Abstract
Rationale: Tracheobronchomalacia (TBM) is a common comorbidity in neonates with bronchopulmonary dysplasia (BPD). However, the effect of TBM on the clinical course of BPD is not well understood.
Objectives: We sought to assess the impact of TBM on outcomes in neonates with BPD in a large, multicenter cohort.
Methods: We performed a cohort study of 974 neonates with BPD admitted to 27 neonatal intensive care units participating in the Children’s Hospital Neonatal Database who had undergone bronchoscopy. In-hospital morbidity for neonates with BPD and TBM (n = 353, 36.2%) was compared with those without TBM (n = 621, 63.8%) using mixed-effects multivariate regression.
Results: Neonates with TBM and BPD had more comorbidities, such as gastroesophageal reflux (odds ratio [OR], 1.65; 95% confidence interval [CI], 1.23–2.29; P = 0.001) and pneumonia (OR, 1.68; 95% CI, 1.21–2.33; P = 0.002), and more commonly required surgeries, such as tracheostomy (OR, 1.55; 95% CI, 1.15–2.11; P = 0.005) and gastrostomy (OR, 1.38; 95% CI: 1.03–1.85; P = 0.03), than those without TBM. Neonates with TBM were hospitalized (118 ± 93 d vs. 105 ± 83 d; P = 0.02) and ventilated (83.1 ± 91.1 d vs. 67.2 ± 71.9 d; P = 0.003) longer than those without TBM. On discharge, neonates with TBM and BPD were more likely to be mechanically ventilated (OR, 1.37; 95% CI, 1.01–1.87; P = 0.045) and possibly less likely to receive oral nutrition (OR, 0.69; 95% CI, 0.47–1.01; P = 0.058).
Conclusions: TBM is common in neonates with BPD who undergo bronchoscopy and is associated with longer and more complicated hospitalizations.
Keywords: tracheobronchomalacia, bronchopulmonary dysplasia, epidemiology
Tracheobronchomalacia (TBM) in children was first described by Holinger and colleagues in 1952 (1) and later defined as weakening of the airway wall due to softening of the cartilaginous rings and decreased tone of the airway smooth muscle. This results in increased airway compliance and reduction of the size of the airway lumen (2). Generalized or localized deformity and narrowing of the intrathoracic airway decreases airflow, increases resistance, and is clinically characterized by retractions, dyspnea, and a prolonged expiratory phase (3). Children can also have recurrent bacterial bronchitis, cyanotic episodes, apnea, and difficulty weaning from ventilatory support (4, 5).
Although TBM is uncommon in the general population (4), the estimated prevalence of TBM in neonates with bronchopulmonary dysplasia (BPD) undergoing bronchoscopy is 10 to 46% (6–9). There is normally an age-related decrease in airway compliance and susceptibility to injury from positive pressure ventilation (10, 11). Airways of preterm animals have increased compliance when compared with airways of term animals (12). Furthermore, positive pressure ventilation can induce airway wall damage (13, 14). Consequently, premature infants are particularly susceptible to developing TBM (15).
Despite the high prevalence of TBM in neonates with BPD, only small, single-center case series have evaluated outcomes in these children (6–9). We aimed to study the risk factors and outcomes of TBM in the setting of BPD in a large, multicenter cohort. We hypothesized that neonates with TBM and BPD would have increased morbidity characterized by surgical interventions, comorbid conditions, longer hospitalizations, and increased need for mechanical ventilation at discharge than other neonates with BPD alone.
Methods
Study Population and Design
We performed a retrospective cohort study of neonates enrolled in the Children’s Hospital Neonatal Database (CHND) from January 1, 2011 until January 1, 2016 (16). The CHND comprises 27 level III and IV neonatal intensive care units (NICUs). Clinical data are prospectively collected by trained chart abstracters, and interrater reliability, measured at least biannually, is greater than 90%. This study was approved by the internal review board at the University of Pennsylvania and the Children’s Hospital of Philadelphia (IRB# 821985 and IRB# 15-011900).
Study Sample
Neonates with BPD in the CHND who had undergone bronchoscopy were eligible for this study. Bronchoscopy is the current gold standard for diagnosing TBM and has high intrarater and interrater reliability (17). A diagnosis of BPD was based on modified NHLBI guidelines (18). For subjects born before 32 weeks postmenstrual age (PMA), mild BPD was defined as treatment with supplemental oxygen for 28 or more days but not at 36 weeks PMA. Moderate BPD included those infants who received supplemental oxygen for 28 or more days and less than 30% supplemental oxygen and less than or equal to 2 L/min (LPM) of flow at 36 weeks PMA. Infants with severe BPD received supplemental oxygen for 28 or more days and greater than or equal to 30% supplemental oxygen, greater than 2 LPM of flow, or positive pressure ventilation at 36 weeks PMA.
For subjects born after 32 weeks gestational age and before 37 weeks gestational age, mild BPD was defined as treatment with supplemental oxygen for 28 or more days but not at 56 days of life. Moderate BPD included those infants who received treatment with supplemental oxygen for 28 or more days and less than 30% supplemental oxygen and less than or equal to 2 LPM of flow at 56 days of life. Infants with severe BPD received supplemental oxygen for 28 or more days and greater than or equal to 30% supplemental oxygen, greater than 2 LPM of flow, or positive pressure ventilation at 56 days of life.
We excluded infants with vascular rings, tracheoesophageal fistulas, and complex congenital heart disease, which can be independently associated with TBM (19), but included subjects with simple cardiovascular defects, such as patent ductus arteriosus, atrial septal defects, and ventricular septal defects. Neonates with congenital diaphragmatic hernia, bronchopulmonary sequestrations, and congenital pulmonary airway malformations were also excluded.
Exposures and Outcomes
A diagnosis of TBM was abstracted from the medical record and recorded as present or not in data collection in the CHND. To confirm the accuracy of this approach (in one center), all bronchoscopy reports from subjects recruited from the Children’s Hospital of Philadelphia were abstracted by one author (E.B.H.) with blinding to the diagnosis in the CHND, and 10% of the reports were randomly selected for reabstraction by a second author (N.L.F.).
The CHND maintains records of demographics, pregnancy and delivery room characteristics, comorbid diagnoses, surgical interventions, and discharge characteristics. The main outcomes for this study were length of stay, duration of mechanical ventilation, death, tracheostomy, gastrostomy, fundoplication, and a physician diagnosis of pneumonia, gastroesophageal reflux disease (GERD), or pulmonary hypertension in the medical record during the NICU hospitalization. Discharge therapies including mechanical ventilation, supplemental oxygen, cardiopulmonary monitoring, and route of feeding, and medications were also studied.
Statistical Analysis
Categorical variables are reported with frequency and percentage and continuous variables with mean and SD. Categorical variables were compared using chi-square or Fisher exact tests, and continuous variables were compared using t test or Wilcoxon rank sum tests where appropriate. To minimize bias, missing data were accounted for using multiple imputation with 20 imputed datasets.
The association of TBM (exposure) with outcomes, including comorbid diagnoses, surgical interventions, discharge respiratory support and route of feeding, and medication use (outcomes) were modeled using mixed-effects logistic regression. The association of TBM with length of stay, PMA at discharge, and ventilator days was modeled using mixed-effects linear regression. Gestational age, admission age, birth weight, BPD severity, sex, race, surfactant use, antenatal steroid use, and insurance status at discharge were included in all multivariate models as fixed effects, and the center was included as a random intercept. We also assessed the effects of additional covariates on the results. Covariates marginally associated with the outcome defined by a P value < 0.10 in univariate analysis were included in the multivariate model. Covariates that altered the effect estimate of TBM by more than 15% or that were independently associated with the outcome of interest were retained in the final model. A sensitivity analysis was performed using only patients who survived the initial hospitalization. All data analyses were performed using Stata 13.1. A P value < 0.05 was considered statistically significant.
Results
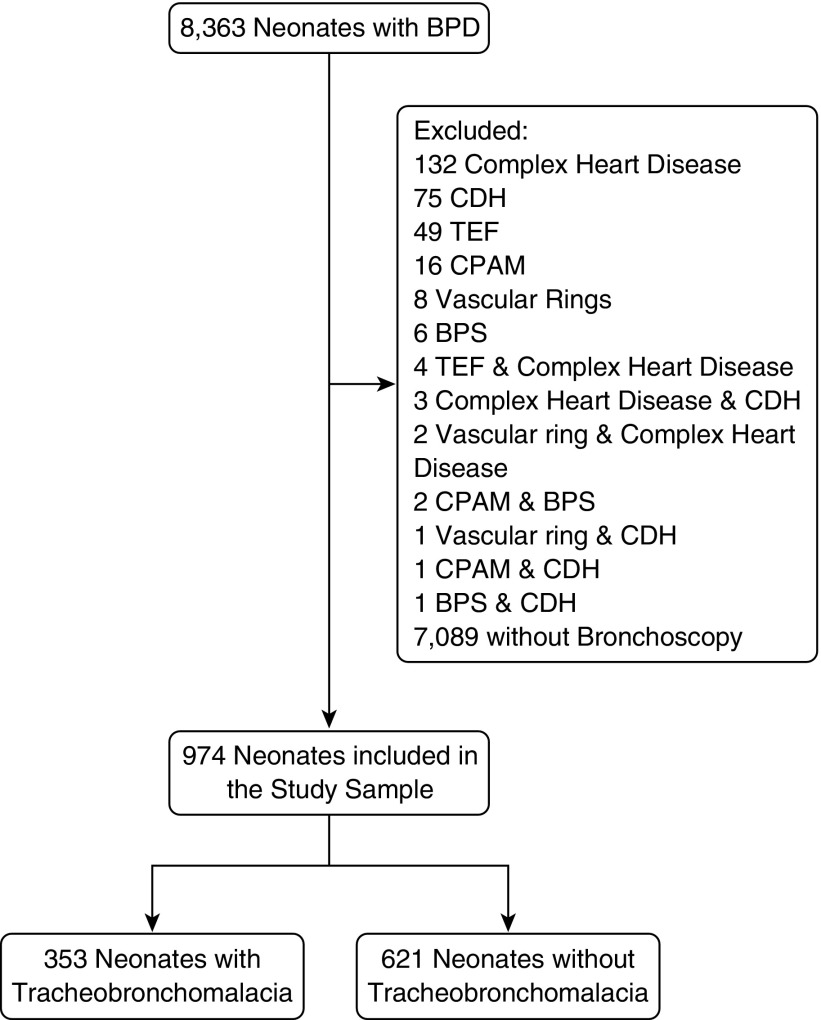
There were 8,363 neonates with BPD in the CHND enrolled between January 2011 and January 2016 (Figure 1). We excluded 132 with complex congenital heart disease, 75 neonates with congenital diaphragmatic hernia, 49 with tracheoesophageal fistula, 16 with congenital pulmonary airway malformations, 8 with vascular rings, 6 with bronchopulmonary sequestrations, and 14 with a combination of exclusionary diagnoses. We then excluded 7,089 neonates who never underwent bronchoscopy, leaving 974 neonates in our study sample.
Figure 1.
Flow chart for the selection of neonates with bronchopulmonary dysplasia (BPD) with and without tracheobronchomalacia. BPS = bronchopulmonary sequestrations; CDH = congenital diaphragmatic hernia; CPAM = congenital pulmonary airway malformations; TEF = tracheoesophageal fistula.
We compared our final study sample to those neonates who were excluded because a bronchoscopy was not performed (Table E1). Those in the study sample were 1 week more advanced in gestational age and were more than 100 g heavier. Neonates in the study sample were admitted to a CHND hospital about 4 weeks later and discharged nearly 10 weeks later than neonates who did not undergo bronchoscopy. Birth and delivery characteristics appeared to be similar between the two groups. Using the bronchoscopy report as the gold standard, the positive predictive value and negative predictive value for a CHND diagnosis of TBM were 95 and 84%, respectively. A subset of 10% of the bronchoscopy reports were reabstracted, and interrater reliability was 93%.
TBM was reported in 353 (36.2%) neonates in the study sample (Table 1). Neonates with TBM were born at a slightly later gestational age and admitted to and discharged from a hospital affiliated with the CHND at an older PMA. During pregnancy, mothers of neonates with TBM were more likely to have received prenatal care, reproductive assistance, and antenatal steroids. Neonates with TBM were more likely develop intrauterine growth restriction and less likely to have pregnancy complicated by placental abruption.
Table 1.
Characteristics of the study cohort stratified by tracheobronchomalacia
| Demographics | TBM (n = 353) | No TBM (n = 621) | P Value |
|---|---|---|---|
| Gestational age, wk | 28.1 ± 4.1 | 27.5 ± 3.9 | 0.02 |
| Admission PMA, wk | 40.5 ± 10.6 | 38.4 ± 10.1 | 0.003 |
| Discharge PMA, wk | 57.4 ± 15.7 | 53.4 ± 11.7 | <0.001 |
| Birth weight, g (n = 968) | 1,100 ± 713 | 1,018 ± 675 | 0.07 |
| Male | 199 (56.4) | 361(58.1) | 0.16 |
| Hispanic ethnicity (n = 909) | 44 (13.1) | 73 (12.7) | 0.95 |
| Maternal race | 0.18 | ||
| White | 185 (52.4) | 290 (46.7) | |
| Black | 111 (30.4) | 220 (35.4) | |
| Other | 44 (12.5) | 74 (11.9) | |
| Stated unknown | 13 (3.7) | 37 (6.0) | |
| Severe BPD | 221 (62.6) | 399 (64.2) | 0.61 |
| Inborn | 5 (1.4) | 8 (1.3) | 0.89 |
| Private insurance (n = 956) | 135 (38.7) | 212 (34.9) | 0.25 |
| Pregnancy characteristics | |||
| Reproductive assistance (n = 850) | 33 (10.3) | 40 (7.6) | 0.18 |
| Prenatal care (n = 946) | 303 (89.4) | 475 (78.2) | <0.001 |
| Antenatal steroids (n = 918) | 223 (67.4) | 356 (60.5) | 0.03 |
| Chorioamnionitis (n = 732) | 37 (14.0) | 52 (11.1) | 0.25 |
| Maternal diabetes (n = 732) | 32 (8.8) | 53 (8.3) | 0.93 |
| Maternal hypertension (n = 732) | 96 (36.4) | 139 (31.8) | 0.21 |
| Intrauterine growth restriction (n = 811) | 23 (7.6) | 18 (3.5) | 0.01 |
| Delivery characteristics | |||
| Malpresentation (n = 903) | 60 (18.5) | 86 (14.9) | 0.16 |
| Placenta previa (n = 903) | 15 (4.6) | 45 (7.8) | 0.07 |
| Abruption (n = 903) | 16 (4.9) | 53 (9.2) | 0.02 |
| Premature rupture of membranes (n = 903) | 80 (24.6) | 155 (26.8) | 0.47 |
| Prolonged rupture of membranes (n = 903) | 41 (12.6) | 66 (11.4) | 0.67 |
| Cesarean section (n = 954) | 244 (70.3) | 417 (68.7) | 0.60 |
| Multiple birth (n = 972) | 69 (19.7) | 133 (21.4) | 0.66 |
| Surfactant (n = 889) | 206 (63.4) | 358 (63.5) | 0.98 |
| Apgar | |||
| 1 min (n = 948) | 4.0 ± 2.3 | 3.8 ± 2.3 | 031 |
| 5 min (n = 922) | 6.4 ± 2.1 | 6.2 ± 2.1 | 0.22 |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; PMA = postmenstrual age; TBM = tracheobronchomalacia.
Data are presented as mean ± SD or n (%).
Comorbidities and Surgical Procedures
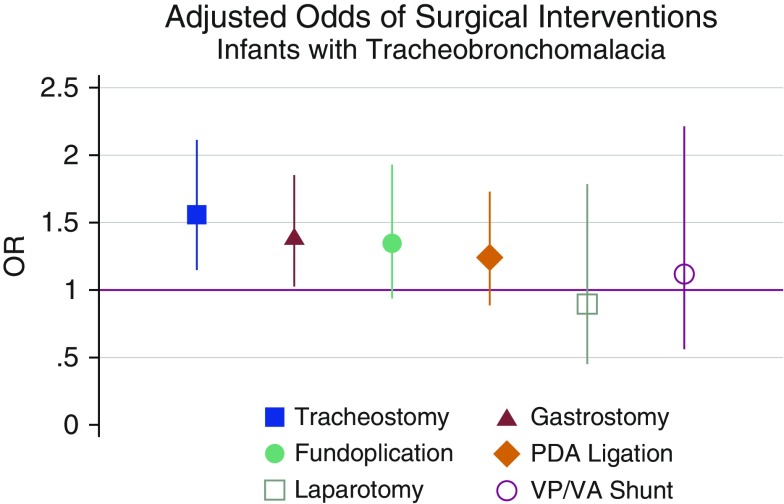
Neonates with TBM were more likely to carry a diagnosis of GERD and pneumonia than neonates without TBM after adjustment for covariates (Table 2). Neonates with TBM more commonly underwent tracheostomy (odds ratio [OR], 1.56; 95% confidence interval [CI], 1.16–2.10; P = 0.004) and gastrostomy (OR, 1.36; 95% CI, 1.02–1.78; P = 0.04) (Figure 2). Other surgical interventions, including fundoplication, patent ductus arteriosus ligation, exploratory laparotomy/laparoscopy, and ventriculoperitoneal/ventriculoatrial shunt, were performed with similar frequency in neonates with and without TBM (Table E2).
Table 2.
Adjusted associations of tracheobronchomalacia with comorbidities
| TBM [n (%)] | No TBM [n (%)] | ORAdjusted (95% CI) | P Value | |
|---|---|---|---|---|
| Gastroesophageal reflux disease | 197 (55.8) | 253 (40.7) | 1.65 (1.23–2.19) | 0.001 |
| Pneumonia | 136 (38.5) | 150 (24.2) | 1.68 (1.21–2.33) | 0.002 |
| Pulmonary hypertension | 87 (24.7) | 127 (20.4) | 1.16 (0.82–1.65) | 0.41 |
| Necrotizing enterocolitis | 28 (7.9) | 75 (12.1) | 0.81 (0.49–1.33) | 0.41 |
| Aspiration | 23 (6.5) | 37 (6.0) | 1.00 (0.55–1.81) | 1.0 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; TBM = tracheobronchomalacia.
All adjusted models are mixed logistic regressions with bronchopulmonary dysplasia severity, race, sex, insurance status, surfactant use, antenatal steroids use, gestational age, admission age, and birth weight as fixed effects and center as a random intercept.
Figure 2.
Adjusted odds ratios (ORs) with 95% confidence interval of surgical interventions for infants with bronchopulmonary dysplasia (BPD) with tracheobronchomalacia. Models are mixed logistic regressions with BPD severity, race, sex, insurance status, surfactant use, antenatal steroids use, gestational age, admission age, and birth weight as fixed effects and center as a random intercept. PDA = patent ductus arteriosus; VP/VA = ventriculoperitoneal/ventriculoatrial.
Timing of Discharge and Mechanical Ventilation
The average hospital length of stay for neonates in the study sample was 110 days (median, 94 d). In univariate analysis, neonates with TBM were hospitalized longer (118 ± 93 vs. 105 ± 83 d; P = 0.02) than those without TBM. Hospital length of stay was nearly 3 weeks longer in neonates with TBM than in those without after adjustment for covariates (P < 0.001) (Figure 3A). Accordingly, neonates with TBM were discharged when they were almost 3 weeks older than neonates without TBM (P < 0.001) (Figure 3B). Most (90%) of the infants received mechanical ventilation during their admission (median, 51 d); however, those with TBM received longer periods of mechanical ventilation (83.1 ± 91.1 vs. 67.2 ± 71.9; P = 0.003), and this difference was even greater after adjustment for covariates (P < 0.001) (Figure 3C).
Figure 3.
Adjusted values with 95% confidence interval for (A) length of stay, (B) postmenstrual age at discharge, and (C) duration of mechanical ventilation for infants with bronchopulmonary dysplasia (BPD) with and without tracheobronchomalacia. Models are mixed linear regressions with BPD severity, race, sex, insurance status, surfactant use, antenatal steroids use, gestational age, admission age, and birth weight as fixed effects and center as a random intercept.
Discharge Support
Neonates with TBM were more likely to continue to receive mechanical ventilation on discharge (OR, 1.36; 95% CI, 1.01–1.87; P = 0.045), but there was no difference in the need for home supplemental oxygen or cardiopulmonary monitoring on the basis of TBM status (Table 3). More than 80% of neonates in our cohort received tube feedings, either gastric or post-pyloric, at discharge, and less than one-third were receiving at least some nutrition by mouth. Neonates with TBM were possibly less likely to be receiving oral feedings at discharge (OR, 0.69; 95% CI, 0.47–1.01; P = 0.058) than those without TBM.
Table 3.
Adjusted associations of tracheobronchomalacia with support at discharge
| TBM [n (%)] | No TBM [n (%)] | ORAdjusted (95% CI) | P Value | |
|---|---|---|---|---|
| Respiratory |
|
|
|
|
| Mechanical ventilation | 182 (51.6) | 277 (45.1) | 1.37 (1.01–1.87) | 0.045 |
| Cardiopulmonary monitoring | 306 (86.7) | 516 (83.9) | 1.25 (0.82–1.90) | 0.31 |
| Supplemental oxygen | 284 (80.4) | 463 (75.3) | 1.23 (0.86–1.75) | 0.25 |
| Feeding | ||||
| Oral feedings | 96 (30.8) | 168 (33.1) | 0.69 (0.48–1.01) | 0.058 |
| Tube feedings | 257 (82.4) | 389 (76.6) | 1.35 (0.90–2.02) | 0.14 |
| Medication | ||||
| Bronchodilators | 121 (34.3) | 163 (26.2) | 1.50 (1.08–2.12) | 0.02 |
| Inhaled steroids | 115 (32.6) | 140 (22.5) | 1.54 (1.09–2.16) | 0.01 |
| Any diuretic | 104 (29.5) | 136 (21.9) | 1.36 (0.97–1.89) | 0.07 |
| Furosemide | 32 (9.1) | 32 (5.2) | 1.93 (1.08–3.42) | 0.03 |
| Spironolactone | 47 (13.3) | 48 (7.7) | 1.67 (1.00–2.77) | 0.05 |
| Chlorothiazide | 76 (21.5) | 112 (18.0) | 1.04 (0.72–1.52) | 0.83 |
| Antireflux therapy | 151 (42.8) | 208 (33.5) | 1.37 (1.03–1.84) | 0.03 |
| Proton pump inhibitor | 113 (32.0) | 158 (25.4) | 1.31 (0.96–1.79) | 0.09 |
| H2 blocker | 42 (11.9) | 55 (8.9) | 1.30 (0.82–2.07) | 0.26 |
| Pulmonary hypertension therapy | 35 (9.9) | 34 (5.5) | 1.72 (1.02–2.89) | 0.04 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; TBM = tracheobronchomalacia.
All adjusted models are mixed logistic regressions with bronchopulmonary dysplasia severity, race, sex, insurance status, surfactant use, antenatal steroids use, gestational age, admission age, and birth weight as fixed effects and center as a random intercept.
A majority of the neonates in both groups was receiving medications at discharge. Infants with TBM were significantly more likely to be prescribed treatment for wheezing, including inhaled bronchodilators (P = 0.02) and inhaled steroids (P = 0.01). Furosemide (P = 0.02), spironolactone (P = 0.02), antireflux therapy (P = 0.03) (specifically PPIs), and therapy targeted for pulmonary hypertension (P = 0.03) were also more commonly prescribed in neonates with TBM at discharge (Table 3).
Sensitivity Analysis
There were 79 (7.9%; 95% CI, 6.2–9.7%) deaths in the study sample before discharge (6.3% [95% CI, 4.0–9.3%] with TBM and 8.8% [95% CI, 6.7–11.2%] without TBM; P = 0.16), which could be a competing risk for many of the outcomes studied. We therefore performed a sensitivity analysis including only subjects who survived to discharge from a CHND hospital. Among survivors, neonates with TBM were more likely to have comorbidities such as GERD and pneumonia. Neonates with TBM were also more likely to undergo tracheostomy (OR, 1.66; 95% CI, 1.22–2.32; P = 0.002). However, there was no difference in odds of gastrostomy tube placement in this survival cohort (Table E3).
The average length of stay for survivors was 109 days (median, 92 d). The length of stay was longer for survivors with TBM (117 ± 93) than those without TBM (105 ± 83) in both univariate and multivariate models. Similarly, neonates with TBM received mechanical ventilation for longer periods of time and were discharged at a greater postmenstrual age (Table E3).
Discussion
In this multicenter cohort of neonates with BPD, we have demonstrated that TBM affects more than one-third of those undergoing bronchoscopy and at least 5% of all neonates with BPD. Neonates with TBM had longer and more complicated hospital courses characterized by increased comorbid diagnoses, including GERD and pneumonia, and more surgical interventions, such as tracheostomy and gastrostomy placement. On discharge, neonates with TBM were more likely to receive many medical therapies and may be more likely to require mechanical ventilation and less likely to receive oral feedings. To our knowledge, this is the largest multicenter cohort study to investigate the independent association of TBM with adverse outcomes in neonates with BPD. The prevalence of TBM and associated adverse outcomes highlights the need for improved diagnostic standards and therapies specific for TBM in neonates with BPD.
The study sample was drawn from more than 8,000 neonates with BPD during a 5-year study period at 27 level III and level IV NICUs and represents ∼10% of the national population with BPD (20). Other studies of TBM in neonates with BPD have been small, single-center reports (6–9), limiting their power to detect important differences in outcomes and to account for the large variability between centers in neonatal practice (21, 22). The use of a multicenter cohort and random effects statistical modeling, however, allows us to account for practice variability and greatly improves the generalizability of these findings. We also had extensive information about many clinically important covariates, lessening the impact of confounding.
The prevalence of TBM in neonates with BPD in our study sample was similar to that in two smaller studies of airway disorders in preterm infants (6, 8). Because the diagnosis of TBM is based on bronchoscopic findings and bronchoscopy is not performed in all neonates, it is difficult to estimate the overall prevalence of TBM with certainty. Clinical diagnosis and radiography have also been used to diagnose TBM; however, there has been variable correlation of these modalities with bronchoscopy, which is considered the gold standard (23).
Infants with BPD and TBM had a longer and more complicated hospital course than those without TBM. Length of stay was increased by more than 3 weeks on average, which is similar in magnitude to the increase in length of stay incurred by neonates who develop necrotizing entercolitis (24). Given the high emotional and medical costs that have long been associated with providing care for premature infants in NICUs (24–26), TBM, if causal, could have a substantial impact on patients and their families from both a quality-of-life standpoint and a financial impact on health systems.
The longer length of stay appeared to be primarily driven by the almost 3 weeks longer duration of invasive mechanical ventilation in neonates with BPD and TBM. Continuous positive airway pressure can reduce dynamic airway collapse and improves respiratory mechanics in neonates with TBM (27, 28). Tracheostomy has also been used to treat TBM by bypassing the collapsible segment of the central airways until the airway matures and was more commonly performed in neonates with BPD and TBM in our study cohort (29, 30). This could suggest that neonates with BPD and TBM need a tracheostomy tube to maintain patency of the airway lumen as the airway grows and becomes more rigid. In addition, neonates with BPD and TBM could potentially benefit from treatment with bethanechol or ipratropium bromide, which can increase tracheal smooth muscle tone (23).
Central airway collapse depends on the rigidity of the central airway and the collapsing transmural airway pressure. Children with obstructive respiratory disease like BPD often use accessory muscles to exhale and have increased peripheral airway resistance. The resulting positive pleural pressure and accentuated resistive intraluminal pressure drop would serve to increase the collapsing transmural airway pressure (23). Neonates with BPD also often require positive pressure ventilation, which can cause airway damage and inflammation, resulting in increased airway collapsibility. Thus, TBM may be a marker for more severe peripheral airway obstruction, which increased duration of respiratory support and hospitalization in the neonates with BPD.
In addition to increased respiratory support, neonates with TBM required more treatment and surgical interventions aimed to protect the airway from aspiration of oral and gastric contents. Gastroesophageal reflux is common in neonates with BPD and has been associated with worsening pulmonary symptoms and variably linked to the pathogenesis of chronic lung disease of prematurity (31–35).
There are several potential limitations of this study. Only neonates undergoing bronchoscopy were included. Not surprisingly, there were some differences in the characteristics of patients in the study sample compared with those excluded, potentially leading to selection bias. We are also not able to discern whether the development of TBM precedes outcomes such as GERD and pneumonia. Future prospective studies with standardized bronchoscopy in neonates with BPD would be necessary to assess generalizability of these results to the larger population and help define the temporal relationship of TBM and outcomes in neonates with BPD.
Misclassification of TBM could have biased the results. Despite the lack of standardized diagnostic criteria for TBM, bronchoscopy has been shown to have high inter- and intrarater reliability in previous report (17). We also showed outstanding accuracy of the recorded diagnosis of TBM in the CHND and documentation in the bronchoscopy reports in our center. Furthermore, if present, misclassification of TBM would likely be nondifferential; thus, the actual associations between TBM and outcomes may be even stronger than we have shown. Our study included a large population; thus, it is unlikely that we have a type II error. Although it is possible that our results were affected by residual or unmeasured confounding, we have attempted to account for many clinically important variables and have performed sensitivity analyses of survivors with nearly identical results.
In conclusion, TBM is a common comorbidity in neonates with BPD that is associated with increased use of medical resources and prolonged hospitalization. Currently, there are no standardized treatments or diagnostic criteria for central airway collapse in neonates with BPD. In future studies of TBM, it will be important to develop standardized diagnostic criteria to better understand disease pathogenesis and monitor response to therapies and interventions.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank National Institutes of Health grants HL-007891 and NIH K24-HL103844 for providing funding support for this work.
The CHNC (http://www.thechnc.org) has partnered with Children’s Hospital Association, Inc. (Overland Park, KS; Alexandria, VA) to design, launch, and maintain the CHND. We are indebted to the following institutions that serve infants and their families and also have invested in and continue to participate in the CHND. For more information about CHNC, please contact Jaqueline Evans, M.D., Chair (exec@thechnc.org). We also thank the site sponsors for their investments in this program. The site sponsors for the CHND are as follows: Children’s Healthcare of Atlanta at Egleston, Atlanta, GA (Francine Dykes, Anthony Piazza); Children’s Healthcare of Atlanta at Scottish Rite, Atlanta, GA (Gregory Sysyn); Children’s of Alabama, Birmingham, AL (Carl Coghill); Le Bonheur Children’s Hospital, Memphis, TN (Ramasubbareddy Dhanireddy); Boston Children’s Hospital, Boston, MA (Anne Hansen); Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL (Karna Murthy); Nationwide Children’s Hospital, Columbus, OH (Kristina Reber); Children’s Medical Center Dallas, Dallas, TX (Rashmin Savani); Children’s Hospital Colorado, Aurora, CO (Theresa Grover); Children’s Hospital of Michigan, Detroit, MI (Girija Natarajan); Cook Children’s Medical Center, Fort Worth, TX (Jonathan Nedrelow, Annie Chi); Texas Children’s Hospital, Houston, TX (Stephen Welty); Children’s Mercy Hospitals & Clinics, Kansas City, MO (Eugenia Pallotto); Arkansas Children’s Hospital, Little Rock, AR (Becky Rodgers, Robert Lyle); Children’s Hospital Los Angeles, Los Angeles, CA (Lisa Kelly [deceased], Steven Chin); UCSF Benioff Children’s Hospital Oakland, Oakland, CA (David Durand, Jeanette Asselin, Priscilla Joe); The Children’s Hospital of Philadelphia, Philadelphia, PA (Jacquelyn Evans, Michael Padula); Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, PA (Beverly Brozanski); St. Louis Children’s Hospital, St. Louis, MO (Joan Rosenbaum, Tasmin Najaf, Amit Mathur, Rakesh Rao); All Children’s Hospital, St. Petersburg, FL (Victor McKay); Rady Children’s Hospital San Diego, San Diego, CA (Mark Speziale); Children’s National Medical Center, Washington, DC (Billie Short); Alfred I. duPont Hospital for Children, Wilmington, DE (Kevin Sullivan); Primary Children’s Hospital, Salt Lake City, UT (Donald Null, Robert DiGeronimo); Children’s Hospital of Wisconsin, Milwaukee, WI (Michael Uhing); Children’s Hospital and Medical Center, Omaha, NE (Lynne Willett, John Grebe); and Florida Hospital for Children, Orlando, FL (Rajan Wadhawan).
Footnotes
Supported by National Institutes of Health grants HL-007891 and K24 H2103844.
Author Contributions: E.B.H., R.T.S., H.Z., H.B.P., and S.M.K. conceived and designed the study. E.B.H., N.L.F., and M.A.P. collected data. E.B.H., R.T.S., and S.M.K. performed data analysis. E.B.H., N.L.F., R.T.S., H.Z., H.B.P., and S.M.K. interpreted the data. E.B.H. drafted the manuscript, and all authors reviewed and revised the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: Children’s Hospitals Neonatal Consortium, Jaqueline Evans, Francine Dykes, Anthony Piazza, Gregory Sysyn, Carl Coghill, Ramasubbareddy Dhanireddy, Anne Hansen, Karna Murthy, Kristina Reber, Rashmin Savani, Theresa Grover, Girija Natarajan, Jonathan Nedrelow, Annie Chi, Stephen Welty, Eugenia Pallotto, Becky Rodgers, Robert Lyle, Lisa Kelly, Steven Chin, David Durand, Jeanette Asselin, Priscilla Joe, Jacquelyn Evans, Michael Padula, Beverly Brozanski, Joan Rosenbaum, Tasmin Najaf, Amit Mathur, Rakesh Rao, Victor McKay, Mark Speziale, Billie Short, Kevin Sullivan, Donald Null, Robert DiGeronimo, Michael Uhing, Lynne Willett, John Grebe, and Rajan Wadhawan
References
- 1.Holinger PH, Johnston KC, Parchet VN, Zimmermann AA. Congenital malformations of the trachea, bronchi and lung. Trans Annu Meet Am Bronchoesophagol Assoc. 1952;58:67–88. [PubMed] [Google Scholar]
- 2.Baxter JD, Dunbar JS. Tracheomalacia. Ann Otol Rhinol Laryngol. 1963;72:1013–1023. doi: 10.1177/000348946307200415. [DOI] [PubMed] [Google Scholar]
- 3.Mair EA, Parsons DS. Pediatric tracheobronchomalacia and major airway collapse. Ann Otol Rhinol Laryngol. 1992;101:300–309. doi: 10.1177/000348949210100403. [DOI] [PubMed] [Google Scholar]
- 4.Boogaard R, Huijsmans SH, Pijnenburg MW, Tiddens HA, de Jongste JC, Merkus PJ. Tracheomalacia and bronchomalacia in children: incidence and patient characteristics. Chest. 2005;128:3391–3397. doi: 10.1378/chest.128.5.3391. [DOI] [PubMed] [Google Scholar]
- 5.Sotomayor JL, Godinez RI, Borden S, Wilmott RW. Large-airway collapse due to acquired tracheobronchomalacia in infancy. Am J Dis Child. 1986;140:367–371. doi: 10.1001/archpedi.1986.02140180101035. [DOI] [PubMed] [Google Scholar]
- 6.Downing GJ, Kilbride HW. Evaluation of airway complications in high-risk preterm infants: application of flexible fiberoptic airway endoscopy. Pediatrics. 1995;95:567–572. [PubMed] [Google Scholar]
- 7.Miller RW, Woo P, Kellman RK, Slagle TS. Tracheobronchial abnormalities in infants with bronchopulmonary dysplasia. J Pediatr. 1987;111:779–782. doi: 10.1016/s0022-3476(87)80267-6. [DOI] [PubMed] [Google Scholar]
- 8.Cohn RC, Kercsmar C, Dearborn D. Safety and efficacy of flexible endoscopy in children with bronchopulmonary dysplasia. Am J Dis Child. 1988;142:1225–1228. doi: 10.1001/archpedi.1988.02150110103030. [DOI] [PubMed] [Google Scholar]
- 9.McCubbin M, Frey EE, Wagener JS, Tribby R, Smith WL. Large airway collapse in bronchopulmonary dysplasia. J Pediatr. 1989;114:304–307. doi: 10.1016/s0022-3476(89)80802-9. [DOI] [PubMed] [Google Scholar]
- 10.Burnard ED, Grauaug A. Dyspnoea and apnoea in the newborn: some results of investigation. Med J Aust. 1965;1:445–455. doi: 10.5694/j.1326-5377.1965.tb71816.x. [DOI] [PubMed] [Google Scholar]
- 11.Bhutani VK, Rubenstein SD, Shaffer TH. Pressure--volume relationships of tracheae in fetal newborn and adult rabbits. Respir Physiol. 1981;43:221–231. doi: 10.1016/0034-5687(81)90104-3. [DOI] [PubMed] [Google Scholar]
- 12.Shaffer TH, Bhutani VK, Wolfson MR, Penn RB, Tran NN. In vivo mechanical properties of the developing airway. Pediatr Res. 1989;25:143–146. doi: 10.1203/00006450-198902000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Penn RB, Wolfson MR, Shaffer TH. Effect of ventilation on mechanical properties and pressure-flow relationships of immature airways. Pediatr Res. 1988;23:519–524. doi: 10.1203/00006450-198805000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Björklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O, Vilstrup CT. Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr Res. 1997;42:348–355. doi: 10.1203/00006450-199709000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Bhutani VK, Rubenstein D, Shaffer TH. Pressure-induced deformation in immature airways. Pediatr Res. 1981;15:829–832. [PubMed] [Google Scholar]
- 16.Murthy K, Dykes FD, Padula MA, Pallotto EK, Reber KM, Durand DJ, Short BL, Asselin JM, Zaniletti I, Evans JR. The Children’s Hospitals Neonatal Database: an overview of patient complexity, outcomes and variation in care. J Perinatol. 2014;34:582–586. doi: 10.1038/jp.2014.26. [DOI] [PubMed] [Google Scholar]
- 17.Majid A, Gaurav K, Sanchez JM, Berger RL, Folch E, Fernandez-Bussy S, Ernst A, Gangadharan SP. Evaluation of tracheobronchomalacia by dynamic flexible bronchoscopy: a pilot study. Ann Am Thorac Soc. 2014;11:951–955. doi: 10.1513/AnnalsATS.201312-435BC. [DOI] [PubMed] [Google Scholar]
- 18.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 19.Carden KA, Boiselle PM, Waltz DA, Ernst A. Tracheomalacia and tracheobronchomalacia in children and adults: an in-depth review. Chest. 2005;127:984–1005. doi: 10.1378/chest.127.3.984. [DOI] [PubMed] [Google Scholar]
- 20.Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. 2014;100:145–157. doi: 10.1002/bdra.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapcharoensap W, Gage SC, Kan P, Profit J, Shaw GM, Gould JB, Stevenson DK, O’Brodovich H, Lee HC. Hospital variation and risk factors for bronchopulmonary dysplasia in a population-based cohort. JAMA Pediatr. 2015;169:e143676. doi: 10.1001/jamapediatrics.2014.3676. [DOI] [PubMed] [Google Scholar]
- 22.Guaman MC, Gien J, Baker CD, Zhang H, Austin ED, Collaco JM Point Prevalence, Clinical Characteristics, and Treatment Variation for Infants with Severe Bronchopulmonary Dysplasia. Point prevalence, clinical characteristics, and treatment variation for infants with severe bronchopulmonary dysplasia. Am J Perinatol. 2015;32:960–967. doi: 10.1055/s-0035-1547326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hysinger EB, Panitch HB. Paediatric tracheomalacia. Paediatr Respir Rev. 2016;17:9–15. doi: 10.1016/j.prrv.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002;109:423–428. doi: 10.1542/peds.109.3.423. [DOI] [PubMed] [Google Scholar]
- 25.McAleese KA, Knapp MA, Rhodes TT. Financial and emotional cost of bronchopulmonary dysplasia. Clin Pediatr (Phila) 1993;32:393–400. doi: 10.1177/000992289303200702. [DOI] [PubMed] [Google Scholar]
- 26.Johnston KM, Gooch K, Korol E, Vo P, Eyawo O, Bradt P, Levy A. The economic burden of prematurity in Canada. BMC Pediatr. 2014;14:93. doi: 10.1186/1471-2431-14-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis S, Jones M, Kisling J, Angelicchio C, Tepper RS. Effect of continuous positive airway pressure on forced expiratory flows in infants with tracheomalacia. Am J Respir Crit Care Med. 1998;158:148–152. doi: 10.1164/ajrccm.158.1.9711034. [DOI] [PubMed] [Google Scholar]
- 28.Panitch HB, Allen JL, Alpert BE, Schidlow DV. Effects of CPAP on lung mechanics in infants with acquired tracheobronchomalacia. Am J Respir Crit Care Med. 1994;150:1341–1346. doi: 10.1164/ajrccm.150.5.7952562. [DOI] [PubMed] [Google Scholar]
- 29.de Trey LA, Dudley J, Ismail-Koch H, Durward A, Bellsham-Revell H, Blaney S, Hore I, Austin CB, Morrison GA. Treatment of severe tracheobronchomalacia: ten-year experience. Int J Pediatr Otorhinolaryngol. 2016;83:57–62. doi: 10.1016/j.ijporl.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Martin WM, Shapiro RS. Long custom-made plastic tracheostomy tube in severe tracheomalacia. Laryngoscope. 1981;91:355–362. [PubMed] [Google Scholar]
- 31.Omari T, Barnett C, Snel A, Davidson G, Haslam R, Bakewell M, Dent J. Mechanism of gastroesophageal reflux in premature infants with chronic lung disease. J Pediatr Surg. 1999;34:1795–1798. doi: 10.1016/s0022-3468(99)90315-9. [DOI] [PubMed] [Google Scholar]
- 32.Jadcherla SR, Peng J, Chan CY, Moore R, Wei L, Fernandez S, DI Lorenzo C. Significance of gastroesophageal refluxate in relation to physical, chemical, and spatiotemporal characteristics in symptomatic intensive care unit neonates. Pediatr Res. 2011;70:192–198. doi: 10.1203/PDR.0b013e31821f704d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoyoux C, Forget P, Lambrechts L, Geubelle F. Chronic bronchopulmonary disease and gastroesophageal reflux in children. Pediatr Pulmonol. 1985;1:149–153. doi: 10.1002/ppul.1950010306. [DOI] [PubMed] [Google Scholar]
- 34.Nobile S, Noviello C, Cobellis G, Carnielli VP. Are infants with bronchopulmonary dysplasia prone to gastroesophageal reflux? A prospective observational study with esophageal pH-impedance monitoring. J Pediatr. 2015;167:279. doi: 10.1016/j.jpeds.2015.05.005. 285.e1. [DOI] [PubMed] [Google Scholar]
- 35.Jadcherla SR, Slaughter JL, Stenger MR, Klebanoff M, Kelleher K, Gardner W. Practice variance, prevalence, and economic burden of premature infants diagnosed with GERD. Hosp Pediatr. 2013;3:335–341. doi: 10.1542/hpeds.2013-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.