatial expression of Eps8 (red fluorescence) and its colocalization with F-actin (green fluorescence) using frozen sections of testes. Cell nuclei were visualized by DAPI. Eps8 is an actin barbed-end capping and bundling protein capable of assembling actin microfilaments to form bundles to support ES function at the Sertoli-spermatid interface (apical ES) and at the Sertoli cell-cell interface (basal ES/BTB). Eps8 (similar to F-actin) appeared as bulb-like ultrastructures at the apical ES, colocalizing with F-actin at the concave (ventral) side of spermatid heads. Following adjudin treatment, a considerable decline in Eps8 expression at the apical ES was detected vs control testes. This thus failed to support actin filament bundles at the apical ES. This decline in robust Eps8 expression was worsened by 12 hours and by 24 and 96 hours (4 days); virtually no Eps8 expression was detected (see also Supplemental Fig. S2 (3.3MB, pdf) ). This trend of time-dependent reduction in Eps8 expression was also found at the basal ES/BTB (see Supplemental Fig. 2 (3.3MB, pdf) ), thereby causing F-actin to fail to localize properly at the basal ES/BTB. Such decline in Eps8 expression thus contributed to the loss of spermatid adhesion, causing spermatid release from the epithelium, mimicking spermiation. Scale bars = 80 and 30 µm in the first micrograph and the corresponding enlarged images shown in insets.
Figure 4.
Adjudin treatment induces considerable changes in the spatiotemporal expression of formin 1 and its colocalization with F-actin at the ES prior to germ cell release from the seminiferous epithelium that mimics spermiation. Adult rats (∼300 g b.w.) were treated with a single dose of adjudin at 50 mg/kg b.w. by oral gavage at time 0 (controls; n = 5 rats). Thereafter, rats (n = 5) were terminated at specified time points for IF microscopy to visualize changes in the spatiotemporal expression of formin 1 (red fluorescence) and its colocalization with F-actin (green fluorescence) using frozen sections of testes. Cell nuclei were visualized by DAPI. Because formin 1 is an actin nucleation protein capable of polymerizing long stretches of actin microfilaments in Sertoli cells to support F-actin organization, its considerable decline in expression at the apical ES thus induced disorganization of F-actin at the site to support spermatid adhesion (see also Supplemental Fig.3 (3.3MB, pdf) ). These changes were noted as soon as 6 and 12 hours after adjudin treatment when formin 1 no longer robustly expressed at the concave side of spermatid heads. Its expression was virtually undetectable by 24 and 96 hours (4 days). Scale bars = 80 and 30 µm in the first micrograph and the corresponding enlarged images shown in insets.
Figure 5.
Adjudin treatment induces considerable changes in the spatiotemporal expression of Arp3 and its colocalization with F-actin at the ES prior to germ cell release from the seminiferous epithelium that mimics spermiation. Adult rats (∼300 g b.w.) were treated with a single dose of adjudin at 50 mg/kg b.w. by oral gavage at time 0 (controls; n = 3 rats). Thereafter, rats (n = 5) were terminated at specified time points for IF microscopy to visualize changes in the spatiotemporal expression of Arp3 (red fluorescence) and its colocalization with F-actin (green fluorescence) using frozen sections of testes. Cell nuclei were visualized by DAPI. Micrographs were enlarged in corresponding insets as indicated by the green, red, or yellow boxed areas. Relative location of the basement membrane was annotated by the dashed white line. F-actin organization was considerably disrupted within 6 hours after adjudin treatment, which continued to worsen. It appeared that F-actin disruption was mediated by considerable changes in spatial expression of Arp3, the barbed-end actin nucleation protein that effectively induced branched actin polymerization, causing the linear and bundled actin microfilaments at the ES to become disassembled and branched, destabilizing the ES. In control testes, Arp3 appeared as bulb-like structures at the concave (ventral) side of spermatid heads, which work together with Eps8 (see Fig. 3) to support endocytic vesicle-based protein trafficking for endocytosis and recycling in stage VII tubules. By 6 hours following adjudin treatment, however, Arp3 no longer appeared as bulb-like structures at the apical ES but considerably diminished (see also Supplemental Fig. 4 (3.3MB, pdf) ); and by 24 and 96 hours (4 days), most of the elongated spermatids were not even supported by F-actin and Arp3, destabilizing spermatid adhesion that led to their eventual release from the epithelium that mimicked spermiation. Scale bars = 80 and 30 µm in the first micrograph and the corresponding enlarged images shown in insets.
Adjudin induces defects in spermiation mediated by MT disruption through changes in the spatiotemporal expression of EB1 and MARK4
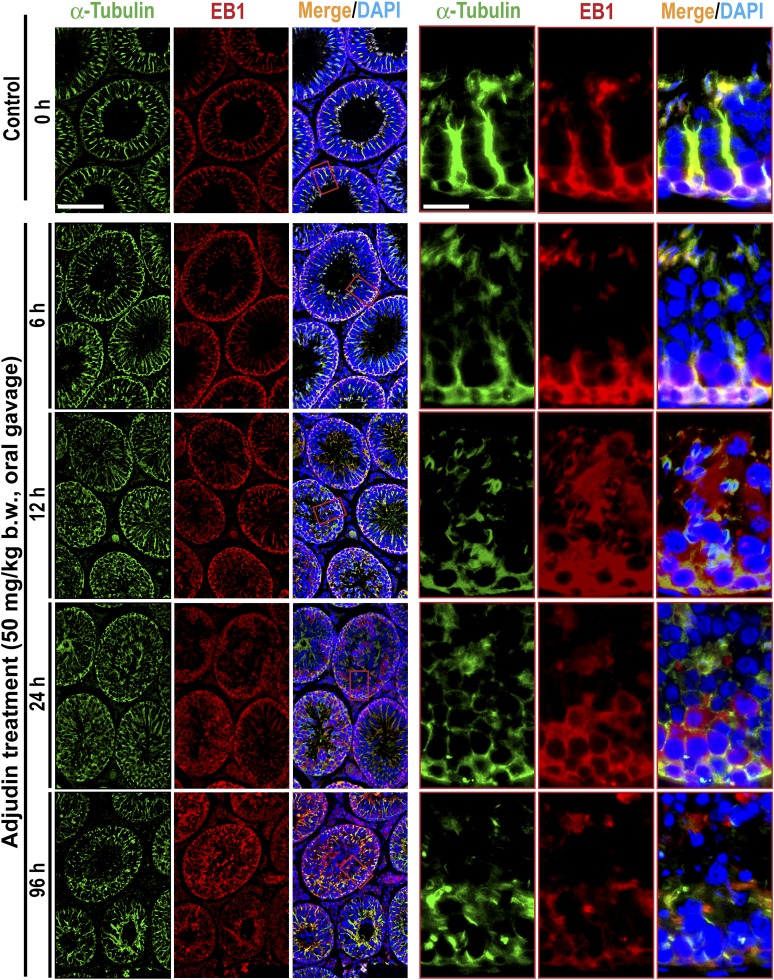
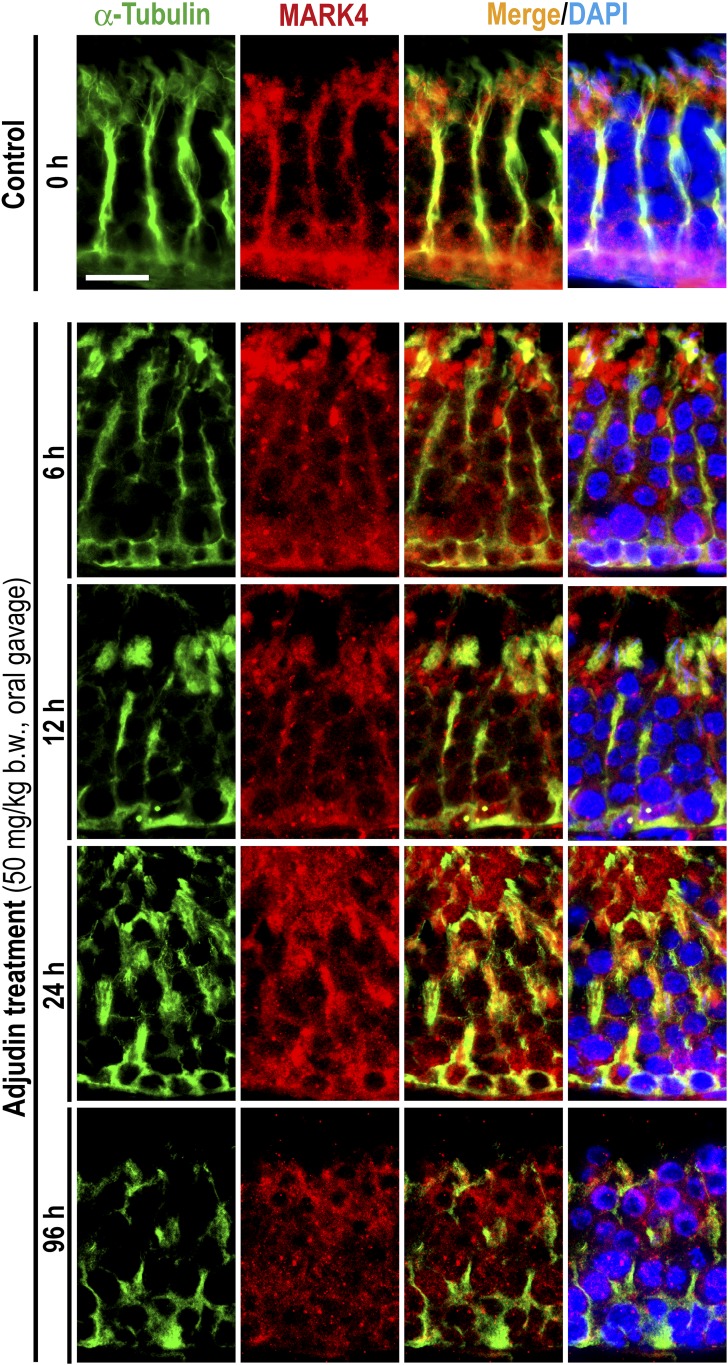
Besides perturbing F-actin organization across the seminiferous epithelium, adjudin was also found to induce gross disruption of MT-based cytoskeleton (Fig. 6). For instance, the conspicuous track-like structures conferred by MTs, as visualized by α-tubulin staining (a building block of MTs), that laid perpendicular to the basement membrane were either truncated or laid parallel to the basement membrane, thereby failing to support the transport of spermatids and organelles (e.g., phagosomes) across the epithelium (Fig. 6), leading to the entrapment of elongated spermatids deep inside the epithelium following adjudin treatment as noted in Fig. 1. Interestingly, these disruptive changes in the organization of MT-based cytoskeleton appeared to be mediated by changes in the spatiotemporal expression of EB1, a +TIP (an MT plus-end tracking protein) protein known to confer MT stability (30, 31) (Fig. 6). Also, the localization of MARK4, a Ser/Thr protein kinase known to induce phosphorylation of MT-associated proteins (MAPs) such as MAP1a, which in turn dissociated from MTs that destabilized MTs, leading to MT disruption (also known as catastrophe) (32, 33), in the seminiferous epithelium was also grossly perturbed (Fig. 7). For instance, EB1 no longer expressed prominently across the epithelium as track-like ultrastructures that laid perpendicular to the basement membrane as found in control testes (Fig. 6). EB1 in adjudin-treated testes also failed to colocalize with MTs but grossly disrupted, including truncation, similar to MTs (Fig. 6). The phenotypes of MARK4 in adjudin-treated testes were also similar to EB1, unlike control testes when MARK4 colocalized with MTs and aligned as track-like ultrastructures that laid perpendicular to the basement membrane; instead, the track-like structures conferred by MARK3 were truncated and grossly disorganized when compared with control testes (Fig. 7).
Figure 6.
Adjudin treatment perturbs the organization of MTs through changes in the spatial expression of EB1. Adult rats (∼300 g b.w.) were treated with a single dose of adjudin at 50 mg/kg b.w. by oral gavage at time 0 (controls; n = 5 rats). Thereafter, rats (n = 5) were terminated at specified time points for IF microscopy to visualize changes in the organization of MTs by staining α-tubulin (green fluorescence; the building blocks of MTs). This disruptive organization of MTs following adjudin treatment appeared to be mediated by changes in the spatial expression of EB1 (red fluorescence), a +TIP protein known to stabilize MTs in Sertoli cells. It was noted that the track-like structures conferred by MTs, known to support the transport of spermatids and other organelles (e.g., residual bodies, phagosomes, endocytic vesicles) and to support actin microfilaments at the ES, across the seminiferous epithelium that laid perpendicular to the basement membrane as found in control testes were considerably disrupted. For instance, within 6 hours after adjudin treatment, these MT-based tracks and the colocalized EB1 were truncated, no longer stretched across the entire epithelium; and by 12 hours, virtually no identifiable track-like structures were found but were considerably truncated. For the residual MTs and the colocalized EB1 that were detected, some were laid in parallel, instead of perpendicular, to the basement membrane. Cell nuclei were visualized by DAPI. Scale bars = 180 and 80 µm in the first micrograph and the corresponding enlarged images shown in insets.
Figure 7.
Adjudin treatment perturbs the organization of MTs through changes in the spatial expression of MARK4. Adults rats (∼300 g b.w.) were treated with a single dose of adjudin at 50 mg/kg b.w. by oral gavage at time 0 (controls; n = 5 rats). Thereafter, rats (n = 5) were terminated at specified time points for IF microscopy to visualize changes in the organization of MTs by staining α-tubulin (green fluorescence) vs MARK4 (red fluorescence). This disruptive organization of MTs following adjudin treatment appeared to be mediated by changes in the spatial expression of MARK4, a Ser/Thr protein kinase known to induce MT catastrophe by phosphorylating MAPs, causing their detachment from MTs, thereby rendering MTs to become less stable in Sertoli cells. The MT-conferred track-like structures necessary to support actin microfilaments at the ES as found in control testes were considerably disrupted following adjudin treatment. For instance, within 6 hours after adjudin treatment, these MT-based tracks that laid across the epithelium and aligned perpendicular to the basement membrane had became less prominent and the colocalized MARK4 were less concentrated to the MT-based tracks but diffusely localized, no longer stretched across the entire epithelium. By 12 hours, most of the tracts were truncated, and by 24 hours, there were virtually no identifiable track-like structures. For the residual MTs and the colocalized MARK4 that were detected, some were laid in parallel, instead of perpendicular, to the basement membrane. Cell nuclei were visualized by DAPI. Scale bar = 80 µm, which applies to all other images.
Adjudin perturbs protein-protein association between actin regulatory proteins and actin vs MT regulatory proteins and MTs
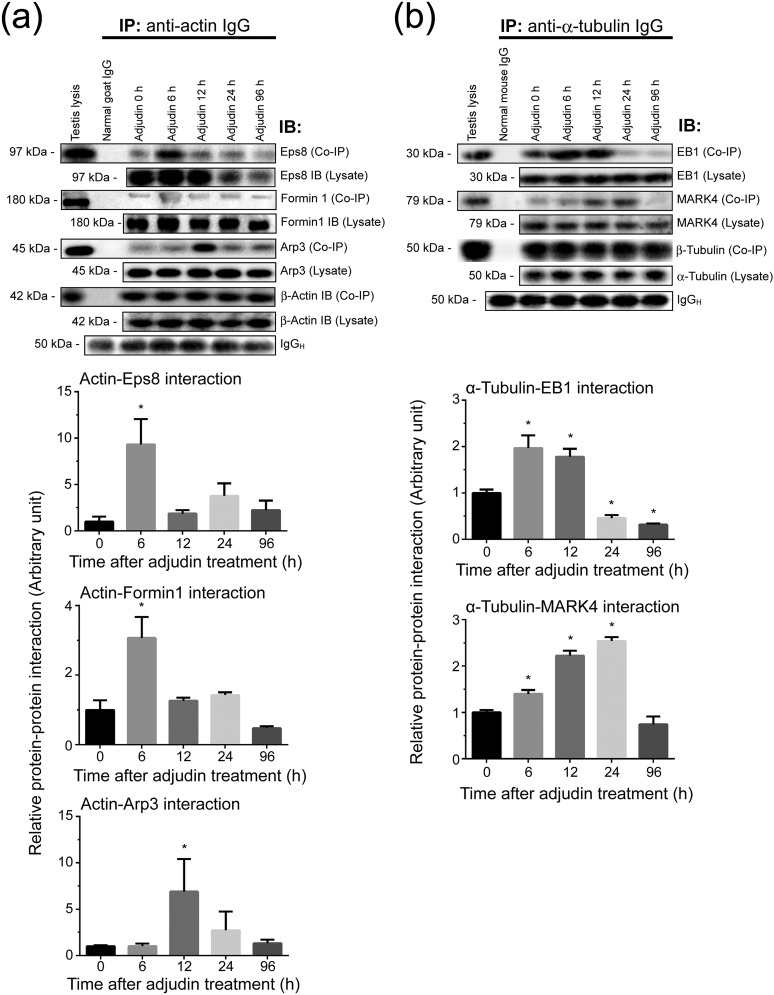
Data shown previously illustrate that the adjudin-induced premature (or unwanted) spermiation was mediated by changes in the spatiotemporal expression of Eps8 (Fig. 3; Supplemental Fig. 2 (3.3MB, pdf) ), formin 1 (Fig. 4; Supplemental Fig. 3 (3.3MB, pdf) ), and Arp3 (Fig. 5; Supplemental Fig. 4 (3.3MB, pdf) ) that regulate F-actin dynamics vs EB1 (Fig. 6) and MARK4 (Fig. 7) that regulate MT dynamics. This thus perturbed the corresponding actin- and MT-based cytoskeletal organization and function. To further expand these observations, Co-IP was performed to assess changes in protein-protein interactions between these regulatory proteins and their corresponding cytoskeletal elements. It was of interest to note that there was a considerable and statistically significant increase in Eps8 and formin 1 association with actin at 6 hours, seemingly suggesting that this was a physiological response of the testis to protect premature release of germ cells that mimicked spermiation following adjudin treatment (Fig. 8). However, this transient increase in actin bundling (and barbed-end capping) and linear actin filament nucleation activities failed to reverse the adjudin-induced actin disorganization due to an increase in Arp3-actin association by 12 hours [Fig. 8(a); Supplemental Fig. 5 (3.3MB, pdf) ], causing actin filament bundles to be converted to an unbundled/branched configuration through the action of Arp3, promoting apical ES disruption that led to premature spermatid release from the testis. Similarly, it was noted that there was an increase in EB1-α-tubulin interaction, attempting by the testis to promote MT stability, but this effort also failed to rescue premature spermatid release because a considerable and statistically significant increase in MARK4-α-tubulin interaction, causing MT catastrophe, leading to spermatid exfoliation [Fig. 8(b); Supplemental Fig. 6 (3.3MB, pdf) ].
Figure 8.
A study by Co-IP to assess changes in the interactions between actin- and MT-based binding and regulatory proteins with the corresponding cytoskeleton during the release of germ cells from the testis in the adjudin model. Lysates of testes (1 mg protein in each Co-IP reaction) from rats were obtained and used for Co-IP (as described in Materials and Methods) following treatment with adjudin (50 mg/kg b.w., oral gavage) at 6, 12, 24, and 96 hours vs 0 hours (control) with n = 3 rats per time point including control. (a) Actin regulatory proteins: Eps8 (an actin barbed-end capping and bundling protein causing actin filaments to assume a bundled configuration as those found at the ES), formin 1 (an actin nucleation protein capable of generating long stretches of actin microfilaments), and Arp3 (a branched actin polymerization protein causing linear actin filaments to become a branched network) were used for this study. Also shown is immunoprecipitated β-actin, which served as the positive control. Uncropped gel images shown herein can be found in Supplemental Fig. 5 (3.3MB, pdf) . (b) MT regulatory proteins: EB1 (a +TIP protein known to stabilize MTs) and MARK4 (a Ser/Thr protein kinase known to induce MT catastrophe) were also used for this study. Also shown is the immunoprecipitated α-tubulin, which was confirmed by using an anti–β-tubulin antibody for immunoblotting (IB) (see Table 1), which served as the positive control. Changes in protein-protein interaction of these selected regulatory proteins with the corresponding β-actin and α-tubulin, the building blocks of actin- and MT-based cytoskeletons, were assessed by Co-IP. In short, anti-actin IgG or anti–α-tubulin IgG, serving as the immunoprecipitating antibody, was incubated with testis lysates obtained from rats treated with adjudin for 6, 12, 24, and 96 hours vs 0 hours (control). Thereafter, immunocomplexes were pulled out by Protein A/G Plus-agarose beads, the interacting proteins with either actin or α-tubulin were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the interacting proteins (and their changes following adjudin treatment) with either actin or MT were visualized by immunoblottings using corresponding specific antibodies (Table 1). Uncropped gel images shown herein can be found in Supplemental Fig. 6 (3.3MB, pdf) . The steady state of the regulatory proteins (i.e., Eps8, formin 1, Arp3, EB1, and MARK4) in testis lysates following adjudin treatment (without Co-IP) was also assessed and annotated as “Lysate.” Representative immunoblot data were shown in the upper panel in either (a) or (b), and the histograms below summarize results of these findings with n = 3 independent experiments using testes from different rats. *P < 0.05 by ANOVA.
Discussion
In rats, mice, and humans, the epithelial cycle of spermatogenesis is composed of 14, 12, and 6 stages, respectively (3, 15, 34, 35). The release of sperm at spermiation in rodents and humans takes place in the seminiferous epithelium in the adluminal compartment near the tubule lumen at late stage VIII and late stage II of the epithelial cycle, respectively (2–4, 35, 36). Thus, fully developed spermatids (i.e., spermatozoa) can enter the epididymis for their maturation. Although this morphological series of events leading up to sperm release at spermiation have been known for over six decades (2–4, 35–37), the underlying molecular mechanism(s) that regulate spermiation remain to be elucidated. Studies in the last two decades have shed new information regarding the biology of spermiation as recently reviewed (1, 6, 10, 38, 39). Emerging evidence has shown that the event of spermiation is regulated by several classes of proteins. First, protein kinases such as p-FAK-Tyr397, p-FAK-Tyr407, and c-Yes (40–45) are putatively regulatory of ES function in the testis, in particular at the apical ES that confers elongating/elongated spermatid adhesion. It is likely that these protein kinases exert their regulatory effects by phosphorylating adhesion protein complexes specific to the apical ES [e.g., α6β1-integrin/laminin-α3/β3/γ3 complex (40, 46–50), nectin-afadin (51–53)], thereby altering the adhesive properties of these adhesion protein complexes that lead to spermatid release from the apical ES site. Second, the involvement of actin- and MT-based cytoskeletons plus the corresponding binding and regulatory proteins, including the downstream nonreceptor protein kinases in mediating changes in cytoskeletal function to support spermiation. Studies have shown that the apical ES is the only anchoring device once it appears at the interface between Sertoli cells and step 8 spermatids in rodents, replacing gap junction and desmosome. In short, the apical ES serves as the only anchoring device until the release of sperm at spermiation (9, 11, 54–57). Furthermore, studies have shown that this is also one of the strongest anchoring junctions in mammalian tissues and cells, considerably stronger than desmosome (7, 58). In this context, it is of interest to note that desmosome maintains the barrier in skin and thought to be an exceptionally strong adhesive junction (59, 60). This unusual adhesive strength of the ES is likely contributed by the array of actin filament bundles that sandwich between the apposing Sertoli-spermatid plasma membrane and the cisternae of the endoplasmic reticulum (5, 8). Additionally, a network of MTs is also located adjacent to the actin filament bundles at the apical ES (5, 10, 61), providing further structural and functional support to apical ES integrity and dynamics. Nonetheless, how these cytoskeletons regulate the release of sperm at spermiation remains unknown. Studies have shown that small G proteins (also known as guanine nucleotide-binding proteins), such as Rac, Rho, Rab, and Cdc42 that are GTPases found in the testis and expressed by Sertoli and/or germ cells, are also involved in the regulation of actin cytoskeletal function of multiple mammalian cells besides Sertoli cells (62–68). For instance, inactivation of Sertoli cell Rho family proteins leads to a disruption of the actin cytoskeleton (63). Also, a recent report has shown that triptolide, a medicinal plant Triterygium wilfordii, is a diterpene triepoxide capable of perturbing Sertoli cell actin organization at the ES by inhibiting Rho GTPases, including RhoA, RhoB, Cdc42, and Rac1 (69). Collectively, these findings illustrate the substantial involvement of G proteins in cytoskeletal organization in the testis and also in spermiation should be carefully evaluated in future studies.
A recent report has demonstrated that spermatid adhesion and spermatid transport are supported by the concerted efforts of the actin- and MT-based cytoskeletons (19). For instance, MTs and F-actin–conferred tracks are working together to support the transport of spermatids and organelles (e.g., residual bodies, phagosomes) across the seminiferous epithelium. The disruption of these cytoskeletons in Sertoli cells across the seminiferous epithelium following adjudin treatment was found to perturb spermatid transport, leading to the entrapment of spermatids in the epithelium, and failed to be released into the tubule lumen (19). Also, F-actin that supported apical ES function was grossly disrupted, failing to support spermatid adhesion, leading to its premature release from the epithelium by mimicking spermiation (19). However, the molecular mechanism(s) underlying these changes remains unknown. Herein, we have used an in vivo model by treating adult rats with adjudin that mimics the release of sperm at spermiation. Premature release of elongated spermatids from the epithelium was found to precede with a surge in association between Eps8 [an actin barbed-end capping and bundling protein (70, 71) that supports actin filament bundles at the ES (27)] and actin. Additionally, a surge in association between formin 1 [an actin nucleator that polymerizes actin monomers into linear microfilaments (72, 73) to support ES function in the testis (28)] and actin was also noted. This increase in association between Eps8 and actin as well as formin 1 and actin was detected by 6 hours after adjudin treatment. Apparently, this is a physiological response of the testis to maintain apical ES function, in an attempt to prevent elongated spermatids from leaving the epithelium prematurely induced by adjudin. Thus, the testis might be trying to generate additional actin microfilaments (through the action of formin 1) so that they can be assembled into bundles (via Eps8) to support apical ES integrity to counteract the disruptive effects of adjudin. Regardless of these efforts, the subsequent dissociation of Eps8 and formin 1 from actin is accompanied by a surge in association between Arp3 [a branched actin nucleation protein (74, 75) that effectively converts linear actin microfilaments into a branched network to destabilize apical ES (29)] and actin by 12 hours. This thus leads to F-actin disorganization to facilitate spermatid release. These changes take place alongside an increase in association between EB1 (an MT stabilizing protein that promotes MT integrity) and α-tubulin by 6 to 12 hours, which is followed by a considerable decline in the EB1 and α-tubulin association. Thereafter, a surge in association between MARK4 [it induces MT catastrophe (33, 76) and a known MT regulator in the testis (24)] and α-tubulin is detected by 12 to 24 hours, leading to MT disruption to facilitate spermatid release from the epithelium. In short, these findings illustrate a simple mechanism is in place in the testis to confer the timely transfer of both actin- and MT-based cytoskeletons from a bundled (or intact) to an unbundled (or disrupted) configuration to support the release of sperm at spermiation via changes in association between the corresponding regulatory proteins that promote actin/MT integrity vs promote actin/MT disorganization.
In summary, the testis is utilizing a simple and seamless mechanism involving two sets of actin and MT regulatory proteins that either promote the assembly of actin filaments or MTs into a bundled/intact configuration compared with a branched/unbundled/disorganized configuration. This thus confers plasticity to the ES to support spermatid adhesion and also spermatid transport during spermiogenesis or spermatid release at spermiation.
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health, Eunice Kennedy Shriver National Insitute of Child Health and Human Development Grants R01 HD056034 and U54 HD029990 Project 5 to C.Y.C.; National Natural Science Foundation of China (NSFC) Grant 81730042 to R.S.G.; NSFC Grant 81601264 to L.L.; Zhejiang Provincial Natural Science Foundation Grant LQ16H04004 to L.L.; Health and Family Planning Commission of Zhejiang Province Grant 2016KYB202 to L.L.; Department of Education of Zhejiang Province Grant Y201534170 to L.L.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- Arp3
- actin-related protein 3
- BTB
- blood-testis barrier
- b.w.
- body weight
- Co-IP
- coimmunoprecipitation
- DAPI
- 4′,6-diamidino-2-phenylindole
- EB1
- end-binding protein 1
- Eps8
- epidermal growth factor receptor pathway substrate 8
- ES
- ectoplasmic specialization
- H&E
- hematoxylin and eosin
- IF
- immunofluorescence
- MAP
- microtubule-associated protein
- MARK
- microtubule affinity-regulating kinase
- MT
- microtubule
- PBS
- phosphate-buffered saline
- v/v
- volume-to-volume ratio.
References
- 1.Cheng CY, Mruk DD. Biochemistry of Sertoli cell/germ cell junctions, germ cell transport, and spermiation in the seminiferous epithelium In: Griswold MD, ed. Sertoli Cell Biology. 2nd ed. Amsterdam: Elsevier; 2015:333–383. [Google Scholar]
- 2.Xiao X, Mruk DD, Wong CKC, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology (Bethesda). 2014;29(4):286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann RP. The cycle of the seminiferous epithelium in humans: a need to revisit? J Androl. 2008;29(5):469–487. [DOI] [PubMed] [Google Scholar]
- 4.Hess RA, Renato de Franca L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. [DOI] [PubMed] [Google Scholar]
- 5.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1(1):14–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell L, Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec. 1976;185(3):259–278. [DOI] [PubMed] [Google Scholar]
- 8.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6(7):380–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778(3):692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang EI, Mruk DD, Cheng CY. Regulation of microtubule (MT)-based cytoskeleton in the seminiferous epithelium during spermatogenesis. Semin Cell Dev Biol. 2016;59:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. [DOI] [PubMed] [Google Scholar]
- 12.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25(5):747–806. [DOI] [PubMed] [Google Scholar]
- 13.Stanton PG. Regulation of the blood-testis barrier. Semin Cell Dev Biol. 2016;59:166–173. [DOI] [PubMed] [Google Scholar]
- 14.Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84(5):851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64(1):16–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.França LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–259. [PubMed] [Google Scholar]
- 17.Chen YM, Lee NPY, Mruk DD, Lee WM, Cheng CY. Fer kinase/FerT and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod. 2003;69(2):656–672. [DOI] [PubMed] [Google Scholar]
- 18.Cheng CY. Toxicants target cell junctions in the testis: insights from the indazole-carboxylic acid model. Spermatogenesis. 2015;4(2):e981485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang EI, Lee WM, Cheng CY. Coordination of actin- and microtubule-based cytoskeletons supports transport of spermatids and residual bodies/phagosomes during spermatogenesis in the rat testis. Endocrinology. 2016;157(4):1644–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl. 2012;35(1):86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng CY, Mruk D, Silvestrini B, Bonanomi M, Wong CH, Siu MKY, Lee NPY, Lui WY, Mo MY. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005;72(4):251–261. [DOI] [PubMed] [Google Scholar]
- 22.Latendresse JR, Warbrittion AR, Jonassen H, Creasy DM. Fixation of testes and eyes using a modified Davidson’s fluid: comparison with Bouin’s fluid and conventional Davidson’s fluid. Toxicol Pathol. 2002;30(4):524–533. [DOI] [PubMed] [Google Scholar]
- 23.Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the sertoli cell blood-testis barrier in male rats: an in vitro study. Endocrinology. 2015;156(2):680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang EI, Xiao X, Mruk DD, Qian XJ, Mok KW, Jenardhanan P, Lee WM, Mathur PP, Cheng CY. Microtubule affinity-regulating kinase 4 (MARK4) is a component of the ectoplasmic specialization in the rat testis. Spermatogenesis. 2012;2(2):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting: an inexpensive alternative to commercially available kits. Spermatogenesis. 2011;1(2):121–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian X, Mruk DD, Wong EW, Lie PP, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in adult rat testes. Endocrinology. 2013;154(5):1907–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23(8):2555–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li N, Mruk DD, Wong CKC, Han D, Lee WM, Cheng CY. Formin 1 regulates ectoplamic specialization in the rat testis through its actin nucleation and bundling activity. Endocrinology. 2015;156(8):2969–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107(25):11411–11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akhmanova A, Steinmetz MO. Control of microtubule organization and dynamics: two ends in the limelight. Nat Rev Mol Cell Biol. 2015;16(12):711–726. [DOI] [PubMed] [Google Scholar]
- 31.Akhmanova A, Steinmetz MO. Microtubule +TIPs at a glance. J Cell Sci. 2010;123(Pt 20):3415–3419. [DOI] [PubMed] [Google Scholar]
- 32.Yu I, Garnham CP, Roll-Mecak A. Writing and reading the tubulin code. J Biol Chem. 2015;290(28):17163–17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol. 2013;217(2):R13–R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Kretser DM, Kerr JB. The cytology of the testis In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW, eds. The Physiology of Reproduction. Vo1 1 New York, NY: Raven Press; 1988:837–932. [Google Scholar]
- 35.Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52(1):198–236. [DOI] [PubMed] [Google Scholar]
- 36.Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3(4):404–417. [DOI] [PubMed] [Google Scholar]
- 37.Russell LD. The perils of sperm release-- ‘let my children go’. Int J Androl. 1991;14(5):307–311. [DOI] [PubMed] [Google Scholar]
- 38.O’Donnell L, O’Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol. 2014;30:45–54. [DOI] [PubMed] [Google Scholar]
- 39.O’Donnell L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis. 2015;4(2):e979623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beardsley A, Robertson DM, O’Donnell L. A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol. 2006;190(3):759–770. [DOI] [PubMed] [Google Scholar]
- 41.Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β 1-integrin and focal adhesion complex-associated proteins. Endocrinology. 2003;144(5):2141–2163. [DOI] [PubMed] [Google Scholar]
- 42.Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA. 2012;109(31):12562–12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan HT, Mruk DD, Li SYT, Mok KW, Lee WM, Wong CKC, Cheng CY. p-FAK-Tyr(397) regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization. Am J Physiol Endocrinol Metab. 2013;305(6):E687–E699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao X, Mruk DD, Cheng CY. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab. 2013;304(2):E145–E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol. 2011;43(4):651–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan HHN, Cheng CY. Laminin α 3 forms a complex with β3 and γ3 chains that serves as the ligand for α 6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281(25):17286–17303. [DOI] [PubMed] [Google Scholar]
- 47.Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70(4):945–964. [DOI] [PubMed] [Google Scholar]
- 48.Salanova M, Ricci G, Boitani C, Stefanini M, De Grossi S, Palombi F. Junctional contacts between Sertoli cells in normal and aspermatogenic rat seminiferous epithelium contain α6β1 integrins, and their formation is controlled by follicle-stimulating hormone. Biol Reprod. 1998;58(2):371–378. [DOI] [PubMed] [Google Scholar]
- 49.Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of β 1 integrin subunit in rat seminiferous epithelium. Biol Reprod. 1992;47(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 50.Koch M, Olson PF, Albus A, Jin W, Hunter DD, Brunken WJ, Burgeson RE, Champliaud MF. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145(3):605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, Nishimune Y, Takai Y. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol. 2002;12(13):1145–1150. [DOI] [PubMed] [Google Scholar]
- 52.Ogita H, Takai Y. Cross-talk among integrin, cadherin, and growth factor receptor: roles of nectin and nectin-like molecule. Int Rev Cytol. 2008;265:1–54. [DOI] [PubMed] [Google Scholar]
- 53.Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275(14):10291–10299. [DOI] [PubMed] [Google Scholar]
- 54.Vogl A, Pfeiffer D, Redenbach D, Grove B. Sertoli cell cytoskeleton In: Russell L, Griswold M, eds. The Sertoli Cell. St. Louis, MO: Clearwater: Cache River Press; 1993:39–86. [Google Scholar]
- 55.Vogl AW. Distribution and function of organized concentrations of actin filaments in mammalian spermatogenic cells and Sertoli cells. Int Rev Cytol. 1989;119:1–56. [DOI] [PubMed] [Google Scholar]
- 56.Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays. 2007;29(1):36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004;15(9):439–447. [DOI] [PubMed] [Google Scholar]
- 58.Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl. 2005;26(3):354–359. [DOI] [PubMed] [Google Scholar]
- 59.Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127(11):2499–2515. [DOI] [PubMed] [Google Scholar]
- 60.Green KJ, Gaudry CA. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol. 2000;1(3):208–216. [DOI] [PubMed] [Google Scholar]
- 61.Russell LD. Form, dimensions, and cytology of mammalian Sertoli cells In: Russell LD, Griswold MD, eds. The Sertoli Cell. St. Louis, MO: Clearwater: Cache River Press; 1993:1–37. [Google Scholar]
- 62.Mayor R, Etienne-Manneville S. The front and rear of collective cell migration. Nat Rev Mol Cell Biol. 2016;17(2):97–109. [DOI] [PubMed] [Google Scholar]
- 63.Freeman EA, Jani P, Millette CE. Expression and potential function of Rho family small G proteins in cells of the mammalian seminiferous epithelium. Cell Commun Adhes. 2002;9(4):189–204. [DOI] [PubMed] [Google Scholar]
- 64.Mruk DD, Lau AS, Conway AM. Crosstalk between Rab GTPases and cell junctions. Contraception. 2005;72(4):280–290. [DOI] [PubMed] [Google Scholar]
- 65.Mruk DD, Lau ASN. RAB13 participates in ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2009;80(3):590–601. [DOI] [PubMed] [Google Scholar]
- 66.Du M, Young J, De Asis M, Cipollone J, Roskelley C, Takai Y, Nicholls PK, Stanton PG, Deng W, Finlay BB, Vogl AW. A novel subcellular machine contributes to basal junction remodeling in the seminiferous epithelium. Biol Reprod. 2013;88(3):60. [DOI] [PubMed] [Google Scholar]
- 67.Ceriani M, Scandiuzzi C, Amigoni L, Tisi R, Berruti G, Martegani E. Functional analysis of RalGPS2, a murine guanine nucleotide exchange factor for RalA GTPase. Exp Cell Res. 2007;313(11):2293–2307. [DOI] [PubMed] [Google Scholar]
- 68.Gómez O, Ballester-Lurbe B, Guasch RM, Pérez-Roger I, García-Roselló E, Terrado J. Analysis of RhoE expression in the testis, epididymis and ductus deferens, and the effects of its deficiency in mice. J Anat. 2014;225(6):583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Zhao F, Lv ZM, Shi WQ, Zhang LY, Yan M. Triptolide disrupts the actin-based Sertoli-germ cells adherens junctions by inhibiting Rho GTPases expression. Toxicol Appl Pharmacol. 2016;310:32–40. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed S, Goh WI, Bu W. I-BAR domains, IRSp53 and filopodium formation. Semin Cell Dev Biol. 2010;21(4):350–356. [DOI] [PubMed] [Google Scholar]
- 71.Upadhyay RD, Kumar AV, Ganeshan M, Balasinor NH. Tubulobulbar complex: cytoskeletal remodeling to release spermatozoa. Reprod Biol Endocrinol. 2012;10(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dominguez R. The WH2 domain and actin nucleation: necessary but insufficient. Trends Biochem Sci. 2016;41(6):478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shekhar S, Pernier J, Carlier MF. Regulators of actin filament barbed ends at a glance. J Cell Sci. 2016;129(6):1085–1091. [DOI] [PubMed] [Google Scholar]
- 74.Pizarro-Cerdá J, Chorev DS, Geiger B, Cossart P. The diverse family of Arp2/3 complexes. Trends Cell Biol. 2017;27(2):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tyler JJ, Allwood EG, Ayscough KR. WASP family proteins, more than Arp2/3 activators. Biochem Soc Trans. 2016;44(5):1339–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McDonald JA. Canonical and noncanonical roles of Par-1/MARK kinases in cell migration. Int Rev Cell Mol Biol. 2014;312:169–199. [DOI] [PubMed] [Google Scholar]
- 77.Tang EI, Xiao X, Mruk DD, Qian XJ, Mok KW, Jenardhanan P, Lee WM, Mathur PP, Cheng CY. Microtubule affinity-regulating kinase 4 (MARK4) is a component of the ectoplasmic specialization in the rat testis. Spermatogenesis. 2012;2(2):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]