Abstract
Two of the most popular bariatric procedures, vertical sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB), are commonly considered metabolic surgeries because they are thought to affect metabolism in a weight loss–independent manner. In support of this classification, improvements in glucose homeostasis, insulin sensitivity, and even discontinuation of type 2 diabetes mellitus (T2DM) medication can occur before substantial postoperative weight loss. The mechanisms that underlie this effect are unknown. However, one of the common findings after VSG and RYGB in both animal models and humans is the sharp postprandial rise in several gut peptides, including the incretin and satiety peptide glucagonlike peptide-1 (GLP-1). The increase in endogenous GLP-1 signaling has been considered a primary pathway leading to postsurgical weight loss and improvements in glucose metabolism. However, the degree to which GLP-1 and other gut peptides are responsible for the metabolic successes after bariatric surgery is continually debated. In this review we discuss the mechanisms underlying the increase in GLP-1 and its potential role in the metabolic improvements after bariatric surgery, including remission of T2DM. Understanding the role of changes in gut peptides, or lack thereof, will be crucial in understanding the critical factors necessary for the metabolic success of bariatric surgery.
We review the role of elevated GLP-1 in the metabolic success of the two most commonly performed bariatric procedures, vertical sleeve gastrectomy and Roux-en-Y gastric bypass.
Despite enormous efforts through lifestyle intervention and pharmaceutical strategies, bariatric surgery remains the most effective treatment for people with severe obesity (1, 2). Beyond weight loss, bariatric procedures result in rapid improvements in glycemia and, for many patients, remission of type 2 diabetes mellitus (T2DM) and other obesity-associated comorbidities. The two most commonly performed bariatric procedures worldwide are the Roux-en-Y gastric bypass (RYGB) and the vertical sleeve gastrectomy (VSG) (3). The RYGB operation bypasses ~95% of the stomach and upper gastrointestinal (GI) tract by creating a small gastric pouch just under the esophagus, then anastomosing the mid- to distal jejunum directly to the small pouch. The remainder of the stomach and the proximal intestine remain in the body and are excluded from nutrient flow, but the distal portion of the duodenum is reattached further down within the jejunum to allow bile acids and digestive enzymes to reach nutrients. In VSG, nearly 80% of the stomach along the greater curvature is removed, creating a narrow tubular stomach, leaving the pylorus structurally intact. In contrast to RYGB, VSG does not involve intestinal rearrangement and thus preserves normal intestinal nutrient flow (4). In a randomized trial, RYGB and VSG compared with medical therapy alone resulted in excess weight loss of ~28%, 25%, and 5% and with T2DM remission of 42%, 37%, and 12%, respectively (5). These data indicate that although the surgeries have drastic differences in anatomical rearrangement, their impact on weight loss and T2DM remission is remarkably greater than that of current medical strategies.
Since the publication of two integral articles nearly 20 years ago (6, 7), research into the mechanisms behind bariatric surgery–induced improvements in body mass and glucose homeostasis has resulted in several theories. Historical hypotheses have focused on the anatomical aspects of the procedures. VSG and RYGB were thought to be “restrictive” because the reduced stomach size would restrict the amount of food that could be ingested at any given meal. Alternatively, surgeries such as RYGB were also thought to be “malabsorptive” in that the intestinal rearrangement would limit macronutrient absorption and calories would be lost during elimination (8, 9). Although these mechanical hypotheses are often still discussed, there are wide-ranging changes in physiology and feeding behavior observed in both animals and humans that cannot be explained by these anatomical rearrangements.
One predominant physiological change after bariatric surgery is the large postprandial increase in a variety of gut-secreted peptides. Many of these peptides have been found to be critical physiological regulators of appetite, energy expenditure, and glucose and lipid metabolism, making them good mechanistic candidates for improved metabolism after surgery. However, the extent to which the increase of any single peptide is the sole mechanism underlying the effect of bariatric procedures is debated. Given the drastic differences in postoperative anatomies but similar outcomes between RYGB and VSG, further scrutiny of intestinal hormone responses between these procedures may provide insight into the hormone-driven mechanisms that underlie metabolic surgical success. This mini-review explores the role of gut peptides, particularly glucagonlike peptide-1 (GLP-1), in the improvement in glycemic control resulting from VSG and RYGB procedures.
Gut Peptide Regulation and Physiology
The intestine secretes a host of gut peptides that respond to nutrient ingestion and regulate glucose and lipid metabolism and satiety. It was originally suggested that the various intestinally derived peptides are produced and secreted by distinct enteroendocrine cells. For example, specialized enteroendocrine K- and I-cells secreted glucose-dependent insulinotropic peptide (GIP) and cholecystokinin (CCK), whereas enteroendocrine L-cells produce preproglucagon-derived peptides [GLP-1, glucagonlike peptide-2 (GLP-2), and oxyntomodulin] and also peptide YY (PYY) (10). However, recent work has challenged this traditional view, because individual enteroendocrine cells have been shown to express multiple gut peptides (11–13). For example, many “L-cells” in the distal jejunum and ileum coexpress GLP-1 and PYY (14), whereas proximal “L-cells” can also produce GLP-1 (15), in addition to secreting CCK, GIP, neurotensin, or secretin (16). The mechanisms that drive enteroendocrine release of these gut peptides into the blood stream are multifactorial and include responses to direct nutrient stimulation, intracellular metabolism, or neuroendocrine mechanisms [see Psichas, et al. (16) for review].
Once released, gut peptides can act locally on afferent neurons innervating the GI tract that signal to the caudal brainstem or enteric neurons, or they can enter the circulation to act on peripheral targets to regulate glucose and lipid metabolism (17). Although many of these peptides work together to induce satiety and improve whole-body glycemia, only a select few have been implicated in the metabolic improvements seen after bariatric procedures. Perhaps because of the timing in which bariatric surgery procedures became more popular alongside developing therapeutics targeted to GLP-1, GLP-1 in particular has been targeted as a mechanism underlying the success of bariatric surgery. In this article, although we briefly summarize the noted changes in other gut peptides, our focus will be on the role of GLP-1 in the success of bariatric surgery.
Gut Peptide Responses to VSG and RYGB
Proximal gut peptides
If postprandial gut peptides are responsible for the success of surgery, then one would predict that the cocktail of peptides initiating these changes would be similar between RYGB and VSG. Given their differing anatomical rearrangements, comparing changes in gut peptides between VSG and RYGB could help narrow down the peptides critical for metabolic success. This reasoning would probably rule out any differences in proximally secreted gut peptides. Although this seems like a rational approach, the data are not as clear as one would predict.
Ghrelin, secreted from gastric and duodenal enteroendocrine cells, is one of the only GI tract–secreted peptides to decrease postprandially and to increase feeding when administered exogenously (18). Given that the majority of the stomach is either bypassed or removed with RYGB and VSG, ghrelin levels would be expected to decrease postoperatively. However, it seems that leaving the stomach tissue in the peritoneal cavity after RYGB is sufficient to maintain ghrelin levels, because multiple studies have demonstrated significantly greater reductions in fasting ghrelin levels after VSG compared with RYGB (19, 20). Whether this decrease in ghrelin is important is in question because mice genetically deficient in ghrelin have normal body weight loss and improvements in glucose tolerance in response to VSG, suggesting the postsurgical decrease in ghrelin is not necessary for metabolic improvements after VSG (21).
A predominant peptide secreted from the duodenum, CCK, promotes gallbladder contraction, slows gastric emptying, and acts as a satiety signal. Although early after surgery (2 weeks) the postprandial increase in CCK is greater in VSG than RYGB, whereas postprandial CCK levels were found to be increased by RYGB (22) 1 (23) and 2 (24) years after surgery. Although more research is needed, if we assume overlapping mechanisms between RYGB and VSG, these data suggest that postsurgical changes in ghrelin and CCK are not a necessary mechanism leading to improvements in weight and glucose regulation after bariatric surgery.
GIP is secreted from enteroendocrine cells located within the proximal gut and is critical for regulation of insulin and gastric secretion and motility. Data on the impact of GIP in postoperative metabolic improvements are conflicting. Postprandial levels of GIP have been reported to be similar 2 weeks after (22) or higher 1 month (25) after RYGB compared to preoperative levels. However, 1 year postoperatively, postprandial GIP levels were found to be lower after RYGB (26) and VSG (20). One possibility for these discrepancies is that postoperative timing of measurements lead to conflicting results. However, GIP is secreted in an active form and is then rapidly degraded by a protease enzyme called dipeptidyl dipeptidase IV (DPPIV). None of these previous studies seemed to measure the active peptide, which may contribute to the conflicting literature. In contrast, the literature does not conflict with regard to the impact of bariatric surgery and responses of GLP-1, a peptide that is also secreted in its active form and is even more rapidly degraded than GIP.
Distal gut peptides
Given that PYY and GLP-1 have been found to be cosecreted, it is not surprising that many clinical and preclinical studies find postprandial increases in both peptides after VSG and RYGB (27–32). Although consistent increases in PYY are seen after surgery, its mechanistic role in the weight loss associated with bariatric surgery has not been studied as extensively as with GLP-1.
The gene encoding GLP-1, preproglucagon, codes multiple peptides in a tissue-specific fashion. Whereas GLP-1 and oxyntomodulin are both thought to regulate satiety and glucose homeostasis, GLP-2 has a more predominant role in regulating intestinal morphology. Nevertheless, as one would predict, oxyntomodulin (29) and GLP-2 (33, 34) have also been reported to be increased by RYGB. Whereas changes in oxyntomodulin after VSG have not been reported, the increase in GLP-2 after VSG has been found to be comparable to that after RYGB (34). Preclincal studies in rats demonstrate that the increase in GLP-2 occurs in parallel with intestinal hypertrophy (35). The role of increased GLP-2 after VSG is unclear because intestinal hypertrophy occurs to a lesser extent, if at all, after VSG (36, 37).
Like GIP, GLP-1 is rapidly degraded by DPPIV, limiting circulating levels. It has been estimated that only 10% to 15% of the secreted GLP-1 reaches the peripheral tissue in the intact form (38). However, in contrast to GIP, GLP-1 has been found to be consistently elevated in response to both RYGB and VSG. Importantly, the effect of both RYGB and VSG on postprandial GLP-1 release is not seen in patients with an equivalent degree of weight loss achieved by caloric restriction (25), highlighting the physiological effect of surgery. Both total and active levels of GLP-1 are increased after surgery. In fact, the absolute concentration of the intact hormone is nearly 10 times higher after RYGB and VSG (23). Although some preclinical (39) and clinical studies (26, 40, 41) demonstrate similar postprandial increases in active GLP-1, some reports have suggested that RYGB increases postprandial GLP-1 to a greater extent than VSG (19, 20). Regardless, it is clear that VSG results in greater postprandial GLP-1 levels compared with the same patients before surgery or compared with overweight controls. Thus, whether these smaller differences in GLP-1 between RYGB and VSG or between VSG and weight loss controls contribute to differences in metabolic outcome remains to be seen.
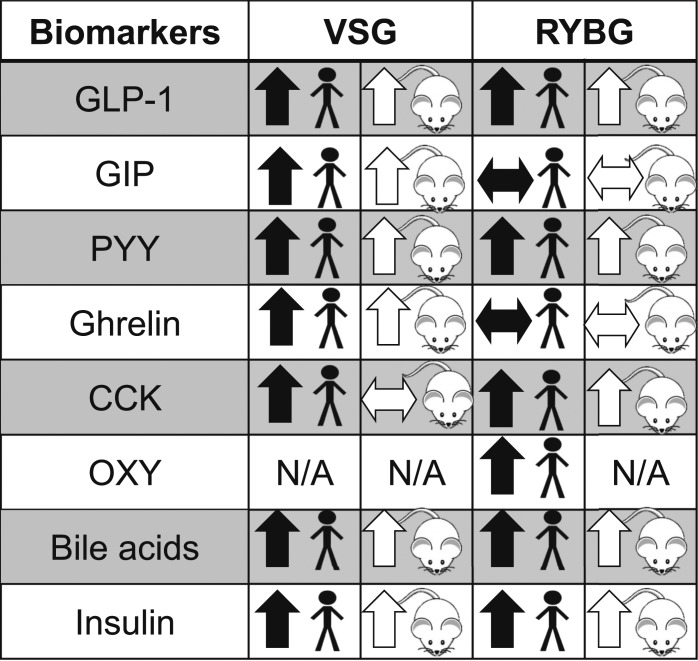
Altogether, these data indicate that changes in GI anatomy can greatly alter the cocktail of postprandial gut peptides (Fig. 1). When RYGB is compared with VSG, it is apparent that the most consistent changes after these two surgeries are in the distal gut peptides, including PYY and the preproglucagon peptides. Although these changes, particularly in PYY and GLP-1, have been found to be associated with greater weight loss, association does not mean causation, and it could be that these changes in gut peptides are simply a physiological marker for bariatric surgery.
Figure 1.
Summary of peptide responses after VSG and RYGB in human and animal models. Horizontal arrow indicates mixed results in the literature. N/A, no change with surgery or not well studied; OXY, oxyntomodulin.
Mechanisms for Surgery-Induced Increases in GLP-1
The mechanisms leading to substantial postprandial release of gut peptides, particularly GLP-1, after bariatric surgery are thought to be largely a consequence of rapid nutrient delivery further down in the GI tract, where the majority of L-cells are located. As one would predict given the change in GI tract anatomy, nutrient delivery into the intestine is extremely rapid after RYGB (41). In fact, early work investigating the physiological mechanisms underlying bariatric surgery used a surgical intervention where a piece of the distal gut was moved up and inserted into the proximal gut. This ileal transposition surgery led to increased GLP-1 and PYY and improved glucose tolerance (42–44). In contrast to these surgeries, VSG increases nutrient delivery to the distal gut by accelerating the gastric emptying rate (45, 46) through increased gastric pressure (46). However, this rapid nutrient entry may be only the start of the process. Although the speed of nutrient delivery may be important after RYGB (47), preclinical research suggests that the gastric emptying rate is not solely responsible for driving increases in GLP-1 after VSG. Notably, nutrient infusion directly into the duodenum at the same rate and volume in rats that had sham or VSG procedures caused similar increases in plasma GLP-1 (46). These data led to the suggestion that intestinal physiology is adapting to rapid nutrient entry by increasing enteroendocrine cell number or increasing nutrient sensitivity of the existing enteroendocrine cell population. One study has shown that VSG in rats promotes increases in L-cell number (36), but another did not (37). The reasons for these discrepant findings after VSG are unclear. One interesting possibility is that differences in prolonged dietary exposure, including chronic exposure to high-fat diets, leads to structural and functional changes in the gut nutrient-sensing pathways (48). For example, the previous study that found changes in cell number maintained animals on a high-fat diet (36), whereas the other study provided a choice between chow and a high-fat diet (37), suggesting that the impact of VSG on intestinal hypertrophy depends on dietary exposure. On the other hand, RYGB is more consistently associated with intestinal hypertrophy regardless of diet exposure (36, 49). Thus, in the case of RYGB, it seems that the increase in enteroendocrine cells, in general, increases the L-cell population, which in turn leads to increased GLP-1 (36).
It has also been postulated that changes in intestinal nutrient-sensing capacity are critical not only for driving changes in gut peptide secretion but also for the overall metabolic success of bariatric procedures. In fact, the intestine is now commonly considered a major site of glucose disposal, thereby stimulating intestinal glucose metabolic pathways toward supporting tissue growth (50). Furthermore, in humans, a study using a uniformly labeled glucose tracer found that glucose absorption was increased after RYGB, and this rapid absorption was associated with the exaggerated release of insulin and GLP-1 (51). Interestingly, active glucose absorption though the intestine has been directly linked to GLP-1 secretion (52).
Another mechanism linking nutrient sensing to the rise in postoperative GLP-1 is the increase in plasma bile acids seen with both RYGB and VSG. These surgeries not only increase total plasma bile acids but also alter the types of bile acids, and this increase is independent of weight loss (53–55). Bile acids act on two different receptors; one is the G-protein–coupled bile acid receptor 1 (TGR5) and the other a nuclear transcription factor, farnesoid X receptor (FXR). The increase in GLP-1 is thought to occur through TGR5 (56). However, the role of bile acids in the surgery-induced rise of GLP-1 remains disputed because GLP-1 responses to VSG were blunted in TGR5 null compared with wild-type mice in one (57) but not another study (58). Although the role of TGR5 remains in question, it is important to note that GLP-1 responses are normal in FXR knockout mice, suggesting that bile acids do not regulate GLP-1 through FXR. As an aside, despite the normal GLP-1 responses, FXR knockout mice do not lose weight or have improvements in glucose tolerance after VSG (59). Thus, bile acid signaling through FXR may be critical for the metabolic success of VSG, but this effect is not mediated via changes in GLP-1.
Collectively, these data suggest that the drive to increase GI peptides, like GLP-1, after surgery is a result of rapid nutrient delivery further down the GI tract, where the majority of L-cells are located. However, the data also indicate that intestinal adaptation in response to chronic exposure to rapid nutrient entry, such as increases in enteroendocrine cell number or nutrient-sensing capability within enteroendocrine cells, contributes to the elevation in postprandial GLP-1 after RYGB or VSG.
Role of GLP-1 in Glucose Homeostasis and T2DM Remission After Bariatric Surgery
Impact of bariatric surgery on glucose homeostasis
Glucose homeostasis is controlled by a complex and integrated system highly dependent on insulin sensitivity and insulin secretion. Clinically, changes in glucose homeostasis are measured by variations in fasting and postprandial glucose and insulin levels, hepatic and skeletal muscle sensitivity, and β-cell responsiveness to glucose. Bariatric surgery affects each of these endpoints. In clinical studies, fasting glucose and insulin are often used in calculating the homeostatic model assessment of insulin resistance (HOMA-IR) as an index of β-cell function and insulin resistance. HOMA-IR has been improved as soon as 5 days after VSG (60) and remains at the same level of improvement as far out as 2 years postoperatively with RYGB and VSG (61).
However, HOMA-IR is a surrogate for studying insulin sensitivity. More complex analyses are vital to increase our understanding of postoperative changes in glucose homeostasis. Hepatic and skeletal muscle insulin sensitivity is best measured via a hyperinsulinemic–euglycemic clamp with glucose tracing techniques. This approach has identified improvements in both fasting hepatic glucose production and hepatic insulin sensitivity in patients with or without T2DM as early as 1 week after RYGB (62), an effect that seems to be maintained for ≥1 year postoperatively (33, 62). Thus, it is clear that hepatic glucose metabolism is improved in patients with or without T2DM after surgery. Both human and rodent studies suggest that early improvements in insulin sensitivity are driven predominantly by the liver, whereas later improvements in skeletal muscle sensitivity come with weight loss (39, 51).
The mechanisms for the early improvements in hepatic glucose production are unknown. However, impaired hepatic insulin action is associated with increases in hepatic fat content. Indeed, both RYGB and VSG produce a dramatic decrease in hepatic fat content and parallel normalization of hepatic glucose production (63). Assuming that reductions in hepatic fat content are unlikely to occur immediately after surgery, an alternative explanation is necessary for the early improvements in hepatic insulin sensitivity. In fact, it has been suggested that the early improvement is due solely to reduced energy intake (64). This issue is an important consideration for the weight loss–independent effects of surgery.
Like those of postprandial GLP-1, the temporal kinetics of insulin are altered postoperatively, resulting in a more rapid peak before returning to a lower fasting level in both rodents and humans. Although these postprandial insulin kinetics may relate directly to GLP-1, in a pooled group of patients who underwent VSG and RYGB, pancreatic fat deposition as assessed by positron emission tomography was found to be reduced alongside improvements in β-cell function (65), suggesting that surgery has effects on the pancreas that are not dependent on a direct effect of GLP-1 on insulin secretion. Thus, bariatric surgery alters every aspect of glucose control from insulin secretion to sensitivity.
Impact of GLP-1 on glucose homeostasis after bariatric surgery
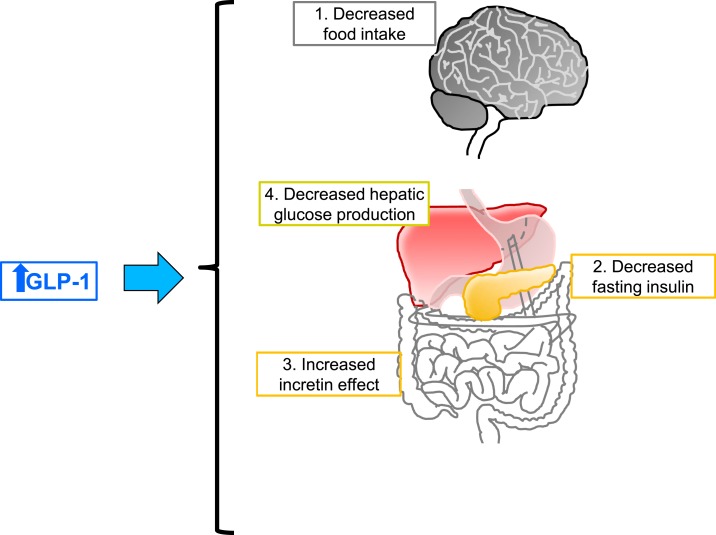
Although the surgery-induced rise in postprandial GLP-1 levels is associated with weight loss and T2DM remission, it remains unknown whether this rise is the cause of postoperative metabolic improvements. Although the effect of GLP-1 on insulin secretion is clear, GLP-1 has been found to act on multiple aspects of glucose control and through a variety of target organs. Some of these effects are summarized in Fig. 2.
Figure 2.
Proposed postprandial GLP-1 action after bariatric surgery. GLP-1 has a wide array of physiological effects. Some effects that are thought to play a role in responses to bariatric surgery include reductions in (1) food intake and, consequently, weight loss, (2) decreased fasting insulin levels, (3) increased incretin effects (greater GLP-1 leads to greater postprandial insulin), and (4) reduced hepatic glucose production. Both clinical and preclinical work support the role of GLP-1 in increasing postprandial insulin levels. However, it is less clear whether GLP-1 directly affects weight loss and T2DM remission associated with surgery.
Decades ago, it was observed that an oral glucose load leads to a much greater insulin excursion than intravenous glucose (66). This phenomenon, dubbed the incretin effect, has been credited to two particular gut peptides, GLP-1 and GIP (67, 68). In nonobese subjects without T2DM, GLP-1 and GIP contribute nearly equally to the incretin effect, stimulating the majority of postprandial insulin release (67). However, in patients with T2DM (69) and, although not to the same degree, in obese patients with normal glucose tolerance (70), the overall incretin effect is reduced. Both RYGB and VSG correct this abnormality and even increase the incretin effect. Given the inconsistent results in the increase in GIP postoperatively, the extent to which GIP contributes to improved glucose tolerance is debatable. Further implicating GLP-1 in the increased insulin response, administration of the GLP-1 receptor antagonist exendin(9-39) has been found to impair insulin response to an oral glucose load in both humans and rodents (39, 71–73). In an effort to determine whether GIP is important after RYGB, one study gave patients who underwent RYGB the DPPIV inhibitor sitagliptin to increase the bioavailability of both GLP-1 and GIP (74). They then combined sitagliptin with the GLP-1 receptor antagonist exendin(9-39) to block GLP-1 signaling and consequently isolate the impact of GIP signaling on glucose tolerance outcomes. Interestingly, sitagliptin did not improve glucose tolerance or β-cell function when GLP-1 receptor signaling was blocked. In contrast, patients with T2DM who have not undergone bariatric procedures fully responded to the DPPIV inhibitor with improved glucose tolerance and insulin secretion, when it was combined with exendin(9-39) (75). Together these data suggest that RYGB shifts the balance of the incretin effect toward GLP-1 and away from GIP.
Although the effect of GLP-1 on insulin secretion after bariatric surgery is clear, GLP-1 has been found to act on multiple aspects of glucose control and through a variety of target organs (Fig. 2). Central nervous system GLP-1 receptors have been shown to be important for regulating body weight and glucose homeostasis, including suppression of hepatic glucose production in rodents (76–78), suggesting that surgically induced increases in GLP-1 could also act centrally to regulate changes in glucose and body weight. To test this hypothesis, exendin(9-39) was infused centrally in rats that had RYGB or sham surgeries (79). Interestingly, the GLP-1 receptor antagonist induced similar increases in body mass regardless of surgery. These data suggest that although these receptors are important for regulating normal body mass, they are not unique to RYGB. Unfortunately, glucose homeostasis was not studied. On the other hand, recent work has demonstrated that downregulation of the β-cell GLP-1 receptor in adult mice by an inducible Cre-loxP system is necessary for surgery-induced improvements in glucose tolerance and glucose-stimulated insulin secretion but not for VSG-induced weight loss (80). These data are consistent with data in humans and rats, where a potent GLP-1 receptor antagonist reduced postprandial insulin secretion after both RYGB (39, 72) and VSG (39). However, when whole-body GLP-1 receptors are knocked out, the animals lose comparable amounts of weight to their wild-type littermates after both VSG (81, 82) and RYGB (79), suggesting that GLP-1 signaling is not necessary for surgery-induced weight loss. Altogether, the data certainly indicate that the increase in postprandial GLP-1 drives acute glucose responses to a meal after bariatric surgery but is not necessary for weight loss.
Role of GLP-1 in T2DM Remission
T2DM is classically characterized by exaggerated insulin secretion in compensation for whole-body insulin resistance and subsequent progressive failure of pancreatic β-cell function. Although this disease is thought to be chronic and progressive, given the aforementioned improvements in insulin secretion and sensitivity it is not surprising that there is a concomitant remission of T2DM for many patients. Although the postprandial GLP-1 response may be necessary for the insulin and glucose responses to a meal after bariatric surgery, whether GLP-1 is responsible for remission of T2DM is unclear. One study found that nutrient-induced GLP-1 response was one of the best predictors of T2DM remission after RYGB (32), whereas another study found that GLP-1 response to a mixed meal in patients 2 years after VSG was similar regardless of postoperative remission, relapse, or no remission of T2DM (83). These subjects also had impaired insulin secretion after a GLP-1 antagonist but limited deterioration of glucose tolerance, suggesting that the increased long-term GLP-1 secretion after VSG is neither sufficient nor critical to maintain normal glucose tolerance in subjects with T2DM. The causative role of GLP-1 in T2DM remission after bariatric surgery will be difficult to tease apart in humans, but other predictive factors such as duration of disease, and thus the degree of β-cell destruction before surgery, may be more critical in determining whether β-cells can recover sufficiently to resolve T2DM (84).
T2DM remission in RYGB and VSG
The overall physiological impact of RYGB and VSG is summarized in Fig. 3. Although this figure summarizes a great number of similarities, whether T2DM remission occurs to the same extent in RYGB and VSG is debatable. Some studies have suggested equal potency between RYGB and VSG (20, 32, 85), whereas others demonstrate that RYGB is more effective than VSG (86–88) at ameliorating T2DM. A small prospective study that compared RYGB and VSG discovered that both procedures resulted in similar improvements in glycemic control and fasting triglyceride levels and in the remission of T2DM (20). A recent 5-year update from the STAMPEDE trial indicated few differences in glycemic, lipid, and metabolic improvements between patients who underwent RYGB and VSG; both surgeries were more effective than intensive medical therapy alone in decreasing or resolving hyperglycemia (86). Some advantages of RYGB over VSG at the 5-year time point included greater weight loss with RYGB and more patients discontinuing T2DM medication (45% with RYGB and 25% with VSG) (86). On the other hand, although RYGB and VSG caused similar reductions in body weight and improvements in HOMA-IR and hemoglobin A1c, RYGB was more effective at improving fasting blood glucose compared with VSG in obese, nondiabetic patients (89), suggesting RYGB and VSG have a similar impact on glucose levels in nondiabetic patients. However, recent studies in patients who underwent RYGB found that estimates of insulin sensitivity with fasting glucose and insulin (including HOMA-IR) or glucose response to an oral glucose load do not readily correlate with direct measures of insulin sensitivity (90), suggesting that there could be methodological concerns about using estimates of insulin sensitivity to compare the efficacy of these two surgeries.
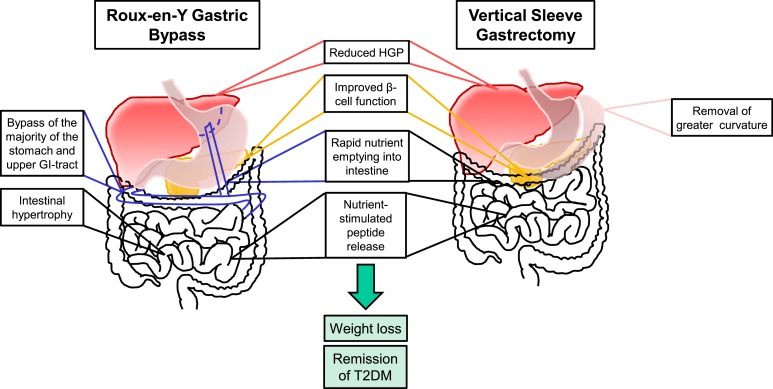
Figure 3.
Similarities and differences in anatomy and outcomes in response to RYGB and VSG. After RYGB (left), a small gastric pouch is made and is anastomosed to the jejunum, bypassing the majority of the stomach and upper intestinal tract. After VSG (right), 80% of the stomach along the greater curvature is removed without intestinal rearrangement to preserve the flow of nutrients through the proximal GI tract. Both surgeries (middle) result in improvements in reduced hepatic glucose production (HGP), increased postprandial insulin secretion, increase nutrient entry rate into the intestine, and increased nutrient-induced gut peptide release, including GLP-1 secretion. Both surgeries also cause weight loss and, in many cases, remission of T2DM.
Altogether, T2DM remission seems to be better after RYGB, suggesting that there are important differences in the impact of each surgery on glucose homeostasis. Elucidating these differences could reveal the mechanisms that underlie surgery-induced improvements in glucose homeostasis.
Weight loss–independent effects on glucose homeostasis after bariatric surgery
It has become increasingly clear that the mechanisms involved in the early glycemic improvements in T2DM remission after bariatric surgery are in addition to the impact of weight loss, given that glucose homeostasis typically improves before weight loss in the first few weeks postoperatively. This phenomenon has given rise to the idea that there are metabolic effects of bariatric surgery that are weight loss independent. That is, weight loss, in and of itself, improves glucose homeostasis, but bariatric surgery causes early, additional responses crucial for the full metabolic effect. Several studies support this hypothesis. One group analyzed multiple studies that compared the endocrine and metabolic responses to either RYGB or low-calorie diets (25). The crucial aspect of this comparison was that both the surgery and dietary intervention subjects had the same degree of weight loss at the time of data collection. They found that the patients who underwent RYGB had improved oral glucose tolerance, sixfold greater GLP-1 levels, and a greater incretin effect (insulin response was greater with oral than with intravenous glucose) compared with the dietary intervention group. In another study, RYGB reduced total and branched-chain amino acids, metabolites that are associated with insulin resistance, compared with patients who had equivalent weight loss through caloric restriction (91). The improvement in glucose metabolism was also evident when compared with an equivalent weight loss group after gastric banding, a form of bariatric surgery in which a band is placed around the proximal stomach (92, 93). Collectively, these data suggest that bariatric surgery, at least RYGB, has metabolic effects that go beyond the impact of weight loss. This effect is more clear in animal studies, where weight-matched controls are easy to achieve by restricting access to food and any changes in diet are controlled for. When this is done, both RYGB and VSG have been shown to result in early improvements in hepatic glucose production, improved glucose tolerance, and improved plasma and hepatic lipids (39, 94).
One argument against this hypothesis is that patients who underwent RYGB were followed 21 days postoperatively alongside a control nonsurgical group that was placed on the same low-calorie diet (500 kcal/d), and both groups demonstrated comparable improvements in insulin sensitivity and β-cell function (95). Regardless of whether the metabolic effects of surgery are indirect, resulting from weight loss, or through an alternative direct factor, it is clear that there are widespread physiological differences in response to weight loss with surgery as opposed to dietary intervention alone. Surgery leads to clear changes in postprandial glucose, insulin, and gut peptide release in addition to increases in bile acids. All these changes probably contribute to a more sustained if not more potent effect on glucose and lipid metabolism than mere calorie restriction.
Differences Between Clinical and Preclinical Results on the Impact of Bariatric Surgery
Animal models of bariatric surgery are a critical tool in determining the mechanisms underlying surgical success. Using traditional genetic techniques, preclinical studies allow us to determine whether a gene is necessary for surgical outcomes. In addition, there is greater ability to control for diet, food intake, or body weight, which is difficult to do in clinical studies. Furthermore, newer technologies are allowing us to manipulate the activity of cell populations on demand (chemogenetics and optogenetics) that will probably be important in understanding changes in the central nervous system and feeding behavior after surgery. However, there are critical species differences from behavior to molecular cell signaling that cannot be ignored.
Qualitatively, rodents and humans have very similar responses to surgery. Both have substantial weight loss, elevated gastric emptying rates, and increased postprandial insulin and GLP-1 levels. Although direct comparisons of RYGB and VSG in rats yield similar weight loss and improvements in glucose tolerance (39), recent data in mice demonstrated that after an initial weight loss, VSG mice continued on a parallel growth trajectory, whereas RYGB animals continued to lose weight for another week and maintained this body weight through 11 weeks postoperatively (96). In the latter study, the initial weight loss and weight regain after VSG in rodents were due to changes in both lean and fat mass, whereas after RYGB, changes in body weight were due predominantly to changes in fat mass. This finding is in contrast to other studies in rats where similar changes in body fat and lean mass were seen after RYGB and VSG (39) and to other mouse models of VSG where differences in body mass over time are due solely to changes in fat mass (59, 82, 97). Furthermore, in humans, postoperative weight loss reflects decreases in both lean and fat mass. As adipose and total body mass decrease in response to the negative energy balance, less muscle mass is necessary for weight bearing, leading to atrophy. In contrast to humans, mice and rats continue to grow throughout their lives, meaning that both fat and lean mass continue to expand. Unlike RYGB in humans, the rodent models of RYGB are associated with increased energy expenditure. However, whether these species differences are due to differences in response to RYGB or to methodological problems in assessing energy expenditure in human and rodent studies is less clear.
Generally speaking, feeding behavior is different between rodents and humans. Whereas humans typically eat three meals a day, rodents tend to eat the majority of their calories at the onset of, and then several smaller meals throughout, their active period (dark cycle). Despite these differences, and despite the caveats that patients are receiving both presurgical and postsurgical dietary counseling, early reductions in food intake drive early surgery-induced weight loss in both humans (98) and rodents (39). In addition, there are clear parallels on the impact of surgery on feeding behavior. Both RYGB and VSG cause animals and humans to ingest smaller, more frequent meals (99–106). Many preclinical experiments maintain animals on the same high-fat diet both before and after surgery, which allows diet-independent analysis of surgical outcomes. Rodents are not faced with the same plethora of food choices as humans. However, both rodents and humans experience a change in food preference, favoring lower-calorie food as opposed to nutrient-dense foods after RYGB and VSG (104, 107, 108). Thus, although there are clearly differences in the ways that laboratory rodents and humans are exposed to food, surgery induces similar changes in feeding behavior, suggesting that these changes are a critical aspect of surgery-induced weight loss.
Finally, although genetic manipulation is one of the strengths of preclinical work, it is important to recognize that transgenic manipulations can induce developmental compensations in parallel pathways that can mask the overall effect of loss of the gene on surgical outcome. There are always important caveats to consider when applying knowledge gained from preclinical models, and thus it is important to consider all these issues when designing studies and interpreting results.
Complications Associated With Bariatric Surgery
An important difference between RYGB and VSG relates to postoperative complications. Generally, complications are less severe for VSG than for RYGB (109), which probably contributes to its increased usage over the years. For VSG, a major complication is gastric reflux, which probably results from the increased gastric pressure. This effect can be severe in some patients and even lead to a subsequent surgical transformation of VSG to RYGB. Although vitamin deficiencies due to malabsorption are common with RYGB, they can also occur with VSG and can be managed to some extent with lifelong vitamin supplementation. Another important complication is the incidence in postoperative hypoglycemia. In a subset of patients, specifically after RYGB, symptoms of postprandial “dumping syndrome” can be debilitating and are characterized by hypoglycemia, hyperinsulinemia, sweating, nausea or vomiting, and heart palpitations. The exact etiology of this type of hypoglycemia is unknown (110). One hypothesis is that this phenomenon is caused by the elevated postprandial GLP-1 and consequently insulin levels after RYGB. Multiple studies have demonstrated that administering exendin(9-39) was sufficient to normalize postprandial insulin and glucose levels and prevent hypoglycemia in patients who underwent RYGB (111, 112). However, considering that GLP-1 levels are also elevated after VSG (though not to the same extent), blocking GLP-1 signaling may be therapeutically sufficient but not the underlying mechanism of dumping syndrome. Lastly, although it is not necessarily a surgical complication per se, some patients do fail to lose weight (113). Thus, the risks for these complications underscore the importance of understanding what mechanisms drive the success of surgery so that other, simpler strategies can be applied or so that we can determine which patients will respond most optimally to surgery.
Conclusions
This review highlights the impact of RYGB and VSG on gut peptide release, particularly GLP-1, and its potential role in the metabolic success of surgery. Largely from preclinical models but also from patients, the data indicate that the postprandial rise in GLP-1 drives the increased insulin and decreased glucose response to a meal. Less clear is the extent to which GLP-1 changes are responsible for T2DM remission. Bariatric surgery success probably results from a range of networking factors rather than a single unifying mechanism. This concept is reflected in recent pharmacotherapies that are aimed at targeting several signaling mechanisms concurrently. This strategy can lead to additivity in weight loss and metabolic improvements, which more closely mirror the effects of metabolic procedures. As evidenced from this review and countless others, bariatric surgeries are now accepted as metabolic surgeries in which weight loss–independent effects on metabolic homeostasis are of equal importance as the weight loss component of the procedure. Postoperative glycemic improvement is a result of multiple contributing factors, which may vary according to the surgical procedure, rodent species, and underlying biology (e.g., sex, genetics, glycemic status) of the patient group studied. Barriers such as health care availability, economic limitations, and surgical invasiveness prohibit bariatric surgery from being widely implemented to solve the worldwide obesity epidemic; therefore, continued preclinical and clinical research will be necessary for understanding the mechanisms of success to find a simpler strategy for weight loss and metabolic health.
Acknowledgments
Financial Support: This work was funded in part by National Institutes of Health Grant R01DK107282.
Acknowledgments
Disclosure Summary: D.S. receives research funding support from the National Institutes of Health, Novo Nordisk, Ethicon Endo-Surgery, and Zafgen. The remaining author has nothing to disclose.
Footnotes
- CCK
- cholecystokinin
- DPPIV
- dipeptidyl dipeptidase IV
- FXR
- farnesoid X receptor
- GI
- gastrointestinal
- GIP
- glucose-dependent insulinotropic peptide
- GLP-1
- glucagonlike peptide-1
- GLP-2
- glucagonlike peptide-2
- HOMA-IR
- homeostatic model assessment of insulin resistance
- PYY
- peptide YY
- RYGB
- Roux-en-Y gastric bypass
- T2DM
- type 2 diabetes mellitus
- TGR5
- G-protein–coupled bile acid receptor 1
- VSG
- vertical sleeve gastrectomy.
References
- 1.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–1585. [DOI] [PubMed] [Google Scholar]
- 2.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, Castagneto M, Bornstein S, Rubino F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–973. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder R, Harrison TD, McGraw SL. Treatment of adult obesity with bariatric surgery. Am Fam Physician. 2016;93(1):31–37. [PubMed] [Google Scholar]
- 4.Nguyen NT, Nguyen B, Gebhart A, Hohmann S. Changes in the makeup of bariatric surgery: a national increase in use of laparoscopic sleeve gastrectomy. J Am Coll Surg. 2013;216(2):252–257. [DOI] [PubMed] [Google Scholar]
- 5.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pories WJ, MacDonald KG Jr, Flickinger EG, Dohm GL, Sinha MK, Barakat HA, May HJ, Khazanie P, Swanson MS, Morgan E, et al. . Is type II diabetes mellitus (NIDDM) a surgical disease? Ann Surg. 1992;215(6):633–642, discussion 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odstrcil EA, Martinez JG, Santa Ana CA, Xue B, Schneider RE, Steffer KJ, Porter JL, Asplin J, Kuhn JA, Fordtran JS. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92(4):704–713. [DOI] [PubMed] [Google Scholar]
- 9.Shin AC, Zheng H, Townsend RL, Patterson LM, Holmes GM, Berthoud H-R. Longitudinal assessment of food intake, fecal energy loss, and energy expenditure after Roux-en-Y gastric bypass surgery in high-fat-fed obese rats. Obes Surg. 2013;23(4):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sjölund K, Sandén G, Håkanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85(5):1120–1130. [PubMed] [Google Scholar]
- 11.Egerod KL, Engelstoft MS, Grunddal KV, Nøhr MK, Secher A, Sakata I, Pedersen J, Windeløv JA, Füchtbauer E-M, Olsen J, Sundler F, Christensen JP, Wierup N, Olsen JV, Holst JJ, Zigman JM, Poulsen SS, Schwartz TW. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153(12):5782–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunddal KV, Ratner CF, Svendsen B, Sommer F, Engelstoft MS, Madsen AN, Pedersen J, Nøhr MK, Egerod KL, Nawrocki AR, Kowalski T, Howard AD, Poulsen SS, Offermanns S, Bäckhed F, Holst JJ, Holst B, Schwartz TW. Neurotensin is coexpressed, coreleased, and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology. 2016;157(1):176–194. [DOI] [PubMed] [Google Scholar]
- 13.Sykaras AG, Demenis C, Cheng L, Pisitkun T, Mclaughlin JT, Fenton RA, Smith CP. Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology. 2014;155(9):3339–3351. [DOI] [PubMed] [Google Scholar]
- 14.Little TJ, Doran S, Meyer JH, Smout AJPM, O’Donovan DG, Wu K-L, Jones KL, Wishart J, Rayner CK, Horowitz M, Feinle-Bisset C. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am J Physiol Endocrinol Metab. 2006;291(3):E647–E655. [DOI] [PubMed] [Google Scholar]
- 15.Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290(3):E550–E559. [DOI] [PubMed] [Google Scholar]
- 16.Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. J Clin Invest. 2015;125(3):908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Côté CD, Zadeh-Tahmasebi M, Rasmussen BA, Duca FA, Lam TKT. Hormonal signaling in the gut. J Biol Chem. 2014;289(17):11642–11649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50(11):2540–2547. [DOI] [PubMed] [Google Scholar]
- 19.Casajoana A, Pujol J, Garcia A, Elvira J, Virgili N, de Oca FJ, Duran X, Fernández-Veledo S, Vendrell J, Vilarrasa N. Predictive value of gut peptides in T2D remission: randomized controlled trial comparing metabolic gastric bypass, sleeve gastrectomy and greater curvature plication. Obes Surg. 2017;27(9):2235–2245. [DOI] [PubMed] [Google Scholar]
- 20.Nosso G, Griffo E, Cotugno M, Saldalamacchia G, Lupoli R, Pacini G, Riccardi G, Angrisani L, Capaldo B. Comparative effects of Roux-en-Y gastric bypass and sleeve gastrectomy on glucose homeostasis and incretin hormones in obese type 2 diabetic patients: a one-year prospective study. Horm Metab Res. 2016;48(5):312–317. [DOI] [PubMed] [Google Scholar]
- 21.Stefater MA, Sandoval DA, Chambers AP, Wilson-Perez HE, Hofmann SM, Jandacek R, Tso P, Woods SC, Seeley RJ. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology 2011;141(3):939–949. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobsen SH, Olesen SC, Dirksen C, Jørgensen NB, Bojsen-Møller KN, Kielgast U, Worm D, Almdal T, Naver LS, Hvolris LE, Rehfeld JF, Wulff BS, Clausen TR, Hansen DL, Holst JJ, Madsbad S. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg. 2012;22(7):1084–1096. [DOI] [PubMed] [Google Scholar]
- 23.Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee W-J, Chen C-Y, Chong K, Lee Y-C, Chen S-C, Lee S-D. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis. 2011;7(6):683–690. [DOI] [PubMed] [Google Scholar]
- 25.Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H, Yapp K, Olivan B. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(7):2479–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3(6):597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dirksen C, Damgaard M, Bojsen-Møller KN, Jørgensen NB, Kielgast U, Jacobsen SH, Naver LS, Worm D, Holst JJ, Madsbad S, Hansen DL, Madsen JL. Fast pouch emptying, delayed small intestinal transit, and exaggerated gut hormone responses after Roux-en-Y gastric bypass. Neurogastroenterol Motil. 2013;25(4):346–e255. [DOI] [PubMed] [Google Scholar]
- 28.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, Restuccia NL, Bessler M. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring). 2006;14(9):1553–1561. [DOI] [PubMed] [Google Scholar]
- 29.Laferrère B, Swerdlow N, Bawa B, Arias S, Bose M, Oliván B, Teixeira J, McGinty J, Rother KI. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab. 2010;95(8):4072–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yousseif A, Emmanuel J, Karra E, Millet Q, Elkalaawy M, Jenkinson AD, Hashemi M, Adamo M, Finer N, Fiennes AG, Withers DJ, Batterham RL. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg. 2014;24(2):241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology. 2010;151(4):1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, Barsotti E, Berta R, Moriconi D, Bellini R, Anselmino M, Ferrannini E. Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab. 2013;98(11):4391–4399. [DOI] [PubMed] [Google Scholar]
- 33.Jørgensen NB, Jacobsen SH, Dirksen C, Bojsen-Møller KN, Naver L, Hvolris L, Clausen TR, Wulff BS, Worm D, Lindqvist Hansen D, Madsbad S, Holst JJ. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303(1):E122–E131. [DOI] [PubMed] [Google Scholar]
- 34.Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, Vidal J. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-en-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26(8):2231–2239. [DOI] [PubMed] [Google Scholar]
- 35.le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, Ghatei MA, Patel A, Bloom SR, Aylwin SJB. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252(1):50–56. [DOI] [PubMed] [Google Scholar]
- 36.Cavin JB, Couvelard A, Lebtahi R, Ducroc R, Arapis K, Voitellier E, Cluzeaud F, Gillard L, Hourseau M, Mikail N, Ribeiro-Parenti L, Kapel N, Marmuse JP, Bado A, Le Gall M. Differences in alimentary glucose absorption and intestinal disposal of blood glucose after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology. 2016;150(2):454–64.e9. [DOI] [PubMed] [Google Scholar]
- 37.Mumphrey MB, Hao Z, Townsend RL, Patterson LM, Berthoud H-R. Sleeve gastrectomy does not cause hypertrophy and reprogramming of intestinal glucose metabolism in rats. Obes Surg. 2015;25(8):1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia. 2005;48(4):612–615. [DOI] [PubMed] [Google Scholar]
- 39.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Pérez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D’Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141(3):950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alamuddin N, Vetter ML, Ahima RS, Hesson L, Ritter S, Minnick A, Faulconbridge LF, Allison KC, Sarwer DB, Chittams J, Williams NN, Hayes MR, Loughead JW, Gur R, Wadden TA. Changes in fasting and prandial gut and adiposity hormones following vertical sleeve gastrectomy or Roux-en-Y gastric bypass: an 18-month prospective study. Obes Surg. 2017;27(6):1563–1572. [DOI] [PubMed] [Google Scholar]
- 41.Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96(7):2227–2235. [DOI] [PubMed] [Google Scholar]
- 42.Koopmans HS, Sclafani A, Fichtner C, Aravich PF. The effects of ileal transposition on food intake and body weight loss in VMH-obese rats. Am J Clin Nutr. 1982;35(2):284–293. [DOI] [PubMed] [Google Scholar]
- 43.Sclafani A. Appetitive behavior after jejunoileal bypass. Int J Obes. 1981;5(5):449–455. [PubMed] [Google Scholar]
- 44.Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288(2):E447–E453. [DOI] [PubMed] [Google Scholar]
- 45.Melissas J, Koukouraki S, Askoxylakis J, Stathaki M, Daskalakis M, Perisinakis K, Karkavitsas N. Sleeve gastrectomy: a restrictive procedure? Obes Surg. 2007;17(1):57–62. [DOI] [PubMed] [Google Scholar]
- 46.Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, Sorrell J, Lemmen L, LaSance K, Woods SC, Seeley RJ, D’Alessio DA, Sandoval DA. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab. 2014;306(4):E424–E432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, Standfield S, Rayner CK, Horowitz M. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22(9):2003–2009. [DOI] [PubMed] [Google Scholar]
- 48.Brandsma E, Houben T, Fu J, Shiri-Sverdlov R, Hofker MH. The immunity–diet–microbiota axis in the development of metabolic syndrome. Curr Opin Lipidol. 2015;26(2):73–81. [DOI] [PubMed] [Google Scholar]
- 49.Mumphrey MB, Patterson LM, Zheng H, Berthoud H-R. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil. 2013;25(1):e70–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341(6144):406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dirksen C, Bojsen-Møller KN, Jørgensen NB, Jacobsen SH, Kristiansen VB, Naver LS, Hansen DL, Worm D, Holst JJ, Madsbad S. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia. 2013;56(12):2679–2687. [DOI] [PubMed] [Google Scholar]
- 52.Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Na(+)-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61(1):187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98(4):E708–E712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Käkelä P, Pääkkönen M, Hallikainen M, Kolehmainen M, Uusitupa M, Moilanen L, Laakso M, Gylling H, Patti ME, Auwerx J, Pihlajamäki J. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obes Surg. 2012;22(9):1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dutia R, Embrey M, O’Brien CS, Haeusler RA, Agénor KK, Homel P, McGinty J, Vincent RP, Alaghband-Zadeh J, Staels B, le Roux CW, Yu J, Laferrère B. Temporal changes in bile acid levels and 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes [published correction appears in Int J Obes (Lond). 2016;40(3):554] Int J Obes. 2015;39(5):806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329(1):386–390. [DOI] [PubMed] [Google Scholar]
- 57.Ding L, Sousa KM, Jin L, Dong B, Kim B-W, Ramirez R, Xiao Z, Gu Y, Yang Q, Wang J, Yu D, Pigazzi A, Schones D, Yang L, Moore D, Wang Z, Huang W. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology. 2016;64(3):760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGavigan AK, Garibay D, Henseler ZM, Chen J, Bettaieb A, Haj FG, Ley RE, Chouinard ML, Cummings BP. TGR5 contributes to glucoregulatory improvements after vertical sleeve gastrectomy in mice. Gut. 2017;66(2):226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rizzello M, Abbatini F, Casella G, Alessandri G, Fantini A, Leonetti F, Basso N. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg. 2010;20(1):50–55. [DOI] [PubMed] [Google Scholar]
- 61.Benaiges D, Flores Le-Roux JA, Pedro-Botet J, Chillarón JJ, Renard M, Parri A, Ramón JM, Pera M, Goday A. Sleeve gastrectomy and Roux-en-Y gastric bypass are equally effective in correcting insulin resistance. Int J Surg. 2013;11(4):309–313. [DOI] [PubMed] [Google Scholar]
- 62.Bojsen-Møller KN, Dirksen C, Jørgensen NB, Jacobsen SH, Serup AK, Albers PH, Hansen DL, Worm D, Naver L, Kristiansen VB, Wojtaszewski JFP, Kiens B, Holst JJ, Richter EA, Madsbad S. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes. 2014;63(5):1725–1737. [DOI] [PubMed] [Google Scholar]
- 63.Immonen H, Hannukainen JC, Iozzo P, Soinio M, Salminen P, Saunavaara V, Borra R, Parkkola R, Mari A, Lehtimäki T, Pham T, Laine J, Kärjä V, Pihlajamäki J, Nelimarkka L, Nuutila P. Effect of bariatric surgery on liver glucose metabolism in morbidly obese diabetic and non-diabetic patients. J Hepatol. 2014;60(2):377–383. [DOI] [PubMed] [Google Scholar]
- 64.Chondronikola M, Harris LLS, Klein S. Bariatric surgery and type 2 diabetes: are there weight loss–independent therapeutic effects of upper gastrointestinal bypass? J Intern Med. 2016;280(5):476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Honka H, Koffert J, Hannukainen JC, Tuulari JJ, Karlsson HK, Immonen H, Oikonen V, Tolvanen T, Soinio M, Salminen P, Kudomi N, Mari A, Iozzo P, Nuutila P. The effects of bariatric surgery on pancreatic lipid metabolism and blood flow. J Clin Endocrinol Metab. 2015;100(5):2015–2023. [DOI] [PubMed] [Google Scholar]
- 66.McIntyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet. 1964;2(7349):20–21. [DOI] [PubMed] [Google Scholar]
- 67.Nauck MA, Bartels E, Orskov C, Ebert R, Creutzfeldt W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab. 1993;76(4):912–917. [DOI] [PubMed] [Google Scholar]
- 68.Vilsbøll T, Krarup T, Madsbad S, Holst JJ. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul Pept. 2003;114(2-3):115–121. [DOI] [PubMed] [Google Scholar]
- 69.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29(1):46–52. [DOI] [PubMed] [Google Scholar]
- 70.Knop FK, Aaboe K, Vilsbøll T, Vølund A, Holst JJ, Krarup T, Madsbad S. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes Metab. 2012;14(6):500–510. [DOI] [PubMed] [Google Scholar]
- 71.Jørgensen NB, Dirksen C, Bojsen-Møller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60(9):2308–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiménez A, Ceriello A, Casamitjana R, Flores L, Viaplana-Masclans J, Vidal J. Remission of type 2 diabetes after Roux-en-Y gastric bypass or sleeve gastrectomy is associated with a distinct glycemic profile. Ann Surg. 2015;261(2):316–322. [DOI] [PubMed] [Google Scholar]
- 74.Svane MS, Bojsen-Møller KN, Nielsen S, Jørgensen NB, Dirksen C, Bendtsen F, Kristiansen VB, Hartmann B, Holst JJ, Madsbad S. Effects of endogenous GLP-1 and GIP on glucose tolerance after Roux-en-Y gastric bypass surgery. Am J Physiol Endocrinol Metab. 2016;310(7):E505–E514. [DOI] [PubMed] [Google Scholar]
- 75.Nauck MA, Kind J, Köthe LD, Holst JJ, Deacon CF, Broschag M, He YL, Kjems L, Foley J. Quantification of the contribution of GLP-1 to mediating insulinotropic effects of DPP-4 inhibition with vildagliptin in healthy subjects and patients with type 2 diabetes using exendin [9-39] as a GLP-1 receptor antagonist. Diabetes. 2016;65(8):2440–2447. [DOI] [PubMed] [Google Scholar]
- 76.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57(8):2046–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burmeister MA, Ayala JE, Smouse H, Landivar-Rocha A, Brown JD, Drucker DJ, Stoffers DA, Sandoval DA, Seeley RJ, Ayala JE. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes. 2017;66(2):372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, Benhamed F, Grémeaux T, Drucker DJ, Kahn CR, Girard J, Tanti JF, Delzenne NM, Postic C, Burcelin R. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest. 2005;115(12):3554–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, Münzberg H, Morrison CD, Drucker DJ, Berthoud H-R. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol. 2014;306(5):R352–R362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garibay D, McGavigan AK, Lee SA, Ficorilli JV, Cox AL, Michael MD, Sloop KW, Cummings BP. β-Cell glucagon-like peptide-1 receptor contributes to improved glucose tolerance after vertical sleeve gastrectomy. Endocrinology. 2016;157(9):3405–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab. 2013;3(2):191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson-Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Pérez-Tilve D, Seeley RJ. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes. 2013;62(7):2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiménez A, Mari A, Casamitjana R, Lacy A, Ferrannini E, Vidal J. GLP-1 and glucose tolerance after sleeve gastrectomy in morbidly obese subjects with type 2 diabetes. Diabetes. 2014;63(10):3372–3377. [DOI] [PubMed] [Google Scholar]
- 84.Aung L, Lee W-J, Chen SC, Ser K-H, Wu C-C, Chong K, Lee Y-C, Chen J-C. Bariatric surgery for patients with early-onset vs late-onset type 2 diabetes. JAMA Surg. 2016;151(9):798–805. [DOI] [PubMed] [Google Scholar]
- 85.Keidar A, Hershkop KJ, Marko L, Schweiger C, Hecht L, Bartov N, Kedar A, Weiss R. Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia. 2013;56(9):1914–1918. [DOI] [PubMed] [Google Scholar]
- 86.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med. 2017;376(7):641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, Kirwan JP, Schauer PR. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes. 2010;34(3):462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee W-J, Chong K, Ser K-H, Lee Y-C, Chen S-C, Chen J-C, Tsai M-H, Chuang L-M. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146(2):143–148. [DOI] [PubMed] [Google Scholar]
- 89.de Barros F, Setúbal S, Martinho JM, Monteiro ABS. Early endocrine and metabolic changes after bariatric surgery in grade III morbidly obese patients: a randomized clinical trial comparing sleeve gastrectomy and gastric bypass. Metab Syndr Relat Disord. 2015;13(6):264–271. [DOI] [PubMed] [Google Scholar]
- 90.Bojsen-Møller KN, Dirksen C, Svane MS, Jørgensen NB, Holst JJ, Richter EA, Madsbad S. Variable reliability of surrogate measures of insulin sensitivity after Roux-en-Y gastric bypass. Am J Physiol Regul Integr Comp Physiol. 2017;312(5):R797–R805. [DOI] [PubMed] [Google Scholar]
- 91.Laferrère B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, Bose M, Teixeira J, Stevens RD, Wenner BR, Bain JR, Muehlbauer MJ, Haqq A, Lien L, Shah SH, Svetkey LP, Newgard CB. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3(80):80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plum L, Ahmed L, Febres G, Bessler M, Inabnet W, Kunreuther E, McMahon DJ, Korner J. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity (Silver Spring). 2011;19(11):2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bose M, Machineni S, Oliván B, Teixeira J, McGinty JJ, Bawa B, Koshy N, Colarusso A, Laferrère B. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity (Silver Spring). 2010;18(6):1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Myronovych A, Kirby M, Ryan KK, Zhang W, Jha P, Setchell KD, Dexheimer PJ, Aronow B, Seeley RJ, Kohli R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity (Silver Spring). 2014;22(2):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, McMahon DJ, Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell function in type 2 diabetic patients. Diabetes. 2013;62(9):3027–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hao Z, Townsend RL, Mumphrey MB, Morrison CD, Münzberg H, Berthoud H-R. RYGB produces more sustained body weight loss and improvement of glycemic control compared with VSG in the diet-induced obese mouse model. Obes Surg. 2017;27(9):2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschöp MH, Sandoval DA, Seeley RJ. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. 2013;144(1):50–52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H; Swedish Obese Subjects Study Scientific Group . Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351(26):2683–2693. [DOI] [PubMed] [Google Scholar]
- 99.Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J, Toure M, Tschop M, Woods SC, Seeley RJ. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138(7):2426–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wilson-Pérez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, Seeley RJ. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes. 2013;37(2):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud H-R. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1273–R1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chambers AP, Wilson-Perez HE, McGrath S, Grayson BE, Ryan KK, D’Alessio DA, Woods SC, Sandoval DA, Seeley RJ. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am J Physiol Endocrinol Metab. 2012;303(8):E1076–E1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Löwenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R1057–R1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laurenius A, Larsson I, Melanson KJ, Lindroos AK, Lönroth H, Bosaeus I, Olbers T. Decreased energy density and changes in food selection following Roux-en-Y gastric bypass. Eur J Clin Nutr. 2013;67(2):168–173. [DOI] [PubMed] [Google Scholar]
- 105.Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB, Lönroth H, Fändriks L, Olbers T. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes. 2012;36(3):348–355. [DOI] [PubMed] [Google Scholar]
- 106.Delin CR, Watts JM, Saebel JL, Anderson PG. Eating behavior and the experience of hunger following gastric bypass surgery for morbid obesity. Obes Surg. 1997;7(5):405–413. [DOI] [PubMed] [Google Scholar]
- 107.Münzberg H, Laque A, Yu S, Rezai-Zadeh K, Berthoud H-R. Appetite and body weight regulation after bariatric surgery. Obes Rev. 2015;16(suppl 1):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hankir MK, Seyfried F, Hintschich CA, Diep T-A, Kleberg K, Kranz M, Deuther-Conrad W, Tellez LA, Rullmann M, Patt M, Teichert J, Hesse S, Sabri O, Brust P, Hansen HS, de Araujo IE, Krügel U, Fenske WK. Gastric bypass surgery recruits a gut PPAR-α-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. 2017;25(2):335–344. [DOI] [PubMed] [Google Scholar]
- 109.Dakour Aridi H, Khazen G, Safadi BY. Comparison of outcomes between laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy in a Lebanese bariatric surgical practice. Obes Surg. 2017. [DOI] [PubMed]
- 110.Øhrstrøm CC, Worm D, Hansen DL. Postprandial hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: an update. Surg Obes Relat Dis. 2017;13(2):345–351. [DOI] [PubMed] [Google Scholar]
- 111.Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6):2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Craig CM, Liu L-F, Deacon CF, Holst JJ, McLaughlin TL. Critical role for GLP-1 in symptomatic post-bariatric hypoglycaemia. Diabetologia. 2017;60(3):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial: a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–234. [DOI] [PubMed] [Google Scholar]