Abstract
Background
Fibroblast growth factor 23 (FGF23), a phosphaturic hormone secreted mainly by osteocytes, maintains serum phosphate levels within a tight range by promoting phosphaturia. Previous studies have mainly focused on the link between FGF23 levels and dietary intake of phosphate but other dietary factors may also influence FGF23 levels.
Objective
The objective of this study was to examine the associations of several potential dietary factors with FGF23 levels.
Methods
This cross-sectional study pooled three populations of young adults with African ancestry and an estimated glomerular filtration rate > 80 ml/min/1.73 m2 (419 in Chicago, IL; 463 in Victoria, Seychelles and 461 in Kumasi, Ghana). FGF23 was measured in fasting plasma samples using a C-terminal assay and diet information was based on two-24 hour recalls. Linear regression was used to examine the association between FGF23 levels and quartiles of calorie-adjusted dietary factors with adjustment for covariates.
Results
Participants were 47% male with mean age of 35.1 ± 6.3 years. Median plasma FGF23 values in RU/ml ranged from 57.7 (interquartile range [IQR] 43.4, 75.3) in the U.S., 42.4 (IQR 33.0, 55.5) in Seychelles and 33.3 (IQR 24.8, 47.6) in Ghana. With adjustment for covariates, increasing quartiles of phosphate, calcium and animal protein intake and decreasing quartiles of vegetable protein, fiber and magnesium were associated with significantly higher FGF23 levels. After further adjustment for dietary factors, significant trends in FGF23 levels were noted only for quartiles of calcium and magnesium intake (P < 0.001). The highest quartiles of calorie-adjusted calcium and magnesium intake were associated with a 0.20 (95% CI 0.10, 0.31) higher and a 0.20 lower (95% CI −0.34, −0.05) standardized log FGF23 level, respectively, compared to the lowest respective quartile.
Conclusions
Dietary factors other than phosphate are associated with FGF23 levels in young adults.
Keywords: FGF23, Fibroblast growth factor 23, Vitamin D, calcium intake, phosphate intake, animal protein, plant protein, casein diet, African-Americans, race, latitude, environment, African ancestry
Names for pubmed-indexing: Kosk, Kramer, Luke, Camacho, Bovet, Forrester, Wolf, Sempos, Melamed, Plange-Rhule, Dugas, Cooper, Durazo-Arvizu
Introduction
Phosphate homeostasis is essential for multiple facets of bone and muscle health. Fibroblast growth factor 23 (FGF23), a phosphaturic hormone secreted mainly by osteocytes, maintains serum phosphate levels within a tight range by downregulating sodium-phosphate co-transporters (types IIa and IIc) in the proximal tubule and promoting phosphaturia.(1–3) FGF23 also inhibits 25-hydroxyvitamin D 1-α- hydroxylase activity, the enzyme that converts 25-hydroxyvitamin D [25(OH)D] to its active form 1,25 dihydroxyvitamin D [1,25(OH)2D]. The reduced conversion of 25(OH)D to the active form of vitamin D reduces gastrointestinal absorption of both phosphate and calcium.(4) In aggregate, the effects of FGF23 help to maintain normal serum phosphate and calcium levels.
Several studies have shown that higher phosphate intake for at least several days leads to compensatory increases in FGF23 levels.(1, 3) Using data from three populations of young adults with African ancestry living in the U.S., Seychelles, and Ghana, we have previously shown that both phosphate intake and FGF3 levels are highest in the U.S(5) and that higher net gastrointestinal phosphate absorption, as reflected by urinary phosphate excretion, is associated with higher FGF23 levels.(5, 6) It is likely that dietary factors other than phosphate also influence FGF23 production either directly or via influencing net gastrointestinal phosphate absorption,(7) but few human studies have examined the association of dietary factors, other than phosphate, with FGF23 levels, especially in populations without kidney disease.(8–10) A more complete picture of the association of diet with FGF23 levels holds strong public health implications because higher plasma FGF23 levels are associated with heightened risk for cardiovascular diseases including left ventricular hypertrophy, atrial fibrillation, reduced ejection fraction, stroke(11–14) and fracture risk in adults with kidney disease.(15, 16)
In this study, we utilized data from three populations of young adults with African ancestry (Maywood, Illinois, USA; Kumasi, Ghana; Cape Town, and Mahé, the main island of Seychelles). Pooling populations with wide variations in environmental exposures including dietary patterns can enhance the elucidation of associations poorly detected in a single population where exposures are more homogenous.(17) Using the pooled sample, we examined the associations of several potential dietary factors with FGF23 levels including calcium, animal and vegetable protein and fiber. We hypothesized that higher dietary intakes of calcium and animal protein and lower intakes of vegetable protein and fiber are associated with higher levels of FGF23 levels.
Methods
Study Population
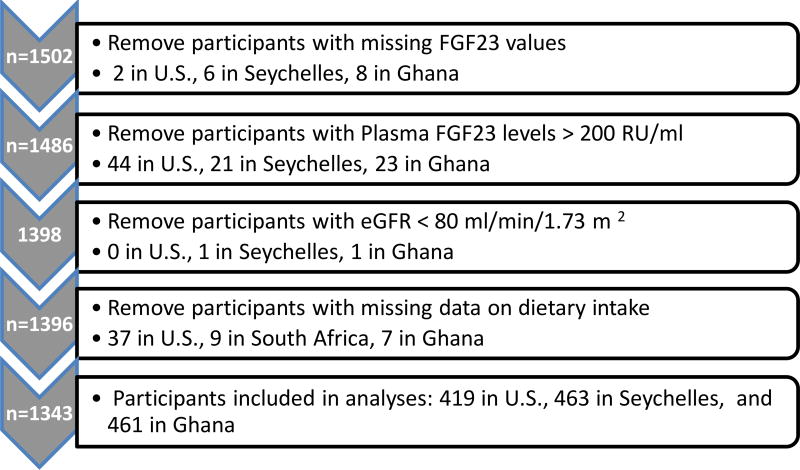
The source population for this study was the Modeling the Epidemiological Transition Study (METS) and 25(OH)D and plasma FGF23 concentrations were measured as part of the Vitamin D Ancillary Study (VIDA). Twenty-five hundred adults, ages 25–45, were enrolled between January, 2010 and December, 2011. Participants were enrolled at each of five sites: Maywood, Illinois, USA; Kingston, Jamaica; Kumasi, Ghana; Cape Town, South Africa; and Mahé, the main island of Seychelles. The sites were chosen to cover a broad range of social and economic development and all participants were primarily of African ancestry. Plasma FGF23 was measured in participants from 3 sites: Maywood, IL (n=502), Seychelles (n=500), and Ghana (n=500). We excluded participants with missing plasma FGF23 levels (2 in U.S., 6 in Seychelles, and 8 in Ghana), and those with an estimated glomerular filtration rate < 80 ml/min/1.73 m2 (0 in U.S., 1 in Seychelles, and 1 in Ghana). Due to potential issues of iron deficiency leading to increases in the C-terminal FGF23 fragment but not intact FGF23 plasma levels, (18) individuals with severe outlying plasma FGF23 levels > 200 RU/ml (44 [41 women] in U.S., 21 [20 women] in Seychelles and 23 [16 women] in Ghana) were excluded. We also excluded individuals with missing dietary data (37 in U.S., 9 in Seychelles, and 7 in Ghana). This left a total analytical sample of 419, 463, and 461 participants from the U.S., Seychelles, and Ghana, respectively (Figure 1). The METS and VIDA studies were approved by the Institutional Review Board or Ethics Committee at each site and written content was obtained from all participants in their native language.
Figure 1.
Flowchart of participant selection
Diet
Dietary data were collected by two 24-hour recalls using the multiple pass method, created by the Medical Research Council of South Africa.(19) All 24-hour recalls were collected by centrally trained interviewers using interview scripts translated into local language. Interviewers recorded participants reported food and amount through various recall styles that captured details including food type, portion size, and preparation and cooking methods. Specific methods have been previously described.(20) Dietary data were sent to the Coordinating Center at Loyola University Chicago where data were entered and analyzed using the Nutrient Data System for Research (NDSR; University of Minneapolis, MN, USA). The Nutrient Data System for Research was modified to accommodate foods and recipes unique to each site using site-specific nutrient databases and previously collected 24-hour recall data. Nutrient values were adjusted for total caloric intake using the residuals of a linear regression model with nutrient intake as the dependent variable and caloric intake as an independent variable.(21, 22) The use of the residual model reduces measurement errors for some nutrients and controls for confounding by the total amount of food consumed.(21, 22) The dietary intakes for each site are shown as absolute values and as mean values per 1000 calories to account for differences in caloric intake across the three sites.
Non-dietary factors
All measurements were undertaken at outpatient clinics located in each of the METS communities. At the baseline clinic visit, weight was measured without shoes to the nearest 0.1 kg using a standard balance (Health-o-meter, Bridgeview, IL). Height was measured using a stadiometer without shoes. Body mass index (BMI) was defined as weight in kg divided by the height in meters squared. Participants were asked to fast 10 hours from the evening prior to the baseline examination for blood sample collections. Cystatin C was measured in fasting serum samples at the St. Gallens lab in Switzerland using nephelometry (Siemens). Glomerular filtration rate was estimated using cystatin C.(23) Total 25-hydroxyvitamin D levels, which reflect the sum of 25(OH)D2 and 25(OH)D3, were measured using a liquid chromatography-tandem mass spectrometric assay at the University of Washington. The calibration of the assay was verified using the NIST standard reference material SRM 972. Inter-assay variability was 6.0% at 11.5 ng/mL and 5.6% at 12.3 ng/mL for 25(OH)D2 and 25(OH)D3, respectively.
Fibroblast Growth Factor-23 levels
Plasma FGF23 levels were measured at the University of Miami Miller School of Medicine using an ELISA that uses 2 antibodies directed against different epitopes within the carboxy-terminal portion of FGF23 and thus capture both the intact hormone and its carboxy-terminal fragments (Immutopics, San Clemente, CA). The lower limit of detection was 12 RU/ml.
Statistical Analysis
We used STATA/IC 13.1 (StataCorp LP, College Station, TX, USA) to perform all statistical analysis. Summary statistics for key baseline characteristics were compared by site. Differences in continuous variables were tested using ANOVA and using Fisher’s exact test for categorical variables tested. If these tests were statistically significant, then the Ghana and Seychelles groups were each compared to the U.S. group. The level of statistical significance was set as P < 0.01 to account for multiple comparisons.
Histograms and boxplots of variables were examined and plasma FGF23 demonstrated a right skewed distribution. The optimal transformation of plasma FGF23 to normality, as determined by the ladder of powers approach, (24) was a log transformation and the histogram of log transformed plasma FGF23 showed a normal distribution. Log transformed plasma FGF23 values were standardized by subtracting each value from the mean and dividing by the standard deviation. Calorie-adjusted nutrient values were categorized into quartiles to decrease the influence of outliers;(21) quartiles were then fitted into generalized linear models with standardized log transformed plasma FGF23 as the dependent variable after pooling all sites. The resulting regression coefficients for each of the calorie-adjusted dietary factor quartiles can be interpreted as the difference in the Z score for log transformed plasma FGF23 levels within a quartile of a dietary factor compared to the lowest quartile after removing the effects of covariates including total caloric intake. We examined dietary factors potentially associated with FGF23 and/or gastrointestinal phosphate absorption which included calcium, phosphate, animal and vegetable protein, total fiber, magnesium, and iron intake. Because the direction of the association of both insoluble fiber and soluble fiber with FGF23 levels was the same, we included total fiber in the model instead of examining insoluble fiber and soluble fiber separately. For each dietary factor, we examined two models. The first model was a parsimonious model that adjusted for age, sex, site, total caloric intake, eGFR and total levels of 25(OH)D. Model 2 adjusted for all variables in Model 1 and also adjusted for the dietary factors calcium, phosphate, animal and vegetable protein, total fiber, magnesium, and iron. Models adjusted for total levels of 25(OH)D due to its influence on gastrointestinal absorption of calcium and phosphate.(25) To evaluate linear trends across calorie adjusted quartiles of dietary factors, we used generalized linear models and fitted the quartiles as a continuous variable and tested whether the slope coefficients differed significantly from zero.
Results
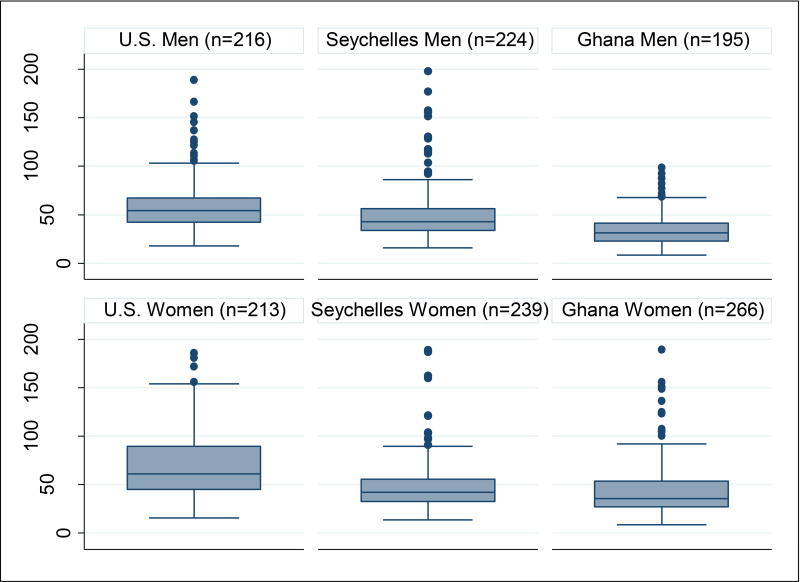
In the pooled sample of 1346 study participants, the mean age was 35.1 (3.6) years and 46.5% were male. Characteristics of the study participants by site are presented in Table 1. The mean age was fairly similar across sites but participants in Seychelles were significantly older than U.S. participants (36.0 (5.6) vs. 34.9 (6.2); P =0.007). Obesity prevalence was highest in the U.S. and lowest in Ghana. The distribution of total 25(OH)D levels ranged from as low as 17.3 (8.0) ng/ml in the U.S. to as high as 30.4 (6.9) ng/ml in Ghana. Median plasma FGF23 values in RU/ml ranged from 57.7 (interquartile range [IQR] 43.4, 75.3) in the U.S., 42.4 (IQR 33.0, 55.5) in Seychelles and 33.3 (IQR 24.8, 47.6) in Ghana. Figure 2 shows the distribution of FGF23 levels by sex and by site. Among men, median FGF23 levels in RU/ml were 54.3 in the U.S. (IQR 42.4–67.2) 42.9 (IQR 27.7–56.2) in Seychelles and 31.2 (IQR 22.9–41.1) in Ghana. Among women, median FGF23 levels in RU/ml ranged from 61.2 (IQR 45.2–89.3) in the U.S., 42.2 (IQR 32.6–55.4) in Seychelles and 35.4 (IQR 27.2–53.7) in Ghana.
Table 1.
Characteristics by site
| United States (n=419) |
Seychelles (n=463) |
Ghana (n=461) |
Overall P- value |
|
|---|---|---|---|---|
| % Male | 51.6 | 48.3 | 42.2 | 0.02 |
| Age (years) | 34.9 (6.2) | †36.0 (5.6) | 34.2 (6.7) | <0.001 |
| Height (cm) | 170.4 (9.0) | †167.4 (8.8) | †162.5 (8.1) | <0.001 |
| Weight (kg) | 92.2 (24.2) | †75.9 (16.9) | †63.4 (11.5) | <0.001 |
| BMI (kg/m2) | 31.8 (8.4) | †27.0 (5.5) | †24.0 (4.5) | <0.001 |
| % BMI ≥30 kg/m2 | 52.2 | †26.1 | †9.9 | <0.001 |
| Estimated GFR (ml/min/1.73m2)c | 134.9 (18.2) | †149.2 (29.1) | †129.8 (19.8) | <0.001 |
| Serum Phosphate (mg/dl) | 3.1 (0.50) | †2.8 (0.45) | †3.4 (0.70) | <0.001 |
| Vitamin D (ng/ml) | 17.3 (8.0) | †29.3 (7.9) | †30.4 (6.9) | <0.001 |
| Plasma FGF23 (RU/mL)a | 57.7 (43.4–75.3) | †42.4 (33.0–55.5) | †33.3 (24.8–47.6) | <0.001b |
P<0.01 compared to U.S.
Data shown as median (interquartile range)
Overall p value calculated by comparing log transformed mean values by site using ANOVA
eGFR calculated using the CKD-Epi equation based on cystatin C(23)
Figure 2.
Boxplot showing Fibroblast Growth Factor 23 (FGF23) Levels by Site and by Sex
Table 2 shows the dietary intakes per day and per 1000 calories by site and in the pooled sample. Total daily energy intake was significantly higher in the U.S. compared to Seychelles and Ghana. Phosphate and calcium intake were lowest in Ghana, while intakes of vegetable protein, fiber and magnesium were highest in Ghana. Table 3 shows the adjusted difference in FGF23 levels by quartiles of calorie-adjusted dietary factors with adjustment for covariates. In model 1 which adjusted for age, sex, site, calories, total 25(OH)D levels and eGFR, significant linear trends in FGF23 levels were noted across quartiles of calorie-adjusted intakes of phosphate, calcium, animal and vegetable protein, iron, total fiber, and magnesium. Increasing quartiles of both calcium, and animal protein were associated with higher FGF23 levels while increasing quartiles of vegetable protein, magnesium and fiber were associated with lower FGF23 levels. After further adjustment for dietary factors, significant linear trends in FGF23 levels remained only for calcium (P < 0.001), and magnesium (P < 0.001). The highest quartile of calorie-adjusted calcium intake was associated with a 0.20 (95% CI 0.10, 0.31) higher standardized log transformed FGF23 level compared to the lowest quartile. In contrast, significantly lower standardized log FGF23 levels were noted in the highest quartile of calorie-adjusted intake of magnesium (−.20 [95% CI −0.34, −0.05)] compared to the lowest quartile.
Table 2.
Dietary characteristics of the three study populations and the pooled sample
| Intake per day | United States (n=419) |
Seychelles (n=463) |
Ghana (n=461) |
Overall P- value |
Pooled sample |
|---|---|---|---|---|---|
| Phosphate intake (mg) | 1179.8 (487.6) | †1142.0 (396.5) | †895.5 (345.1) | <0.001 | 1068.6 (429.7) |
| Calcium intake (mg) | 655.6 (342.5) | †545.2 (251.0) | †334.3 (144.7) | <0.001 | 507.1 (287.9) |
| Animal Protein (g) | 62.4 (31.0) | 60.3 (27.6) | †28.7 (20.4) | <0.001 | 50.1 (30.7) |
| Vegetable Protein (g) | 24.0 (10.6) | †22.4 (8.7) | †30.8 (12.5) | <0.001 | 25.8 (11.4) |
| Carbohydrates (g) | 265.5 (109.4 | †303.4 (78.5) | †234.9 (79.1) | <0.001 | 268.0 (93.8) |
| Fat (g) | 97.90 (42.0) | †60.9 (27.9) | †48.8 (26.7) | <0.001 | 67.9 (38.3) |
| Total Fiber (g) | 14.3 97.1) | †13.5 (6.8) | †25.1 (9.7) | <0.001 | 17.8 (9.6) |
| Soluble Fiber (g) | 4.7 (2.5) | †3.9 (2.1) | †6.1 (2.8) | <0.001 | 4.9 (2.6) |
| Insoluble fiber (g) | 9.6 (5.4) | 9.6 (5.2) | †19.0 (7.5) | <0.001 | 12.8 (7.6) |
| Magnesium (mg) | 237.3 (103.5) | †259.3 (89.9) | †340.0 (120.6) | <0.001 | 12.8 (7.6) |
| Total Vitamin D (µg) | 1.9 (2.2) | †2.8 (2.0) | †2.4 (6.0) | <0.001 | 4.8 (8.7) |
| Iron (mg) | 14.6 (7.5) | †13.2 (4.7) | 14.3 (4.7) | <0.001 | 14.0 (5.8) |
| Calories | 2323.2 (886.2) | †1855.5 (589.9) | †1863.2 (496.2) | <0.001 | 2003.4 (702.9) |
| Intake per 1000 calories | |||||
| Phosphate (mg) | 518.4 (131.2) | †621.7 (129.5) | †478.1 (114.8) | <0.001 | 540.1 (139.3) |
| Calcium (mg) | 294.2 (132.6) | 296.5 (110.0) | †181.8 (69.5) | <0.001 | 256.1 (119.0) |
| Animal Protein (g) | 27.3 (10.2) | †32.8 (11.6) | †15.2 (9.3) | <0.001 | 25.0 (12.8) |
| Vegetable Protein (g) | 10.6 (3.6) | †12.3 (3.6) | †16.5 (4.6) | <0.001 | 13.2 (4.7) |
| Carbohydrate (g) | 115.7 (24.2) | †128.2 (24.4) | †165.3 (26.8) | <0.001 | 137.1 (32.8) |
| Fat (g) | 41.5 (7.9) | †32.3 (8.7) | †25.3 (10.4) | <0.001 | 32.7 (11.2) |
| Total Fiber (g) | 6.5 (3.0) | †7.5 (3.4) | †13.5 (4.0) | <0.001 | 9.3 (4.7) |
| Soluble fiber (g) | 2.1 (1.1) | 2.1 (1.3) | †3.3 (1.3) | <0.001 | 2.5 (1.3) |
| Insoluble fiber (g) | 4.3 (2.3) | †5.4 (2.7) | †10.2 (3.0) | <0.001 | 6.7 (3.7) |
| Magnesium (mg) | 105.4 (35.9) | †142.7 (34.7) | †181.7 (38.0) | <0.001 | 144.6 (47.6) |
| Vitamin D (µg) | 1.9 (2.2) | †2.8 (2.0) | †2.4 (6.0) | <0.001 | 2.4 (3.9) |
| Iron (mg) | 6.5 (2.8) | †7.2 (2.0) | †7.8 (1.7) | <0.001 | 7.2 (2.3) |
Table 3.
Adjusted difference in standardized log transformed fibroblast growth factor 23 levels by quartiles of calorie-adjusted dietary factors
| Phosphate | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
| Range (mg/day) | 116.0–849.4 | 851.0–971.2 | 972.0–1124.8 | 1125.0–2304.3 | |
| Median (mg/day) | 741.6 | 916.6 | 1048.6 | 1278.3 | |
| FGF23 median (RU/ml) | 42.2 | 41.8 | 43.7 | 45.2 | |
| Model 1 | Referent | 0.03 (−0.07, 0.13) | 0.08 (−0.01, 0.18) | 0.12 (0.03, 0.21) | 0.006 |
| Model 2 | Referent | 0.02 (−0.09, 0.12) | −0.02 (−0.14, 0.09) | 0.02 (−0.12, 0.16) | 0.6 |
| Calcium | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
| Intake Range (mg/day) | 470.7–859.9 | 860.0–970.0 | 971.0–1101.3 | 1102.0–2061.0 | |
| Quartile Median (mg/d) | 775.3 | 914.7 | 1025.0 | 1240.2 | |
| FGF23 median | 38.4 | 42.1 | 44.5 | 49.5 | |
| Model 1 | Referent | 0.09 (−0.003, 0.18) | 0.17 (0.08, 0.27) | 0.29 (0.20, 0.38) | <0.001 |
| Model 2 | Referent | 0.04 (−0.05, 0.13) | 0.10 (0.002, 0.20) | 0.20 (0.10, 0.31) | <0.001 |
| Animal Protein | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
| Intake Range (mg/day) | 0–36.1 | 36.2–48.6 | 48.7–62.3 | 62.4–166.8 | |
| Quartile Median (mg/d) | 24.5 | 42.4 | 54.6 | 75.9 | |
| FGF23 median (RU/ml) | 37.5 | 44.4 | 47.7 | 45.7 | |
| Model 1 | Referent | 0.19 (0.10, 0.29) | 0.32 (0.22, 0.42) | 0.24 (0.15, 0.33) | <0.001 |
| Model 2 | Referent | 0.11 (0.003, 0.21) | 0.17 (0.04, 0.29) | 0.11 (−0.03, 0.25) | 0.2 |
| Vegetable Protein | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
| Intake Range (mg/day) | 0–14.3 | 14.4–18.6 | 18.7–24.0 | 24.1–75.7 | |
| Quartile Median (mg/d) | 11.5 | 16.4 | 21.0 | 29.8 | |
| FGF23 median (RU/ml) | 49.4 | 46.9 | 40.5 | 37.9 | |
| Model 1 | Referent | −0.03 (−0.13, 0.06) | −0.17 (−0.27, −0.08) | −0.24 (−0.33, −0.15) | <0.001 |
| Model 2 | Referent | 0.04 (−0.06, 0.14) | 0.01 (−0.10, 0.13) | 0.08 (−0.06, 0.21) | 0.2 |
| Total Fiber | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
| Range (g/day) | 2.1–25.7 | 25.8–29.2 | 29.3–34.9 | 35.0–81.8 | |
| Median (g/day) | 23.3 | 27.4 | 31.7 | 40.9 | |
| FGF23 Median (RU/ml) | 49.4 | 49.6 | 43.1 | 35.0 | |
| Model 1 | Referent | −0.03 (−0.13, 0.07) | −0.14 (−0.23, −0.04) | −0.37 (−0.46, −0.28) | <0.001 |
| Model 2 | Referent | 0.01 (−0.09, 0.11) | −0.03 (−0.15, 0.09) | −0.14 (−0.27, −0.01) | 0.08 |
| Magnesium | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
| Range (mg/day) | 73.7 –346.4 | 346.8–384.9 | 385.5–438.8 | 438.9 – 891.5 | |
| Median (mg/day) | 310.5 | 367.7 | 413.4 | 498.3 | |
| FGF23 Median (RU/ml) | 52.2 (39.4–74.5) | 48.5 (37.0–64.3) | 42.5 (31.4–56.3) | 36.4 (27.0–51.5) | |
| Model 1 | Referent | −.03 (−0.15, 0.08) | −0.19 (−0.29, −0.09) | −0.37 (−0.46, −0.27) | <0.001 |
| Model 2 | Referent | 0.02 (−0.11, 0.14) | −0.08 (−0.22, 0.05) | −0.20 (−0.34, −0.05) | <0.001 |
| Iron | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
| Range | 8.2–15.6 | 15.6–17.4 | 17.5–19.7 | 19.8–66.2 | |
| Median (mg/d) | 14.2 | 16.5 | 18.7 | 21.9 | |
| FGF23 median (RU/ml) | 50.0 | 41.6 | 39.2 | 42.0 | |
| Model 1 | Referent | −0.12 (−0.22, −0.02) | −0.20 (−0.30, −0.10) | −0.17 (−0.26, −0.08) | <0.001 |
| Model 2 | Referent | −0.11 (−0.21, −0.01) | −0.14 (−0.25, −0.03) | −0.10 (−0.21, 0.01) | 0.08 |
Dietary factors in model were adjusted for caloric intake using the residual method. Model 1 adjustes for age, sex, calories, serum 25-hydroxyvitamin D levels and estimated glomerular filtration rate based on cystatin C; Model 2 adjusts for age, sex, site, calories, serum vitamin D levels, estimated glomerular filtration rate based on cystatin C and all dietary factors listed in table.
Discussion
We used data from young adults of African ancestry living in three different environments to examine the association of calorie-adjusted dietary intakes with FGF23 levels. Overall, we found that higher calcium and animal protein intakes were associated with higher FGF23 levels while higher intakes of fiber, magnesium and iron were associated with lower FGF23 levels. We did not find a significant association between calorie-adjusted intakes of vegetable protein or phosphate after mutual adjustment for dietary factors. Phosphate intake is poorly quantified using standard nutrition surveys due to the often hidden sources of phosphate in foods and may explain the very weak association we noted between dietary phosphate intake and FGF23 levels.(26) A few studies have shown lower FGF23 levels with increasing plant protein intake,(27, 28) but findings have not been consistent(29) which could be due to the complexity of dietary factors that may influence FGF23 production and difficulties in quantifying vegetable intake due to consumption of vegetables in mixed dishes.(30) However, we did note decreasing FGF23 levels across increasing quartiles of vegetable protein intake prior to adjustment for dietary factors consistent with previous studies.(27) Phosphate content in plant protein is mainly in the form of phytic acid, otherwise known as inositol hexakisphosphate, the principal storage form of phosphate in grains, nuts and seeds.(31) In order to absorb the phosphate from phytic acid, the enzyme phytase is needed to remove the phosphate from the inositol group. Because phytase activity is low in humans,(31) net gastrointestinal absorption of phosphate in plant protein sources is much lower than phosphate obtained from animal protein sources or from foods preserved with inorganic phosphate.(32) Higher quartiles of animal protein intake were associated with significantly higher FGF23 levels prior to adjustment for dietary factors, which is also consistent with previous studies.(28)
Our findings of incremental increases in FGF23 levels across quartiles of dietary calcium intake are consistent with results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Germany, a cohort of 2134 middle-aged adults. Among EPIC participants, higher dietary calcium intake was associated with higher odds of having plasma FGF23 levels > 90 RU/ml.(8) In addition, Kobayashi et al. reported a positive correlation between plasma FGF23 levels and serum calcium levels and the dose of calcium gluconate given to 18 patients after parathyroidectomy for treatment of primary hyperparathyroidism.(33) While short-term calcium loading has not been shown to increase plasma FGF23 levels,(34) calcium-based phosphate binder use for several months in patients with non-dialysis dependent chronic kidney disease consistently increases FGF23 levels while use of non-calcium based phosphate binder use does not.(35–37) The potential link between calcium intake and plasma FGF23 levels is also supported by experiments in parathyroidectomized Wistar rats whereby increases in dietary calcium intake for 10 days increased both serum calcium and FGF23 levels.(38)
We noted significantly lower FGF23 levels in the highest vs. lowest calorie-adjusted magnesium intake quartiles even with adjustment for calcium intake. Over 50 years ago, magnesium deficiency was linked with increased urinary phosphate excretion(39, 40) due to its activation of the calcium sensing receptor on the parathyroid gland resulting in decreased parathyroid hormone (PTH) production. Animal studies have demonstrated that magnesium stimulates renal phosphate absorption via type IIa sodium-phosphate transporters in a PTH dependent manner, but existing animal studies do not consistently demonstrate that altering the magnesium content of the diet alters FGF23 levels. (41–43) To our knowledge, no previous study has examined the association between magnesium intake and FGF23 levels in humans. Dietary iron intake was also associated with FGF23 levels with a lower iron intake associated with higher FGF23 levels. These findings are consistent with the known effects of iron deficiency impairing the FGF23 cleavage and increasing in the C-terminal FGF23 fragment.(18, 44)
Our study focused on young adults of African ancestry living in three different environments. The pooling of data from three populations with marked differences in dietary patterns led to wide variability in diet and this variability may have facilitated discernment of associations between dietary factors and FGF23 levels. A noteworthy limitation of this study is the use of self-reported dietary intakes. Across all three of these study sites, 24-hour dietary recalls have been shown to significantly underestimate dietary energy intake and potentially other nutrients.(20) There is evidence to suggest, however, that relative intakes may be preserved.(20) Another limitation is lack of information on serum calcium and magnesium levels. Study participants were young and overall healthy and hypocalcemia and hypomagnesemia would be uncommon in any of the sites. We could only measure levels of plasma C-terminal FGF23 rather than intact plasma FGF23, but results from the two assays have yielded qualitatively similar associations in most settings.(45) Another limitation is lack of information on serum iron or ferritin levels. We excluded individuals with FGF23 levels > 200 RU/ml due to potential issues of iron deficiency. The majority of the individuals with FGF23 values > 200 RU/ml were women who were likely premenopausal given their age.
Conclusion
Bone derived plasma FGF23 levels are associated with several dietary factors including intakes of calcium, and magnesium in young adults with African ancestry. The link between FGF23 with several dietary factors indicates that phosphate restriction alone -without consideration of other dietary factors- may not be effective for lowering FGF23 levels. The findings from this study should be confirmed in other study populations.
Acknowledgments
This study was supported in part by funding from the National Institutes of Health 3 (RD), 5R01DK080763-04 (AL), and by Loyola University Chicago – Intramural Award LU#204006.
Abbreviations
- FGF23
Fibroblast Growth Factor 23
- 25(OH)D
25-hydroxyvitamin D
- 1, 25(OH)D
1, 25-dihydroxyvitamin D
- METS
Modeling the Epidemiologic Transition Study
- VIDA
Vitamin D Ancillary Study
- BMI
Body Mass Index
- IQR
Interquartile Range
Footnotes
Disclosures: D. Kosk (no conflicts of interest), H. Kramer (no conflicts of interest), A. Luke (no conflicts of interest), P. Camacho (no conflicts of interest), P. Bovet (no conflicts of interest), T. Forrester (no conflicts of interest), M. Wolf (no conflicts of interest), C. Sempos (no conflicts of interest), M. Melamed (no conflicts of interest), J Plange-Rhule (no conflicts of interest), L. Dugas (no conflicts of interest), R. Cooper (no conflicts of interest), and R. Durazo-Arvizu (no conflicts of interest).
Data were presented in poster form at the American Public Health Association Meeting October 2015, Chicago IL (DK)
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institutes of Health or the US Department of Health and Human Services.
Author Contributions:
Dominique Kosk, Holly Kramer, Ramon Durazo-Arvizu, Amy Luke, Richard Cooper and Myles Wolfe all participated in the design of the research project. Amy Luke, Myles Wolf, Lara Dugas, Richard Cooper, Jacob Plange Rhule, Terrence Forrester and Pascal Bovet were all directly involved in the design of the main study and all authors including Chris Sempos and Michal Melamed provided scientific input for the manuscript and revisions. Dominique Kosk, Ramon Durazo-Arvizu, and Holly Kramer conducted the analysis. All authors contributed and wrote the paper. Dominique Kosk, Holly Kramer and Ramon Durazo-Arvizu are responsible for the final content.
References
- 1.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. Journal of Clinical Endocrinology & Metabolism. 2005;90:1519–24. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 2.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. Journal of Clinical Endocrinology & Metabolism. 2006;91:3144–9. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 3.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. Journal of Bone & Mineral Research. 2006;21:1187–96. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 4.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukomoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Min Res. 2003;19:429. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 5.Yuen SN, Kramer H, Luke A, Bovet P, Plange-Rhule J, Forrester T, Wolf M, Camacho P, Harders R, Dugas L, Cooper R, Durazo-Arvizu R. Fibroblast growth factor (FGF-23) levels differ across populations by degree of industrialization. J Clin Endocrinology and Metabolism. doi: 10.1210/jc.2015-3558. Epub 2016 Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eckberg K, Kramer H, Wolf M, Durazo-Arvizu R, Tayo B, Luke A, Cooper R. Impact of westernization on fibroblast growth factor 23 levels among individuals of African ancestry. Nephrology Dialysis Transplantation. 2015;30:630–5. doi: 10.1093/ndt/gfu342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torre M, Rodriguez AR, Saura-Calixto F. Effects of dietary fiber and phytic acid on mineral availability. Crit. Rev. Food Sci. Nutr. 1991;30:1–22. doi: 10.1080/10408399109527545. [doi] [DOI] [PubMed] [Google Scholar]
- 8.di Giuseppe R, Kuhn T, Hirche F, Buijsse B, Dierkes J, Fritsche A, Kaaks R, Boeing H, Stangl GI, Weikert C. Potential Predictors of Plasma Fibroblast Growth Factor 23 Concentrations: Cross-Sectional Analysis in the EPIC-Germany Study. PLoS ONE [Electronic Resource] 2015;10:e0133580. doi: 10.1371/journal.pone.0133580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baia LC, Van den Berg E, Vervloet MG, Heilberg IP, Navis G, Bakker SJ, Geleijnse JM, Kromhout D, Soedamah-Muthu SS, De Borst MH NIGRAM consortium. Fish and omega-3 fatty acid intake in relation to circulating fibroblast growth factor 23 levels in renal transplant recipients. Nutr. Metab. Cardiovasc. Dis. 2014;24:1310–6. doi: 10.1016/j.numecd.2014.06.006. [doi] [DOI] [PubMed] [Google Scholar]
- 10.Vervloet MG, van Ittersum FJ, Buttler RM, Heijboer AC, Blankenstein MA, ter Wee PM. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clinical Journal of The American Society of Nephrology: CJASN. 2011;6:383–9. doi: 10.2215/CJN.04730510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyamura M, Fujita S, Morita H, Sakane K, Okamoto Y, Sohmiya K, Hoshiga M, Ishizaka N. Circulating Fibroblast Growth Factor 23 Has a U-Shaped Association With Atrial Fibrillation Prevalence. Circulation Journal. 2015;79:1742–8. doi: 10.1253/circj.CJ-15-0413. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez OM, Wolf M, Taylor EN. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the Health Professionals Follow-up Study. Clinical Journal of The American Society of Nephrology: CJASN. 2011;6:2871–8. doi: 10.2215/CJN.02740311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright CB, Dong C, Stark M, Silverberg S, Rundek T, Elkind MS, Sacco RL, Mendez A, Wolf M. Plasma FGF23 and the risk of stroke: the Northern Manhattan Study (NOMAS) Neurology. 2014;82:1700–6. doi: 10.1212/WNL.0000000000000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seiler S, Cremers B, Rebling NM, Hornof F, Jeken J, Kersting S, Steimle C, Ege P, Fehrenz M, Rogacev KS, Scheller B, Bohm M, Fliser D, Heine GH. The phosphatonin fibroblast growth factor 23 links calcium-phosphate metabolism with left-ventricular dysfunction and atrial fibrillation. Eur. Heart J. 2011;32:2688–96. doi: 10.1093/eurheartj/ehr215. [DOI] [PubMed] [Google Scholar]
- 15.Lane NE, Parimi N, Corr M, Yao W, Cauley JA, Nielson CM, Ix JH, Kado D, Orwoll E Osteoporotic Fractures in Men (MrOS) Study Group. Association of serum fibroblast growth factor 23 (FGF23) and incident fractures in older men: the Osteoporotic Fractures in Men (MrOS) study. J. Bone Miner. Res. 2013;28:2325–32. doi: 10.1002/jbmr.1985. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda E, Yoshida M, Sasaki S. Applicability of fibroblast growth factor 23 for evaluation of risk of vertebral fracture and chronic kidney disease-mineral bone disease in elderly chronic kidney disease patients. BMC Nephrol. 2012;13:122. doi: 10.1186/1471-2369-13-122. 2369-13-122. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper R, Forrester T, Ogunbiyi O, Muffinda J. Angiotensinogen levels and obesity in four black populations. ICSHIB Investigators. J. Hypertens. 1998;16:571–5. doi: 10.1097/00004872-199816050-00003. [DOI] [PubMed] [Google Scholar]
- 18.Braithwaite V, Jarjou LM, Goldberg GR, Prentice A. Iron status and fibroblast growth factor-23 in Gambian children. Bone. 2012;50:1351–6. doi: 10.1016/j.bone.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steyn NP, Nel JH, Parker WA, Ayah R, Mbithe D. Dietary, social, and environmental determinants of obesity in Kenyan women. Scand. J. Public Health. 2011;39:88–97. doi: 10.1177/1403494810384426. [doi] [DOI] [PubMed] [Google Scholar]
- 20.Orcholski L, Luke A, Plange-Rhule J, Bovet P, Forrester TE, Lambert EV, Dugas LR, Kettmann E, Durazo-Arvizu RA, Cooper RS, Schoeller DA. Under-reporting of dietary energy intake in five populations of the African diaspora. Br. J. Nutr. 2015;113:464–72. doi: 10.1017/S000711451400405X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 22.Curhan GC, Willet WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women. Archives of Internal Medicine. 2004;164:885–891. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 23.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS CKD-EPI I. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012;367:20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tukey J. On the comparative anatomy of transformations. Annals of Mathematical Sciences. 1957;28:602–632. [Google Scholar]
- 25.Walling MW. Intestinal Ca and phosphate transport: differential responses to vitamin D3 metabolites. Am. J. Physiol. 1977;233:E488–94. doi: 10.1152/ajpendo.1977.233.6.E488. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan CM, Leon JB, Sehgal AR. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. Journal of Renal Nutrition. 2007;17:350–4. doi: 10.1053/j.jrn.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scialla JJ, Appel LJ, Wolf M, Yang W, Zhang X, Sozio SM, Miller ER, 3rd, Bazzano LA, Cuevas M, Glenn MJ, Lustigova E, Kallem RR, Porter AC, Townsend RR, Weir MR, Anderson CA, Chronic Renal Insufficiency Cohort-CRIC Study Group Plant protein intake is associated with fibroblast growth factor 23 and serum bicarbonate levels in patients with chronic kidney disease: the Chronic Renal Insufficiency Cohort study. Journal of Renal Nutrition. 2012;22:379–388.e1. doi: 10.1053/j.jrn.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clinical Journal of The American Society of Nephrology: CJASN. 2011;6:257–64. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moorthi RN, Armstrong CL, Janda K, Ponsler-Sipes K, Asplin JR, Moe SM. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am. J. Nephrol. 2014;40:582–91. doi: 10.1159/000371498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DJ, Holowaty EJ. Brief, validated survey instruments for the measurement of fruit and vegetable intakes in adults: a review. Prev. Med. 2003;36:440–7. doi: 10.1016/s0091-7435(02)00040-3. doi: S0091743502000403 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Iqbal TH, Lewis KO, Cooper BT. Phytase activity in the human and rat small intestine. Gut. 1994;35:1233–6. doi: 10.1136/gut.35.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalantar-Zadeh K, Gutekunst L, Mehrotra R, Kovesdy CP, Bross R, Shinaberger CS, Noori N, Hirschberg R, Benner D, Nissenson AR, Kopple JD. Understanding sources of dietary phosphorus in the treatment of patients with chronic kidney disease. Clinical Journal of The American Society of Nephrology: CJASN. 2010;5:519–30. doi: 10.2215/CJN.06080809. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi K, Imanishi Y, Miyauchi A, Onoda N, Kawata T, Tahara H, Goto H, Miki T, Ishimura E, Sugimoto T, Ishikawa T, Inaba M, Nishizawa Y. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. European Journal of Endocrinology. 2006;154:93–9. doi: 10.1530/eje.1.02053. [DOI] [PubMed] [Google Scholar]
- 34.Wesseling-Perry K, Wang H, Elashoff R, Gales B, Juppner H, Salusky IB. Lack of FGF23 response to acute changes in serum calcium and PTH in humans. Journal of Clinical Endocrinology & Metabolism. 2014;99:E1951–6. doi: 10.1210/jc.2014-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isakova T, Ix JH, Sprague SM, Raphael KL, Fried L, Gassman JJ, Raj D, Cheung AK, Kusek JW, Flessner MF, Wolf M, Block GA. Rationale and Approaches to Phosphate and Fibroblast Growth Factor 23 Reduction in CKD. Journal of the American Society of Nephrology. 2015;26:2328–39. doi: 10.1681/ASN.2015020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM. Effects of phosphate binders in moderate CKD. Journal of the American Society of Nephrology. 2012;23:1407–15. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khouzam NM, Wesseling-Perry K, Salusky IB. The role of bone in CKD-mediated mineral and vascular disease. Pediatric Nephrology. 2015;30:1379–88. doi: 10.1007/s00467-014-2919-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez-Ortiz ME, Lopez I, Munoz-Castaneda JR, Martinez-Moreno JM, Ramirez AP, Pineda C, Canalejo A, Jaeger P, Aguilera-Tejero E, Rodriguez M, Felsenfeld A, Almaden Y. Calcium deficiency reduces circulating levels of FGF23. Journal of the American Society of Nephrology. 2012;23:1190–7. doi: 10.1681/ASN.2011101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginn HE, Shanbour LL. Phosphaturia in magnesium-deficient rats. Am. J. Physiol. 1967;212:1347–50. doi: 10.1152/ajplegacy.1967.212.6.1347. [DOI] [PubMed] [Google Scholar]
- 40.Massry SG, Coburn JW, Kleeman CR. Evidence for suppression of parathyroid gland activity by hypermagnesemia. J. Clin. Invest. 1970;49:1619–29. doi: 10.1172/JCI106379. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortega B, MacWilliams JR, Dey JM, Courtright VB. Hyperphosphatemia, hypocalcemia and increased serum potassium concentration as distinctive features of early hypomagnesemia in magnesium-deprived mice. Magnes. Res. 2015;28:126–35. doi: 10.1684/mrh.2015.0394. [doi] [DOI] [PubMed] [Google Scholar]
- 42.Matsuzaki H, Kajita Y, Miwa M. Magnesium deficiency increases serum fibroblast growth factor-23 levels in rats. Magnes. Res. 2013;26:18–23. doi: 10.1684/mrh.2013.0331. [doi] [DOI] [PubMed] [Google Scholar]
- 43.Thumfart J, Jung S, Amasheh S, Kramer S, Peters H, Sommer K, Biber J, Murer H, Meij I, Querfeld U, Wagner CA, Muller D. Magnesium stimulates renal phosphate reabsorption. Am. J. Physiol. Renal Physiol. 2008;295:F1126–33. doi: 10.1152/ajprenal.00353.2007. [doi] [DOI] [PubMed] [Google Scholar]
- 44.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, Zumbrennen-Bullough KB, Sun CC, Lin HY, Babitt JL, Wolf M. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2015 doi: 10.1038/ki.2015.290. [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf M. Forging forward with 10 burning questions on FGF23 in kidney disease. Journal of the American Society of Nephrology. 2010;21:1427–35. doi: 10.1681/ASN.2009121293. [DOI] [PubMed] [Google Scholar]