Abstract
Rationale: Higher social support is associated with a better quality of life and functioning in adults with chronic obstructive pulmonary disease (COPD).
Objectives: To determine the association between structural and functional social support and self-care behaviors in adults with COPD.
Methods: This was a longitudinal study using data from the CASCADE (COPD Activity: Serotonin Transporter, Cytokines, and Depression) study, which was focused on depression and functioning in COPD. Physical activity was measured with a validated accelerometer at baseline, year 1, and year 2. Additional self-care behaviors included pulmonary rehabilitation attendance, smoking status, receipt of influenza and/or pneumococcal vaccinations, and medication adherence. Structural social support indicators included living status, being partnered, number of close friends/relatives, and presence of a family caregiver. Functional social support was measured with the Medical Outcomes Social Support Survey (MOSSS). Mixed-effects and logistic regression models were used.
Results: A total of 282 participants with Global Initiative for Chronic Obstructive Lung Disease stage II to IV COPD were included (age, 68 ± 9 yr; 80% men; FEV1% predicted, 45 ± 16). For physical activity, participants who lived with others accrued 903 more steps per day than those who lived alone (95% confidence interval [CI], 373–1,433; P = 0.001); increases in the MOSSS total score were associated with more steps per day (β = 10; 95% CI, 2–18; P = 0.02). The odds of pulmonary rehabilitation participation were more than 11 times higher if an individual had a spouse or partner caregiver compared with not having a caregiver (odds ratio [OR], 11.03; 95% CI, 1.93–62.97; P < 0.01). Higher functional social support (MOSSS total score) was associated with marginally lower odds of smoking (OR, 0.99; 95% CI, 0.98–1.00; P = 0.03) and higher odds of pneumococcal vaccination (OR, 1.02; 95% CI, 1.00–1.03; P = 0.02). Social support was not associated with influenza vaccination or medication adherence.
Conclusions: Structural social support, which was measured by reports of living with others and having a caregiver, was respectively associated with higher levels of physical activity and greater participation in pulmonary rehabilitation in adults with COPD. Our findings reinforce the critical importance of the social environment in shaping patients’ success with self-care.
Clinical Trial registered with clinicaltrials.gov (NCT01074515).
Keywords: physical activity, smoking status, pulmonary rehabilitation, vaccination, medication adherence
Chronic obstructive pulmonary disease (COPD) is a progressive disease, and it is the third leading cause of death in the United States (1). Management of COPD is typically focused on reducing exacerbation risks and on relieving the impact of symptoms on physical functioning and well-being (2). Patients and their families are primarily responsible for undertaking a number of self-care behaviors to successfully manage COPD and other chronic conditions (3–5). Self-management is a term that is used to describe the process of taking responsibility for one’s own day-to-day care to maintain well-being (6). For adults with COPD, engaging in regular physical activity, quitting smoking, participating in pulmonary rehabilitation, receiving vaccinations, and adhering to medication are core behaviors to improve health outcomes (2).
Self-management is influenced by a number of factors, including social support (7). Social support is the individual’s experience of being cared for and loved, having a sense of being valued and needed by other people, and being part of a mutually supportive network (8, 9). Social support has been conceptualized as having two domains: structural and functional. Structural social support describes the characteristics of the social network that surrounds a person and his/her interactions within this network (e.g., marital status and living arrangements) (10). In contrast, functional, or perceived, social support describes the specific functions provided to a person by his/her social network. It can be described using five dimensions: emotional, informational, tangible, affectionate, and positive social interaction (11).
Studies show that higher levels of social support are associated with better self-care behaviors in other chronic diseases, such as diabetes, chronic heart disease, and chronic kidney disease (7, 12). Only a few studies of participants with COPD have reported findings on the effect of social support on self-care behaviors. For example, two studies found that functional social support from family members helped participants manage their COPD (13, 14). However, none of these studies have systematically examined the association of both structural and functional support on self-care behaviors in adults with COPD.
Therefore, the purpose of this study is to determine the association between the individual components of structural and functional social support and self-care behaviors (physical activity, smoking status, participating in pulmonary rehabilitation, receiving vaccinations, and adhering to inhaler or nebulizer medication) in adults with COPD. Some of the results have been previously reported in the form of an abstract (15).
Methods
Study Design/Settings
This secondary analysis used data from the COPD Activity: Serotonin Transporter, Cytokines, and Depression (CASCADE) study at three time points (baseline, year 1, and year 2). The CASCADE study was a multisite prospective observational study of participants with COPD who were followed for 2 years to study the biological causes and functional consequences of depression. It was approved by the institutional review boards of the three clinical sites, which included one academic medical center and two Veterans Affairs Health Care Systems in the United States. The study was registered with ClinicalTrials.gov (NCT01074515).
Participants
The CASCADE study recruited participants from queries of medical records and pulmonary function tests, chest clinics from the three medical centers, a research database maintained by the investigators, pulmonary rehabilitation programs, Better Breathers support groups, community pulmonary medicine practices, advertisements, the study web site, and other referrals. The inclusion criteria were: (1) clinical diagnosis of COPD, (2) post-bronchodilator FEV1/FVC less than 70%, (3) moderate to very severe disease with an FEV1 less than 80% predicted, (4) age 40 years or older, (5) current or past cigarette smoking (>10 pack-years), (6) stable disease with no acute exacerbations of COPD in the past 4 weeks, and (7) ability to speak, read, and write English.
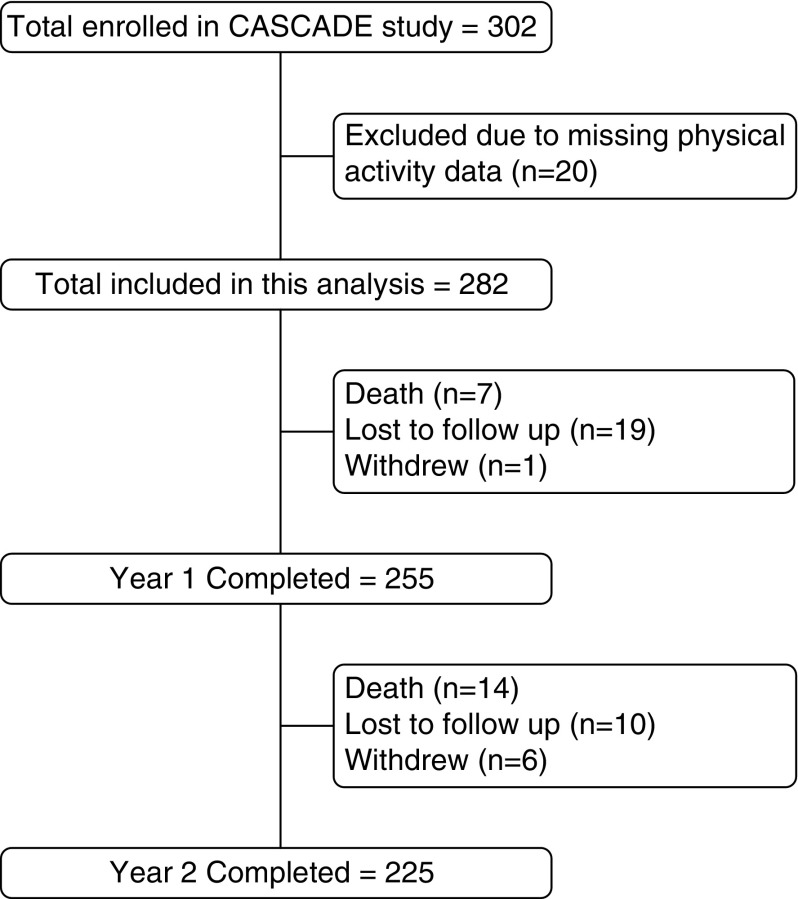
Because the CASCADE study was focused on depression and inflammation, we excluded participants with any of the following conditions: other chronic lung diseases (e.g., asthma, bronchiectasis, cystic fibrosis, or idiopathic pulmonary fibrosis), uncompensated heart failure (with exacerbation in the past 4 wk), primary pulmonary vascular disease, chronic antibiotic use or ongoing infection, autoimmune disease, lung cancer or metastatic cancer, chronic renal failure requiring dialysis, chronic uncompensated liver disease, HIV/AIDS, or chronic oral prednisone use, bipolar disease, psychotic disorders, or dementia. For this analysis, we further excluded participants from the CASCADE cohort who did not have objective physical activity measurement at baseline (Figure 1).
Figure 1.
Study sample flowchart. CASCADE = COPD Activity: Serotonin Transporter, Cytokines, and Depression; COPD = chronic obstructive pulmonary disease.
Procedures
All CASCADE study participants provided informed consent before their first clinic assessment, which included prebronchodilator and post-bronchodilator spirometry and completion of questionnaires. At the end of the clinic visit, participants were asked to wear an activity monitor for 7 days. Two days after this clinic visit, a trained mental-health professional completed a depression and anxiety assessment by telephone. These procedures were repeated 1 and 2 years later.
Measures
Demographics
Demographic data included self-reported age, sex, race, education level, employment status, and household income. Alcohol use was measured with the Audit-C, a three-item alcohol screen, which scored on a scale of 0 to 12 (scores of 0 reflect no alcohol use). In men, a score of 4 or more is considered positive for hazardous drinking; in women, a score of 3 or more is considered positive (16). Spirometry was performed by research coordinators following American Thoracic Society standards (17), and post-bronchodilator values were used in the analyses. Disease severity measures included the BODE Index (18) and oxygen use. The BODE Index is a 10-point scale multidimensional grading system that includes weighted scores for body mass index, airflow obstruction (FEV1), dyspnea (Modified Medical Research Council Scale), and exercise capacity (6-minute-walk test). Comorbidities were measured by self-report using the Charlson Comorbidity Index (19), and participants were categorized as having no comorbidities or one or more comorbidities. Psychological symptoms were measured with the Hospital Anxiety and Depression Scale (HADS) (20).
Social support
Structural social support was measured using three questions: (1) whether participants live alone or live with others, (2) whether they are partnered, and (3) the number of close friends and relatives. Midway through the study, we added an additional question regarding the presence of a family/friend caregiver (which family member or friend is most involved in your care now?) and thus have complete data on this variable only at the year 2 assessment. Functional social support was measured with the Medical Outcomes Social Support Scale (MOSSS) (11). The MOSSS has 20 questions that can be summarized into a total score and four subscales measuring different dimensions of perceived support: emotional/informational, tangible, affectionate, and positive social interaction.
Self-care behaviors
Physical activity was measured with a Stepwatch 3 Activity Monitor (SAM; OrthoCare Innovations, Washington, DC) fastened above the right ankle. The SAM is a highly accurate research-grade accelerometer previously validated in adults with COPD (21). Participants were asked to wear the SAM during waking hours for 7 days. Total step count per day was the primary physical activity variable.
Other self-care behaviors were based on self-reported responses to four yes/no questions: “In the past week, have you smoked any cigarettes, even a puff?” “Have you ever participated in an exercise program for your lungs (pulmonary rehabilitation)?” “In the last year, did you receive an influenza vaccination (flu shot)?” “Have you received a pneumonia vaccination in the past (Pneumovax)?”
Adherence to inhaler or nebulizer medications was measured with four questions about carelessness, forgetting, stopping medication when feeling better, and using less of the medication than prescribed when feeling better in the past 3 months (22, 23) using a 5-point Likert scale from 1 (most of the time) to 5 (none of the time). An adherence score was created by summing responses to these four questions (score range: 4–20). Participants were considered fully adherent if they scored a total of 20 points.
Statistical Analysis
Descriptive statistics were used to describe the data over 2 years of follow-up. With the exception of physical activity, none of the other five self-care behaviors changed significantly at year 1 and 2. Therefore, we used only baseline data to examine the unadjusted and adjusted cross-sectional associations between social support variables (with the exception of caregiver status) and the outcomes of current smoking status, participation in pulmonary rehabilitation, receipt of vaccinations, and adherence with inhaler or nebulizer medication using linear or logistic regression models. One of the structural social support variables, caregiver status, was only ascertained at year 2, and therefore models with caregiver status only used year 2 study data. Each social support variable was included in separate models. Baseline cross-sectional analyses were adjusted for age, sex, race, education level, income, employment status, alcohol use, BODE, home oxygen use, Charlson Comorbidity Index, HADS-depression, and HADS-anxiety. Due to the smaller sample size for the cross-sectional model of caregiver support and outcomes using the data from year 2, we adjusted for a more limited set of covariates, demographic (age, sex, employment, household income) and disease severity (BODE Index, oxygen use, and Charlson Comorbidity Index).
For physical activity, mixed-effects longitudinal unadjusted and adjusted models were used to examine relationships between measures of structural/functional social support and physical activity over 2 years. The models contained assessment period (time) as a fixed factor and subject as the random factor. The β coefficient in this model uses the data from all three time points to provide an overall estimated effect of social support on physical activity. Demographics (age, sex, race, education level, income, employment status, and alcohol use) were fixed (time-invariant) covariates collected only at baseline; disease severity variables (BODE, home oxygen use, and Charlson Comorbidity Index) and psychological variables (HADS-depression, HADS-anxiety) were assessed at all three time points and were treated as time-varying covariates. With the mixed-effects models, we were able to include data for all participants who contributed data for at least one follow-up assessment.
All analyses were conducted using Stata 14.0 (StataCorp LP, College Station, TX). A P value < 0.05 was considered statistically significant for all tests.
Results
Sample Characteristics
A total of 302 participants were enrolled in the CASCADE study; however, 20 participants were excluded from this analysis due to unusable physical activity data, leaving 282 participants in our baseline study cohort (Figure 1). Participant characteristics at baseline, year 1, and year 2 are shown in Table 1. The sample included mostly white men (80%), with a mean age of 68 ± 9 years. One-third of the participants used supplemental oxygen, and half had one or more comorbidities; the mean FEV1% predicted was 45 ± 16 and BODE Index was 4 ± 2. Self-reported symptoms of depression and anxiety were low.
Table 1.
Sample characteristics
| Variables | Baseline (n = 282) | Year 1 (n = 255) | Year 2 (n = 225) |
|---|---|---|---|
| Age, yr | 67.7 ± 8.6 | 68.6 ± 8.6 | 69.6 ± 8.6 |
| Sex, male | 226 (80) | 203 (80) | 181 (80) |
| Race, white | 246 (87) | 225 (88) | 201 (89) |
| Education, some college or more | 218 (77) | 197 (77) | 176 (78) |
| Income, ≥$20K/yr | 172 (62) | 161 (64) | 141 (64) |
| Currently employed | 42 (15) | 39 (15) | 25 (11) |
| Alcohol misuse* | 54 (19) | 44 (17) | 39 (17) |
| FEV1% predicted | 44.9 ± 15.7 | 46.5 ± 16.7 | 44.9 ± 17.3 |
| BODE Index (↓0–10) | 3.7 ± 2.3 | 3.5 ± 2.3 | 3.4 ± 2.4 |
| O2 supplementation | 98 (34) | 91 (36) | 93 (41) |
| Charlson Comorbidity Index ≥ 1 | 138 (49) | 121 (48) | 109 (48) |
| HADS-Depression (↓0–21) | 4.2 ± 4.1 | 3.2 ± 4.0 | 3.0 ± 3.3 |
| HADS-Anxiety (↓0–21) | 5.0 ± 3.9 | 3.6 ± 3.8 | 3.3 ± 3.6 |
| Structural social support | |||
| Marital status: partnered | 163 (58) | 147 (58) | 124 (55) |
| Live with others | 211 (75) | 190 (75) | 162 (72) |
| Report having ≥6 close friends or relatives | 125 (44) | 112 (45) | 108 (48) |
| Presence of unpaid caregiver† | n = 215 | ||
| Partner | N/A | N/A | 117 (54) |
| Other (child, sibling, friend) | N/A | N/A | 76 (35) |
| None | N/A | N/A | 22 (10) |
| Functional social support | |||
| MOSSS, total (↑0–100) | 68.4 ± 27.8 | 73.8 ± 24.9 | 73.5 ± 25.1 |
| Emotional/informational | 66.7 ± 28.3 | 71.8 ± 25.1 | 72.8 ± 25.8 |
| Tangible | 70.5 ± 29.4 | 73.6 ± 26.9 | 73.2 ± 29.1 |
| Affectionate | 72.0 ± 32.3 | 78.6 ± 28.9 | 76.7 ± 30.1 |
| Positive social interaction | 70.6 ± 30.2 | 76.1 ± 27.0 | 75.4 ± 27.0 |
| Self-care behaviors | |||
| Physical activity, total steps/d | 6,001.5 ± 3,341.8 | 5,830.6 ± 3,260.7 | 5,527.7 ± 3,121.0 |
| Current smoker | 86 (29) | 67 (25) | 60 (25) |
| Participation in pulmonary rehabilitation | 83 (28) | 84 (32) | 82 (35) |
| Received influenza vaccination in the last year | 262 (87) | 234 (88) | 201 (85) |
| Received pneumococcal vaccination in the past | 252 (84) | 223 (84) | 200 (85) |
| Full adherence with inhaler or nebulizer medication | 81 (29) | 77 (31) | 70 (32) |
Definition of abbreviations: BODE = body mass index, obstruction, dyspnea, exercise; HADS = Hospital Anxiety and Depression Scale; MOSSS = Medical Outcomes Social Support Survey; N/A = not applicable.
Data are presented as n (%) or mean ± SD. Direction of arrows represents scores reflecting better health: ↑ indicates that higher scores are better health; ↓ indicates that lower scores are better health.
Possible alcohol misuse based on an Audit C score ≥4 in men or ≥3 in women.
Because this question was added after the study started, we only have data for the second year of follow-up.
Overall, participants had relatively high levels of structural and functional social support. More than half were married or partnered, 75% were living with others, and 90% reported having a family caregiver. The mean total score on the MOSSS was 68 ± 28.
Participants accrued a mean of 6,002 ± 3,342 steps per day at baseline, with reductions of 474 ± 221 over the 2 years of follow-up. At baseline, 29% were still smoking, 28% had attended a pulmonary rehabilitation program in the past, nearly all had received the influenza or pneumococcal vaccination, and 29% were reported being fully adherent to their inhaler or nebulizer medications.
Social Support and Physical Activity
The longitudinal unadjusted and adjusted linear mixed models examining the association between social support and physical activity over 2 years are summarized in Table 2. We did not find an interaction between functional or structural social support with time when predicting physical activity. The unadjusted models showed that living with others and having higher levels of perceived social support (total, emotional/informational, and positive social interaction) were significantly associated with higher step counts. In adjusted analyses, participants who lived with others accrued 903 more steps per day than those who lived alone (β = 903; 95% CI, 373–1,433; P = 0.001). Similarly, perceived social support remained significantly associated with higher step counts in the adjusted models. A 1-point increase in MOSSS total score was associated with 10 more steps per day (β = 10; 95% CI, 2–18; P = 0.02).
Table 2.
Associations between social support and physical activity
| Models* | Unadjusted |
Adjusted† |
||||
|---|---|---|---|---|---|---|
| β | P Value | 95% CI | β | P Value | 95% CI | |
| Structural social support | ||||||
| Live with others | 779.7 | <0.01 | 227.2 to 1,372.3 | 903.0 | <0.01 | 372.8 to 1,433.2 |
| Married/partnered | 454.8 | 0.12 | −113.1 to 1,022.8 | 490.1 | 0.07 | −31.5 to 1,011.8 |
| ≥6 Friends and relatives | −117.5 | 0.50 | −459.6 to 224.6 | −149.4 | 0.40 | −497.1 to 198.4 |
| Caregiver‡ | ||||||
| No caregiver | Ref | Ref | Ref | Ref | Ref | Ref |
| Spouse or partner | −388.9 | 0.61 | −1,881.8 to 1,103.9 | 453.3 | 0.48 | −803.8 to 1,710.4 |
| Other | −1,061.7 | 0.18 | −2,617.7 to 494.2 | −3.3 | 0.99 | −1,281.0 to 1,274.4 |
| Functional social support: MOSSS scores | ||||||
| Total | 11.8 | <0.01 | 3.5 to 20.2 | 10.1 | 0.02 | 1.9 to 18.3 |
| Emotional and informational | 10.9 | <0.01 | 3.0 to 18.8 | 9.1 | 0.02 | 1.3 to 16.8 |
| Tangible | 6.0 | 0.12 | −1.5 to 13.5 | 5.9 | 0.11 | −1.3 to 13.2 |
| Affectionate | 5.0 | 0.16 | −1.9 to 11.9 | 3.8 | 0.27 | −2.9 to 10.5 |
| Positive social interaction | 9.0 | 0.01 | 1.9 to 16.1 | 7.9 | 0.03 | 0.8 to 14.9 |
Definition of abbreviations: BODE = body mass index, obstruction, dyspnea, exercise; CI = confidence interval; HADS = Hospital Anxiety and Depression Scale; MOSSS = Medical Outcomes Study Social Support Scale.
Boldface type indicates values with P < 0.05.
Each social support measure was modeled separately.
Adjusted for year, age, sex, race, education level, income, employment status, alcohol use, BODE, home oxygen use, Charlson Comorbidity Index, HADS-depression, and HADS-anxiety.
Only year 2 data used for caregiver analyses. Reference group of caregiver was participants without caregiver. Model adjusted for age, sex, income, employment status, BODE, home oxygen use, and Charlson Comorbidity Index.
We also examined whether the effect of overall perceived social support (MOSSS total score) was associated with physical activity after adjusting for structural support (living situation) in the same model over 2 years. The unadjusted analysis showed that both living with others and having higher levels of total functional social support were significantly and independently associated with higher step counts. However, after adjusting for covariates, only living with others remained associated with higher step counts over the 2-year period (β = 812; 95% CI, 264–1,359; P = 0.004).
The cross-sectional year 2 analysis showed that the presence of a family caregiver did not have an effect on step counts.
Social Support and Pulmonary Rehabilitation
Logistic regression analyses showed that living with others, being married or partnered, and having six or more close friends or relatives were not associated with previous participation in pulmonary rehabilitation (Table 3). However, the odds of participation in pulmonary rehabilitation were more than five times higher if a participant had a caregiver compared with no caregiver (OR, 5.51–5.75; P < 0.05). After adjusting for covariates, the odds of participation in pulmonary rehabilitation was 11 times higher when participants had a spouse or partner as their caregiver compared with those without a caregiver (OR = 11.03; 95% CI, 1.93–62.97; P < 0.01). Functional social support was not associated with attending pulmonary rehabilitation.
Table 3.
Associations between social support and pulmonary rehabilitation
| Models* | Unadjusted |
Adjusted† |
||||
|---|---|---|---|---|---|---|
| OR | P Value | 95% CI | OR | P Value | 95% CI | |
| Structural social support | ||||||
| Live with others | 0.92 | 0.79 | 0.51 to 1.67 | 1.29 | 0.49 | 0.64 to 2.60 |
| Married/partnered | 1.14 | 0.64 | 0.67 to 1.92 | 1.43 | 0.28 | 0.75 to 2.72 |
| ≥6 Close friends and relatives | 1.38 | 0.23 | 0.82 to 2.32 | 1.53 | 0.16 | 0.84 to 2.77 |
| Caregiver‡ | ||||||
| No caregiver | Ref | Ref | Ref | Ref | Ref | Ref |
| Spouse or partner | 5.75 | 0.02 | 1.28 to 25.84 | 11.03 | <0.01 | 1.93 to 62.97 |
| Other | 5.51 | 0.03 | 1.20 to 25.39 | 4.34 | 0.10 | 0.77 to 24.31 |
| Functional social support: MOSSS scores | ||||||
| Total | 1.00 | 0.49 | 0.99 to 1.01 | 1.00 | 0.52 | 0.99 to 1.01 |
| Emotional and informational | 1.01 | 0.34 | 1.00 to 1.01 | 1.01 | 0.27 | 1.00 to 1.02 |
| Tangible | 1.00 | 0.81 | 0.99 to 1.01 | 1.00 | 0.97 | 0.99 to 1.01 |
| Affectionate | 1.01 | 0.23 | 1.00 to 1.01 | 1.00 | 0.32 | 1.00 to 1.01 |
| Positive social interaction | 1.00 | 0.47 | 1.00 to 1.01 | 1.00 | 0.38 | 0.99 to 1.01 |
Definition of abbreviations: BODE = body mass index, obstruction, dyspnea, exercise; CI = confidence interval; HADS = Hospital Anxiety and Depression Scale; MOSSS = Medical Outcomes Study Social Support Scale; OR = odds ratio.
Boldface type indicates values with P < 0.05.
Each social support measure was modeled separately.
Adjusted for age, sex, race, education level, income, employment status, alcohol use, BODE, home oxygen use, Charlson Comorbidity Index, HADS-depression, and HADS-anxiety.
Only year 2 data used for caregiver analyses. Reference group of caregiver was participants without caregiver. Model adjusted for age, sex, income, employment status, BODE, home oxygen use, and Charlson Comorbidity Index.
Social Support and Current Smoking Status
The unadjusted logistic regression analyses showed that none of the structural social support measures were associated with current smoking status except for presence of a caregiver (Table 4). However, this association was no longer significant in the adjusted model. Functional social support had a statistically significant but modest relationship with smoking status (OR, 0.99; 95% CI, 0.98–1.0; P = 0.03) in the adjusted model.
Table 4.
Associations between social support and current smoking status
| Models* | Unadjusted |
Adjusted† |
||||
|---|---|---|---|---|---|---|
| OR | P Value | 95% CI | OR | P Value | 95% CI | |
| Structural social support | ||||||
| Live with others | 1.01 | 0.97 | 0.55 to 1.86 | 0.79 | 0.51 | 0.39 to 1.60 |
| Married/partnered | 0.87 | 0.60 | 0.51 to 1.48 | 0.91 | 0.76 | 0.48 to 1.71 |
| ≥6 Close friends and relatives | 0.71 | 0.21 | 0.41 to 1.21 | 0.92 | 0.79 | 0.49 to 1.72 |
| Caregiver‡ | ||||||
| No caregiver | Ref | Ref | Ref | Ref | Ref | Ref |
| Spouse or partner | 0.32 | 0.02 | 0.12 to 0.82 | 0.52 | 0.25 | 0.17 to 1.59 |
| Other | 0.19 | <0.01 | 0.07 to 0.53 | 0.37 | 0.10 | 0.11 to 1.20 |
| Functional social support: MOSSS scores | ||||||
| Total | 0.99 | <0.01 | 0.98 to 1.00 | 0.99 | 0.03 | 0.98 to 1.00 |
| Emotional and informational | 0.99 | 0.03 | 0.98 to 1.00 | 0.99 | 0.11 | 0.98 to 1.00 |
| Tangible | 0.99 | <0.01 | 0.98 to 0.99 | 0.98 | <0.01 | 0.97 to 1.00 |
| Affectionate | 0.99 | 0.04 | 0.98 to 1.00 | 0.99 | 0.14 | 0.98 to 1.00 |
| Positive social interaction | 0.99 | 0.03 | 0.98 to 1.00 | 0.99 | 0.06 | 0.98 to 1.00 |
Definition of abbreviations: BODE = body mass index, obstruction, dyspnea, exercise; CI = confidence interval; HADS = Hospital Anxiety and Depression Scale; MOSSS = Medical Outcomes Study Social Support Scale; OR = odds ratio.
Boldface type indicates values with P < 0.05.
Each social support measure was modeled separately.
Adjusted for age, sex, race, education level, income, employment status, alcohol use, BODE, home oxygen use, Charlson Comorbidity Index, HADS-depression, and HADS-anxiety.
Only year 2 data used for caregiver analyses. Reference group of caregiver was participants without caregiver. Model adjusted for age, sex, income, employment status, BODE, home oxygen use, and Charlson Comorbidity Index.
Social Support and Vaccinations
Although there was no relationship between structural or perceived social support and influenza vaccination, perceived social support was associated with marginally higher odds of pneumococcal vaccination in adjusted models (Table 5).
Table 5.
Associations between social support and pneumococcal vaccination
| Models* | Unadjusted |
Adjusted† |
||||
|---|---|---|---|---|---|---|
| OR | P Value | 95% CI | OR | P Value | 95% CI | |
| Structural social support | ||||||
| Live with others | 1.17 | 0.67 | 0.57 to 2.43 | 1.23 | 0.62 | 0.53 to 2.85 |
| Married/partnered | 1.39 | 0.32 | 0.72 to 2.66 | 0.98 | 0.96 | 0.46 to 2.11 |
| ≥6 Close friends and relatives | 1.60 | 0.17 | 0.82 to 3.16 | 1.26 | 0.57 | 0.57 to 2.77 |
| Caregiver‡ | ||||||
| No caregiver | Ref | Ref | Ref | Ref | Ref | Ref |
| Spouse or partner | 0.91 | 0.89 | 0.24 to 3.40 | 0.60 | 0.49 | 0.14 to 2.54 |
| Other | 0.89 | 0.87 | 0.23 to 3.49 | 0.83 | 0.80 | 0.19 to 3.59 |
| Functional social support: MOSSS scores | ||||||
| Total | 1.02 | <0.01 | 1.01 to 1.03 | 1.02 | 0.02 | 1.00 to 1.03 |
| Emotional and informational | 1.02 | <0.01 | 1.01 to 1.03 | 1.02 | <0.01 | 1.01 to 1.03 |
| Tangible | 1.02 | <0.01 | 1.01 to 1.03 | 1.02 | 0.02 | 1.00 to 1.03 |
| Affectionate | 1.01 | <0.01 | 1.01 to 1.02 | 1.01 | 0.04 | 1.00 to 1.02 |
| Positive social interaction | 1.01 | 0.02 | 1.00 to 1.02 | 1.01 | 0.07 | 1.00 to 1.02 |
Definition of abbreviations: BODE = body mass index, obstruction, dyspnea, exercise; CI = confidence interval; HADS = Hospital Anxiety and Depression Scale; MOSSS = Medical Outcomes Study Social Support Scale; OR = odds ratio.
Boldface type indicates values with P < 0.05.
Each social support measure was modeled separately.
Adjusted for age, sex, race, education level, income, employment status, alcohol use, BODE, home oxygen use, Charlson Comorbidity Index, HADS-depression, and HADS-anxiety.
Only year 2 data used for caregiver analyses. Reference group of caregiver was participants without caregiver. Model adjusted for age, sex, income, employment status, BODE, home oxygen use, and Charlson Comorbidity Index.
Social Support and Adherence with Inhaler or Nebulizer Medication
There was no significant association between structural or perceived social support and adherence with inhaler or nebulizer medication.
Discussion
We found that the type and level of social support had differing effects on core self-care behaviors in adults with COPD. Living with others had a stronger association with physical activity than functional social support and was associated with a clinically meaningful increase of more than 900 steps per day (24) compared with living alone. Having a spouse or partner caregiver was associated with more than a tenfold increase in pulmonary rehabilitation participation compared with having no caregiver. Functional but not structural social support had a small beneficial relationship with smoking behavior and pneumococcal vaccination of unclear clinical significance. Neither structural nor functional support was associated with influenza vaccination or adherence to inhaler or nebulizer medications.
Because higher levels of physical activity have been shown to be associated with lower risk of exacerbations, hospitalizations, and all-cause mortality in COPD (25–28), our finding that living with others and having a higher perception of overall social support is positively associated with physical activity is especially important. To the best of our knowledge, this is the first longitudinal study on the relationship between structural and functional social support and physical activity in adults with COPD.
It is important to note that participants who reported living with others rated far higher levels of functional social support across all four MOSSS subscales (+15 to 27 points) than those who lived alone and that the presence of a caregiver had no influence on physical activity. Thus, it is reasonable to surmise that the physical proximity of living with others matters more in terms of opportunities for positive social interactions, which results in greater engagement in both self-care and social activities in and outside the home. In an earlier study, Donesky and colleagues found that living with others was associated with a higher frequency, duration, and continuity of walking in adults with COPD (29), but that study did not measure functional social support. More recently, Mesquita and colleagues reported on a cross-sectional study of cohabitating patient–family member dyads in the Netherlands and found that adults with COPD who lived with more active family members had higher levels of physical activity than those living with an inactive family member (30). Furthermore, loneliness has been shown to be associated with lower levels of physical activity in healthy older adults (31). Our findings are novel and extend the limited evidence base, in that we examined the effects of both structural and functional social support on changes in physical activity over time.
We did not find an interaction between functional or structural social support with time when predicting physical activity, suggesting that the rate of change in physical activity did not vary by level of social support over 2 years of follow-up. At all three time points, higher social support was associated with increased physical activity; however, higher social support does not protect adults with COPD from declines in physical activity over time. Although physical activity is expected to decline with disease progression (32), living with others may be one of many important factors that contribute to maintaining patients’ daily physical activities through greater opportunities for reciprocal social interactions. It is therefore important to consider how to incorporate social support and interactions into interventions that aim to increase physical activity. More detailed study of these processes is warranted, because optimizing living arrangements is potentially modifiable in some situations.
Pulmonary rehabilitation is integral in the management of COPD (2). However, participation in, and adherence to, pulmonary rehabilitation remains very poor (33, 34). There was a high rate of participation in pulmonary rehabilitation in our sample, which is not an accurate reflection of uptake in the general U.S. COPD population and likely reflects our recruitment efforts for the main study that included pulmonary rehabilitation programs. We found that although living with others and being partnered were not associated with having participated in a pulmonary rehabilitation program before enrollment in our study, having a spouse or partner as a caregiver had a strong relationship with participation. This is not surprising, because our question specifically asked if participants had a family or friend who is most involved in their care. Our finding is consistent with previous studies reporting that lack of encouragement and support from family and friends were associated with nonparticipation and nonadherence to pulmonary rehabilitation (35–37). We recognize there are a number of other barriers to participation in pulmonary rehabilitation beyond social support (e.g., transportation challenges, access, insurance coverage, motivation) that we were unable to account for in our analysis.
Smoking is the main cause of COPD (2) and is associated with an increased risk of COPD exacerbations (38). Accordingly, identifying factors associated with smoking cessation is a high priority. In this study, we found that higher functional but not structural social support was related to decreased likelihood of being a current smoker. Previous studies have found that smoking cessation in adults with COPD is more related to worse disease severity, use of smoking cessation medications, and having health insurance (39). It is unclear how best to incorporate social or family support to help adults with COPD quit smoking (40), although there is some evidence to suggest that people with high levels of partner support and perceived social support are more successful at quitting smoking (41–43). Our results suggest that functional social support, especially tangible social support, plays a positive role in smoking cessation.
Influenza and pneumococcal vaccination are important primary and secondary prevention strategies to prevent exacerbations in adults with COPD (2, 44–46). The lack of variation in influenza vaccination in our study may partly explain why we did not find any relationship between social support and influenza vaccination, as 85% of the sample reported receiving an influenza vaccination. Interestingly, Burns and colleagues found that community-dwelling older adults who lived with others were more likely to report influenza vaccination than those living alone (47). We found that higher functional social support was associated with modest higher odds of pneumococcal vaccination.
Pharmacological treatment helps to reduce symptoms and exacerbations in COPD (2). We found that neither structural nor functional social support was associated with adherence to inhaler or nebulizer medication. Using the same adherence measure, Khdour and colleagues also found that neither marital status nor living arrangements was associated with medication adherence in COPD (48). However, in another large study that relied on both subjective (self-reporting) and objective (canister weight change) measurement of adherence over a 2-year period, participants who were married were more likely to adhere to their inhalers than unmarried individuals (49). In a cross-sectional study using pharmacy refill data, Trivedi and colleagues found that caregivers, especially spouses, improved medication adherence in individuals with COPD compared with those without caregivers (50). These inconsistent findings may be due to the different adherence measurements used across studies and the fact that we used a self-report measure of adherence.
Limitations
Several limitations are worth noting. The majority of our sample were men, most of whom had attended some college, many with a history of military service, who were relatively active and had low levels of depression and anxiety, and thus our findings may not generalize to the larger COPD population. Although we measured both structural and functional social support, we did not measure other potentially important dimensions of social relationships, such as family conflict and cohesion, that might have adverse influences on self-care behaviors (51). In addition, because most of the dependent variables did not change over time, we were only able to examine cross-sectional relationships between social support and self-care behaviors, with the exception of physical activity. Finally, the lack of correction for multiple comparisons may have resulted in spurious associations in these analyses, but we were careful to not overinterpret our findings.
Conclusions
Structural social support measured by reports of living with others and having a caregiver were, respectively, associated with higher levels of physical activity and greater participation in pulmonary rehabilitation in adults with COPD. Our findings reinforce the critical importance of the social environment in shaping patients’ success with self-care. Although our study should be replicated in larger, more representative samples, we believe that efforts to engage patients in these core self-care behaviors must, at the very least, routinely assess for and tangibly assist patients in marshalling the necessary social support to maximize their chances of effecting positive change.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank all the study participants.
Footnotes
Supported in part by National Institutes of Health grants 5R01HL093146 and UL1RR025014, the Department of Veterans Affairs (V.S.F.), and resources from the VA Puget Sound Health Care System, Seattle, Washington. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Author Contributions: Z.C. contributed to data acquisition, analysis, and interpretation and preparation of the manuscript and served as principal author. V.S.F. contributed to the study design, data analysis and interpretation, and preparation of the manuscript. B.B. contributed to the preparation of the manuscript. K.P. contributed to data acquisition, analysis, and interpretation. H.Q.N. contributed to the study design, data analysis and interpretation, and preparation of the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.National Heart, Lung, and Blood Institute. What is COPD? 2017 April [accessed 2017 May 20]. Available from: http://www.nhlbi.nih.gov/health/health-topics/topics/copd.
- 2.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease 2017 report: GOLD executive summary. Respirology. 2017;22:575–601. doi: 10.1111/resp.13012. [DOI] [PubMed] [Google Scholar]
- 3.Jordan RE, Majothi S, Heneghan NR, Blissett DB, Riley RD, Sitch AJ, Price MJ, Bates EJ, Turner AM, Bayliss S, et al. Supported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysis. Health Technol Assess. 2015;19:1–516. doi: 10.3310/hta19360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nici L, Bontly TD, Zuwallack R, Gross N. Self-management in chronic obstructive pulmonary disease: time for a paradigm shift? Ann Am Thorac Soc. 2014;11:101–107. doi: 10.1513/AnnalsATS.201306-150FR. [DOI] [PubMed] [Google Scholar]
- 5.Deek H, Hamilton S, Brown N, Inglis SC, Digiacomo M, Newton PJ, Noureddine S, MacDonald PS, Davidson PM FAMILY Project Investigators. Family-centred approaches to healthcare interventions in chronic diseases in adults: a quantitative systematic review. J Adv Nurs. 2016;72:968–979. doi: 10.1111/jan.12885. [DOI] [PubMed] [Google Scholar]
- 6.Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 7.Gallant MP. The influence of social support on chronic illness self-management: a review and directions for research. Health Educ Behav. 2003;30:170–195. doi: 10.1177/1090198102251030. [DOI] [PubMed] [Google Scholar]
- 8.Cassel J. The contribution of the social environment to host resistance. Am J Epidemiol. 1976;104:107–123. doi: 10.1093/oxfordjournals.aje.a112281. [DOI] [PubMed] [Google Scholar]
- 9.Cobb S. Presidential address-1976: social support as a moderator of life stress. Psychosom Med. 1976;38:300–314. doi: 10.1097/00006842-197609000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Barrera M., Jr Distinctions between social support concepts, measures, and models. Am J Community Psychol. 1986;14:413–445. [Google Scholar]
- 11.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 12.Vassilev I, Rogers A, Sanders C, Kennedy A, Blickem C, Protheroe J, Bower P, Kirk S, Chew-Graham C, Morris R. Social networks, social capital and chronic illness self-management: a realist review. Chronic Illn. 2011;7:60–86. doi: 10.1177/1742395310383338. [DOI] [PubMed] [Google Scholar]
- 13.Xiaolian J, Chaiwan S, Panuthai S, Yijuan C, Lei Y, Jiping L. Family support and self-care behavior of Chinese chronic obstructive pulmonary disease patients. Nurs Health Sci. 2002;4:41–49. doi: 10.1046/j.1442-2018.2002.00100.x. [DOI] [PubMed] [Google Scholar]
- 14.Kara Kaşikçi M, Alberto J. Family support, perceived self-efficacy and self-care behaviour of Turkish patients with chronic obstructive pulmonary disease. J Clin Nurs. 2007;16:1468–1478. doi: 10.1111/j.1365-2702.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Fan VS, Pike KC, Lee J, Yee N, Borson S, et al. The impact of social support on changes in physical activity over 2-years in patients with COPD [abstract] Am J Respir Crit Care Med. 2016;193:A4524. [Google Scholar]
- 16.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 17.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 19.D’Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49:1429–1433. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 20.Ng T-P, Niti M, Tan W-C, Cao Z, Ong K-C, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167:60–67. doi: 10.1001/archinte.167.1.60. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen HQ, Burr RL, Gill DP, Coleman K. Validation of the StepWatch device for measurement of free-living ambulatory activity in patients with chronic obstructive pulmonary disease. J Nurs Meas. 2011;19:76–90. doi: 10.1891/1061-3749.19.2.76. [DOI] [PubMed] [Google Scholar]
- 22.Brooks CM, Richards JM, Kohler CL, Soong S-J, Martin B, Windsor RA, Bailey WC. Assessing adherence to asthma medication and inhaler regimens: a psychometric analysis of adult self-report scales. Med Care. 1994;32:298–307. doi: 10.1097/00005650-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Demeyer H, Burtin C, Hornikx M, Camillo CA, Van Remoortel H, Langer D, Janssens W, Troosters T. The minimal important difference in physical activity in patients with COPD. PLoS One. 2016;11:e0154587. doi: 10.1371/journal.pone.0154587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61:772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waschki B, Kirsten A, Holz O, Müller KC, Meyer T, Watz H, Magnussen H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140:331–342. doi: 10.1378/chest.10-2521. [DOI] [PubMed] [Google Scholar]
- 27.Moy ML, Gould MK, Liu IA, Lee JS, Nguyen HQ. Physical activity assessed in routine care predicts mortality after a COPD hospitalisation. ERJ Open Res. 2016;2:00062–2015. doi: 10.1183/23120541.00062-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen HQ, Chu L, Amy Liu I-L, Lee JS, Suh D, Korotzer B, Yuen G, Desai S, Coleman KJ, Xiang AH, et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:695–705. doi: 10.1513/AnnalsATS.201401-017OC. [DOI] [PubMed] [Google Scholar]
- 29.Donesky D, Janson SL, Nguyen HQ, Neuhaus J, Neilands TB, Carrieri-Kohlman V. Determinants of frequency, duration, and continuity of home walking in patients with COPD. Geriatr Nurs. 2011;32:178–187. doi: 10.1016/j.gerinurse.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Mesquita R, Nakken N, Janssen DJA, van den Bogaart EHA, Delbressine JML, Essers JMN, Meijer K, van Vliet M, de Vries GJ, Muris JWM, et al. Activity levels and exercise motivation in COPD patients and their resident loved ones. Chest. 2017;151:1028–1038. doi: 10.1016/j.chest.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Hawkley LC, Thisted RA, Cacioppo JT. Loneliness predicts reduced physical activity: cross-sectional & longitudinal analyses. Health Psychol. 2009;28:354–363. doi: 10.1037/a0014400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33:262–272. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]
- 33.Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis. 2011;8:89–99. doi: 10.1177/1479972310393756. [DOI] [PubMed] [Google Scholar]
- 34.Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. Eur Respir J. 2006;27:788–794. doi: 10.1183/09031936.06.00130605. [DOI] [PubMed] [Google Scholar]
- 35.Arnold E, Bruton A, Ellis-Hill C. Adherence to pulmonary rehabilitation: a qualitative study. Respir Med. 2006;100:1716–1723. doi: 10.1016/j.rmed.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Hayton C, Clark A, Olive S, Browne P, Galey P, Knights E, Staunton L, Jones A, Coombes E, Wilson AM. Barriers to pulmonary rehabilitation: characteristics that predict patient attendance and adherence. Respir Med. 2013;107:401–407. doi: 10.1016/j.rmed.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Young P, Dewse M, Fergusson W, Kolbe J. Respiratory rehabilitation in chronic obstructive pulmonary disease: predictors of nonadherence. Eur Respir J. 1999;13:855–859. doi: 10.1034/j.1399-3003.1999.13d27.x. [DOI] [PubMed] [Google Scholar]
- 38.Au DH, Bryson CL, Chien JW, Sun H, Udris EM, Evans LE, Bradley KA. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457–463. doi: 10.1007/s11606-009-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilberink SR, Jacobs JE, Schlösser M, Grol RP, de Vries H. Characteristics of patients with COPD in three motivational stages related to smoking cessation. Patient Educ Couns. 2006;61:449–457. doi: 10.1016/j.pec.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Luker KA, Chalmers KI, Caress AL, Salmon MP. Smoking cessation interventions in chronic obstructive pulmonary disease and the role of the family: a systematic literature review. J Adv Nurs. 2007;59:559–568. doi: 10.1111/j.1365-2648.2007.04379.x. [DOI] [PubMed] [Google Scholar]
- 41.Mermelstein R, Cohen S, Lichtenstein E, Baer JS, Kamarck T. Social support and smoking cessation and maintenance. J Consult Clin Psychol. 1986;54:447–453. doi: 10.1037//0022-006x.54.4.447. [DOI] [PubMed] [Google Scholar]
- 42.Murray RP, Johnston JJ, Dolce JJ, Lee WW, O’Hara P Lung Health Study Research Group. Social support for smoking cessation and abstinence: the Lung Health Study. Addict Behav. 1995;20:159–170. doi: 10.1016/s0306-4603(99)80001-x. [DOI] [PubMed] [Google Scholar]
- 43.Westmaas JL, Bontemps-Jones J, Bauer JE. Social support in smoking cessation: reconciling theory and evidence. Nicotine Tob Res. 2010;12:695–707. doi: 10.1093/ntr/ntq077. [DOI] [PubMed] [Google Scholar]
- 44.Wongsurakiat P, Maranetra KN, Wasi C, Kositanont U, Dejsomritrutai W, Charoenratanakul S. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination: a randomized controlled study. Chest. 2004;125:2011–2020. doi: 10.1378/chest.125.6.2011. [DOI] [PubMed] [Google Scholar]
- 45.Alfageme I, Vazquez R, Reyes N, Muñoz J, Fernández A, Hernández M, Merino M, Perez J, Lima J. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax. 2006;61:189–195. doi: 10.1136/thx.2005.043323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee TA, Weaver FM, Weiss KB. Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. J Gen Intern Med. 2007;22:62–67. doi: 10.1007/s11606-007-0118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burns VE, Ring C, Carroll D. Factors influencing influenza vaccination uptake in an elderly, community-based sample. Vaccine. 2005;23:3604–3608. doi: 10.1016/j.vaccine.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 48.Khdour MR, Hawwa AF, Kidney JC, Smyth BM, McElnay JC. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD) Eur J Clin Pharmacol. 2012;68:1365–1373. doi: 10.1007/s00228-012-1279-5. [DOI] [PubMed] [Google Scholar]
- 49.Rand CS, Nides M, Cowles MK, Wise RA, Connett J The Lung Health Study Research Group. Long-term metered-dose inhaler adherence in a clinical trial. Am J Respir Crit Care Med. 1995;152:580–588. doi: 10.1164/ajrccm.152.2.7633711. [DOI] [PubMed] [Google Scholar]
- 50.Trivedi RB, Bryson CL, Udris E, Au DH. The influence of informal caregivers on adherence in COPD patients. Ann Behav Med. 2012;44:66–72. doi: 10.1007/s12160-012-9355-8. [DOI] [PubMed] [Google Scholar]
- 51.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23:207–218. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.