Abstract
Objective:
To add quantitative muscle MRI to the clinical trial toolbox for facioscapulohumeral muscular dystrophy (FSHD) by correlating it to clinical outcome measures in a large cohort of genetically and clinically well-characterized patients with FSHD comprising the entire clinical spectrum.
Methods:
Quantitative MRI scans of leg muscles of 140 patients with FSHD1 and FSHD2 were assessed for fatty infiltration and TIRM hyperintensities and were correlated to multiple clinical outcome measures.
Results:
The mean fat fraction of the total leg musculature correlated highly with the motor function measure, FSHD clinical score, Ricci score, and 6-minute walking test (correlation coefficients −0.845, 0.835, 0.791, −0.701, respectively). Fat fraction per muscle group correlated well with corresponding muscle strength (correlation coefficients up to −0.82). The hamstring muscles, adductor muscles, rectus femoris, and gastrocnemius medialis were affected most frequently, also in early stage disease and in patients without leg muscle weakness. Muscle involvement was asymmetric in 20% of all muscle pairs and fatty infiltration within muscles showed a decrease from distal to proximal of 3.9%. TIRM hyperintense areas, suggesting inflammation, were found in 3.5% of all muscles, with and without fatty infiltration.
Conclusions:
We show a strong correlation between quantitative muscle MRI and clinical outcome measures. Muscle MRI is able to detect muscle pathology before clinical involvement of the leg muscles. This indicates that quantitative leg muscle MRI is a promising biomarker that captures disease severity and motor functioning and can thus be included in the FSHD trial toolbox.
Facioscapulohumeral muscular dystrophy (FSHD) is one of the commonest forms of adult muscular dystrophy.1 It is characterized by slowly progressive weakness of the facial and shoulder girdle muscles, followed by leg and trunk muscles.2 Disease course and severity are highly variable, even within families, ranging from asymptomatic gene carriers to wheelchair-bound patients. Since the discovery of the (epi)genetic origin of the disease, new therapeutic strategies are being developed and clinical trials are at the horizon. Consequently, there is an urgent need for a clinical trial toolbox, including patient registries, biomarkers, and clinical outcome measures.3 While clinical outcome measures provide direct measures of patient functioning or ability, they may not be sensitive enough to capture changes in this slowly progressive disorder. Especially for early phase clinical trials, biomarkers that detect subtle changes in muscle pathology are required. Quantitative muscle MRI may provide such a biomarker4; however, evidence of its correlation to clinical outcome measures still has to be established. Previous studies on this correlation have mostly used qualitative or ordinal, and thus nonlinear, MRI data.5–11 Studies that have used quantitative MRI comprised small cohorts with a maximum of 50 patients and none of them included patients across the entire FSHD clinical spectrum.12–16
We enrolled a large cohort of patients with genetically confirmed and clinically heterogeneous FSHD. This study presents baseline data on quantitative MRI of the leg muscles and its correlation to multiple clinical outcome measures to determine the value of muscle MRI as a component of the FSHD trial toolbox.
METHODS
Patients.
We included patients with genetically confirmed FSHD 18 years and older at the neurology department of the Radboud University Medical Center, Nijmegen, the Netherlands, between 2014 and 2015. Asymptomatic gene carriers (individuals who reported no symptoms, with or without signs of FSHD on clinical examination) with at least one affected family member were also included. We included patients with FSHD1 and patients with FSHD2 to compare the groups. Exclusion criteria were contraindications for MRI (e.g., claustrophobia or metallic implants).
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the regional medical ethics committee. All patients signed informed consent.
Clinical outcome measures.
In all patients, multiple clinical outcome measures were scored. The motor function measure (MFM) is a 32-item scale assessing the severity of the motor deficit in neuromuscular diseases; outcomes range from 0% to 100%, in which 100% implies no motor deficits.17 The 6-minute walking test (6-MWT) is used to evaluate functional capacity in neuromuscular disorders and indicates the maximum distance that a patient is able to walk in 6 minutes.18,19 The clinical severity score by Ricci et al.20 (Ricci score) indicates clinical severity on a 10-point scale in which 0 indicates no muscle weakness and 10 indicates wheelchair dependency. The Ricci score assumes a fixed sequence of muscle involvement and is less suitable for patients with an atypical disease course. We therefore included the FSHD clinical score, which evaluates strength and functionality of 6 muscle regions separately.21 This score ranges from 0 to 15, where 0 indicates no muscle weakness and 15 severe muscle weakness in all muscle groups. Manual muscle testing was scored using a 6-point Medical Research Council (MRC) gradation ranging from 0 (no muscle contraction) to 5 (maximal muscle strength) for hip flexion, hip abduction, knee flexion, knee extension, foot dorsal flexion, and foot plantar flexion.22 The maximum isometric contraction of the knee extensors was assessed using fixed dynamometry, scored in Newtons. All clinical scores were obtained by one trained physician (K.M.).
MRI acquisition.
All MRI examinations were performed on the same 3T MRI system (TIM Trio; Siemens, Erlangen, Germany) using a scanning protocol adapted from previous studies.14,15 Participants were placed feet first supine in the scanner. Scout images were made in 3 orthogonal directions to position imaging slices for subsequent scans. A transverse Dixon sequence was acquired around the upper and lower leg (field of view 271 × 435 mm, matrix size 200 × 320, repetition time 10 ms, echo time 2.45/3.675 ms, number of slices 144, slice thickness 5 mm, slice gap 0 mm, flip angle [FA] 3°). For the TIRM sequence, the inversion time was selected to null the fat signals (field of view 271 × 435, matrix size 160 × 256, repetition time 4,000 ms, echo time 40 ms, inversion time 220 ms, number of slices 72, slice thickness 5 mm, slice gap 5 mm, FA 150°).
MRI analysis.
The water and fat image of the Dixon sequence were used to create a fat fraction map using MATLAB according to the following equation: 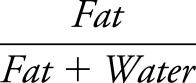 . The fat fraction map was used to draw region of interest (ROI) per muscle using ImageJ software (figure 1).23 All drawn ROIs were checked by a second clinician, and interrater variability was assessed on a subset of 10 scans (380 muscles). Muscle cross-sectional area and fat fractions were calculated per ROI. We evaluated 12 upper leg muscles and 7 lower leg muscles. ROIs were drawn at 2 different slices using the localizer sequences: in the upper leg at one-third and two-thirds of the distance between the spina iliaca anterior superior and the upper edge of the patella. In the lower leg, we used slices at two-thirds and 5/6 of the distance between the lateral malleolus and the lower edge of the patella for the gastrocnemius and one-third and two-thirds of the distance between these landmarks for the other lower leg muscles. TIRM images were visually assessed for the presence of signal hyperintensity in each muscle by a radiologist and the first author (K.M.). When there was discrepancy between the 2 scores, another author (C.G.C.H.) also assessed the image.
. The fat fraction map was used to draw region of interest (ROI) per muscle using ImageJ software (figure 1).23 All drawn ROIs were checked by a second clinician, and interrater variability was assessed on a subset of 10 scans (380 muscles). Muscle cross-sectional area and fat fractions were calculated per ROI. We evaluated 12 upper leg muscles and 7 lower leg muscles. ROIs were drawn at 2 different slices using the localizer sequences: in the upper leg at one-third and two-thirds of the distance between the spina iliaca anterior superior and the upper edge of the patella. In the lower leg, we used slices at two-thirds and 5/6 of the distance between the lateral malleolus and the lower edge of the patella for the gastrocnemius and one-third and two-thirds of the distance between these landmarks for the other lower leg muscles. TIRM images were visually assessed for the presence of signal hyperintensity in each muscle by a radiologist and the first author (K.M.). When there was discrepancy between the 2 scores, another author (C.G.C.H.) also assessed the image.
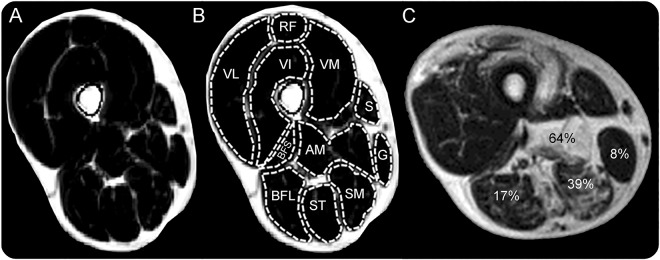
Figure 1. Example of transverse Dixon fat fraction map of upper leg muscles of the right leg.
(A) Dixon fat fraction map of upper leg muscles without fatty infiltration. (B) Regions of interest per muscle. (C) Examples of different degrees of fatty infiltration per muscle. AM = adductor magnus; BFL = biceps femoris long head; BFS = biceps femoris short head; G = gracilis; RF = rectus femoris; S = sartorius; SM = semimembranosus; ST = semitendinosus; VI = vastus intermedius; VL = vastus lateralis; VM = vastus medialis.
Statistical analyses.
All statistical analyses were performed using IBM SPSS Statistics (SPSS Inc., Chicago, IL), version 22. Spearman rho analyses were used to calculate bivariate correlations, independent t test to compare means. A χ2 test was used to search for an association between TIRM positivity and sex. Simple linear regression analyses were used for the relation between duration of symptoms and mean fat fraction and clinical outcome measures. Interrater variability was assessed using intraclass correlation coefficient (CC). p Values ≤0.05 were considered statistically significant.
RESULTS
Patients.
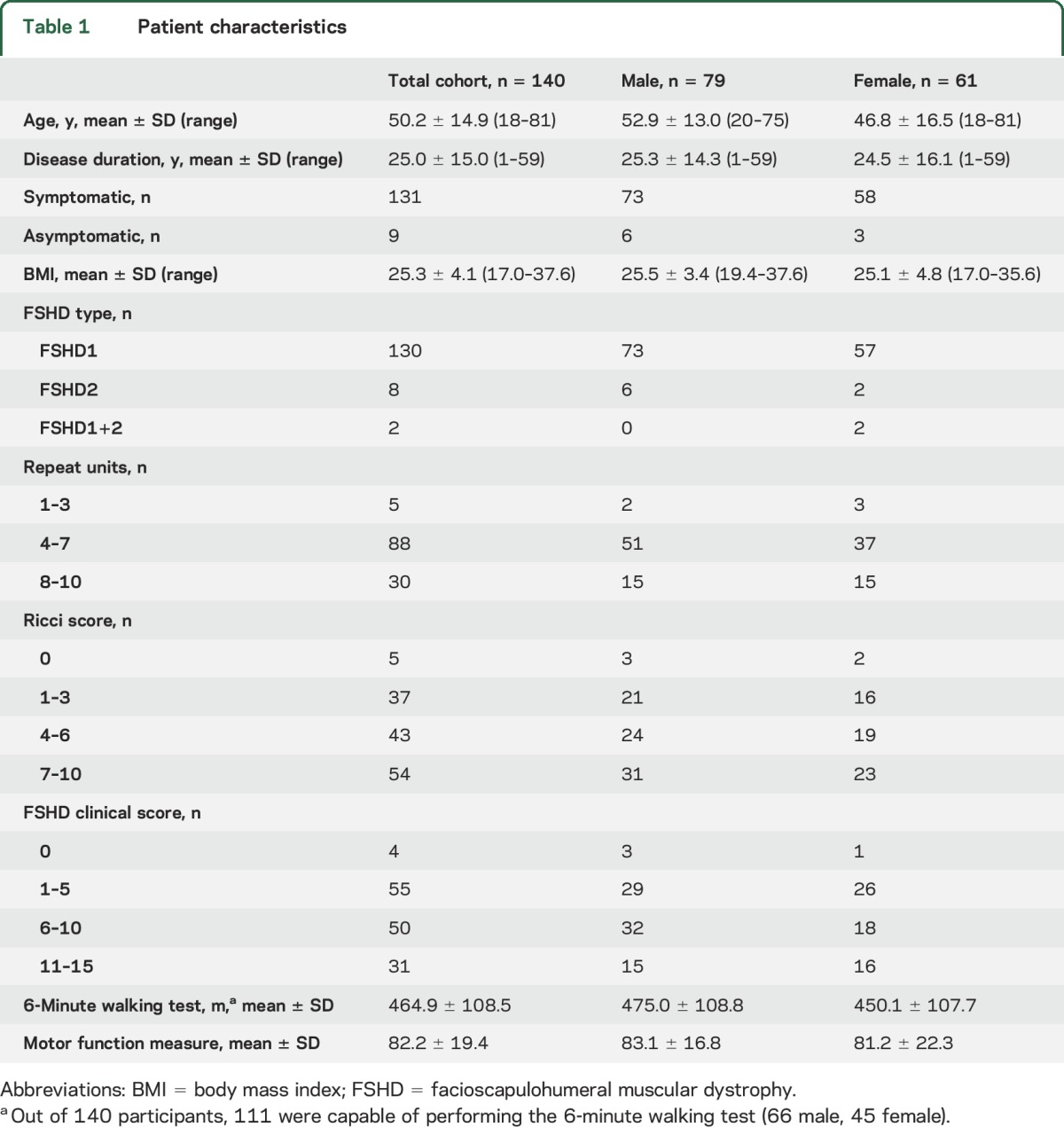
A total of 140 patients with genetically confirmed FSHD participated in this study. Patient characteristics are listed in table 1.
Table 1.
Patient characteristics
Clinical correlations.
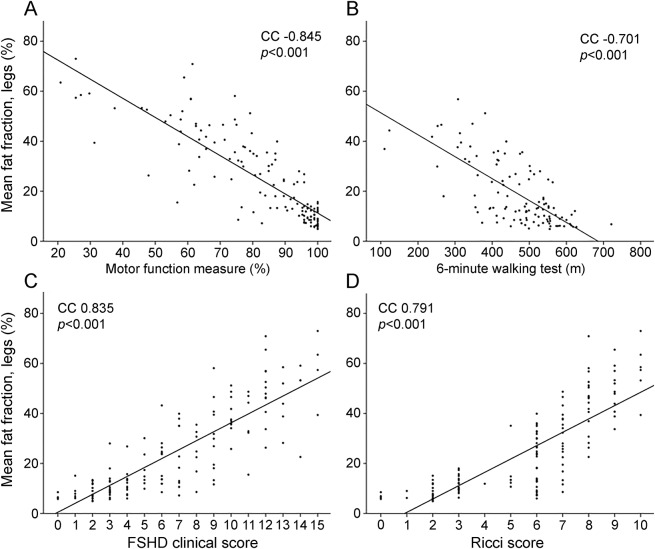
The mean fat fraction correlated highly and statistically significantly with all clinical outcome measures (figure 2). MFM showed the strongest correlation (CC −0.845). MRC scores of knee flexion, knee extension, and foot dorsal flexion correlated highly with the mean fat fraction of the corresponding muscle groups (CC right leg −0.74, −0.77, −0.69, respectively; and left leg −0.74, −0.58, −0.76, respectively; all p < 0.001). The correlation for the plantar flexors was weak (CC right leg −0.38, left leg −0.43; p < 0.001), as was the correlation between quantitative isometric knee extensor strength and corresponding fat fraction of quadriceps muscles (right leg CC −0.42, left leg −0.37; p < 0.001).
Figure 2. Correlation coefficient (CC) between mean fat fraction and clinical outcome measures.
(A) Motor function measure. (B) The 6-minute walking test. (C) facioscapulohumeral muscular dystrophy (FSHD) clinical score. (D) Ricci score. Each dot represents the score of one patient. The 6-minute walking test could only be performed by 111 of 140 patients.
Notably, the rectus femoris was often fatty infiltrated, while the rest of the quadriceps muscles were normal. Knee extension strength was tested in the seated position. In this position, knee extension is primarily driven by the vastus muscles, because the rectus femoris is already shortened. We therefore also correlated knee extension strength to the mean fat fraction of the 3 vastus muscles, leaving out the rectus femoris. Correlation coefficients then increased for manual muscle testing (right leg −0.82, left leg −0.79; p < 0.001) and quantitative muscle testing (right leg −0.51, left leg −0.46; p < 0.001).
Nine asymptomatic gene carriers were included. Four had no signs of FSHD on examination, 2 had mild scapular winging, 2 had a combination of mild scapular winging and minimal facial weakness, and 1 had unilateral mild hamstring weakness. Two asymptomatic gene carriers with signs of FSHD showed fatty infiltration of leg muscles: one had a rectus femoris muscle with a fat fraction of 23.5%; another had elevated fat fractions in the rectus femoris, adductor longus, and soleus muscles of 36.2%, 67.0%, and 23.3%, respectively.
In patients without any functional involvement of the leg muscles (Ricci scores <2.5, n = 43), specific muscles showed elevated mean fat fractions: semimembranosus (19.7% ± 19.5%), rectus femoris (16.3%, ±19.0), adductor longus (13.9%, ±14.7), tibialis anterior (11.7%, ±8.4), adductor magnus (11.1%, ±8.6). Three patients had mild weakness of the dorsiflexors of the ankle on manual muscle testing and one 32-year-old woman showed knee extensor weakness on quantitative muscle assessment (227 N). Excluding these patients from the analyses did not change the results. Regression analysis was performed to assess associations between disease duration and clinical measures. In this cohort, 10 years longer disease duration was associated with 7.5% (95% confidence interval [CI] 5.9–9.1) higher mean fat fraction for the total legs, 7.7% (95% CI 5.8–9.5) lower score on the MFM, 44.8 (95% CI 32.2–57.5) meters less on the 6-MWT, and 1.7 (95% CI 1.3–2.1) and 0.8 (95% CI 0.6–1.1) points higher on the FSHD clinical score and Ricci score, respectively.
Pattern of muscle involvement.
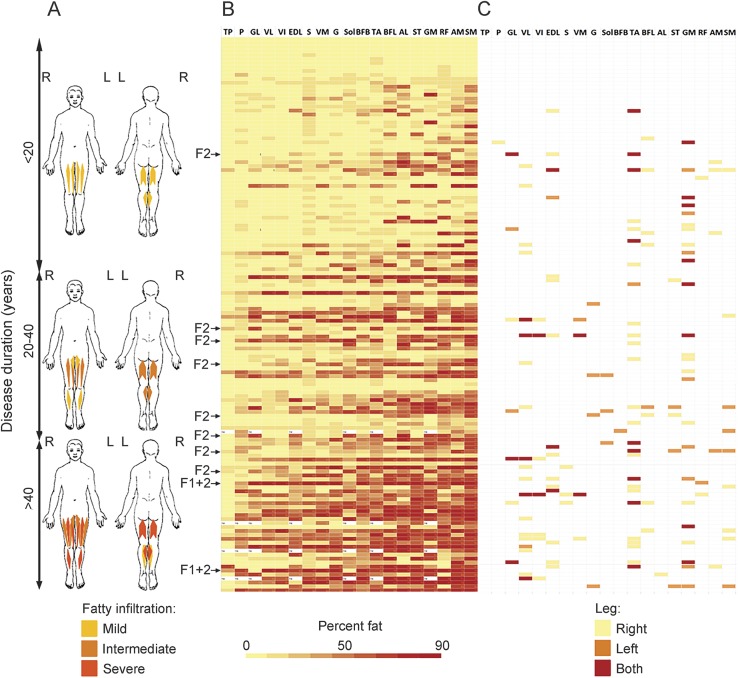
Fat fraction per muscle ranged from 2% to 98%. Of all 5,243 leg muscles, 46.4% showed hardly any fatty infiltration (fat fractions <10%). About a quarter (23.5%) was severely fatty infiltrated (fat fractions >60%). Strong interrater agreement was found for the assessment of fatty infiltration (intraclass correlation coefficient 0.992; p < 0.01). The most often and most severely affected muscles were the semimembranosus (mean fat fraction 41.8%, ±27.6), adductor magnus (33.8%, ±27.4), rectus femoris (31.5%, ±26.9), gastrocnemius medialis (31.4%, ±27.9), semitendinosus (31.0%, ±27.7), and adductor longus (29.6%, ±27.8). The tibialis posterior, peroneus, and gastrocnemius lateralis were least severely affected (mean fat fractions 8.7% ± 9.2%, 16.1% ± 15.6%, and 16.9% ± 21.0%, respectively). Mean fat fractions for other muscles are included in table e-1 at Neurology.org. Fat fractions of all muscles sorted on duration of symptoms show that in this cohort the muscles that are most severely affected in later disease stages are also the ones that are affected early (figure 3).
Figure 3. Pattern of muscle involvement.
(A) Visualization of mean degree of fatty infiltration in leg muscles in patients with increasing duration of symptoms. (B) Heatmap of fat fraction per muscle for each patient individually. Each row represents a patient ordered progressively by duration of symptoms. Each column represents a muscle ordered progressively by degree of fatty infiltration. (C) Heatmap of TIRM-positive muscles per patient. AL = adductor longus; AM = adductor magnus; BFB = biceps femoris short head; BFL = biceps femoris long head; EDL = extensor digitorum longus; F1+2 = facioscapulohumeral muscular dystrophy types 1 and 2 combined; F2 = facioscapulohumeral muscular dystrophy type 2; G = gracilis; GL = gastrocnemius lateralis; GM = gastrocnemius medialis; P = peroneus; RF = rectus femoris; S = sartorius; SM = semimembranosus; Sol = soleus; ST = semitendinosus; TA = tibialis anterior; TP = tibialis posterior; VI = vastus intermedius; VL = vastus lateralis; VM = vastus medialis.
Asymmetric involvement in at least one muscle pair, defined as a difference of >10% in fat fractions between sides, was found in 80.7% (113/140) of all patients. However, most muscles were symmetrically affected; i.e., 20.1% of all muscle pairs showed asymmetry in fat fraction between sides. Of those muscle pairs with asymmetry, there was right-sided preference in 53.0%.
Facioscapulohumeral muscular dystrophy type 2.
In 8 patients with FSHD2, we found a similar pattern of muscle involvement and asymmetry compared to FSHD1. Most severely affected were semimembranosus, rectus femoris, adductor longus, adductor magnus, gastrocnemius medialis, and tibialis anterior. Two individuals with combined FSHD1 and FSHD2 had a very severe phenotype with mean fat fraction of 65.5% and 73.0%, which was much higher than the mean total fat fraction of 23.8% of the patients with FSHD1 and 31.6% of the patients with FSHD2.
TIRM hyperintensity.
In 68 out of 140 patients (48.6%), we found at least one muscle with a hyperintense signal on TIRM sequence (TIRM-positive). A total of 5,240 muscles were assessed for TIRM hyperintensity and 186 were positive (3.5%) (figure 3). The muscles that were positive most frequently were the tibialis anterior (12.9%), gastrocnemius medialis (11.8%), and vastus lateralis (8.9%). The tibialis posterior, peroneus, and adductor longus rarely displayed TIRM hyperintensity (0%–0.4%). The presence of TIRM hyperintensity per muscle is reported in table e-1. Out of 186 TIRM-positive muscles, 29 (15.6%) had a normal fat fraction (<10%) and 23 (12.4%) a high fat fraction (>60%). In 73.1% of TIRM-positive muscles, the TIRM hyperintense area was found directly adjacent to a fatty infiltrated area. TIRM-positive muscles were most frequently found in patients with moderately severe disease (Ricci score 4–8). None was found in patients with severity scores of 0 or 1. A total of 62.9% of TIRM-positive muscles were found in the right leg, although the difference with the number in the left leg was nonsignificant (p = 0.157), except for the vastus lateralis (p = 0.001), tibialis anterior (p = 0.000), and extensor digitorum longus (p = 0.012). Significantly more male patients had TIRM-positive muscles than did female patients: 45 men vs 23 women (χ2 = 5.1; p = 0.024). In addition, the mean number of TIRM-positive muscles was significantly higher in men than in women (1.6 vs 0.9; p = 0.04). They did not significantly differ in age, disease duration, FSHD type, repeat fragment size, or scores on clinical outcome measures.
Proximal-distal gradient.
Overall, there was a mean decrease of fat fraction within muscles from distal to proximal of 3.9%, ±3.7. A decrease from distal to proximal of >10% was found in 33.2% of the muscles (711/2,140). The adductor magnus, semimembranosus, tibialis anterior, and rectus femoris showed the largest distal to proximal gradient (mean decreases 9.3% ± 14.7%, 7.2% ± 18.4%, 6.9% ± 12.4%, and 4.9% ± 14.8%, respectively). A total of 162 of 2,140 (7.6%) individual muscles showed an increase of >10% from distal to proximal. In some muscles, we observed a gradient in fatty infiltration from distal to proximal, with a TIRM-positive front in unaffected muscle tissue proximally adjacent to fatty infiltrated muscle tissue (figure 4).
Figure 4. Distal to proximal gradient in fatty infiltration with TIRM-positive front in unaffected muscle tissue.
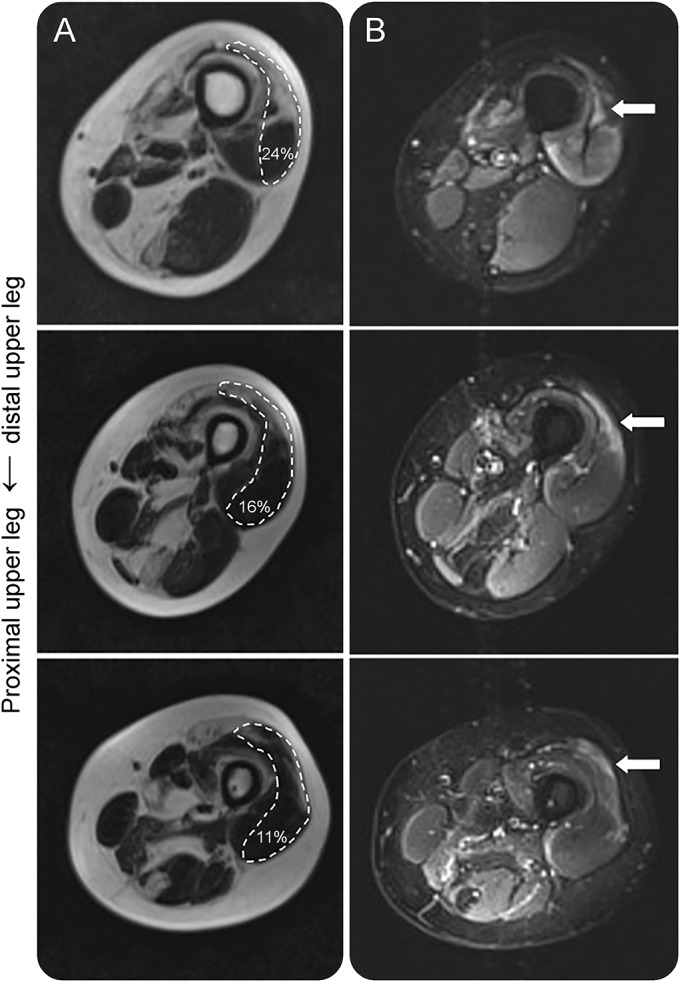
(A) Dixon fat images show fatty infiltration of the vastus medialis lateralis of the left leg with a distal to proximal decrease in fat fraction. (B) Corresponding TIRM images show a front of TIRM hyperintensity in normal-appearing muscle tissue, adjacent to the fatty infiltrated muscle tissue.
DISCUSSION
This study provides a thorough description of the relation of quantitative muscle MRI with clinical measures in a large cohort of patients with FSHD comprising the entire clinical spectrum. It demonstrates that the mean total leg fat fraction on MRI correlates strongly with measurements of strength, clinical severity scores, and measures of overall functional activity, and thus validates MRI as a biomarker of disease severity. MRI was able to detect muscle changes in patients without any loss of leg muscle function, especially in the hamstring muscles, adductor muscles, and rectus femoris. Possibly, we did not find a loss of leg muscle function in these patients because of compensation by other muscles (of the same group), compensation within the muscle on a molecular level, or because limited fatty infiltration does not restrict the functional capacity of the muscle. Our finding of early MRI changes in individuals without leg muscle weakness suggests that MRI might be used to identify individuals and muscles at risk for clinical progression.
The hamstrings, adductor muscles, and gastrocnemius medialis were the most frequently affected muscles. Most often spared were the tibialis posterior, gastrocnemius lateralis, and peroneus. The most frequently and most severely affected muscles were also the muscles that were affected in patients in early disease stages. Our findings support the hypothesis that muscles become affected in a sequential order, and that muscles that become affected in later stages progress at a similar pace as early affected muscles. The pattern of involvement in fatty infiltration is in concordance with findings in a large qualitatively assessed FSHD cohort, although this cohort included only asymptomatic gene carriers or patients with a Ricci score of 5 and higher, indicating that no mildly affected patients were included.5 We found a similar frequency of asymmetric muscle involvement as previous studies,5,16 but we did not find a clear right side predominance for fatty infiltration in the lower extremities.
Recently, hyperintensities on TIRM sequence in FSHD muscles have been proposed to represent muscle edema, which is sometimes interpreted as active muscle inflammation,15,24,25 and a marker for disease activity. The prevalence of TIRM-hyperintense muscles in this study (3.5%) is comparable to prevalences in the literature (3%–7%). In addition, we found a similar proportion of TIRM-positive muscles without fatty infiltration. TIRM-positive areas were often found near fatty infiltrated regions. In some muscles, there was a TIRM-positive front located proximally to the fatty infiltration, supporting the hypothesis that in many muscles fatty infiltration starts distally in the muscle. However, the muscles that were most frequently TIRM-positive were not the same muscles as those that were most severely or frequently fatty infiltrated. In addition, in mildly affected patients, fatty infiltrated muscles were more frequently observed than TIRM-positive muscles. These findings could indicate variation in the pathogenic mechanism, including different durations of the TIRM-positive phase, TIRM-positive areas that reverse to normal muscle tissue, and the occurrence of fatty infiltration without preceding TIRM positivity. The proportion of TIRM-positive muscles was higher in men than in women.5 This suggests that there are sex-specific disease differences, possibly related to differences in reproductive hormones.
In all patients, we found a combination of normal and fatty infiltrated muscles, and in some also TIRM-positive muscles, supporting the hypothesis that FSHD is a muscle per muscle disease with separate muscles affected in different stages of disease. Longitudinal studies are required to further explore this hypothesis and its underlying mechanisms. Muscles of the trunk and upper limb could also be included in future studies.
The unique value of this study lies in the use of quantitative analysis of muscle fat fractions in a large genetically confirmed and clinically diverse cohort. For use as a biomarker, quantitative measurement is essential, since linear values can be used for parametric statistical analyses in contrast to ordinal scores.26 This might be more sensitive to change over time compared to functional measures in this slowly progressive disorder. A previous study showed that quantitative assessment has a larger sensitivity and better accuracy compared to a semiquantitative visual score.16 In addition, it can provide information on the pattern of involvement not only between but also within different muscles. The single-center, single-evaluator design of this study avoids interrater variability and increases the reliability of correlations between fat fractions and clinical measures. However, a limitation of this design is the decrease in the external validity. Results regarding disease duration should be interpreted with caution since age at disease onset was collected retrospectively.
For clinical trials, we propose quantitative muscle MRI assessment in addition to clinical outcome measures that directly measure patient functioning, like the motor function measure. The motor function measures, however, showed a ceiling effect in mildly affected patients. Although a good reliability and validity of the scale is suggested for patients with FSHD, its psychometric properties, including its target population, should be evaluated in more detail.27 Qualitative MRI evaluations will remain useful, for example in assessing the presence of TIRM hyperintensities and detecting subtle muscular changes that do not show in the clinical examination of the patient.
This study shows a strong correlation of quantitative muscle MRI with different clinical outcome measures, especially with the motor function measure. Since quantitative muscle MRI also has good clinimetric properties, it is a promising biomarker representative of disease severity. For clinical trials, we propose to include quantitative muscle MRI and the motor function measure in the FSHD trial toolbox.
ACKNOWLEDGMENT
The authors thank the patients who took part in this study; the Radiology Department of the Radboud University Medical Center for support, particularly Prof. Arend Heerschap and Linda Heskamp; and Prof. Stephen Tapscott for critically reading the manuscript and making suggestions for improvement.
GLOSSARY
- 6-MWT
6-minute walking test
- CC
correlation coefficient
- CI
confidence interval
- FA
flip angle
- FSHD
facioscapulohumeral muscular dystrophy
- MFM
motor function measure
- MRC
Medical Research Council
- ROI
region of interest
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Karlien Mul: study concept and design, acquisition of data, analysis and interpretation of data, drafting of manuscript and figures. Sanne Vincenten: acquisition of data, analysis and interpretation of data, drafting of manuscript and figures. Nicol Voermans: critical revision of manuscript for intellectual content. Richard Lemmers: performing and interpretation of genetic analyses, critical revision of manuscript for intellectual content. Patrick van der Vliet: performing and interpretation of genetic analyses, critical revision of manuscript for intellectual content. Silvère van der Maarel: obtained funding for the study, critical revision of manuscript for intellectual content. George Padberg: critical revision of manuscript for intellectual content. Corinne Horlings: study concept and design, analysis and interpretation of data, drafting of manuscript and figures. Baziel van Engelen: obtained funding for the study, study supervision, study concept and design, interpretation of data, critical revision of manuscript for intellectual content.
STUDY FUNDING
This study was funded by the Prinses Beatrix Spierfonds and Stichting Spieren voor Spieren.
DISCLOSURE
K. Mul, S. Vincenten, N. Voermans, R. Lemmers, and P. van der Vliet report no disclosures relevant to the manuscript. S. van der Maarel: consultant for Atyr-Pharma, receives grants from the NIH National Institute of Neurologic Disorders and Stroke (P01NS069539), the Prinses Beatrix Spierfonds, the European Union Framework Programme 7 (agreement 2012–305121, NEUROMICS), the FSH Society, Stichting Spieren voor Spieren, the FSHD Global Research Foundation, FSHD Stichting, and Friends of FSH Research. G. Padberg: consultant for Atyr-Pharma and Facio Therapies. C. Horlings reports no disclosures relevant to the manuscript. B. van Engelen receives grants from Prinses Beatrix Spierfonds, Association Francaise contre les Myopathies, Stichting Spieren voor Spieren, FSHD Stichting, and NWO Dutch Organisation for scientific research. Go to Neurology.org for full disclosures
REFERENCES
- 1.Deenen JC, Arnts H, van der Maarel SM, et al. Population-based incidence and prevalence of facioscapulohumeral dystrophy. Neurology 2014;83:1056–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mul K, Lassche S, Voermans NC, Padberg GW, Horlings CG, van Engelen BG. What's in a name? The clinical features of facioscapulohumeral muscular dystrophy. Pract Neurol 2016;16:201–207. [DOI] [PubMed] [Google Scholar]
- 3.Tawil R, Shaw DW, van der Maarel SM, Tapscott SJ. Clinical trial preparedness in facioscapulohumeral dystrophy: outcome measures and patient access: 8–9 April 2013, Leiden, The Netherlands. Neuromuscul Disord 2014;24:79–85. [DOI] [PubMed] [Google Scholar]
- 4.Tawil R, Padberg GW, Shaw DW, van der Maarel SM, Tapscott SJ, Participants FW. Clinical trial preparedness in facioscapulohumeral muscular dystrophy: clinical, tissue, and imaging outcome measures 29–30 May 2015, Rochester, New York. Neuromuscul Disord 2016;26:181–186. [DOI] [PubMed] [Google Scholar]
- 5.Tasca G, Monforte M, Ottaviani P, et al. Magnetic resonance imaging in a large cohort of facioscapulohumeral muscular dystrophy patients: pattern refinement and implications for clinical trials. Ann Neurol Epub 2016 Mar 19. [DOI] [PubMed]
- 6.Tasca G, Monforte M, Iannaccone E, et al. Upper girdle imaging in facioscapulohumeral muscular dystrophy. PLoS One 2014;9:e100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerevini S, Scarlato M, Maggi L, et al. Muscle MRI findings in facioscapulohumeral muscular dystrophy. Eur Radiol 2015;26:693–705. [DOI] [PubMed] [Google Scholar]
- 8.Regula JU, Jestaedt L, Jende F, Bartsch A, Meinck HM, Weber MA. Clinical muscle testing compared with whole-body magnetic resonance imaging in facio-scapulo-humeral muscular dystrophy. Clin neuroradiology 2015;26:445–455. [DOI] [PubMed] [Google Scholar]
- 9.Leung DG, Carrino JA, Wagner KR, Jacobs MA. Whole-body magnetic resonance imaging evaluation of facioscapulohumeral muscular dystrophy. Muscle Nerve 2015;52:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsen DB, Gideon P, Jeppesen TD, Vissing J. Leg muscle involvement in facioscapulohumeral muscular dystrophy assessed by MRI. J Neurol 2006;253:1437–1441. [DOI] [PubMed] [Google Scholar]
- 11.Rijken NH, van der Kooi EL, Hendriks JC, et al. Skeletal muscle imaging in facioscapulohumeral muscular dystrophy, pattern and asymmetry of individual muscle involvement. Neuromuscul Disord 2014;24:1087–1096. [DOI] [PubMed] [Google Scholar]
- 12.Andersen G, Dahlqvist JR, Vissing CR, Heje K, Thomsen C, Vissing J. MRI as outcome measure in facioscapulohumeral muscular dystrophy: 1-year follow-up of 45 patients. J Neurol 2017;264:438–447. [DOI] [PubMed] [Google Scholar]
- 13.Dahlqvist JR, Vissing CR, Thomsen C, Vissing J. Severe paraspinal muscle involvement in facioscapulohumeral muscular dystrophy. Neurology 2014;83:1178–1183. [DOI] [PubMed] [Google Scholar]
- 14.Janssen B, Voet N, Geurts A, van Engelen B, Heerschap A. Quantitative MRI reveals decelerated fatty infiltration in muscles of active FSHD patients. Neurology 2016;86:1700–1707. [DOI] [PubMed] [Google Scholar]
- 15.Janssen BH, Voet NB, Nabuurs CI, et al. Distinct disease phases in muscles of facioscapulohumeral dystrophy patients identified by MR detected fat infiltration. PLoS One 2014;9:e85416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lareau-Trudel E, Le Troter A, Ghattas B, et al. Muscle quantitative MR imaging and clustering analysis in patients with facioscapulohumeral muscular dystrophy type 1. PLoS One 2015;10:e0132717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berard C, Payan C, Hodgkinson I, Fermanian J; Group MFMCS. A motor function measure for neuromuscular diseases: construction and validation study. Neuromuscul Disord 2005;15:463–470. [DOI] [PubMed] [Google Scholar]
- 18.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 19.Eichinger K, Heatwole C, Heininger S, et al. Validity of the 6 minute walk test in facioscapulohumeral muscular dystrophy. Muscle Nerve 2017;55:333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricci E, Galluzzi G, Deidda G, et al. Progress in the molecular diagnosis of facioscapulohumeral muscular dystrophy and correlation between the number of KpnI repeats at the 4q35 locus and clinical phenotype. Ann Neurol 1999;45:751–757. [DOI] [PubMed] [Google Scholar]
- 21.Lamperti C, Fabbri G, Vercelli L, et al. A standardized clinical evaluation of patients affected by facioscapulohumeral muscular dystrophy: the FSHD clinical score. Muscle Nerve 2010;42:213–217. [DOI] [PubMed] [Google Scholar]
- 22.Council MR. Aids to the Investigation of Peripheral Nerve Injuries. London: Her Majesty's Stationary Office; 1976. [Google Scholar]
- 23.Rasband WS. ImageJ, US. In: National Institutes of Health. Bethesda: National Institutes of Health; 1997–2016. [Google Scholar]
- 24.Tasca G, Pescatori M, Monforte M, et al. Different molecular signatures in magnetic resonance imaging-staged facioscapulohumeral muscular dystrophy muscles. PLoS One 2012;7:e38779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frisullo G, Frusciante R, Nociti V, et al. CD8(+) T cells in facioscapulohumeral muscular dystrophy patients with inflammatory features at muscle MRI. J Clin Immunol 2011;31:155–166. [DOI] [PubMed] [Google Scholar]
- 26.Stucki G, Daltroy L, Katz JN, Johannesson M, Liang MH. Interpretation of change scores in ordinal clinical scales and health status measures: the whole may not equal the sum of the parts. J Clin Epidemiol 1996;49:711–717. [DOI] [PubMed] [Google Scholar]
- 27.de Lattre C, Rippert P, Hmaroun D, Sacconi S, Poirot I, Vuillerot C. The motor function measure (MFM) in the facioscapulohumeral dystrophy (FSHD) population: description and responsiveness. Ann Phys Rehabil Med 2016;59S:e84–e85. [Google Scholar]