Abstract
Diacylglycerol kinases (DGKs) regulate the balance between diacylglycerol (DAG) and phosphatidic acid. DGKζ is highly abundant in skeletal muscle and induces fiber hypertrophy. We hypothesized that DGKζ influences functional and metabolic adaptations in skeletal muscle and whole-body fuel utilization. DAG content was increased in skeletal muscle and adipose tissue, but unaltered in liver of DGKζ KO mice. Linear growth, body weight, fat mass, and lean mass were reduced in DGKζ KO versus wild-type mice. Conversely, male DGKζ KO and wild-type mice displayed a similar robust increase in plantaris weight after functional overload, suggesting that DGKζ is dispensable for muscle hypertrophy. Although glucose tolerance was similar, insulin levels were reduced in high-fat diet (HFD)-fed DGKζ KO versus wild-type mice. Submaximal insulin-stimulated glucose transport and p-Akt Ser473 were increased, suggesting enhanced skeletal muscle insulin sensitivity. Energy homeostasis was altered in DGKζ KO mice, as evidenced by an elevated respiratory exchange ratio, independent of altered physical activity or food intake. In conclusion, DGKζ deficiency increases tissue DAG content and leads to modest growth retardation, reduced adiposity, and protection against insulin resistance. DGKζ plays a role in the control of growth and metabolic processes, further highlighting specialized functions of DGK isoforms in type 2 diabetes pathophysiology.
Keywords: diacylglycerol kinase ζ, diabetes, diacylglycerol, diet, dietary lipids, lipid kinases, muscle, obesity
Diacylglycerol (DAG) is a precursor for triglyceride biosynthesis that functions as a second messenger with important signaling roles (1). The intracellular concentration and subcellular localization of DAG influences growth, development, and metabolism, with levels tightly controlled by the balance between the rates of synthesis and degradation (1). DAG promotes signal transduction through activation of conventional and novel protein kinase C (PKC) isoforms (2). Enzymes controlling intracellular DAG levels include lipid phosphate phosphatases, phospholipase C, phospholipase D, and DAG kinases (DGKs). DGKs terminate DAG signaling by phosphorylating DAG to produce phosphatidic acid (PA), which also acts as a second messenger. Thus, DGKs play a central lipid metabolizing role in regulating the balance between DAG and PA, thereby modulating the spatial and functional segregation of these lipid species, as well as regulating the concentration of these lipid second messengers at specific intracellular sites, such as the plasma membrane, endoplasmic reticulum, Golgi apparatus, and nuclei (3, 4). Ten mammalian DGK isozymes (α, β, γ, δ, ε, ζ, η, θ, ι, and κ) have been identified and classified into five subgroups based on primary structure (5). DGK isoforms have distinct biological functions depending upon the cellular location and/or interacting proteins (4). Understanding the control of DAG synthesis and degradation through specific DGK isoforms may provide insight into diseases as diverse as cancer, neurodegenerative or immunological disorders, and diabetes (6, 7).
The role of specific DGK isoforms in metabolic disease is emerging. Type I DGK isoforms, DGKα and DGKγ, play an essential role in insulin secretion (8), which may be dependent on calcium-binding EF hand motifs (4); thus, the role of these isoenzymes in insulin secretion may be related to glucose-induced calcium signaling. DGKδ, a type II DGK isoform, is implicated in the development of peripheral insulin resistance and obesity, with reduced expression and total DGK activity in skeletal muscle from type 2 diabetic patients and animal models of the disease (9). Type II DGK isoenzymes (δ, η, and κ) are characterized by a pleckstrin homology domain at the N terminus that may be important for subcellular localization (4). Elevated levels of glucose (9) or monounsaturated free fatty acids (10) reduce DGKδ abundance and total DGK activity, implicating that systemic factors associated with type 2 diabetes attenuate DGKδ signaling and localization. Overexpression of DGKδ in myotubes enhances glucose uptake (11), whereas DGKδ haploinsufficiency in mice leads to peripheral insulin resistance, metabolic inflexibility, and age-dependent obesity, concomitant with DAG-induced PKC activity and attenuation of insulin signaling (9). The type III isoenzyme, DGKε, contains a hydrophobic segment to confer membrane localization (4). DGKε influences skeletal muscle levels of unsaturated and saturated DAG species and alters glucose tolerance and whole-body lipid oxidation (12). Thus, abundance, localization, and substrate specificity of each DGK isoform, as well as the balance between DAG and PA species, may influence cellular processes as diverse as insulin secretion, glucose metabolism, and energy homeostasis.
The role of type IV (DGKζ and DGKι) and type V (DGKθ) DGKs in metabolic disease is uncharted. DGKζ and DGKι have a myristoylated alanine-rich C kinase substrate (MARCKS) homology phosphorylation site domain that functions as a localization signal, as well as four ankyrin repeats and a carboxy terminal PDZ binding domain (4). DGKζ is abundantly expressed in brain, skeletal muscle, heart, and pancreas (13). Skeletal and cardiac tissue-specific DGKζ variants are predominantly localized in the nucleus (14, 15), consistent with a role for this isoenzyme in myogenic differentiation (15) and cardiac and skeletal muscle hypertrophy (16, 17). While DGKζ has been proposed as a potential therapeutic target to block cardiac dysfunction and prevent congestive heart failure (18), the role of this isoenzyme in insulin resistance and type 2 diabetes is unknown. The aim of this study was to determine the role of DGKζ in functional and metabolic adaptions in skeletal muscle and whole-body glucose and energy homeostasis. As overexpression of DGKζ induces skeletal muscle fiber hypertrophy (16), we hypothesized that ablation of DGKζ may influence functional and metabolic properties of skeletal muscle.
EXPERIMENTAL PROCEDURES
Animals
DGKζ KO mice were generated on a mixed C57BL/6×129X1/SvJ background, as described earlier (19) and kindly provided by Dr. Matthew K. Topham (University of Utah, Salt Lake City, UT). We studied DGKζ KO mice on an inbred strain (C57BL/6) generated by successive backcross breeding. The expression of DGKα, -δ, -ε, and -ι, DGK isoforms known to be expressed in skeletal muscle and adipose tissue (20), was unaltered between DGKζ KO and wild-type littermates (data not shown). Thus, other DGK isoforms do not appear to compensate for the loss of DGKζ in this model. Age- and sex-matched wild-type littermates were used as controls. Animals were maintained in a temperature controlled facility on a 12/12 light/dark cycle with free access to food and water. Mice were fed normal chow or high-fat diet (HFD; 54.8% energy from fat) from 5 weeks of age for 12 weeks, as described (12). Body weight was measured weekly. For terminal experiments, animals were anesthetized and liver, gonadal fat, and skeletal muscle were collected for biochemical assays, as described below. Several types of skeletal muscle were sampled. In mouse models, extensor digitorum longus (EDL) and gastrocnemius are fast twitch muscles, predominantly composed of type IIB and IIDB fibers, whereas soleus is a slow twitch muscle, predominantly composed of type IIA fibers (21). We used EDL and gastrocnemius interchangeably and soleus, as these muscle types provide a broad representation of the muscles of the whole body in the mouse. For assays requiring large amounts of tissue, we selected gastrocnemius over EDL. The regional animal ethical committee (Stockholm, Sweden) approved all experimental procedures.
Glucose tolerance test
Glucose (2 g/kg body weight) was administered by intraperitoneal injection to 4 h-fasted (15 weeks of age) chow- or HFD-fed mice. Blood was sampled via the tail vein to assess glucose (One Touch Ultra glucose meter; Lifescan, Milpitas, CA) and insulin (Insulin ELISA kit; Crystal Chem Inc., Downers Grove, IL) concentration.
Body composition
Lean mass and fat mass were assessed in conscious mice (14 weeks of age) using the EchoMRI-100 system (Echo Medical Systems, Houston, TX).
Whole-body energy homeostasis
Food intake, oxygen consumption (VO2), carbon dioxide production (VCO2), respiratory exchange ratio (RER), and locomotor activity were measured using a Comprehensive Lab Animal Monitoring System (Columbus Instruments, Columbus, OH) as described (9).
Ex vivo lipolysis in gonadal adipose tissue
Mice were fasted for 4 h, anesthetized (Avertin, 2,2,2-tribromo ethanol 99% and tertiary amyl alcohol, at 15–17 μl/g body weight, ip), and gonadal adipose tissue was collected. Tissue (∼20 mg) was incubated in the absence or presence of isoprenaline (10−6 M) for 90 min at 37°C in D-PBS supplemented with 2% RIA-grade BSA. The tissue was removed and the glycerol concentration in the medium was determined. Glycerol release into the medium was analyzed as a marker of lipolysis after stimulation with isoprenaline. Glycerol was measured using a Zenbio glycerol analysis kit (Research Triangle Park, NC).
Skeletal muscle glucose transport assay
Incubation medium was prepared from Krebs-Henseleit bicarbonate buffer containing 5 mmol/l HEPES and 0.1% BSA (RIA grade). Mice (17 weeks of age) were fasted for 4 h and subsequently anesthetized (Avertin, 2,2,2-tribromo ethanol 99% and tertiary amyl alcohol at 15–17 μl/g body weight, ip). EDL was incubated in the absence or presence of insulin (0.36 nmol/l, Actrapid; Novo Nordisk, Bagsværd, Denmark) to assess 2-deoxy-glucose uptake as described (22).
Euglycemic-hyperinsulinemic clamp
Glucose turnover rate was measured in conscious HFD-fed mice (17 weeks of age) under basal and euglycemic-hyperinsulinemic conditions, as described (9). Hepatic glucose production was determined by subtracting the average glucose infusion rate at the steady state from the glucose utilization.
Induction of skeletal muscle hypertrophy
Functional overload was performed by surgical bilateral removal of soleus and gastrocnemius muscles, as described (23, 24). Sham-operated mice in which the plantaris, soleus, and gastrocnemius muscles were separated from each other with a forceps were used as a control. Fourteen days after the surgery, fed mice were anesthetized with Avertin (0.02 ml/g; 2.5% solution of 99% 2,2,2-tribromo ethanol and tertiary amyl alcohol) and plantaris muscles were weighed and stored at −80°C until further processing. Plantaris muscle was studied because this particular muscle undergoes marked hypertrophy after surgical removal of the gastrocnemius and soleus muscles from the mouse leg.
DAG content
Total tissue DAG content was determined using a mouse DAG ELISA kit (Cusabio, Wuhan, China).
Immunoblot analysis
Tissues were homogenized and processed for Western blot analysis, as described (9). Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse IgG was obtained from Bio-Rad Laboratories (Hercules, CA). Rabbit polyclonal antibodies to Akt (#9272), adipose triglyceride lipase (ATGL) (#2138), hormone-sensitive lipase (HSL) (#4107), phospho-HSL-Ser660 (#4137), phospho-acetyl-CoA carboxylase (ACC)-Ser79 (#3661), phospho-Akt-Ser473 (#9271), and phospho-Akt-Thr308 (#4056) were from Cell Signaling Technology, Inc. (Beverly, MA). DAG O-acyltransferase (DGAT)1 (#ab54037) and DGAT2 (#ab102831) were from Abcam (Cambridge, UK). FASN (#ST1549) and comparative gene identification-58 (CGI58) (#ABS1616) were from Merck Millipore (Darmstadt, Germany). Myogenin antibody (#sc-52903) was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Proteins were visualized using enhanced chemiluminescence (GE Healthcare Europe GmbH, Germany) and quantified by densitometry.
Statistical analysis
Statistical analysis was performed using Student’s t-test, two-way ANOVA, or repeated measures two-way ANOVA. Post hoc analysis with Sidak’s testing was performed when a main effect was detected. All data are reported as mean ± SEM. Differences between groups were considered statistically significant at P < 0.05.
RESULTS
DGKζ alters DAG content in skeletal muscle and adipose tissue, but not liver
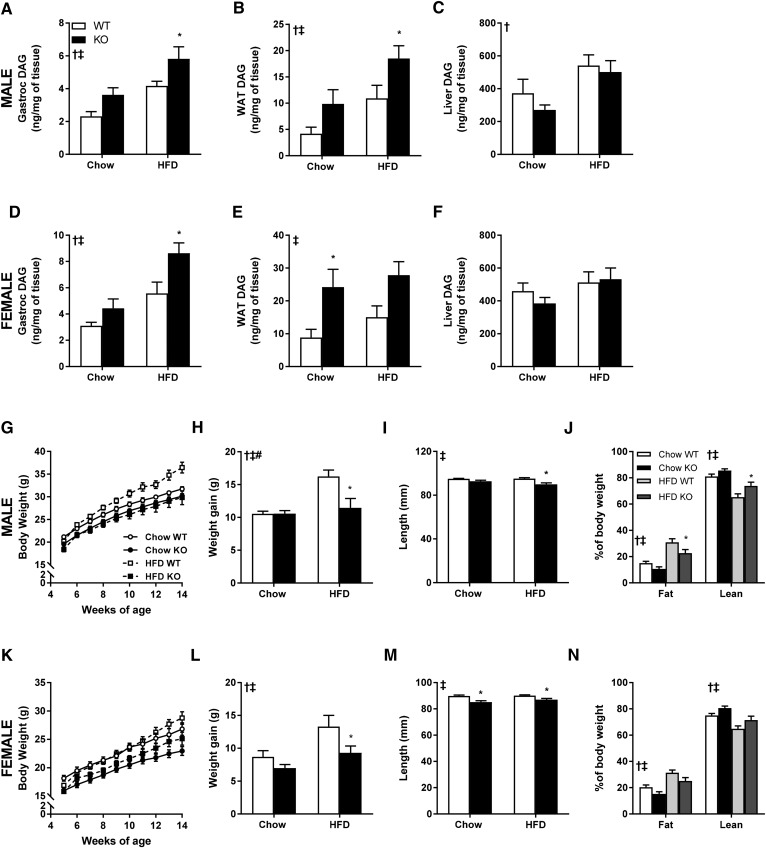
Total DAG content was determined in gastrocnemius muscle, gonadal adipose tissue, and liver from male and female DGKζ KO and wild-type mice fed either chow or HFD (Fig. 1A–F). DAG content was increased in skeletal muscle (Fig. 1A, D) and gonadal adipose tissue (Fig. 1B, E), but unaltered in liver (Fig. 1C, F), from male and female DGKζ KO mice irrespective of diet. The tissue-specific changes in DAG content in DGKζ KO versus wild-type mice correspond with the mRNA profile of DGKζ (13, 14, 25), with high levels in skeletal muscle, modest levels in gonadal adipose tissue, and low levels in liver.
Fig. 1.
Tissue-specific DAG content and body composition of DGKζ KO and wild-type mice. DAG content in gastrocnemius muscle (A, D), gonadal white adipose tissue (WAT) (B, E), and liver (C, F) from male (A–C) and female (D–F) DGKζ KO and wild-type mice kept on chow or HFD (n = 8–10 mice). Body weight growth curves (G, K), body weight gain (H, L), snout to anus length (I, M), and MRI-measured body composition (J, N) at age 16 weeks after 11 weeks on HFD from male (G–J) and female (K–N) DGKζ KO and wild-type mice (n = 6–15 mice). Two-way ANOVA or repeated measures two-way ANOVA with Sidak’s post hoc testing. ‡P < 0.05 overall genotype difference, †P < 0.05 overall diet effect, #P < 0.05 interaction; *P < 0.05 versus wild-type mice on same diet.
DGKζ influences body weight and growth curves
Body weight of chow- or HFD-fed male and female DGKζ KO and wild-type mice was determined at 5 weeks of age and measured once a week for 10 weeks (Fig. 1G, K). Irrespective of diet, body weight of DGKζ KO mice was reduced compared with age- and sex-matched wild-type mice. Over this 10 week period, DGKζ KO mice gained less weight on HFD as compared with wild-type mice, irrespective of sex (Fig. 1H, L). Differences in body weight were more pronounced in female DGKζ KO mice compared with male DGKζ KO mice, particularly under chow-fed conditions. Linear growth (snout to anus length) was reduced in male and female DGKζ KO mice (Fig. 1I, M). Body composition analysis was performed at 14 weeks of age. Fat mass was reduced in both male and female DGKζ KO mice irrespective of diet (Fig. 1J, N). Lean body mass was reduced in chow-fed female DGKζ KO mice, but not chow-fed male DGKζ KO mice (Fig. 1J, N). Thus, DGKζ deficiency is associated with growth retardation, a moderate reduction in lean mass, and a profound reduction in adiposity, even in HFD-challenged mice.
HSL protein abundance and phosphorylation in white adipose tissue
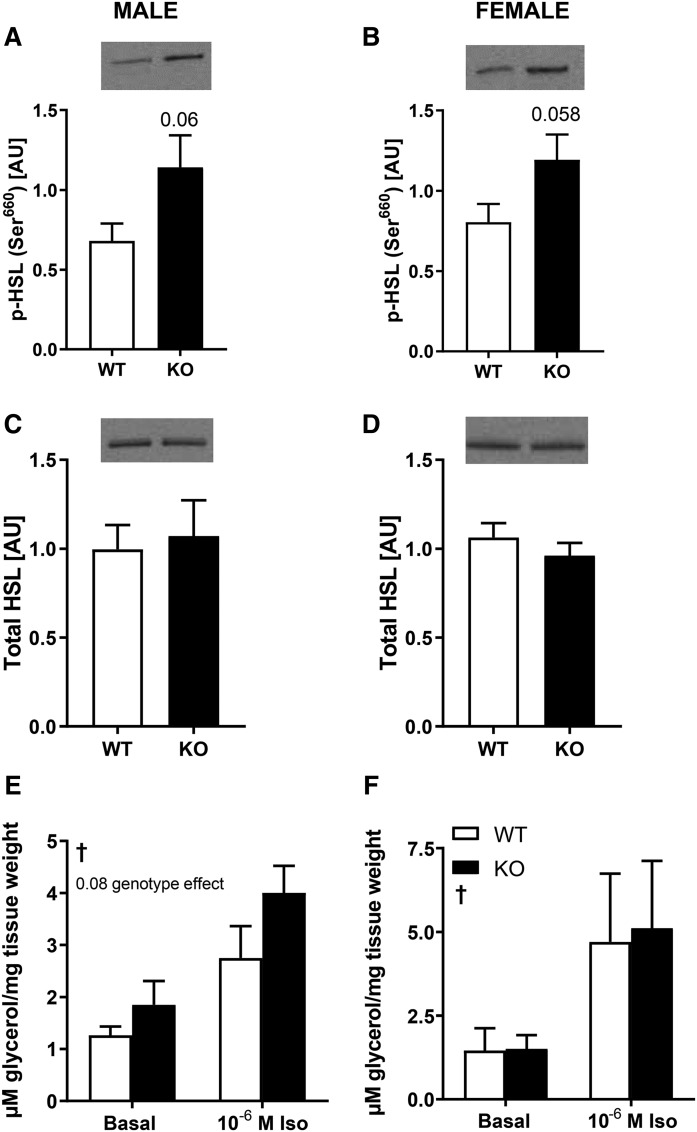
To gain insight into the mechanism for the reduction in adiposity in DGKζ KO mice, abundance and phosphorylation of proteins controlling lipolysis were assessed in adipose tissue from 4 h-fasted HFD-fed male and female DGKζ KO and wild-type mice. HSL is a key enzyme involved in the regulation of lipolysis in white adipose tissue. Phosphorylation of HSL on Ser660, a major PKA phosphorylation site, tended to increase in white gonadal adipose tissue from DGKζ KO mice (Fig. 2A, B), whereas protein abundance of HSL was unaltered (Fig. 2C, D). Ex vivo lipolysis was assessed in gonadal adipose tissue (Fig. 2E, F). A trend for increased basal and isoprenaline-stimulated lipolysis was observed in male, but not female, DGKζ KO mice. These findings indirectly suggest a modest increase in lipolytic activity. Protein abundance of ATGL, a lipid droplet-bound coactivator (CGI58), and enzymes involved in triglyceride synthesis (DGAT1 and DGAT2) were unaltered in gonadal white adipose tissue from DGKζ KO mice (data not shown). Protein abundance of ACC and FASN, two key enzymes involved in lipogenesis, as well as ACC Ser79 phosphorylation, was unaltered in gonadal adipose tissue from DGKζ KO mice (data not shown).
Fig. 2.
HSL and lipolysis in adipose tissue from DGKζ KO and wild-type mice. Western blot analysis of abundance of p-HSL Ser660 and total HSL protein in gonadal adipose tissue from HFD male (A, C) and female (B, D) DGKζ KO and wild-type mice (n = 7–12 mice). Student’s t-test. Gonadal adipose tissue was obtained from 17-week-old 4 h-fasted DGKζ KO and wild-type mice after 12 weeks on HFD and incubated ex vivo in the absence (basal) or presence of isoprenaline (10−6 M) for measurement of glycerol release into the medium in male (E) and female (F) mice (n = 5–9 mice). Two-way ANOVA with Sidak’s post hoc testing. †P < 0.05 overall isoprenaline effect.
Role of DGKζ in skeletal muscle growth and hypertrophy
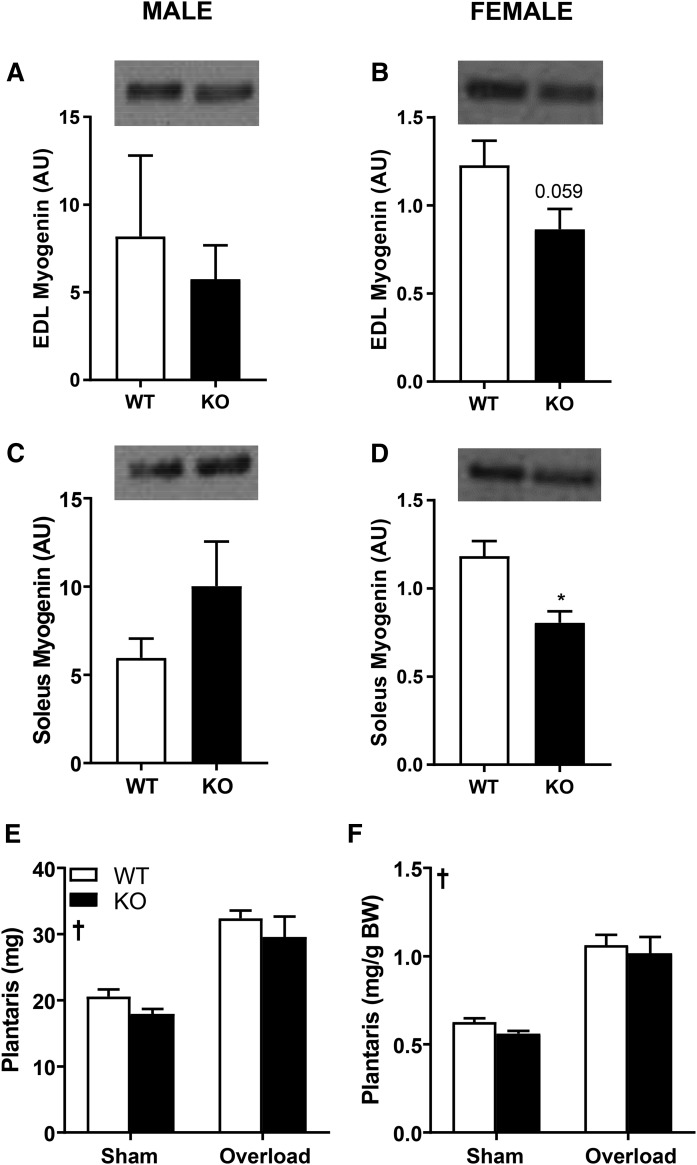
As body weight and lean body mass were reduced in DGKζ KO mice, we hypothesized that DGKζ may play a critical role in skeletal muscle growth and hypertrophy. Thus, we measured protein abundance of myogenin in skeletal muscle, given the requirement of this transcription factor in myocyte fusion and myotube formation. Interestingly, myogenin KO mice have reduced body size, implicating a role for this myogenic regulatory factor in regulating body homeostasis (26). We found myogenin abundance was reduced in EDL and soleus muscle from chow-fed female DGKζ KO mice, but not chow-fed male DGKζ KO mice (Fig. 3A–D). To investigate the role of DGKζ in the dynamic control of skeletal muscle mass, male DGKζ KO and wild-type mice were subjected to functional overload to induce muscle hypertrophy. DGKζ KO and wild-type mice displayed a robust increase in absolute and relative plantaris weight compared with respective control sham-operated mice (Fig. 3E, F). Plantaris muscle weight was unaltered between DGKζ KO and wild-type mice after functional overload. Our results suggest that DGKζ is dispensable for skeletal muscle hypertrophy following a 14-day functional overload. Plantaris muscles of control sham-operated DGKζ KO mice were slightly lighter as compared with wild-type control mice. This difference was not preserved following functional overload, indicating that plasticity and the hypertrophic response of skeletal muscle is retained in DGKζ KO mice.
Fig. 3.
Myogenin abundance and effects of functional overload on plantaris muscle weight. Western blot analysis of myogenin abundance in EDL (A, B) and soleus (C, D) muscle in male (A, C) and female (B, D) DGKζ KO and wild-type mice on chow diet (n = 5–13 mice). Student’s t-test, *P < 0.05 versus wild-type mice. Male DGKζ KO and wild-type mice had 14 days of functional overload on chow diet. Absolute (E) and relative to body weight (F) plantaris muscle weight (n = 6 mice). Two-way ANOVA with Sidak’s post hoc testing. †P < 0.05 surgery effect.
Role of DGKζ in glucose homeostasis
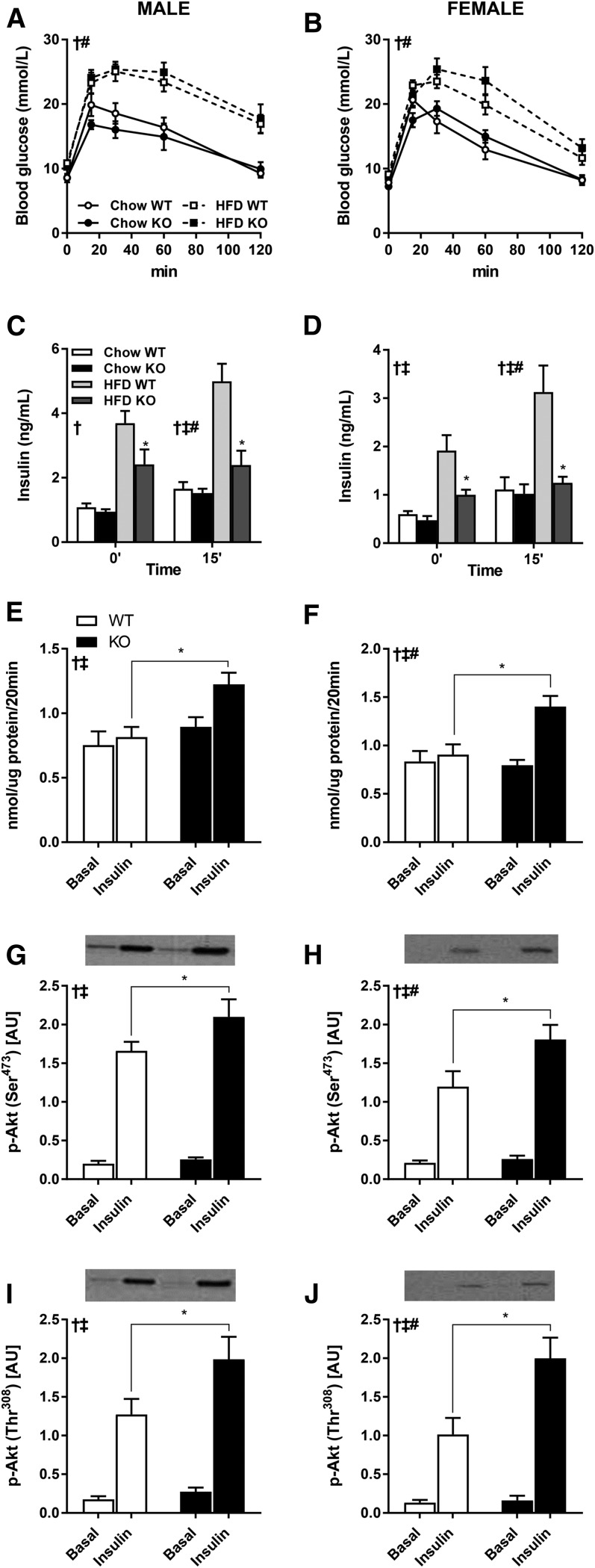
Elevated DAG content in skeletal muscle, adipose tissue, and liver has been implicated in development of insulin resistance (27). Thus, we determined the role of DGKζ in the control of glucose homeostasis in male and female chow-fed mice. We found that fasting glucose levels and glucose tolerance curves were similar between DGKζ KO and wild-type mice irrespective of sex (Fig. 4A, B). Insulin levels measured in the fasting state and 15 min into the glucose tolerance test were also similar between DGKζ KO and wild-type mice irrespective of sex (Fig. 4C, D). Thus, DGKζ is dispensable for normal glucose homeostasis in chow-fed mice, despite the elevation of total DAG content in skeletal muscle and gonadal adipose tissue. We next determined the role of DGKζ in the control of glucose tolerance in HFD-fed male and female mice. Similar to our findings in chow-fed mice, glucose tolerance was unaltered between HFD-fed DGKζ KO and wild-type mice irrespective of sex (Fig. 4A, B). However, insulin levels measured in the fasting state and 15 min into the glucose tolerance test were reduced in male and female HFD-fed DGKζ KO versus wild-type mice (Fig. 4C, D), suggesting that insulin sensitivity is enhanced.
Fig. 4.
Glucose tolerance and skeletal muscle glucose uptake and signal transduction. Intraperitoneal glucose tolerance tests were performed in 4 h-fasted 15-week-old DGKζ KO and wild-type mice, after 10 weeks on HFD (n = 7–16). Plasma glucose (A, B) and insulin (C, D) concentration in male (A, C) and female (B, D) mice were assessed during the intraperitoneal glucose tolerance tests. EDL muscle was obtained from 17-week-old 4 h-fasted DGKζ KO and wild-type mice after 12 weeks on HFD and incubated ex vivo in the absence (basal) or presence of insulin (0.36 nmol/l) for measurement of glucose transport (E, F) and Akt Ser473 and Thr308 phosphorylation (G–J) in male (E, G, I) and female (F, H, J) mice (n = 9–13 mice). Two-way ANOVA or repeated measures two-way ANOVA with Sidak’s post hoc testing. C, D: ‡P < 0.05 overall genotype difference, †P < 0.05 overall diet effect, #P < 0.05 interaction; *P < 0.05 versus wild-type mice on same diet. E–J: ‡P < 0.05 overall genotype difference, †P < 0.05 overall insulin effect, #P < 0.05 interaction; *P < 0.05 versus wild-type mice of same condition.
To test the hypothesis that DGKζ deficiency alters insulin sensitivity, we measured insulin-stimulated glucose transport and signal transduction in isolated skeletal muscle from HFD-fed DGKζ KO and wild-type mice. EDL muscle was incubated ex vivo in the presence or absence of a sub-maximal dose of insulin (0.36 nmol/l) and glucose transport was assessed. Basal glucose transport was similar between HFD-fed DGKζ KO and wild-type mice, irrespective of sex (Fig. 4E, F). Insulin-stimulated glucose uptake was impaired in EDL muscle from HFD-fed male and female wild-type mice, consistent with our earlier finding that HFD impairs insulin sensitivity of glucose transport in skeletal muscle (28). In contrast, insulin-stimulated glucose transport was enhanced in EDL muscle from HFD-fed male and female DGKζ KO mice (Fig. 4E, F). Consistent with our results for glucose transport, insulin-stimulated phosphorylation of Akt on Ser473 (Fig. 4G, H) and Thr308 (Fig. 4I, J) was enhanced in EDL muscle from HFD-fed male and female DGKζ KO mice. Collectively, our results suggest that DGKζ deficiency protects against peripheral insulin resistance in HFD-fed mice, despite elevated DAG content in peripheral tissues.
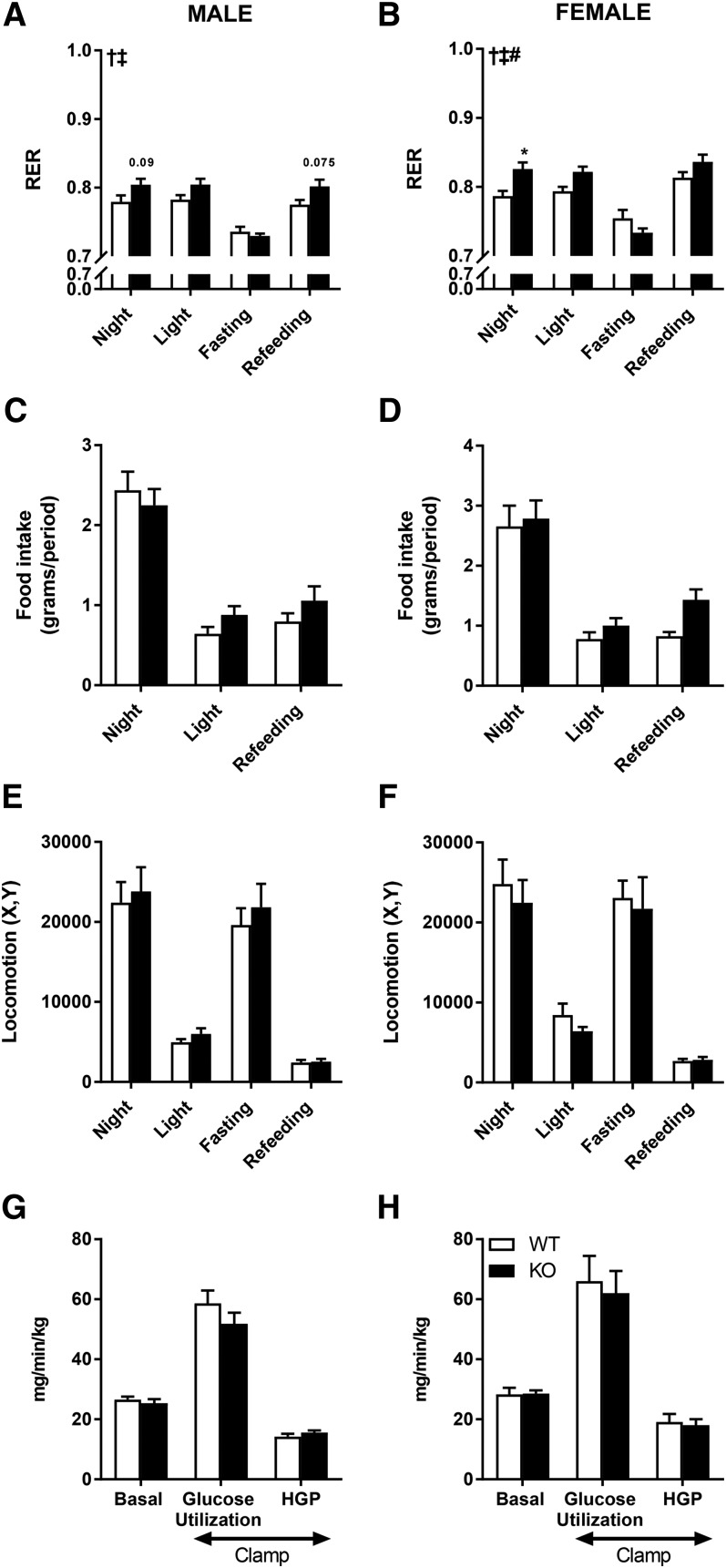
DGKζ drives whole body carbohydrate metabolism
We next measured several metabolic parameters in vivo in male and female HFD-fed mice over 3 days, including food consumption, locomotor activity, VO2, VCO2, and RER. RER was elevated in DGKζ KO mice compared to wild-type mice during the light and dark phases, but not during a 12 h fasting period. VO2 was higher in DGKζ KO mice (data not shown). These data suggest that energy expenditure is upregulated in DGKζ KO mice, with carbohydrates serving as a preferred energy substrate during rest and active phases, respectively (Fig. 5A, B). Food intake [recorded as absolute amount (Fig. 5C, D) or as a percentage of total body weight (data not shown)] and locomotor activity (Fig. 5E, F) were unaltered between HFD-fed DGKζ KO and wild-type mice, irrespective of sex. Basal and insulin-mediated peripheral glucose utilization and hepatic glucose production in conscious mice assessed in vivo during a euglycemic-hyperinsulinemic clamp were similar between genotypes (Fig. 5G, H). We also found that tissue weight, as well as mRNA expression of UCP1 and PGC1α, was unaltered in brown adipose tissue of wild-type versus DGKζ KO mice (data not shown). These results suggest that the elevated energy expenditure in DGKζ KO mice throughout the light and dark phases is independent of changes in physical activity, food intake, and thermogenesis, but may occur from a metabolic switch that promotes increased glucose oxidation.
Fig. 5.
Whole-body energy and glucose homeostasis. Energy homeostasis in DGKζ KO mice on HFD. RER (A, B), food intake (C, D), and locomotor activity (E, F) were assessed in male (A, C, E) and female (B, D, F) DGKζ KO and wild-type mice at 16 weeks of age and after 11 weeks on HFD (n = 8–14). Whole-body glucose utilization in DGKζ KO and wild-type mice. Basal and insulin-stimulated (clamp) whole-body glucose utilization and hepatic glucose production (HGP) were determined during a euglycemic-hyperinsulinemic clamp in 4 h-fasted mice at age 17 weeks after 12 weeks on HFD in male (G) and female (H) mice (n = 6–7). Two-way ANOVA with Sidak’s post hoc testing. ‡P < 0.05 overall genotype effect, †P < 0.05 overall diet effect, #P < 0.05 interaction; *P < 0.05 versus wild-type mice of same time point.
DISCUSSION
DGKζ has been implicated in several pathophysiological conditions, including cancer, cardiovascular disorders, autoimmune diseases, and obesity (29), but the physiological role of this enzyme in functional and metabolic adaptations of skeletal muscle and whole-body glucose and energy homeostasis is unknown. Here, we provide evidence that life-long deficiency of DGKζ leads to modest growth retardation, reduced adiposity, and protection against peripheral insulin resistance in mice fed a HFD, despite elevated DAG content in peripheral tissues.
DGK isoforms have unique subtype-specific structural and functional domains, suggesting that each subfamily plays a distinct physiological role. DGKζ is characterized by the presence of two C1 domains, a MARCKS homology phosphorylation site domain that contains a nuclear localization signal and acts as a substrate for conventional PKCs, a conserved catalytic domain that contains a nuclear export signal, and four ankyrin repeats (30). The presence of nuclear localization and nuclear export signals suggest that DGKζ plays a role in the regulation of nuclear events and gene expression, either by acting as a signal transducer, interacting as a component of large protein complexes that bring together lipid metabolism enzymes, or by directly altering the local concentration of DAG and PA. Our finding that DGKζ influences body weight, lean body mass, and adiposity support a role of this isoform in growth, development, and metabolism.
DGKζ affects cell cycle and skeletal muscle differentiation (15, 31–33). In mouse myoblasts, DGKζ expression is increased during myogenic differentiation and associated with the nuclear matrix, implicating a role in DNA replication, gene expression, and protein phosphorylation (33). Gene silencing of DGKζ impairs skeletal muscle differentiation, as evidenced by reduced protein abundance of myogenin (33). Conversely, gene silencing of DGKδ, a type II subfamily member, did not alter skeletal muscle differentiation (33) or lean body mass (9), indicating DGK isoform-specific regulation. We found that myogenin expression was reduced in skeletal muscle from chow-fed female DGKζ KO mice, but not male DGKζ KO mice. Myogenin is essential for skeletal muscle development and viability during fetal development, but dispensable when deleted after birth (26). An earlier description of another cohort of these DGKζ KO mice suggests muscle to body weight ratio, muscle fiber type distribution, and histological appearance are unaltered (16). While these findings are inconsistent with our results, several differences exist between these studies. For example, we studied male and female mice on a C57BL/6 background at 15–17 weeks of age, whereas earlier workers (16) studied 8- to 10-week-old mice on a mixed C57BL/6×129X1/SvJ background of an undetermined sex and results were compared against wild-type FVB/N and C57BL/6 mice, rather than wild-type littermates.
DGKζ overexpression attenuates cardiac hypertrophy in response to G-protein-coupled receptor agonists or pressure overload (34–36) and inhibits myocardial atrophy in streptozotocin-induced diabetic mice (17). Skeletal muscle hypertrophy involves an increase in mass and cross-sectional area of individual skeletal muscle fibers. Given the proposed role of DGKζ in skeletal muscle differentiation and cell growth (32), we subjected DGKζ KO mice to functional overload in order to determine the requirement of DGKζ for hypertrophy in mature adult skeletal muscle. Functional overload mimics effects of resistance exercise training and drives a hypertrophic response on muscle growth. Overexpression of DGKζ in tibialis anterior muscle leads to an activation of PA-mTOR signaling and increases skeletal muscle fiber size (16). While DGKζ is a component of a mechanosensitive signaling cascade that regulates skeletal muscle mass, we found that DGKζ was not required for skeletal muscle hypertrophy following a 14 day functional overload. While DGKζ appears to play a role in myogenic differentiation and growth, skeletal muscle hypertrophy arising from functional overload clearly involves the interplay between multiple signal transducers and pathways to ensure skeletal muscle plasticity (37).
HFDs are commonly used in animal models as an environmental stressor to rapidly increase body weight and reduce whole-body insulin sensitivity. In skeletal muscle, chronic HFD impairs insulin signal transduction and glucose uptake in wild-type mice (28). We found that DGKζ deficiency protects against peripheral insulin resistance in HFD-fed mice, despite elevated DAG content in peripheral tissues. Insulin sensitivity was increased in DGKζ KO mice in isolated skeletal muscle and during the glucose tolerance test. Conversely, insulin responsiveness, as determined during the euglycemic-hyperinsulinemic clamp, was similar between DGKζ KO and wild-type mice, suggesting that the maximal rate of glucose uptake was unaltered. The metabolic phenotype and increased insulin sensitivity of DGKζ KO mice was unexpected, given our earlier observation that deficiency of DGKδ, a member of the type II subfamily of DGKs, also increased DAG content; but in this model, peripheral insulin sensitivity, insulin signaling, and glucose transport were reduced and age-dependent obesity was apparent (9). Deficiency of the type III subfamily member, DGKε, also influences the balance of DAG species implicated in the development of peripheral insulin resistance in skeletal muscle (12). Although insulin sensitivity was unaltered in DGKε-deficient mice, whole-body RER was reduced and abundance of mitochondrial markers was increased, despite the elevated DAG levels, indicating a greater reliance on fat oxidation and intracellular lipid metabolism (12). These results with isoform-specific DGK KO mouse models challenge the notion that elevated levels of total DAG content in skeletal muscle and adipose tissue directly impair whole-body glucose and energy homeostasis.
Our finding that DGKζ KO mice are protected against the development of insulin resistance, despite elevated tissue levels of DAG is a paradox. Several observational studies in type 2 diabetic or obese individuals suggest that total DAG content in skeletal muscle is not consistently elevated despite severe insulin resistance (38–40). Moreover, skeletal muscle total DAG content is elevated in insulin-sensitive endurance-trained athletes (34, 39). Thus chain length, the degree of fatty acid saturation, and the intracellular localization of specific DAG species, rather than elevations in total DAG content, may play an important role in the development of insulin resistance (41–43). Moreover, multiple isomers of DAG exist, and not all DAG species appear to provoke insulin resistance (42). Thus, we cannot exclude the possibility that DGKζ KO mice may accumulate 1,3-DAG, an isomer that promotes insulin sensitivity (44), rather than 1,2-DAG, an isomer that activates PKC (45), which could also explain the apparent paradox. Alterations in the intracellular localization of PA may also influence signal transduction and skeletal muscle metabolism (46). DGK isoforms appear to influence growth and metabolism by distinct mechanisms depending on structural diversity and relative tissue-specific abundance, as well as localization and particular lipid species metabolized.
DGKζ KO mice are protected against the development of insulin resistance on a HFD. These metabolic improvements may be partly related to the reduction in fat mass, possibly due to altered HSL activity and lipolysis in gonadal adipocytes. We also found a shift in RER, suggesting increased energy expenditure in DGKζ-deficient mice, with carbohydrates serving as the preferred energy substrate during both the light and dark cycle. The apparent greater reliance for carbohydrate as an energetic source in DGKζ-deficient mice is corroborated by the increased insulin-stimulated glucose transport in skeletal muscle and reduced insulin levels during the glucose tolerance test, suggesting that DGKζ deficiency enhances insulin sensitivity. The trend for increased p-HSL Ser660 abundance and lipolysis in adipose tissue, suggesting increased overall lipolysis, concomitant with increased glucose uptake in skeletal muscle, may appear paradoxical; however, increase of fatty oxidation promotes an increase in acetyl-CoA, which is transformed into citrate. A fraction of citrate is transported to the cytosol where it inhibits PFK-1 activity, leading to decreased glucose transport and oxidation (47, 48). Our results suggest that DGKζ deficiency in adipose tissue and skeletal muscle influences glucose and energy homeostasis. Nevertheless DGKζ is also expressed in distinct regions of the brain, in particular hypothalamic neurons, and via its ankyrin repeats, interacts with the cytoplasmic portion of the leptin receptor (Ob-Rb) (49). Paradoxically, an inverse relationship between hypothalamic DGKζ mRNA levels and body fat has been reported in HFD-fed rodents, leading to the hypothesis that reductions in DGK activity in the hypothalamus may lead to obesity. The role of hypothalamic DGKζ is likely to be complex given that we observed that DGKζ deficiency was associated with leanness and enhanced skeletal muscle insulin sensitivity, rather than the predicted (49) obesity and insulin resistance phenotype. Notably, the augmentation in whole-body energy expenditure occurred without alterations in physical activity or behavioral defects. Our results suggest that a simultaneous increase in both glucose and fatty acid oxidation in peripheral tissues contributes to the enhanced insulin sensitivity in DGKζ KO mice. Given our results, complementary approaches to examine the tissue-specific role of DGKζ, particularly in brain, skeletal muscle, heart, and pancreas, may be warranted. However, our study of whole-body DGKζ KO mice provides important insight to the role of this gene in the regulation and whole-body metabolism and growth.
In conclusion, life-long deficiency of DGKζ leads to modest growth retardation, reduced adiposity, and protection against peripheral insulin resistance in HFD-fed mice. DGKζ ablation increases DAG content in peripheral tissues controlling glucose and energy homeostasis. Moreover, DGKζ plays a role in skeletal muscle differentiation, likely due to a fundamental role during development. Thus, DGKζ plays a role in the control of growth and metabolic processes and further highlights specialized functions of DGK isoforms in glucose and energy homeostasis and type 2 diabetes pathophysiology.
Acknowledgments
The authors are grateful to Dr. Matthew K. Topham (University of Utah, Salt Lake City, UT) for providing the DGKζ KO mouse model.
Footnotes
Abbreviations:
- ACC
- acetyl-CoA carboxylase
- ATGL
- adipose triglyceride lipase
- DAG
- diacylglycerol
- DGAT
- diacylglycerol O-acyltransferase
- DGK
- diacylglycerol kinase
- EDL
- extensor digitorum longus
- HFD
- high-fat diet
- HSL
- hormone-sensitive lipase
- MARCKS
- myristoylated alanine-rich C kinase substrate
- PA
- phosphatidic acid
- PKC
- protein kinase C
- RER
- respiratory exchange ratio
- VCO2
- carbon dioxide production
- VO2
- oxygen consumption
This work was supported by the Novo Nordisk Foundation, Strategic Research Program in Diabetes at Karolinska Institutet, European Research Council Ideas Program Grant ERC-2008-AdG233285, Swedish Research Council Grant 2011-3550, Swedish Diabetes Foundation Grant DIA2012-082, the Swedish Society for Medical Research, and Swedish Foundation for Strategic Research Grant SRL10-0027. The authors have no relevant conflicts of interest to disclose.
REFERENCES
- 1.Carrasco S., and Merida I.. 2007. Diacylglycerol, when simplicity becomes complex. Trends Biochem. Sci. 32: 27–36. [DOI] [PubMed] [Google Scholar]
- 2.Ron D., and Kazanietz M. G.. 1999. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 13: 1658–1676. [PubMed] [Google Scholar]
- 3.Topham M. K. 2006. Signaling roles of diacylglycerol kinases. J. Cell. Biochem. 97: 474–484. [DOI] [PubMed] [Google Scholar]
- 4.Topham M. K., and Epand R. M.. 2009. Mammalian diacylglycerol kinases: molecular interactions and biological functions of selected isoforms. Biochim. Biophys. Acta. 1790: 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakane F., Imai S., Kai M., Yasuda S., and Kanoh H.. 2007. Diacylglycerol kinases: why so many of them? Biochim. Biophys. Acta. 1771: 793–806. [DOI] [PubMed] [Google Scholar]
- 6.Sakane F., Imai S., Kai M., Yasuda S., and Kanoh H.. 2008. Diacylglycerol kinases as emerging potential drug targets for a variety of diseases. Curr. Drug Targets. 9: 626–640. [DOI] [PubMed] [Google Scholar]
- 7.Sakane F., Mizuno S., and Komenoi S.. 2016. Diacylglycerol kinases as emerging potential drug targets for a variety of diseases: an update. Front. Cell Dev. Biol. 4: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneko Y. K., and Ishikawa T.. 2015. Diacylglycerol signaling pathway in pancreatic beta-cells: an essential role of diacylglycerol kinase in the regulation of insulin secretion. Biol. Pharm. Bull. 38: 669–673. [DOI] [PubMed] [Google Scholar]
- 9.Chibalin A. V., Leng Y., Vieira E., Krook A., Bjornholm M., Long Y. C., Kotova O., Zhong Z., Sakane F., Steiler T., et al. 2008. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell. 132: 375–386. [DOI] [PubMed] [Google Scholar]
- 10.Sakiyama S., Usuki T., Sakai H., and Sakane F.. 2014. Regulation of diacylglycerol kinase delta2 expression in C2C12 skeletal muscle cells by free fatty acids. Lipids. 49: 633–640. [DOI] [PubMed] [Google Scholar]
- 11.Wada Y., Sakiyama S., Sakai H., and Sakane F.. 2016. Myristic acid enhances diacylglycerol kinase delta-dependent glucose uptake in myotubes. Lipids. 51: 897–903. [DOI] [PubMed] [Google Scholar]
- 12.Mannerås-Holm L., Schönke M., Brozinick J. T., Vetterli L., Bui H. H., Sanders P., Nascimento E. B. M., Björnholm M., Chibalin A. V., and Zierath J. R.. 2017. Diacylglycerol kinase ε deficiency preserves glucose tolerance and modulates lipid metabolism in obese mice. J. Lipid Res. 58: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunting M., Tang W., Zimmerman G. A., McIntyre T. M., and Prescott S. M.. 1996. Molecular cloning and characterization of a novel human diacylglycerol kinase zeta. J. Biol. Chem. 271: 10230–10236. [PubMed] [Google Scholar]
- 14.Ding L., McIntyre T. M., Zimmerman G. A., and Prescott S. M.. 1998. The cloning and developmental regulation of murine diacylglycerol kinase zeta. FEBS Lett. 429: 109–114. [DOI] [PubMed] [Google Scholar]
- 15.Evangelisti C., Riccio M., Faenza I., Zini N., Hozumi Y., Goto K., Cocco L., and Martelli A. M.. 2006. Subnuclear localization and differentiation-dependent increased expression of DGK-zeta in C2C12 mouse myoblasts. J. Cell. Physiol. 209: 370–378. [DOI] [PubMed] [Google Scholar]
- 16.You J. S., Lincoln H. C., Kim C. R., Frey J. W., Goodman C. A., Zhong X. P., and Hornberger T. A.. 2014. The role of diacylglycerol kinase zeta and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J. Biol. Chem. 289: 1551–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilim O., Takeishi Y., Kitahara T., Arimoto T., Niizeki T., Sasaki T., Goto K., and Kubota I.. 2008. Diacylglycerol kinase zeta inhibits myocardial atrophy and restores cardiac dysfunction in streptozotocin-induced diabetes mellitus. Cardiovasc. Diabetol. 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niizeki T., Takeishi Y., Kitahara T., Arimoto T., Koyama Y., Goto K., Mende U., and Kubota I.. 2008. Diacylglycerol kinase zeta rescues G alpha q-induced heart failure in transgenic mice. Circ. J. 72: 309–317. [DOI] [PubMed] [Google Scholar]
- 19.Zhong X. P., Hainey E. A., Olenchock B. A., Jordan M. S., Maltzman J. S., Nichols K. E., Shen H., and Koretzky G. A.. 2003. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nat. Immunol. 4: 882–890. [DOI] [PubMed] [Google Scholar]
- 20.Mannerås-Holm L., Kirchner H., Björnholm M., Chibalin A. V., and Zierath J. R.. 2015. mRNA expression of diacylglycerol kinase isoforms in insulin-sensitive tissues: effects of obesity and insulin resistance. Physiol. Rep. 3: e12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Augusto V., Padovani C. R., and Rocha Campos G. E.. 2004. Skeletal muscle fiber types in C57Bl6J mice. Braz. J. Morphol. Sci. 21: 89–94. [Google Scholar]
- 22.Hansen P. A., Gulve E. A., and Holloszy J. O.. 1994. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J. Appl. Physiol. (1985). 76: 979–985. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin K. M., Valdez V., Schrader L. F., and Herrick R. E.. 1981. Effect of functional overload on substrate oxidation capacity of skeletal muscle. J. Appl. Physiol. 50: 1272–1276. [DOI] [PubMed] [Google Scholar]
- 24.Bodine S. C., and Baar K.. 2012. Analysis of skeletal muscle hypertrophy in models of increased loading. Methods Mol. Biol. 798: 213–229. [DOI] [PubMed] [Google Scholar]
- 25.Goto K., and Kondo H.. 1996. A 104-kDa diacylglycerol kinase containing ankyrin-like repeats localizes in the cell nucleus. Proc. Natl. Acad. Sci. USA. 93: 11196–11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meadows E., Cho J. H., Flynn J. M., and Klein W. H.. 2008. Myogenin regulates a distinct genetic program in adult muscle stem cells. Dev. Biol. 322: 406–414. [DOI] [PubMed] [Google Scholar]
- 27.Samuel V. T., and Shulman G. I.. 2012. Mechanisms for insulin resistance: common threads and missing links. Cell. 148: 852–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zierath J. R., Houseknecht K. L., Gnudi L., and Kahn B. B.. 1997. High-fat feeding impairs insulin-stimulated GLUT4 recruitment via an early insulin-signaling defect. Diabetes. 46: 215–223. [DOI] [PubMed] [Google Scholar]
- 29.Shulga Y. V., Topham M. K., and Epand R. M.. 2011. Regulation and functions of diacylglycerol kinases. Chem. Rev. 111: 6186–6208. [DOI] [PubMed] [Google Scholar]
- 30.Rincón E., Gharbi S. I., Santos-Mendoza T., and Mérida I.. 2012. Diacylglycerol kinase ζ: at the crossroads of lipid signaling and protein complex organization. Prog. Lipid Res. 51: 1–10. [DOI] [PubMed] [Google Scholar]
- 31.Topham M. K., Bunting M., Zimmerman G. A., McIntyre T. M., Blackshear P. J., and Prescott S. M.. 1998. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-zeta. Nature. 394: 697–700. [DOI] [PubMed] [Google Scholar]
- 32.Evangelisti C., Astolfi A., Gaboardi G. C., Tazzari P., Pession A., Goto K., and Martelli A. M.. 2009. TIS21/BTG2/PC3 and cyclin D1 are key determinants of nuclear diacylglycerol kinase-zeta-dependent cell cycle arrest. Cell. Signal. 21: 801–809. [DOI] [PubMed] [Google Scholar]
- 33.Evangelisti C., Tazzari P. L., Riccio M., Fiume R., Hozumi Y., Fala F., Goto K., Manzoli L., Cocco L., and Martelli A. M.. 2007. Nuclear diacylglycerol kinase-zeta is a negative regulator of cell cycle progression in C2C12 mouse myoblasts. FASEB J. 21: 3297–3307. [DOI] [PubMed] [Google Scholar]
- 34.Amati F., Dube J. J., Alvarez-Carnero E., Edreira M. M., Chomentowski P., Coen P. M., Switzer G. E., Bickel P. E., Stefanovic-Racic M., Toledo F. G., et al. 2011. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes. 60: 2588–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada M., Takeishi Y., Arimoto T., Niizeki T., Kitahara T., Goto K., Walsh R. A., and Kubota I.. 2007. Diacylglycerol kinase zeta attenuates pressure overload-induced cardiac hypertrophy. Circ. J. 71: 276–282. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi H., Takeishi Y., Seidler T., Arimoto T., Akiyama H., Hozumi Y., Koyama Y., Shishido T., Tsunoda Y., Niizeki T., et al. 2005. Adenovirus-mediated overexpression of diacylglycerol kinase-zeta inhibits endothelin-1-induced cardiomyocyte hypertrophy. Circulation. 111: 1510–1516. [DOI] [PubMed] [Google Scholar]
- 37.Egerman M. A., and Glass D. J.. 2014. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 49: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anastasiou C. A., Kavouras S. A., Lentzas Y., Gova A., Sidossis L. S., and Melidonis A.. 2009. Diabetes mellitus is associated with increased intramyocellular triglyceride, but not diglyceride, content in obese humans. Metabolism. 58: 1636–1642. [DOI] [PubMed] [Google Scholar]
- 39.Perreault L., Bergman B. C., Hunerdosse D. M., and Eckel R. H.. 2010. Altered intramuscular lipid metabolism relates to diminished insulin action in men, but not women, in progression to diabetes. Obesity (Silver Spring). 18: 2093–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jocken J. W., Moro C., Goossens G. H., Hansen D., Mairal A., Hesselink M. K., Langin D., van Loon L. J., and Blaak E. E.. 2010. Skeletal muscle lipase content and activity in obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 95: 5449–5453. [DOI] [PubMed] [Google Scholar]
- 41.Bergman B. C., Perreault L., Hunerdosse D. M., Koehler M. C., Samek A. M., and Eckel R. H.. 2010. Increased intramuscular lipid synthesis and low saturation relate to insulin sensitivity in endurance-trained athletes. J. Appl. Physiol. (1985). 108: 1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritter O., Jelenik T., and Roden M.. 2015. Lipid-mediated muscle insulin resistance: different fat, different pathways? J. Mol. Med. (Berl.). 93: 831–843. [DOI] [PubMed] [Google Scholar]
- 43.Bergman B. C., Hunerdosse D. M., Kerege A., Playdon M. C., and Perreault L.. 2012. Localisation and composition of skeletal muscle diacylglycerol predicts insulin resistance in humans. Diabetologia. 55: 1140–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito S., Hernandez-Ono A., and Ginsberg H. N.. 2007. Dietary 1,3-diacylglycerol protects against diet-induced obesity and insulin resistance. Metabolism. 56: 1566–1575. [DOI] [PubMed] [Google Scholar]
- 45.Balogh A., Csuka O., Teplan I., and Keri G.. 1995. Phosphatidylcholine could be the source of 1,2-DAG which activates protein kinase C in EGF-stimulated colon carcinoma cells (HT29). Cell. Signal. 7: 793–801. [DOI] [PubMed] [Google Scholar]
- 46.Shad B. J., Smeuninx B., Atherton P. J., and Breen L.. 2015. The mechanistic and ergogenic effects of phosphatidic acid in skeletal muscle. Appl. Physiol. Nutr. Metab. 40: 1233–1241. [DOI] [PubMed] [Google Scholar]
- 47.Randle P. J., Garland P. B., Hales C. N., and Newsholme E. A.. 1963. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1: 785–789. [DOI] [PubMed] [Google Scholar]
- 48.Randle P. J., Garland P. B., Newsholme E. A., and Hales C. N.. 1965. The glucose fatty acid cycle in obesity and maturity onset diabetes mellitus. Ann. N. Y. Acad. Sci. 131: 324–333. [DOI] [PubMed] [Google Scholar]
- 49.Liu Z., Chang G. Q., and Leibowitz S. F.. 2001. Diacylglycerol kinase zeta in hypothalamus interacts with long form leptin receptor. Relation to dietary fat and body weight regulation. J. Biol. Chem. 276: 5900–5907. [DOI] [PubMed] [Google Scholar]