Abstract
Primary aldosteronism (PA) is a common form of endocrine hypertension that is characterized by the excessive production of aldosterone relative to suppressed plasma renin levels. PA is usually caused by either a unilateral aldosterone-producing adenoma or bilateral adrenal hyperplasia. Somatic mutations have been identified in several genes that encode ion pumps and channels that may explain the aldosterone excess in over half of aldosterone-producing adenomas, whereas the pathophysiology of bilateral adrenal hyperplasia is largely unknown. A number of mouse models of hyperaldosteronism have been described that recreate some features of the human disorder, although none replicate the genetic basis of human PA. Animal models that reproduce the genotype–phenotype associations of human PA are required to establish the functional mechanisms that underlie the endocrine autonomy and deregulated cell growth of the affected adrenal and for preclinical studies of novel therapeutics. Herein, we discuss the differences in adrenal physiology across species and describe the genetically modified mouse models of PA that have been developed to date.
We discuss the differences in adrenal steroidogenesis across species and review the genetically modified mouse models of primary aldosteronism developed to date.
Primary aldosteronism (PA) is the most common form of endocrine hypertension, characterized by the excessive production of aldosterone relative to suppressed plasma renin, and is associated with an increased risk of cardiovascular and cerebrovascular complications (1, 2). PA is predominantly caused by either of two main subtypes: unilateral aldosterone excess, due to an aldosterone-producing adenoma, or bilateral aldosterone excess, caused by bilateral adrenal hyperplasia. Unilateral PA is treated by adrenalectomy, whereas patients with bilateral PA are usually treated pharmacologically with a mineralocorticoid receptor antagonist (3).
Major advances have been made in our understanding of the pathogenesis of aldosterone-producing adenomas with the discovery of somatic mutations in a number of genes that can explain the overproduction of aldosterone in over half of patients with a unilateral aldosterone-producing adenoma. The affected genes encode ion pumps and channels (KCNJ5, ATP1A1, ATP2B3, and CACNA1D) or key players in the control of cell growth (CTNNB1 and PRKACA) (4). Mutations in several of the affected genes (KCNJ5, ATP2B3, and CACNA1D) have been shown to cause an increase in intracellular Ca2+ concentration that results in an increase in transcription of the gene encoding aldosterone synthase (CYP11B2) via the activation of Ca2+ signaling (5–7). In contrast, mutations in ATP1A1 (Na+/K+-ATPase 1-Leu104Arg and Val332Gly) result in intracellular acidification that may cause the overproduction of aldosterone (8). The pathogenesis of bilateral adrenal hyperplasia, however, remains obscure, mainly because molecular studies are hampered as a result of the limited availability of resected adrenal specimens from patients with bilateral adrenal hyperplasia who are treated pharmacologically.
Animal models of PA would be useful to study mechanisms that control cell growth and autonomous aldosterone production and for preclinical testing of novel therapeutics. In this review, we discuss adrenal physiology in different animal species and describe the currently available mouse models of hyperaldosteronism.
Physiology of the Adrenal Gland in Different Species
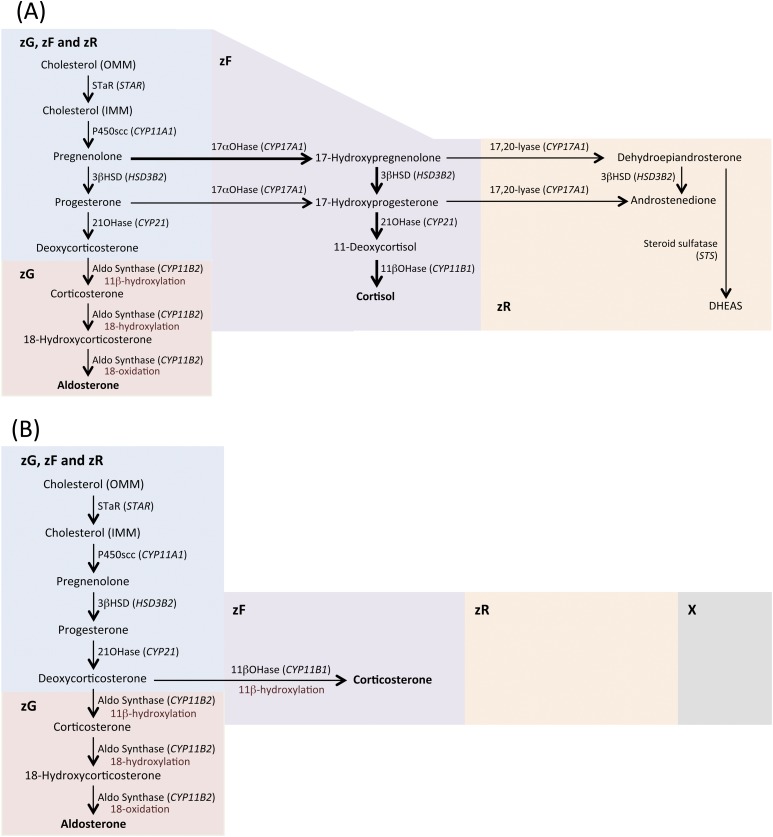
The human adrenal cortex comprises three morphologically distinct zones: the zona glomerulosa (zG), zona fasciculata (zF), and zona reticularis (zR), which have specific functional properties for the production of mineralocorticoids, glucocorticoids, and androgens, respectively (referred to as “functional zonation”). In the normal human adrenal gland, the restricted synthesis of aldosterone in the zG and of cortisol in the zF is a result of the adrenal expression of CYP11B2 exclusively in the zG for aldosterone synthesis and the expression of CYP17 and the gene encoding 11β-hydroxylase (CYP11B1) in the zF for cortisol production (9, 10) (Fig. 1).
Figure 1.
Cholesterol is mobilized from a store in the outer mitochondrial membrane (OMM) and is transferred by steroidogenic acute regulatory protein (StAR) to the inner mitochondrial membrane (IMM), where it is converted to pregnenolone by P450scc, the first and rate-limiting step of steroidogenesis (9, 10). Aldosterone biosynthesis is restricted to the zG, where aldosterone synthase is localized. (A) In humans, the activity of 17α-hydroxylase (17αOHase)/17,20 lyase (CYP17A1) is a key enzyme for the synthesis of both cortisol and androgens; the main biosynthetic pathway for cortisol synthesis is via the 17α-hydroxylation of pregnenolone, indicated by thicker arrows. (B) Mice, like other rodents, do not express CYP17A1 and, as a result, the major glucocorticoid synthesized is corticosterone (instead of cortisol), which is produced from deoxycorticosterone by 11β-hydroxylase [11βOHase (Cyp11b1)] in the zF. (A and B) The absence of CYP17A1 expression in mice means that adrenal androgens cannot be synthesized as in humans in the zR, and mice do not have a distinguishable zR. (B) Young mice have an X-zone that degenerates by the first pregnancy in females and by sexual maturity in males. Figure 1 includes data from Stowasser and Gordon (10). 21OHase, 21-hydroxylase; 3βHSD, 3β-hydroxysteroid dehydrogenase; Aldo synthase, aldosterone synthase; DHEAS, dehydroepiandrosterone sulfate; STS, steroid sulfatase.
The adrenal cortices of pigs, dogs, and cattle are similarly functionally organized to those of humans with aldosterone biosynthesis restricted to cells of the zG and cortisol production in the zF. Mice display a different morphological zonation compared with humans. The CYP17 gene encoding 17α-hydroxylase and 17,20-lyase is not expressed in mice, as in other rodents, and therefore, mice produce corticosterone instead of cortisol and do not synthesize adrenal androgens. A functional zR is thus absent in mice, and this zone is difficult to distinguish histologically (Fig. 1; Table 1).
Table 1.
Comparative Physiology of the Adrenal Cortex: From Mice to Humans
| Function | Humans | Cows, Pigs, Dogs, and Sheep | Mice and Rats |
|---|---|---|---|
| Key gene for mineralocorticoid biosynthesis in zG | CYP11B2 | CYP11Ba | Cyp11b2 |
| Principal mineralocorticoid | Aldosterone | Aldosterone | Aldosterone |
| Key gene or genes for glucocorticoid biosynthesis in zF | CYP11B1 | CYP11Ba | Cyp11b1 |
| CYP17 | CYP17 | ||
| Principal glucocorticoid | Cortisol | Cortisol | Corticosterone |
| Adrenal androgen synthesis | Present | Present | Absent |
Expressed in zG and zF.
The adrenals of young mice have a layer of juxtamedullary cortical cells (the X-zone) with an undefined function (11). The X-zone degenerates during the first pregnancy of female mice, disappearing by the 12th day of gestation, and by puberty in male mice. In the absence of pregnancy, the X-zone nonetheless degenerates in females but slowly over a variable period that may last up to 200 days (11). The adrenals of female mice have a notably higher weight and total volume of the cortex and medulla than those of males from the age of five weeks that is maintained into adulthood (12).
In humans, the final three steps in the biosynthesis of aldosterone involve the conversion of deoxycorticosterone by the successive action of the 11β-hydroxylase, 18-hydroxylase, and 18-methyloxidase activities of a single enzyme, aldosterone synthase, encoded by CYP11B2 (9, 10). An 11β-hydroxylase in the zF, encoded by a distinct but highly homologous gene to CYP11B2, called CYP11B1, converts 11-deoxycortisol to cortisol. Humans, mice, rats, hamsters, guinea pigs, and monkeys express two CYP11B enzymes, whereas cows, sheep, horses, pigs, and dogs express a single CYP11B enzyme that catalyzes the final step(s) in the synthesis of both cortisol and aldosterone (13–18) (Table 1). Despite the presence of a single CYP11B in these species, functional zonation with aldosterone production in the zG and cortisol synthesis in the zF is maintained by an unknown mechanism, which presumably involves the presence of zone-specific factors that modulate CYP11B activity.
Regulation of Aldosterone Synthesis in Humans and Other Species
The principal function of aldosterone is to maintain fluid and electrolyte balance, promoting Na+ retention and K+ secretion in the distal nephron of the kidney, thereby controlling blood pressure (10). In zG cells, angiotensin II (Ang II) stimulates phospholipase C and triggers inositol triphosphate-dependent Ca2+ release from the endoplasmic reticulum, whereas high plasma K+ concentrations or long-term stimulation by Ang II result in membrane depolarization and the activation of L- and T-type Ca2+ channels (19). The increase in intracellular Ca2+ leads to an increase in expression of the gene encoding aldosterone synthase (CYP11B2) and aldosterone production (17). Small changes in plasma K+, as low as 1 mM, can double aldosterone secretion (20). The basis of this sensitivity is the high background K+ conductance with a membrane potential close to the K+ equilibrium potential. At low plasma K+ concentrations, the membrane voltage is hyperpolarized, and small increases in K+ are sufficient to depolarize slightly the membrane and activate T-type Ca2+ channels (21–23). Therefore, mechanisms that regulate the membrane potential of the zG cell play a crucial role in the control of aldosterone production and usually depend on the equilibrium potential and ion conductance across the plasma membrane.
The negative membrane voltage is mainly determined by two-pore domain K+ channels, the tandem of P domains in weak, inwardly rectifying, K+ channel–related, acid-sensitive K+ channels (TASK channels), which generate the aforementioned background or “leak” K+ currents. K+ inwardly rectifying channels [G protein–activated inwardly rectifying K+ channels (GIRK)] also regulate the membrane potential of zG cells, and GIRK4 (encoded by KCNJ5) may function in normal adrenal physiology, in addition to its pathological role in aldosterone production via gain-of-function mutations, as described previously.
Different species use different K+ channels to maintain the membrane potential of the zG cell (24) and distinct differences between species have been reported for the expression of some key K+ channels (25). TASK-1 is expressed in the zG and zF in both humans and rats. TASK3 is localized to the zG in the human and rat adrenal with high expression in the rat and low expression in the human (25). GIRK4 (KCNJ5) is strongly expressed in the zG of the human adrenal but is undetectable in the rat zG and zF (25). Thus, the distinct expression patterns of K+ channels between species suggests an evolutionary divergence in the regulation of aldosterone production and indicates that rats and probably also mice may not be ideal models to study human pathological conditions of aldosterone excess.
Mouse Models of Primary Aldosteronism
Several genetically modified mouse models of hyperaldosteronism have been reported that display the main biochemical features of PA but do not reproduce the adrenal tumorigenesis or hyperplasia associated with this condition. Mouse models of hyperaldosteronism are discussed further later and are summarized in Table 2.
Table 2.
Mouse Models of Hyperaldosteronism
| Mouse Model | Description | Phenotype | Reference |
|---|---|---|---|
| Kcnk3−/− | K+ channel 2 pore domain, member 3 (Task1) deletion | Elevated plasma aldosterone relative to suppressed plasma renin; aberrant Cyp11b1 expression in zF in females and in prepubertal males; decreased plasma K+; elevated aldosterone and high blood pressure remediable by dexamethasone | Heitzmann, et al. (26) |
| Kcnk3−/−/Dkk3−/− | Task1 and Dickkopf WNT signaling pathway inhibitor 3 deletion | Increased expression Cyp11b2; hyperaldosteronism phenotype extended to adult males | El Wakil, et al. (27) |
| Kcnk3−/−/Kcnk9−/− | Task1 and -3 deletion | Elevated plasma aldosterone relative to suppressed plasma renin; elevated aldosterone not suppressible by high salt diet; hyperaldosteronism phenotype extended to adult males | Davies, et al. (28) |
| Kcnk9−/− | K+ channel 2 pore domain, member 9 (Task3) deletion | Mild overproduction of aldosterone relative to suppressed plasma renin; aldosterone not suppressible by high salt diet; salt-sensitive high blood pressure | Guagliardo, et al. (29) |
| Penton, et al. (30) | |||
| Kcnmb1−/− | K+ channel, Ca2+-activated, subunit β deletion | Elevated plasma aldosterone, high blood pressure, renal K+ retention | Grimm, et al. (31) |
| Kcnma1−/− | K+ channel, Ca2+-activated, subunit α deletion | Elevated plasma aldosterone, high blood pressure, decreased plasma K+, increased vascular tone | Sausbier, et al. (32) |
| ΔCat | Constitutive activation of β catenin in adrenal cortex | Elevated plasma aldosterone; adrenal hyperplasia; development of adrenal carcinoma | Berthon, et al. (33) |
| ApcMin/+ | Expression of a truncated form of tumor suppressor protein Apc | Elevated plasma aldosterone and corticosterone; elevated plasma volume and high blood pressure | Bhandaru, et al. (34) |
| Cry1−/−/Cry2−/− | Deletion of core components of circadian clock (Cry1 and Cry2) | Elevated plasma aldosterone; salt-sensitive high blood pressure; increased expression of Hsd3b6 | Doi, et al. (35) |
| Tgfb1L/L | Reduced expression (≈10% normal) of Tgfb1 mRNA | Increased aldosterone-to-renin ratio (6× normal), elevated blood pressure; decreased urinary water and electrolyte output | Kakoki, et al. (36) |
| Enu | Random mutagenesis screen using N-ethyl-N-nitrosourea | Increased aldosterone-to-renin ratio; increased Cyp11b2 gene expression | Spyroglou, et al. (37) |
| Cyp11b2 hi/hi | Substitution of unstable 3′UTR of Cyp11b2 with more stable 3′UTR of bovine GH | Modest increase in Cyp11b2 expression; salt-sensitive high blood pressure | Makhanova (38) |
| hAS+/− | Human CYP11B1 promoter fused to coding region of human CYP11B2 | Elevated plasma aldosterone; salt-sensitive high blood pressure; increased CYP11B2 gene expression | Gu, et al. (39) |
Abbreviations: 3′UTR, untranslated region; Apc, adenomatous polyposis coli gene; Cry1, cryptochrome-1 gene; Cry2, cryptochrome-2 gene; Dkk3, dickkopf family gene; Enu, N-ethyl-N-nitrosourea; GH, growth hormone; hAS, human aldosterone synthase; Hsd3b6, gene encoding 3β- and steroid δ-isomerase 6 (hydroxy-δ-5-steroid dehydrogenase); Tgfb1, transforming growth factor β1; ΔCat, constitutively activated β-catenin.
Mouse Models With Deletions of TASK Channels
Electrophysiological recordings of zG cells have emphasized the importance of leak-type K+ channels of the two-pore domain family in conferring background K+ conductance. In rodents, two members of the two-pore domain family, Task1 (Kcnk3) and Task3 (Kcnk9), are the dominant leak-type channels. Female mice with the Task1 gene deleted display hyperaldosteronism with low plasma renin activity and a decrease in plasma K+. Systolic blood pressure is increased and responds to administration of a mineralocorticoid receptor antagonist (26). The Cyp11b2 gene is expressed aberrantly exclusively in the zF, and the elevated aldosterone is suppressed by dexamethasone (26). Male Task1−/− mice and heterozygous females do not exhibit a phenotype. Mice of both sexes at postnatal day 18 exhibit an abnormal distribution of the Cyp11b2 enzyme. Castrated male Task1−/− display an abnormal expression of Cyp11b2, similar to females, and estradiol administration decreases the level of Cyp11b2 expression but not the zonal distribution. Female Task1−/− injected with testosterone display a normal zonation indicating the role of androgens (26).
Gene expression analysis of the adrenals of male and female Task1−/− revealed a limited number of differentially expressed genes, mostly involved in signaling cascades, and one of these belongs to the dickkopf family (Dkk3) which is expressed in the zG of the adrenal and inhibits aldosterone secretion in cultured adrenal cells (27). Inactivation of Dkk3 in male Task1−/− extended the hyperaldosteronism phenotype to male mice. The zonal distribution of the double-deleted Dkk3−/− and Task1−/− was preserved in contrast to female Task1−/− mice.
TASK1 (KCNK3) is expressed in both mouse and human adrenals. In the human adrenocortical NCI-H295R cell line, downregulation of TASK1 by a small interfering RNA increased aldosterone production (40). Genome-wide association studies have identified KCNK3 single nucleotide polymorphism (SNP) variants associated with blood pressure in humans (41). The KCNK3 SNP (rs1275988) is associated with hypertension in African Americans and a nearby SNP (rs13394970) is associated with hypertension in Hispanics, which was found in the Multi-Ethnic Study of Atherosclerosis, which comprised 7840 individuals. Aldosterone levels and plasma renin activity, in a subset of 1653 participants, were also found associated with KCNK3 rs2586886 (41). The functional significance of these SNPs on channel function is unknown, and whereas in the mouse, complete deletion of Kcnk3 in females is required for the phenotype, an alteration determined by a polymorphism is of unknown significance in humans.
Gene deletion of both the Task1 and Task3 in male mice ablates background K+ currents, resulting in marked depolarization of the membrane potential in zG cells (28). Task1−/−, Task3−/− mice excrete higher urinary aldosterone at all levels of sodium intake. The mice also have normal or low renin activity, and aldosterone production fails to normalize on high sodium intake or after the administration of the Ang receptor blocker candesartan (28). Adrenal zonation is unaltered, and Task1−/−, Task3−/− mice may represent a model of idiopathic PA.
Task3−/− mice display mild aldosterone overproduction and a decreased plasma renin concentration and fail to suppress aldosterone excretion on a high sodium diet. These mice are hypersensitive to Ang II and have high blood pressure (29, 30). The baseline membrane potential of zG cells was not different from that of wild-type mice, even though the mice were slightly hypokalemic. Although there was a small increase in Cyp11b2 mRNA, there was no increase in aldosterone synthase expression. A recent study indicates that TASK3 is not located in the plasma membrane but is located in the mitochondrial membrane and plays a role in mitochondrial membrane potential (42). It has been suggested that the lack of plasma membrane depolarization, unchanged expression of the Cyp11b2 protein, but increased production of aldosterone might result from an increase in the activity of the late pathway of aldosterone biosynthesis (43) (conversion of deoxycorticosterone to aldosterone in the mitochondria). Neonatal Task3−/− mice display a severe phenotype with a strong increase in plasma aldosterone, corticosterone, and progesterone that decreases with age (44), with a significant increase in Cyp11b2 mRNA for the neonatal mice that normalizes in adults. There is a marked increase in adrenal renin expression and concentration, mainly in the zF that decreases with age (44).
Mouse Models With Deletions of Large Ca2+-Activated K+ Channel Subunit Deletions
Ca2+-activating big K+ channels (BK) are composed of an α pore (BKα) and one to four β subunits. There are multiple splice variants of BKα, and the cell-specific function of BK channels is determined by the BKα splice variant in association with one of the four β subunits (45). Under normal conditions, the BKα/β1 channel opens in response to local elevations in intracellular Ca2+ (Ca2+ sparks), leading to a compensatory vasorelaxation, but in a study performed with the BKβ1 gene inactivated (Kcnmb1−/−) in male mice, channel activity is uncoupled from Ca2+ sparks, leading to hypertension (31). The BKβ−/− mice retain fluid, which is enhanced on a high K+ diet, and administration of the mineralocorticoid receptor antagonist eplerenone corrected the fluid retention and nearly normalized blood pressure (31, 46). Plasma aldosterone concentrations are increased and exacerbated by K+ loading, as the adrenal is highly sensitive to K+ retention by the renal-connecting tubules of the BKβ1−/− mice (31). The increased aldosterone secretion is a result of an increased sensitivity of the zG to K+ (31). Whereas BKα is highly expressed in the zG and weakly in the adrenal medulla, BKβ1 appears to be expressed in the medulla only (31, 32). In the adrenal medulla, BKα/β may function in hyperpolarization of the membrane potential of chromaffin cells to inhibit Ca2+-mediated catecholamine release, analogous to the role of this channel in other tissues. In zG cells, catecholamines induce increases in cyclic adenosine monophosphate levels that have been demonstrated to stimulate Ca2+ influx via L-type Ca2+ channels (47), a known pathway leading to increased aldosterone production. The hypertension in the BKβ1−/− model is a result of both the elevation of aldosterone levels and the abnormal vasorelaxation of the vascular smooth muscle cells.
Gene deletion of the BKα (Kcnma1−/−) resulted in a small, but significant increase in blood pressure with normal heart rate, a gender-independent decrease in plasma K+ concentration, and an elevation of plasma aldosterone with normal renin or serum corticotropin levels (32). The elevation of the blood pressure was a result of the hyperaldosteronism and the vascular dysfunction that resulted from the gene deletion.
Transgenic Mouse Models With Constitutively Activated β-Catenin
The Wnt/β-catenin signaling is essential for embryonic development and cell proliferation, but constitutive activation is associated with a variety of cancers (48). In the absence of extracellular Wnt ligands, the N-terminal domain of β-catenin is phosphorylated on serines/threonine residues by a multi-protein destruction complex composed of casein kinase I, glycogen synthase kinase 3β, axin, and adenomatous polyposis coli (APC), which results in ubiquitylation and proteosomal degradation. The binding to the Frizzled/lipoprotein receptor-related protein receptor inhibits the destruction complex, and β-catenin is stabilized and undergoes nuclear translocation and activation of lymphoid enhancer factor/T cell factor transcription factors, resulting in increased gene expression (49).
β-Catenin plays a role in adrenal development (50), activated β-catenin has been shown to occur in adrenal cortical carcinomas and adenomas (48), and activating mutations of β-catenin are found in some human aldosterone-producing adenomas (51). Transgenic mice with an adrenal-restricted constitutive activation of β-catenin (ΔCat) display adrenal hyperplasia and dysplasia that produces profound changes in zonal identity and causes hyperaldosteronism in females, with some of the mice also developing adrenal cancers at 17 months of age (33). Disease progression was slow in male ΔCat, mice and the study was largely performed in females; however, males also displayed adrenal hyperplasia but no signs of malignancy.
Transgenic Mouse Models With a Truncated Form of Adenomatous Polyposis Coli
As described previously, APC is a component of the destruction complex of β-catenin. Mice that express a defective mutant of Apc, which lacks the C-terminal portion of the gene (apcMin/+), develop multiple intestinal tumors (52). These mice also exhibit hypertension with an increase in plasma volume, a marked increase in fractional urinary excretion of K+, a decrease in urinary Na+ excretion, and an increase in plasma aldosterone and corticosterone concentrations (34).
The aldosterone-responsive serum/glucocorticoid-regulated kinase (SGK) 1 is also stimulated by β-catenin; therefore, stabilization of β-catenin would be expected to upregulate SGK1. Considerable evidence indicates that SGK causes an increase in the expression of cell-surface epithelial Na+ channels, which function in the regulation of Na+ and fluid reabsorption in the kidney and colon (53, 54). ApcMin/+/sgk−/− mice display increased aldosterone but not corticosterone levels compared with apcMin/+ mice, and the hypertension of apcMin/+ mice is ablated in the double-transgenic model (34). This effect is attributed to the impaired Na+ retention determined by the sgk deletion, whereas aldosterone secretion is directly affected by Apc-dependent signaling (34). This suggests that the apcMin/+ mice are a model of PA, whereas the double apcMin/+/sgk−/− transgenic model is a model of both primary and secondary aldosteronism. The normalization of hypertension by deletion of the sgk gene likely reflects the role of Sgk in epithelial Na+ channels expression. The previous study on apcMin/+ and apcMin/+/sgk−/− mice was performed using sex-matched mice of 3 months of age. No gender-related phenotype differences were reported.
The potential relevance of APC variants in some patients with PA is indicated by a case report of a young patient with severe hypertension and PA in a background of familial adenomatous polyposis with a germline heterozygous APC mutation (55). The patient displayed bilateral macronodular adrenal hyperplasia with lateralized aldosterone secretion. Molecular analysis and histopathology of the resected adrenal showed three nodules with one expressing CYP11B2 and carrying a somatic KCNJ5 mutation. This nodule and an additional nodule had a somatic biallelic APC inactivation that potentially triggered cell proliferation, with the KCNJ5 mutation providing the second genetic hit to drive the CYP11B2 overexpression (55).
Mouse Models With Deletion of Cryptochrome-1 and Cryptochrome-2
Behavior, metabolism, and physiology are subject to a well-controlled, daily rhythm, regulated by a molecular oscillator called the circadian clock. Alteration of the circadian rhythm in shift workers, flight crews, and individuals with sleep disorders has a higher than average incidence of cardiovascular disorders (56). Disruption of the circadian clock in cryptochrome (Cry) null mice that lack the core clock components Cry1−/−/Cry2−/− results in hyperaldosteronism with salt-sensitive hypertension and renal damage (57, 58). Age- and gender-related variations were eliminated by the inclusion of only male mice, aged 12 to 16 weeks. The hyperaldosteronism is reportedly a result of dysregulation of the 3β-hydroxysteroid dehydrogenase b6 (Hsd3b6), which is overexpressed in the zG of the mouse adrenal. The expression of the aldosterone synthase is unchanged, and it is believed that overexpression of the Hsd3b6 allows further substrate to become available for the synthesis of aldosterone (35, 57). The Hsd3b6 homolog in humans is the HSD3B1 isozyme, which is expressed in the zG of the human adrenal (58).
Period 1 (Per1) is another core component of the circadian clock, and Per1 and Cry2 can mediate opposing effects on target genes (59). Mice with reduced Per1 levels in vivo display decreased plasma aldosterone concentrations and a reduction in Hsd3b6 expression (60). This may be accounted for by an increase in Cry2 expression as Per1 levels decrease, which causes a dysregulation of Hsd3b6 gene expression.
Mouse Models With Differential Levels of Transforming Growth Factor β1 expression
Polymorphisms of the transforming growth factor β1 (TGFB1) gene located in the sequence coding the signal peptide are associated with a reduced risk of hypertension in a European population (61), and a different polymorphism that affects a different amino acid in the signal peptide has been associated with hypertension in an Asian population (62). The 3′ untranslated region of the Tgfb1 gene was manipulated to increase or decrease the stability of the encoded mRNA. Male mice were then created to express Tgfb1 in five grades, from 10% to 300% that of normal. Plasma aldosterone and corticosterone concentrations increased as Tgfb1 gene expression levels decreased. Mice with the lowest Tgfb1 expression (Tgfb1L/L) had ∼200% higher plasma aldosterone and corticosterone concentrations compared with wild-type, whereas the plasma aldosterone concentration of mice with the highest expression of Tgfb1 (Tgfb1H/H) was approximately halved. Expression of Cyp11b2 and Cyp11b1 followed the changes in Tgfb1 expression as predicted. The animals with decreased Tgfb1 had higher plasma volumes and elevated blood pressure and lower levels of Ang II (36), and the hypertension was corrected with spironolactone.
Mouse Models Produced Using a Random Mutagenesis Screen
In attempt to find novel genetic loci associated with PA, a large-scale mutation screen was performed in mice injected with N-ethyl-N-nitrosourea (an alkylating agent that causes ethylation of nucleic acids, resulting in point mutations) to introduce randomly mutations into the mouse genome (37). As expected, the resulting mutagenesis caused a phenotypic spectrum from total loss of function of some genes to gain of function and generated mouse lines with elevated plasma aldosterone concentrations in males but not in females. Exome next-generation sequencing identified eight mutated genes that were common between the F1 and F5 generations of the mice with high plasma aldosterone concentrations. Although animals carry more than one mutated gene, an attempt was made to correlate the expression of mutated genes with aldosterone levels, and animals carrying mutations in the Sspo, Dguok, Hoxaas2, and Clstn3 genes displayed higher aldosterone levels (63). Histological examination of the adrenal did not reveal any adenomas or hyperplasia but exhibited an increased staining for the Cyp11b2 enzyme that was more pronounced in the middle and inner areas of the adrenal cortex compared with wild-type animals, suggesting an altered zonation of Cyp11b2. Further studies will be necessary to characterize each individual gene in the regulation of aldosterone.
Mouse Models With an Increased Expression of the Aldosterone Synthase Gene
The biosynthesis of aldosterone depends on the transcriptionally regulated last enzyme in the pathway, aldosterone synthase (CYP11B2) (19). Male and female mice were generated with increased stability of the Cyp11b2 mRNA by substitution of the 3′untranslated region with the more stable 3′ untranslated sequence of the bovine growth hormone mRNA (38). Cyp11b2hi/hi mice displayed a slight increase in Cyp11b2 mRNA expression on a normal sodium diet with normal plasma aldosterone concentrations that were higher than those of wild-type animals on a high sodium diet (38). The Cyp11b2hi/hi mice on a modest high-salt diet infused with Ang II had a higher blood pressure, cardiac hypertrophy, and oxidative stress than wild-type animals (38). There were no reported phenotype differences related to gender. The modest elevation of aldosterone synthase expression in humans could result in the development of hypertension in societies that consume a high sodium diet.
Transgenic mice were produced with the promoter region of the human CYP11B1 gene fused to the coding region of the human CYP11B2 gene (39). The heterozygous mice, called hAS+/−, were shown to be hyperaldosteronemic and hypertensive when administered a high salt diet, and as expected, the plasma K+ was lower and Na+ higher than the wild-type mice. The high salt-induced hypertension was normalized by the administration of fadrozole, an aldosterone synthase inhibitor. These mice could be useful for the study of the cardiovascular and renal effects of endogenous-elevated aldosterone.
Perspectives and Conclusions
Mouse models of human disease often fail to represent the whole clinical spectrum. Although several mouse models of hyperaldosteronism have been described over recent years, they have not reproduced a genetic alteration shown to cause PA in humans and do not display the tumor formation or hyperplasia that reflect the pathophysiology of this disease. These models are, however, valuable to study mechanisms of fluid and electrolyte homeostasis and the regulation of aldosterone production, as well as providing potential targets for the pharmacological treatment of conditions where aldosterone production is elevated, as in PA.
Humans and rodents display some notable differences in adrenal physiology with alternative patterns of adrenal steroid production and distinct differences in the expression of K+ channels that maintain the membrane potential of zG cells. This divergence underscores the dubious suitability of available mouse models to resemble human pathophysiology. Further refined mouse models with inducible, zone-specific introduction of point mutations that have been observed in human disease could represent one possible approach to fill this gap. Furthermore, the adrenal physiology of larger animals, such as pigs, that are useful to model complex disease traits, could also resemble more closely that of humans and may offer the possibility to recapitulate genetic hits in ion pumps and channels that have been shown to cause the human disorder. Yet, pigs express a single CYP11B enzyme for aldosterone and cortisol synthesis instead of the two enzymes expressed in humans (CYP11B2 and CYP11B1). Notwithstanding this complexity, the sequencing of the pig genome by the Swine Genome Sequencing Project and advances in genome-editing technologies render feasible the production of a pig knockin model of PA with an adrenal-expressing human CYP11B1 and CYP11B2 to provide data that can be translated to the human condition.
Acknowledgments
Financial Support: This work was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program [Grant Agreement No. 694913 (to M.R.)]; Deutsche Forschungsgemeinschaft [within the CRC/Transregio 205/1 “The Adrenal: Central Relay in Health and Disease” (to M.R. and T.A.W.), and Grant RE 752/20-1 (to M.R.)]; Else Kröner-Fresenius Stiftung in support of the German Conns Registry-Else-Kröner Hyperaldosteronism Registry [2013_A182 and 2015_A171 (to M.R.)]; and National Heart, Lung, and Blood Institute [Grant R01 HL27255 (to C.E.G.-S.)] and National Institute of General Medical Sciences [Grant U54 GM115428 (to C.E.G.-S.)] from the US National Institutes of Health. C.E.G.-S. was a Visiting Fellow with the Center for Advanced Studies of the Ludwig Maximilian University of Munich, Germany.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ang II
- angiotensin II
- APC
- adenomatous polyposis coli
- BK
- big K+ channels
- Cry
- cryptochrome
- CYP11B1
- gene encoding 11β-hydroxylase
- CYP11B2
- gene encoding aldosterone synthase
- Dkk3
- dickkopf family gene
- GIRK
- G protein–activated inwardly rectifying K+ channels
- Hsd3b6
- 3β-hydroxysteroid dehydrogenase b6
- PA
- primary aldosteronism
- Per1
- period 1
- SGK
- serum/glucocorticoid-regulated kinase
- SNP
- single nucleotide polymorphism
- TASK channels
- tandem of P domains in weak, inwardly rectifying, K+ channel–related, acid-sensitive K+ channels
- TGFB1
- transforming growth factor β1
- zF
- zona fasciculata
- zG
- zona glomerulosa
- zR
- zona reticularis
- ΔCat
- constitutively activated β-catenin.
References
- 1.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–1248. [DOI] [PubMed] [Google Scholar]
- 2.Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98(12):4826–4833. [DOI] [PubMed] [Google Scholar]
- 3.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr, Endocrine S. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes-Rosa FL, Boulkroun S, Zennaro MC. Somatic and inherited mutations in primary aldosteronism. J Mol Endocrinol. 2017;59(1):R47–R63. [DOI] [PubMed] [Google Scholar]
- 5.Choi M, Scholl UI, Yue P, Björklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RPK. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tauber P, Penton D, Stindl J, Humberg E, Tegtmeier I, Sterner C, Beuschlein F, Reincke M, Barhanin J, Bandulik S, Warth R. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology. 2014;155(4):1353–1362. [DOI] [PubMed] [Google Scholar]
- 7.Tauber P, Aichinger B, Christ C, Stindl J, Rhayem Y, Beuschlein F, Warth R, Bandulik S, Tauber P, Aichinger B, Christ C, Stindl J, Rhayem Y, Beuschlein F, Warth R, Bandulik S. Cellular pathophysiology of an adrenal adenoma-associated mutant of the plasma membrane Ca(2+)-ATPase ATP2B3. Endocrinology. 2016;157(6):2489–2499. [DOI] [PubMed] [Google Scholar]
- 8.Stindl J, Tauber P, Sterner C, Tegtmeier I, Warth R, Bandulik S. Pathogenesis of adrenal aldosterone-producing adenomas carrying mutations of the Na(+)/K(+)-ATPase. Endocrinology. 2015;156(12):4582–4591. [DOI] [PubMed] [Google Scholar]
- 9.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stowasser M, Gordon RD. Primary aldosteronism: changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiol Rev. 2016;96(4):1327–1384. [DOI] [PubMed] [Google Scholar]
- 11.Burrows H. Sex hormones of the adrenal cortex In: Biological Actions of Sex Hormones. Cambridge, UK: Cambridge University Press; 2013:440–456. [Google Scholar]
- 12.Bielohuby M, Herbach N, Wanke R, Maser-Gluth C, Beuschlein F, Wolf E, Hoeflich A. Growth analysis of the mouse adrenal gland from weaning to adulthood: time- and gender-dependent alterations of cell size and number in the cortical compartment. Am J Physiol Endocrinol Metab. 2007;293(1):E139–E146. [DOI] [PubMed] [Google Scholar]
- 13.Schiffer L, Anderko S, Hannemann F, Eiden-Plach A, Bernhardt R. The CYP11B subfamily. J Steroid Biochem Mol Biol. 2015;151:38–51. [DOI] [PubMed] [Google Scholar]
- 14.Bülow HE, Bernhardt R. Analyses of the CYP11B gene family in the guinea pig suggest the existence of a primordial CYP11B gene with aldosterone synthase activity. Eur J Biochem. 2002;269(15):3838–3846. [DOI] [PubMed] [Google Scholar]
- 15.Ogishima T, Mitani F, Ishimura Y. Isolation of two distinct cytochromes P-45011 beta with aldosterone synthase activity from bovine adrenocortical mitochondria. J Biochem. 1989;105(4):497–499. [DOI] [PubMed] [Google Scholar]
- 16.Boon WC, Coghlan JP, McDougall JG. Late steps of aldosterone biosynthesis: sheep are not rats. Clin Exp Pharmacol Physiol Suppl. 1998;25(S1):S21–S27. [DOI] [PubMed] [Google Scholar]
- 17.Robic A, Faraut T, Prunier A. Pathways and genes involved in steroid hormone metabolism in male pigs: a review and update. J Steroid Biochem Mol Biol. 2014;140:44–55. [DOI] [PubMed] [Google Scholar]
- 18.Sanders K, Mol JA, Kooistra HS, Slob A, Galac S. New insights in the functional zonation of the canine adrenal cortex. J Vet Intern Med. 2016;30(3):741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol. 2012;350(2):151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spät A. Glomerulosa cell--a unique sensor of extracellular K+ concentration. Mol Cell Endocrinol. 2004;217(1-2):23–26. [DOI] [PubMed] [Google Scholar]
- 21.Lotshaw DP. Effects of K+ channel blockers on K+ channels, membrane potential, and aldosterone secretion in rat adrenal zona glomerulosa cells. Endocrinology. 1997;138(10):4167–4175. [DOI] [PubMed] [Google Scholar]
- 22.Lotshaw DP. Characterization of angiotensin II-regulated K+ conductance in rat adrenal glomerulosa cells. J Membr Biol. 1997;156(3):261–277. [DOI] [PubMed] [Google Scholar]
- 23.Lotshaw DP. Role of membrane depolarization and T-type Ca2+ channels in angiotensin II and K+ stimulated aldosterone secretion. Mol Cell Endocrinol. 2001;175(1-2):157–171. [DOI] [PubMed] [Google Scholar]
- 24.Guagliardo NA, Yao J, Hu C, Barrett PQ. Minireview: aldosterone biosynthesis: electrically gated for our protection. Endocrinology. 2012;153(8):3579–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen AX, Nishimoto K, Nanba K, Rainey WE. Potassium channels related to primary aldosteronism: expression similarities and differences between human and rat adrenals. Mol Cell Endocrinol. 2015;417:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heitzmann D, Derand R, Jungbauer S, Bandulik S, Sterner C, Schweda F, El Wakil A, Lalli E, Guy N, Mengual R, Reichold M, Tegtmeier I, Bendahhou S, Gomez-Sanchez CE, Aller MI, Wisden W, Weber A, Lesage F, Warth R, Barhanin J. Invalidation of TASK1 potassium channels disrupts adrenal gland zonation and mineralocorticoid homeostasis. EMBO J. 2008;27(1):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Wakil A, Bandulik S, Guy N, Bendahhou S, Zennaro MC, Niehrs C, Mari B, Warth R, Barhanin J, Lalli E. Dkk3 is a component of the genetic circuitry regulating aldosterone biosynthesis in the adrenal cortex. Hum Mol Genet. 2012;21(22):4922–4929. [DOI] [PubMed] [Google Scholar]
- 28.Davies LA, Hu C, Guagliardo NA, Sen N, Chen X, Talley EM, Carey RM, Bayliss DA, Barrett PQ. TASK channel deletion in mice causes primary hyperaldosteronism. [published correction appears in Proc Natl Acad Sci USA. 2008;105(36):13696]. Proc Natl Acad Sci USA. 2008;105(6):2203–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guagliardo NA, Yao J, Hu C, Schertz EM, Tyson DA, Carey RM, Bayliss DA, Barrett PQ. TASK-3 channel deletion in mice recapitulates low-renin essential hypertension. Hypertension. 2012;59(5):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penton D, Bandulik S, Schweda F, Haubs S, Tauber P, Reichold M, Cong LD, El Wakil A, Budde T, Lesage F, Lalli E, Zennaro MC, Warth R, Barhanin J. Task3 potassium channel gene invalidation causes low renin and salt-sensitive arterial hypertension. Endocrinology. 2012;153(10):4740–4748. [DOI] [PubMed] [Google Scholar]
- 31.Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC. Hypertension of Kcnmb1-/- is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci USA. 2009;106(28):11800–11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, Abdullah U, Krippeit-Drews P, Feil R, Hofmann F, Knaus HG, Kenyon C, Shipston MJ, Storm JF, Neuhuber W, Korth M, Schubert R, Gollasch M, Ruth P. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 2005;112(1):60–68. [DOI] [PubMed] [Google Scholar]
- 33.Berthon A, Sahut-Barnola I, Lambert-Langlais S, de Joussineau C, Damon-Soubeyrand C, Louiset E, Taketo MM, Tissier F, Bertherat J, Lefrançois-Martinez AM, Martinez A, Val P. Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum Mol Genet. 2010;19(8):1561–1576. [DOI] [PubMed] [Google Scholar]
- 34.Bhandaru M, Kempe DS, Rotte A, Rexhepaj R, Kuhl D, Lang F. Hyperaldosteronism, hypervolemia, and increased blood pressure in mice expressing defective APC. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R571–R575. [DOI] [PubMed] [Google Scholar]
- 35.Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16(1):67–74. [DOI] [PubMed] [Google Scholar]
- 36.Kakoki M, Pochynyuk OM, Hathaway CM, Tomita H, Hagaman JR, Kim HS, Zaika OL, Mamenko M, Kayashima Y, Matsuki K, Hiller S, Li F, Xu L, Grant R, Bertorello AM, Smithies O. Primary aldosteronism and impaired natriuresis in mice underexpressing TGFβ1. [published correction appears in Proc Natl Acad Sci USA. 2013;110(17):7097]. Proc Natl Acad Sci USA. 2013;110(14):5600–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spyroglou A, Wagner S, Gomez-Sanchez C, Rathkolb B, Wolf E, Manolopoulou J, Reincke M, Bidlingmaier M, Hrabé de Angelis M, Beuschlein F. Utilization of a mutagenesis screen to generate mouse models of hyperaldosteronism. Endocrinology. 2011;152(1):326–331. [DOI] [PubMed] [Google Scholar]
- 38.Makhanova N, Hagaman J, Kim HS, Smithies O. Salt-sensitive blood pressure in mice with increased expression of aldosterone synthase. Hypertension. 2008;51(1):134–140. [DOI] [PubMed] [Google Scholar]
- 39.Gu H, Ma Z, Wang J, Zhu T, Du N, Shatara A, Yi X, Kowala MC, Du Y. Salt-dependent blood pressure in human aldosterone synthase-transgenic mice. Sci Rep. 2017;7(1):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nogueira EF, Gerry D, Mantero F, Mariniello B, Rainey WE. The role of TASK1 in aldosterone production and its expression in normal adrenal and aldosterone-producing adenomas. Clin Endocrinol (Oxf). 2010;73(1):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung J, Barrett PQ, Eckert GJ, Edenberg HJ, Xuei X, Tu W, Pratt JH. Variations in the potassium channel genes KCNK3 and KCNK9 in relation to blood pressure and aldosterone production: an exploratory study. J Clin Endocrinol Metab. 2012;97(11):E2160–E2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao J, McHedlishvili D, McIntire WE, Guagliardo NA, Erisir A, Coburn CA, Santarelli VP, Bayliss DA, Barrett PQ. Functional TASK-3-like channels in mitochondria of aldosterone producing zona glomerulosa cells. Hypertension. 2017;70(2):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez-Sanchez CE, Kuppusamy M, Gomez-Sanchez EP. Of mice and man and the regulation of aldosterone secretion. Hypertension. 2017;70(2):240–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bandulik S, Tauber P, Penton D, Schweda F, Tegtmeier I, Sterner C, Lalli E, Lesage F, Hartmann M, Barhanin J, Warth R. Severe hyperaldosteronism in neonatal Task3 potassium channel knockout mice is associated with activation of the intraadrenal renin-angiotensin system. Endocrinology. 2013;154(8):2712–2722. [DOI] [PubMed] [Google Scholar]
- 45.Holtzclaw JD, Grimm PR, Sansom SC. Role of BK channels in hypertension and potassium secretion. Curr Opin Nephrol Hypertens. 2011;20(5):512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimm PR, Sansom SC. BK channels and a new form of hypertension. Kidney Int. 2010;78(10):956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durroux T, Gallo-Payet N, Payet MD. Effects of adrenocorticotropin on action potential and calcium currents in cultured rat and bovine glomerulosa cells. Endocrinology. 1991;129(4):2139–2147. [DOI] [PubMed] [Google Scholar]
- 48.El Wakil A, Lalli E. The Wnt/beta-catenin pathway in adrenocortical development and cancer. Mol Cell Endocrinol. 2011;332(1-2):32–37. [DOI] [PubMed] [Google Scholar]
- 49.Kim A, Giordano TJ, Kuick R, Serecky K, Hammer GD. Wnt/betacatenin signaling in adrenocortical stem/progenitor cells: implications for adrenocortical carcinoma. Ann Endocrinol (Paris). 2009;70(3):156. [DOI] [PubMed] [Google Scholar]
- 50.Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD. Targeted disruption of beta-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135(15):2593–2602. [DOI] [PubMed] [Google Scholar]
- 51.Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Björklund P. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016;6(1):19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247(4940):322–324. [DOI] [PubMed] [Google Scholar]
- 53.Kamynina E, Staub O. Concerted action of ENaC, Nedd4-2, and Sgk1 in transepithelial Na(+) transport. Am J Physiol Renal Physiol. 2002;283(3):F377–F387. [DOI] [PubMed] [Google Scholar]
- 54.Wulff P, Vallon V, Huang DY, Völkl H, Yu F, Richter K, Jansen M, Schlünz M, Klingel K, Loffing J, Kauselmann G, Bösl MR, Lang F, Kuhl D. Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest. 2002;110(9):1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vouillarmet J, Fernandes-Rosa F, Graeppi-Dulac J, Lantelme P, Decaussin-Petrucci M, Thivolet C, Peix JL, Boulkroun S, Clauser E, Zennaro MC. Aldosterone-producing adenoma with a somatic KCNJ5 mutation revealing APC-dependent familial adenomatous polyposis. J Clin Endocrinol Metab. 2016;101(11):3874–3878. [DOI] [PubMed] [Google Scholar]
- 56.Portaluppi F. The circadian organization of the cardiovascular system in health and disease. Indian J Exp Biol. 2014;52(5):395–398. [PubMed] [Google Scholar]
- 57.Nugrahaningsih DA, Emoto N, Vignon-Zellweger N, Purnomo E, Yagi K, Nakayama K, Doi M, Okamura H, Hirata K. Chronic hyperaldosteronism in cryptochrome-null mice induces high-salt- and blood pressure-independent kidney damage in mice. Hypertens Res. 2014;37(3):202–209. [DOI] [PubMed] [Google Scholar]
- 58.Doi M, Satoh F, Maekawa T, Nakamura Y, Fustin JM, Tainaka M, Hotta Y, Takahashi Y, Morimoto R, Takase K, Ito S, Sasano H, Okamura H. Isoform-specific monoclonal antibodies against 3β-hydroxysteroid dehydrogenase/isomerase family provide markers for subclassification of human primary aldosteronism. J Clin Endocrinol Metab. 2014;99(2):E257–E262. [DOI] [PubMed] [Google Scholar]
- 59.Richards J, All S, Skopis G, Cheng KY, Compton B, Srialluri N, Stow L, Jeffers LA, Gumz ML. Opposing actions of Per1 and Cry2 in the regulation of Per1 target gene expression in the liver and kidney. Am J Physiol Regul Integr Comp Physiol. 2013;305(7):R735–R747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richards J, Cheng KY, All S, Skopis G, Jeffers L, Lynch IJ, Wingo CS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention. Am J Physiol Renal Physiol. 2013;305(12):F1697–F1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cambien F, Ricard S, Troesch A, Mallet C, Générénaz L, Evans A, Arveiler D, Luc G, Ruidavets JB, Poirier O. Polymorphisms of the transforming growth factor-beta 1 gene in relation to myocardial infarction and blood pressure. The Etude Cas-Témoin de l’Infarctus du Myocarde (ECTIM) Study. Hypertension. 1996;28(5):881–887. [DOI] [PubMed] [Google Scholar]
- 62.Niu W. Evaluation of transforming growth factor beta-1 gene 869T/C polymorphism with hypertension: a meta-analysis. Int J Hypertens. 2011;2011:934265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez-Rivas LG, Rhayem Y, Sabrautzki S, Hantel C, Rathkolb B, Hrabě de Angelis M, Reincke M, Beuschlein F, Spyroglou A. Genetic characterization of a mouse line with primary aldosteronism. J Mol Endocrinol. 2017;58(2):67–78. [DOI] [PubMed] [Google Scholar]